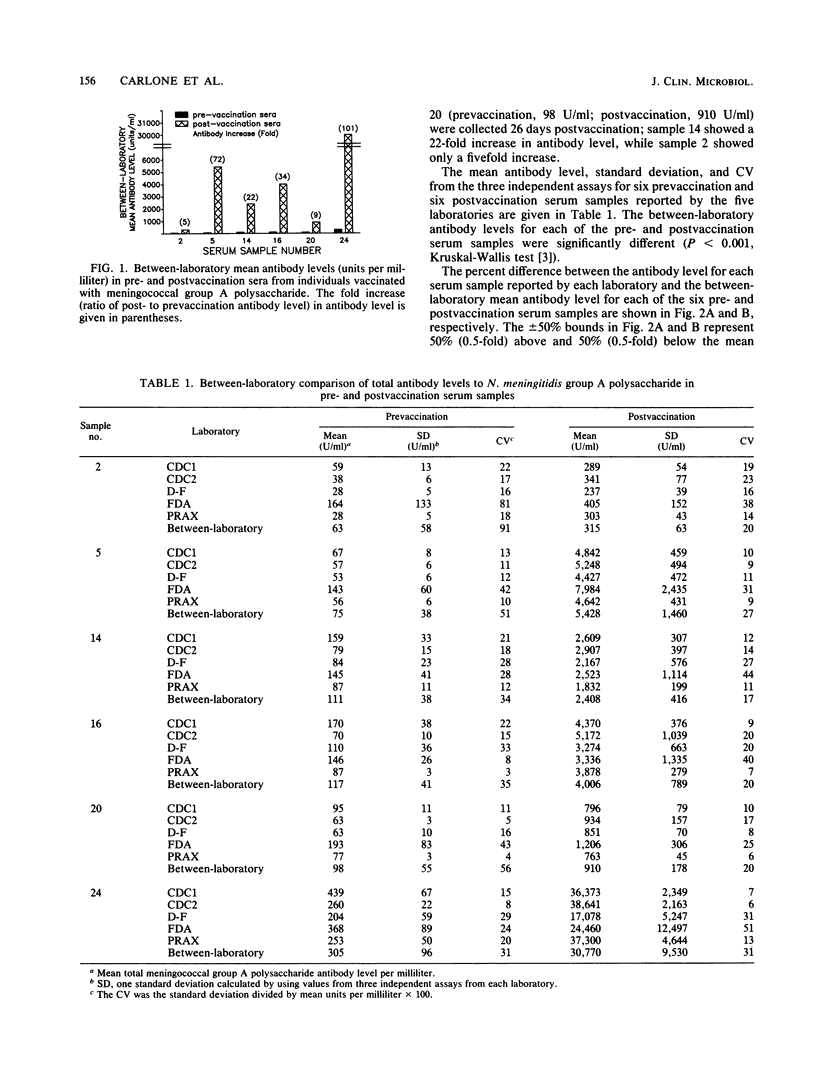
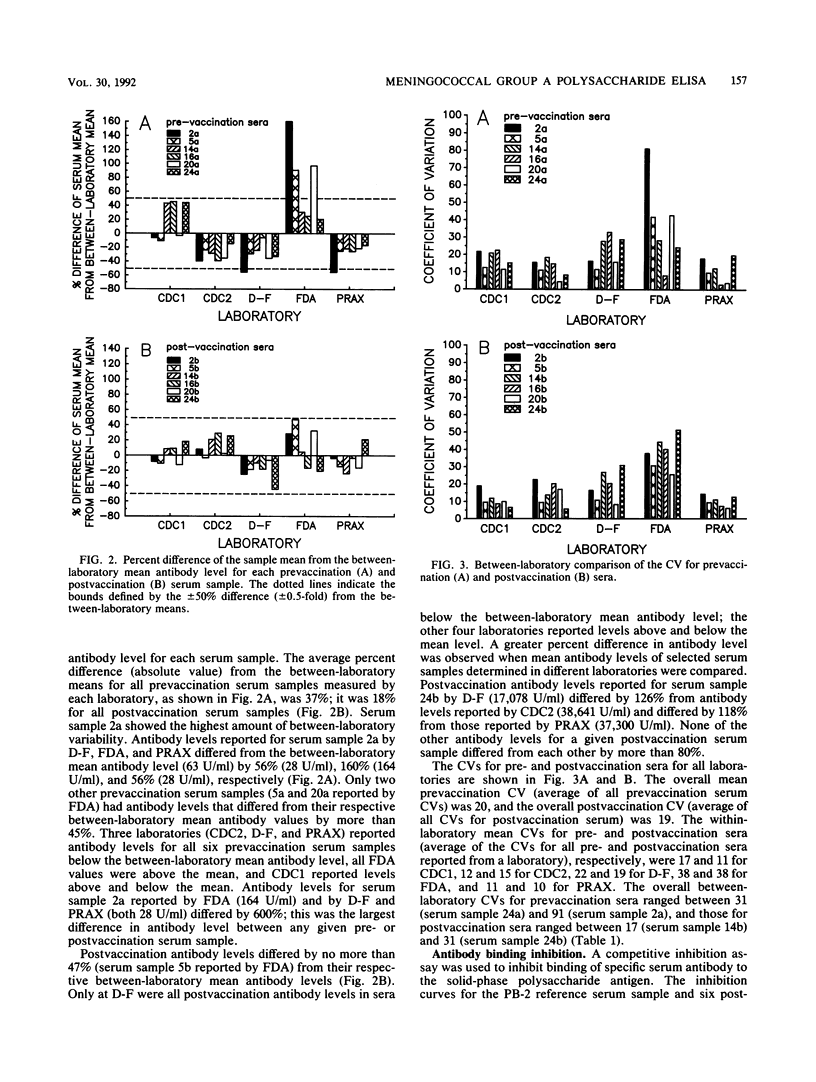
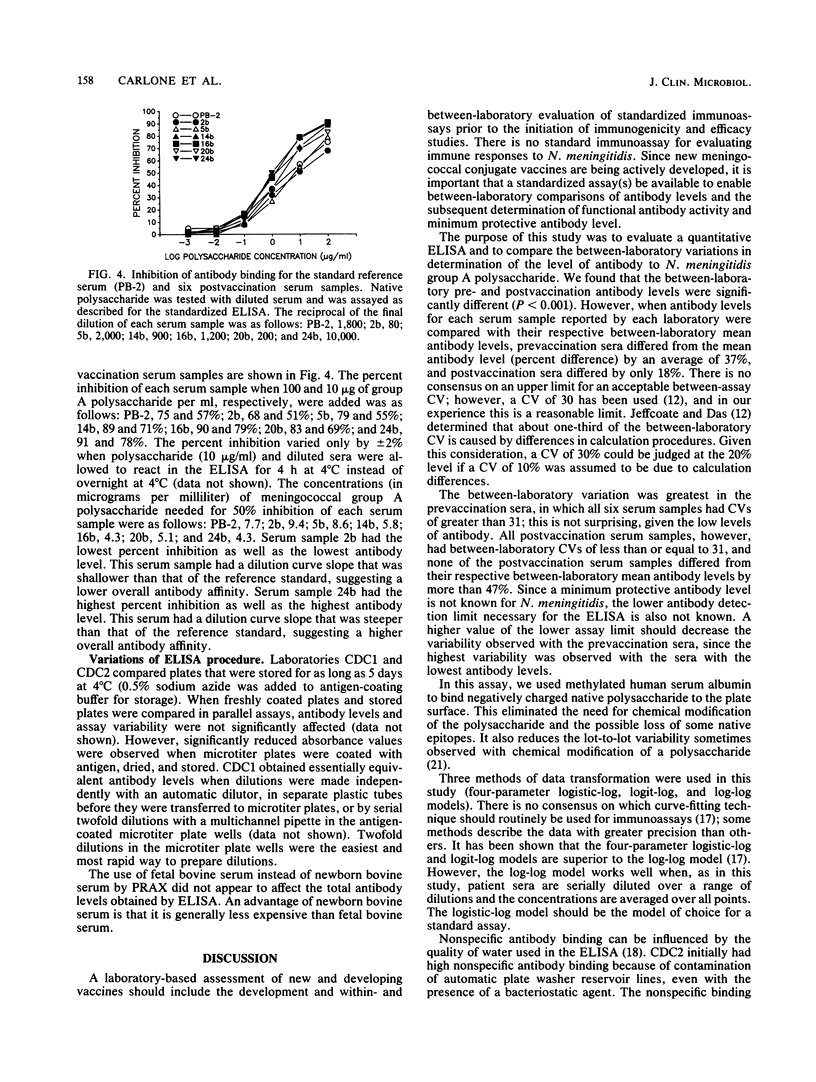
Abstract
There is no standard immunoassay for evaluating immune responses to meningococcal vaccines. We developed an enzyme-linked immunosorbent assay to measure total levels of antibody to Neisseria meningitidis group A capsular polysaccharide. Five laboratories measured the antibody levels in six paired pre- and postvaccination serum samples by using the enzyme-linked immunosorbent assay. Methylated human serum albumin was used to bind native group A polysaccharide to microtiter plate surfaces. The between-laboratory coefficients of variation for pre- and postvaccination sera had ranges of 31 to 91 and 17 to 31, respectively. The mean laboratory coefficients of variation for pre- and postvaccination sera, respectively, were 17 and 11 (Molecular Biology Laboratory, Centers for Disease Control), 12 and 15 (Immunodiagnostic Methods Laboratory, Centers for Disease Control), 22 and 19 (Dana-Farber Cancer Institute), 38 and 38 (Bacterial Polysaccharide Laboratory, U.S. Food and Drug Administration), and 11 and 10 (Praxis Biologics, Inc.). Standardization of this enzyme-linked immunosorbent assay should allow interlaboratory comparison of meningococcal vaccine immunogenicity, thus providing a laboratory-based assessment tool for evaluating meningococcal vaccines.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakere G., Frasch C. E. Specificity of antibodies to O-acetyl-positive and O-acetyl-negative group C meningococcal polysaccharides in sera from vaccinees and carriers. Infect Immun. 1991 Dec;59(12):4349–4356. doi: 10.1128/iai.59.12.4349-4356.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuvery E. C., Kayhty M. H., Leussink A. B., Kanhai V. Comparison of radioimmunoassay and enzyme-linked immunosorbent assay in measurement of antibodies to Neisseria meningitidis group A capsular polysaccharide. J Clin Microbiol. 1984 Oct;20(4):672–676. doi: 10.1128/jcm.20.4.672-676.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuvery E. C., van Delft R. W., Tiesjema R. H., Nagel J. The enzyme-linked immunosorbent assay of meningococcal and some related Escherichia coli polysaccharides. J Biol Stand. 1983 Jul;11(3):195–204. doi: 10.1016/s0092-1157(83)80006-7. [DOI] [PubMed] [Google Scholar]
- Black C. M., Plikaytis B. D., Wells T. W., Ramirez R. M., Carlone G. M., Chilmonczyk B. A., Reimer C. B. Two-site immunoenzymometric assays for serum IgG subclass infant/maternal ratios at full-term. J Immunol Methods. 1988 Jan 21;106(1):71–81. doi: 10.1016/0022-1759(88)90273-6. [DOI] [PubMed] [Google Scholar]
- Brandt B. L., Wyle F. A., Artenstein M. S. A radioactive antigen-binding assay for Neisseria meningitidis polysaccharide antibody. J Immunol. 1972 Apr;108(4):913–920. [PubMed] [Google Scholar]
- Frasch C. E., Zahradnik J. M., Wang L. Y., Mocca L. F., Tsai C. M. Antibody response of adults to an aluminum hydroxide-adsorbed Neisseria meningitidis serotype 2b protein-group B polysaccharide vaccine. J Infect Dis. 1988 Oct;158(4):710–718. doi: 10.1093/infdis/158.4.710. [DOI] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969 Jun 1;129(6):1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Liu T. Y., Artenstein M. S. Human immunity to the meningococcus. 3. Preparation and immunochemical properties of the group A, group B, and group C meningococcal polysaccharides. J Exp Med. 1969 Jun 1;129(6):1349–1365. doi: 10.1084/jem.129.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Rey M., Triau R., Sparks K. J. Quantitative determination of the human immune response to immunization with meningococcal vaccines. J Clin Invest. 1972 Jan;51(1):89–96. doi: 10.1172/JCI106801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffcoate S. L., Das R. E. Interlaboratory comparison of radioimmunoassay results. Variation produced by different methods of calculation. Ann Clin Biochem. 1977 Sep;14(5):258–260. doi: 10.1177/000456327701400170. [DOI] [PubMed] [Google Scholar]
- Käyhty H. Comparison of passive hemagglutination, bactericidal activity, and radioimmunological methods in measuring antibody responses to Neisseria meningitidis group A capsular polysaccharide vaccine. J Clin Microbiol. 1980 Aug;12(2):256–263. doi: 10.1128/jcm.12.2.256-263.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käyhty H., Mäkelä P. H., Ruoslahti E. Radioimmunoassay of capsular polysaccharide antigens of groups A and C meningococci and Haemophilus influenzae type b in cerebrospinal fluid. J Clin Pathol. 1977 Sep;30(9):831–833. doi: 10.1136/jcp.30.9.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinonen M., Frasch C. E. Class-specific antibody response to group B Neisseria meningitidis capsular polysaccharide: use of polylysine precoating in an enzyme-linked immunosorbent assay. Infect Immun. 1982 Dec;38(3):1203–1207. doi: 10.1128/iai.38.3.1203-1207.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- Plikaytis B. D., Turner S. H., Gheesling L. L., Carlone G. M. Comparisons of standard curve-fitting methods to quantitate Neisseria meningitidis group A polysaccharide antibody levels by enzyme-linked immunosorbent assay. J Clin Microbiol. 1991 Jul;29(7):1439–1446. doi: 10.1128/jcm.29.7.1439-1446.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruslin F. H., To S. E., Winston R., Rodman T. C. Caveats and suggestions for the ELISA. J Immunol Methods. 1991 Mar 1;137(1):27–35. doi: 10.1016/0022-1759(91)90390-2. [DOI] [PubMed] [Google Scholar]
- Roberts R. B. The relationship between group A and group C meningococcal polysaccharides and serum opsonins in man. J Exp Med. 1970 Mar 1;131(3):499–513. doi: 10.1084/jem.131.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severin W. P. Latex agglutination in the diagnosis of meningococcal meningitis. J Clin Pathol. 1972 Dec;25(12):1079–1082. doi: 10.1136/jcp.25.12.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siber G. R., Priehs C., Madore D. V. Standardization of antibody assays for measuring the response to pneumococcal infection and immunization. Pediatr Infect Dis J. 1989 Jan;8(1 Suppl):S84–S91. [PubMed] [Google Scholar]
- Sippel J. E., Prato C. M., Girgis N. I., Edwards E. A. Detection of Neisseria meningitidis group A, Haemophilus influenzae type b, and Streptococcus pneumoniae antigens in cerebrospinal fluid specimens by antigen capture enzyme-linked immunosorbent assays. J Clin Microbiol. 1984 Aug;20(2):259–265. doi: 10.1128/jcm.20.2.259-265.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramont E. C., Artenstein M. S. Latex agglutination test for measurement of antibodies to meningococcal polysaccharides. Infect Immun. 1972 Mar;5(3):346–351. doi: 10.1128/iai.5.3.346-351.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. I., Greenberg D. P., Anderson P. W., Burkart K. S., Christenson P. D., Gordon L. K., Kayhty H., Kuo J. S., Vella P. Variable quantitation of Haemophilus influenzae type b anticapsular antibody by radioantigen binding assay. J Clin Microbiol. 1988 Jan;26(1):72–78. doi: 10.1128/jcm.26.1.72-78.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Dalrymple J. M., Artenstein M. S. Analysis of parameters affecting the solid phase radioimmunoassay quantitation of antibody to meningococcal antigens. J Immunol. 1976 Nov;117(5 PT2):1788–1798. [PubMed] [Google Scholar]



