Abstract
Toll-like receptor 4 (TLR4), the signal-transducing molecule of the LPS receptor complex, plays a fundamental role in the sensing of LPS from Gram-negative bacteria. Activation of TLR4 signaling pathways by LPS is a critical upstream event in the pathogenesis of Gram-negative sepsis, making TLR4 an attractive target for novel antisepsis therapy. To validate the concept of TLR4-targeted treatment strategies in Gram-negative sepsis, we first showed that TLR4−/− and myeloid differentiation primary response gene 88 (MyD88)−/− mice were fully resistant to Escherichia coli–induced septic shock, whereas TLR2−/− and wild-type mice rapidly died of fulminant sepsis. Neutralizing anti-TLR4 antibodies were then generated using a soluble chimeric fusion protein composed of the N-terminal domain of mouse TLR4 (amino acids 1–334) and the Fc portion of human IgG1. Anti-TLR4 antibodies inhibited intracellular signaling, markedly reduced cytokine production, and protected mice from lethal endotoxic shock and E. coli sepsis when administered in a prophylactic and therapeutic manner up to 13 h after the onset of bacterial sepsis. These experimental data provide strong support for the concept of TLR4-targeted therapy for Gram-negative sepsis.
Keywords: endotoxic shock, Gram-negative bacteria, lipopolysaccharide, TLR4
The incidence of sepsis is rising, and the mortality remains high, reaching 25%–30% in patients with severe sepsis and 50%–60% in those who develop septic shock (1). Despite initial encouraging results, the benefits of most new antisepsis therapies (e.g., drotrecogin-alpha activated, corticosteroids, intensive insulin therapy, and vasopressin) remain uncertain (2). Thus, identification of new treatment options for septic patients remains imperative.
Endotoxin (LPS) is a major component of the outer membrane of Gram-negative bacteria and a critical actor in the pathogenesis of Gram-negative sepsis (3). Sensing of LPS by innate immune cells is vital for host defenses against Gram-negative bacteria. This multistep recognition process is initiated by the binding of LPS to the LPS-binding protein (LBP) that conveys LPS to a cell surface receptor complex composed of CD14, MD-2, and Toll-like receptor (TLR4) (4–10). LPS binds to CD14 and is then delivered to the MD-2–TLR4 complex (11). Structural studies of the interactions among the LPS antagonists lipid IVa, eritoran (E5564), MD-2, and TLR4 have revealed that LPS binds to an hydrophobic internal pocket of MD-2 that itself is bound to the concave surface of the N-terminal and central domains of TLR4 (12, 13). Binding of LPS to the MD-2–TLR4 complex causes TLR4 dimerization and sets off intracellular signaling initiated by the Toll/IL-1 receptor (TIR) domain–containing adaptor molecules MyD88, TIR domain–containing adaptor–inducing IFN-β (TRIF), TIR domain–containing adaptor protein (TIRAP), and TRIF-related adapter molecule (TRAM) (14). The TIRAP-MyD88–dependent signaling pathway activates NF-κB and the MAPKs (ERK-1/2, JNK, and p38), resulting in the expression of numerous genes encoding cytokines and other inflammatory molecules. The TRAM-TRIF–dependent signaling pathway activates IFN response factor 3, inducing the production of type I IFN. Cytokines, chemokines, and type I IFN are critical to the host antimicrobial defense response.
Regulation of innate immune responses is a delicate balancing act, and dysregulated innate immune reactions, by either default or excess, have dramatic consequences for the infected host, as seen in severe sepsis. Given its central role in the pathogenesis of Gram-negative sepsis, TLR4 is a target of choice for the development of novel antisepsis therapies. Here we report that anti-TLR4 antibodies raised against the ectodomain of TLR4 improved survival in experimental models of Gram-negative bacterial sepsis when administered both prophylactically and therapeutically.
Results
TLR4 and MyD88 Are Critical Effector Molecules in Escherichia coli Sepsis.
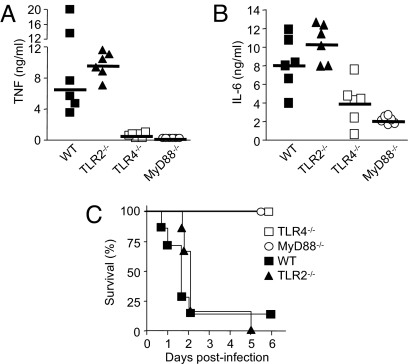
To validate the concept of immunomodulation of the TLR4 activation pathway as a treatment strategy for Gram-negative sepsis, we studied cytokine production profiles and survivals of wild-type (WT), TLR4−/−, TLR2−/−, and MyD88−/− mice in a model of lethal peritonitis induced by E. coli, the most common cause of Gram-negative sepsis (15). Given the critical role played by TLR2 in the sensing of Gram-positive bacteria and some Gram-negative bacteria (16, 17), we used TLR2−/− mice as controls. At 4 h after bacterial challenge, very high concentrations of bioactive TNF were detected in the circulation of the WT and TLR2−/− mice (median, 6.5 ng/mL vs. 9.7 ng/mL; P = .5) (Fig. 1A). In contrast, TNF was either strikingly reduced or undetectable in the TLR4−/− and MyD88−/− mice (0.5 and 0 ng/mL, respectively; P = .002). Likewise, circulating levels of bioactive IL-6 were much higher in the WT and TLR2−/− mice (8.0 and 10.6 ng/mL; P = .31) than in the TLR4−/− and MyD88−/− mice (4.2 and 2.0 ng/mL; P = .04 and .002) (Fig. 1B). Blunted proinflammatory responses were associated with full survival of the TLR4−/− and MyD88−/− mice, whereas all but 1 of the WT and TLR2−/− mice died (P < .001) (Fig. 1C). This indicates that the activation of TLR4, but not of TLR2, is critical to the host response to E. coli sepsis.
Fig. 1.
TLR4-deficient and MyD88-deficient mice are protected from lethal Gram-negative bacterial sepsis. (A–C) WT, TLR2−/−, TLR4−/−, and MyD88−/− C57BL/6 mice were injected i.p. with 2 × 109 cfu of E. coli O18 and treated with antibiotics as described in Materials and Methods. Plasma concentrations of TNF (A) and IL-6 (B) were measured 4 h after bacterial challenge. The horizontal line represents the median cytokine concentration (TNF: P < .005 for TLR4−/− or MyD88−/− vs. WT or TLR2−/−, P = .15 for TLR4−/− vs. MyD88−/−, and P = .48 for WT vs. TLR2−/−; IL-6: P < .05 and < .005 for TLR4−/− and MyD88−/− vs. WT or TLR2−/−, P = .13 for TLR4−/− vs. MyD88−/−, and P = .31 for WT vs. TLR2−/−). (C) Survival of TLR4−/−, MyD88−/−, TLR2−/−, and WT mice (P < .001). Data points are from 1 experiment (n = 6 to 7 mice per treatment groups).
Chimeric Mouse TLR4–Human Fc Fusion Protein.
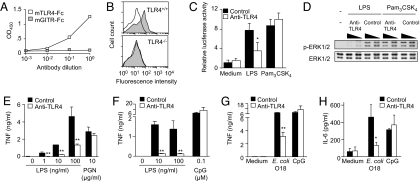
TLR4 is composed of N-terminal, central, and C-terminal domains. MD-2 binds to the concave surface of the N-terminal and central TLR4 domains (12, 18). To obtain anti-TLR4 antibodies, we first generated a soluble recombinant chimeric protein composed of the N-terminal half of the mouse TLR4 ectodomain (amino acids 1–334) fused to the Fc domain of human IgG1 (mTLR4-Fc). The recombinant mTLR4-Fc protein was produced in HEK 293T cells to ensure posttranscriptional modifications. In the presence of serum as a source of soluble MD-2, LPS was shown to bind to mTLR4-Fc (Fig. S1A). mTLR4-Fc also was shown to inhibit LPS-induced TNF release in a whole-blood assay (Fig. S1B). Together, these data indicate that the recombinant mTLR4-Fc protein expresses TLR4 domains critical for the binding of MD-2–LPS complexes, and that mTLR4-Fc acts as a decoy soluble receptor capable of inhibiting the activation of membrane-bound TLR4–MD2 receptor complex by LPS.
Anti-TLR4 Antibodies Inhibit Innate Immune Responses Induced by LPS and Gram-Negative Bacteria.
mTLR4-Fc was used to generate high titers of rabbit anti-mouse TLR4 antibodies, which were purified through a 3-step procedure as described in Materials and Methods. Specificity was confirmed by demonstrating that anti-TLR4 antibodies recognized mTLR4-Fc but not an irrelevant chimeric fusion protein (mGITR-Fc) by ELISA (Fig. 2A), and also by the staining of WT but not TLR4−/− mouse peritoneal macrophages by flow cytometry (Fig. 2B). We then studied the capacity of anti-TLR4 antibodies to inhibit responses of innate immune cells stimulated with LPS in vitro. Compared with control antibodies, anti-TLR4 antibodies strongly inhibited LPS-induced intracellular signal transduction, as demonstrated by the luciferase reporter activity driven by NF-κB in RAW 264.7 macrophages (Fig. 2C) and by phosphorylation of ERK-1/2 in bone marrow–derived macrophages (Fig. 2D). Anti-TLR4 antibodies also markedly inhibited LPS- and E. coli–induced TNF and IL-6 production by RAW 264.7 macrophages and by mouse whole blood (Fig. 2E–H and data not shown). In contrast, anti-TLR4 antibodies did not affect signal transduction or cytokine production by macrophages or by whole blood stimulated with other TLR ligands, such as Pam3CSK4 (Fig. 2C and D), peptidoglycan (Fig. 2E), and cytosine guanine dinucleotide (CpG) oligonucleotides (ODNs) (Fig. 2F–H). The biological activity of anti-TLR4 antibodies also was demonstrated through a proof-of-principle type experiment demonstrating that immunoneutralization of TLR4 activity, like TLR4 deficiency, increased circulating bacterial counts and mortality in nonsevere E. coli peritonitis and Klebsiella pneumoniae pneumonia models (Fig. S2). Together, these results provide compelling evidence that anti-TLR4 antibodies recognize membrane-bound TLR4 and inhibit innate immune responses of cells stimulated with LPS or Gram-negative bacteria in vitro and in vivo.
Fig. 2.
Anti-TLR4 antibodies bind to TLR4 and inhibit the activation of macrophages induced by LPS. (A) Anti-TLR4 antibodies binding to immobilized mTLR4-Fc but not to mGITR-Fc by ELISA. (B) Flow cytometry analysis of anti-TLR4 antibodies (gray area) binding to WT (i.e., TLR4+/+) (Upper) but not to TLR4−/− (Lower) thioglycollate-elicited mouse peritoneal macrophages. Background staining using control antibodies is shown in white. (C) NF-κB activity in RAW 264.7 macrophages transiently transfected with a trimeric κB site luciferase reporter vector and preincubated for 30 min with anti-TLR4 and control antibodies (100 μg/mL) before stimulation with LPS (10 ng/mL) or Pam3CSK4 (2 μg/mL) for 18 h. Data on relative luciferase activity are expressed as mean ± SD of 4 replicates from 1 representative experiment. *P = .001 for anti-TLR4 versus control antibodies. (D) Western blot analyses of phosphorylated-ERK1/2 (p-ERK1/2) and total ERK1/2 expression in bone marrow–derived macrophages preincubated for 20 min with 10 or 100 μg/mL of anti-TLR4 or control antibodies before stimulation with LPS (1 ng/mL) and Pam3CSK4 (1 μg/mL) for 20 min. (E–H) TNF and IL-6 production by RAW 264.7 macrophages (E) or mouse whole blood (F–H) preincubated for 30 min with anti-TLR4 or control antibodies (100 μg/mL) before stimulation with 100 ng/mL of LPS, 10 μg/mL of PGN, 0.1 μM CpG ODN (CpG), or 106 cfu/mL of heat-killed E. coli O18 for 4 h. Data are expressed as mean ± SD of triplicates from 1 representative experiment. *.005 < P < .05 and **P < .005for anti-TLR4 versus control antibodies.
Anti-TLR4 Antibodies Protect Against Lethal Endotoxemia.
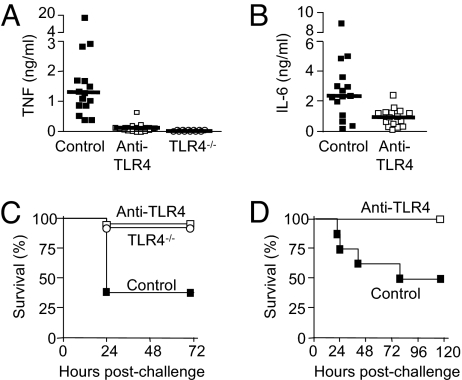
Affording protection against lethal endotoxemia is important in patients with fulminant meningococcemia associated with high levels of circulating endotoxin (19). We explored the protective capacity of the anti-TLR4 antibodies in a model of endotoxemia in D-galactosamine–sensitized mice. Consistent with the results observed in vitro, anti-TLR4 antibodies given i.p. 15 min before an LPS challenge almost completely eliminated TNF production (P < .0001) (Fig. 3A) and strongly reduced IL-6 production (P = .005) (Fig. 3B). Of note, the amount of TNF produced by mice treated with anti-TLR4 antibodies was comparable to that produced by TLR4−/− mice. Prevention of cytokine release by anti-TLR4 was associated with improved survival (controls, 94% in anti-TLR4 and 92% in TLR4−/−, compared with 37% in controls; P = .0001) (Fig. 3C). Time-course analyses of the magnitude and duration (up to 60 h) of the inhibition of cytokine production and protection afforded by a single dose of anti-TLR4 antibodies against lethal endotoxemia (Table S1) suggests the possibility that anti-TLR4 treatment also could work when given after the LPS challenge. Indeed, as shown in Fig. 3D, anti-TLR4 treatment remained fully protective when given up to 4 h after LPS exposure (P = .025). Anti-TLR4 antibodies did not protect mice from toxic shock induced by Pam3CSK4, a Gram-positive lipopeptide and activator of TLR1-TLR2 heterodimers (Fig. S3), providing evidence of TLR4 specificity.
Fig. 3.
Anti-TLR4 antibodies inhibit cytokine production and protect mice from lethal endotoxemia when administered prophylactically and therapeutically. (A–C) Mice injected i.v. with 40 mg/kg of anti-TLR4 or control antibodies and TLR4−/− mice were sensitized with D-galactosamine 15 min before an i.v. injection of 50 ng of E. coli O111:B4 LPS. Plasma concentrations of TNF (A) and of IL-6 (B) were measured 1 h after LPS injection. The horizontal line represents the median cytokine concentration. Control versus anti-TLR4 antibodies, P < .0001 for TNF and P = .005 for IL-6. (C) Prophylaxis. Survival of mice treated with anti-TLR4 versus control antibodies (n = 19 and 21 mice per treatment group; P = .0001) and TLR4−/− mice (n = 8). Data points are from 4 independent experiments for antibody evaluation. (D) Therapy. Survival of BALB/c mice treated with anti-TLR4 or control antibodies (40 mg/kg i.p.) 4 h after i.p. injection of 1 mg of E. coli O111:B4 LPS (P = .025). Data points are from 1 experiment (n = 8 mice per treatment group).
Anti-TLR4 Antibodies Protect Against Lethal Live E. coli Sepsis.
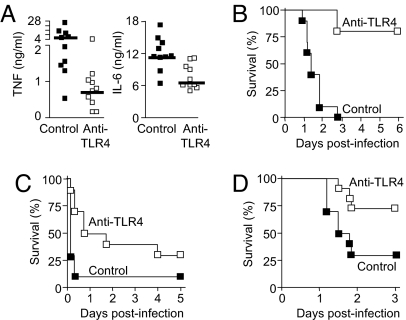
We studied the impact of anti-TLR4 antibodies in a classical Gram-negative bacterial sepsis model induced by an i.p. injection of live E. coli, the most frequent cause of bacterial sepsis in humans (15). Prophylactic administration of anti-TLR4 antibodies led to a 5-fold reduction in the median circulating TNF level (4.2 ng/mL in controls vs. 0.8 ng/mL in anti-TLR4; P < .005), a 2-fold reduction of IL-6 (11.1 vs. 6.3 ng/mL; P < .005) (Fig. 4A), and a striking increase in survival (0 vs. 80%; P < .0001) (Fig. 4B).
Fig. 4.
Prophylactic and therapeutic administration of anti-TLR4 antibodies protect mice from lethal Gram-negative bacterial sepsis. (A–D) BALB/c mice were injected i.p. with anti-TLR4 or control antibodies (160 mg/kg for A–C and 200 mg/kg for D) given before (prophylactically; A and B) or after (therapeutically; C and D) an i.p. injection of a high (2 × 109 cfu) inoculum (A–C) or low (2 × 105 cfu) inoculum (D) of E. coli O18. (A) Plasma concentrations of TNF and IL-6 were measured 4 h after the bacterial challenge. The horizontal line represents the median cytokine concentration. P < .005 for TNF and IL-6. (B–D) Survival of mice treated prophylactically (B) (at −4, −0.5, and + 4 h) or therapeutically either early (+1 and + 4 h) (C) or late (+13 h) (D). P < .0001, .02, and .03, respectively. Data points are from 1 experiment (n = 10–12 mice per treatment group).
To test anti-TLR4 antibodies in a condition mimicking their clinical use in patients with sepsis, we administered therapy after the onset of infection in 2 different severity models. In the first model, mice were challenged with a high E. coli inoculum (2 × 109 cfu), which caused a fulminant, rapidly lethal sepsis. Delayed (+1 h) administration of anti-TLR4 was associated with increased survival rate (30% vs. 10%; P = .02) and prolonged survival time (median time to death, 30 h in anti-TLR4 mice vs. 4 h in control mice; P = .008) (Fig. 4C). In the second model, mice were challenged with a lower E. coli inoculum (2 × 105 cfu), which caused an acute but less fulminant course of sepsis. Initiation of anti-TLR4 therapy as much as 13 h after the onset of infection, at which point clinical signs of sepsis were established and circulating levels of endotoxin were elevated (mean ± SD, 13.1 ± 15.2 ng/mL; range, 2.91–45.7 ng/mL; n = 7), remained associated with improved survival (75% vs. 30%; P = .03) (Fig. 4D). Together, these results demonstrate that anti-TLR4 antibodies are highly efficacious as adjunctive therapy for E. coli sepsis, with a window of clinical application including both prophylactic and therapeutic intervention modalities.
Discussion
Major breakthroughs in our understanding of the pathogenesis of Gram-negative sepsis are providing new treatment opportunities for severe sepsis and septic shock. For example, TLR4 and MD-2 have recently emerged as critical sensors of LPS (4–6, 20). As the signal-transducing component of the LPS receptor complex, TLR4 is a very attractive target for new antisepsis therapy. Here we provide compelling experimental evidence supporting the efficacy of anti-TLR4 adjunctive therapy for Gram-negative sepsis. Using a recombinant chimeric fusion protein composed of the N-terminal and central domains (amino acids 1–334) of the extracellular part of TLR4 and the Fc portion of human IgG1, we produced anti-TLR4 antibodies that inhibited LPS-induced intracellular signaling and cytokine production and protected mice from lethal endotoxic shock and E. coli bacterial sepsis, even when treatment was delayed for several hours after endotoxemia or the onset of sepsis. Resolution of the crystal structures of the human and mouse TLR4—MD-2 complexes has provided an explanation for the mode of action of these anti-TLR4 antibodies (12). Based on the identification of the residues implicated in the contact between TLR4 and MD-2 and present in the chimeric mTLR4-Fc immunogen, anti-TLR4 antibodies likely impede the binding of the MD-2–LPS complex to TLR4.
The protective effects of the anti-TLR4 therapy were impressive and in some respects unique. Previous studies conducted with anti-LBP or anti-CD14 antibodies in experimental models of endotoxic shock and Gram-negative bacterial sepsis uniformly failed to show protection when treatment was administered after LPS (anti-LBP) or simultaneously with or shortly after bacterial challenge (anti-LBP and anti-CD14) (21–23). In contrast, anti-TLR4 antibodies were found to prevent death from endotoxic shock even when treatment was delayed for as much as 4 h after the LPS challenge (Fig. 3D). These findings provide strong support for an anti-TLR4 treatment strategy in patients with fulminant meningococcemia associated with high levels of circulating endotoxin in whom anti-LPS (i.e., recombinant bactericidal/permeability-increasing protein) and anti-sepsis (i.e., activated protein C) therapies have failed (24, 25). Unlike monoclonal antibodies raised against TLR4–MD-2, which work only when administered prophylactically in bacterial sepsis (26, 27), anti-TLR4 antibodies afforded remarkable protection against lethal E. coli sepsis when treatment was delayed for as much as 13 h after the onset of infection (Fig. 4D), offering a much broader window of therapeutic intervention.
Some Gram-negative endotoxin species also are sensed by TLR2 (28–30), and several bacterial components (i.e., peptidoglycan, lipopeptides, flagellin, CpG DNA motifs) are recognized by other members of the TLR family besides TLR4, including TLR1, TLR2, TLR5, TLR6, and TLR9. These properties support the potential need for combined anti-TLR therapies. Along these lines, Spiller et al. (27) recently proposed the need for dual blockade of TLR2 and TLR4–MD-2 to protect against Gram-negative sepsis when therapy is initiated after the onset of infection. Challenging the concept of a need for dual TLR2 and TLR4–MD-2 targeted therapy (27), our findings demonstrate that TLR2 clearly was not a key player in the pathogenesis of Gram-negative sepsis. Indeed, unlike the TLR4−/− mice, the TLR2−/− mice produced an abundant amount of cytokines during E. coli sepsis and had a rapidly fatal clinical course identical to that of WT mice (Fig. 1), an observation consistent with recent in vitro data indicating that TLR4–MD-2 is the main recognition system for enterobacteria like E. coli and K. pneumoniae (29). Furthermore, the sole blockade of TLR4 was sufficient to protect against Gram-negative sepsis caused by E. coli, even when therapy was administered long after the start of sepsis. Although somewhat overlooked, prophylactic anti-TLR4 monotherapy also has been shown to be protective against lethal E. coli infection (27), suggesting that administration of repeated doses of anti-TLR4 antibody might increase survival when given therapeutically, as shown in the present study. Other plausible reasons for the divergent results between our study and the study of Spiller et al. (27) could include the much broader antibody repertoire of polyclonal antibodies; the use of different E. coli and mouse strains, bacterial inocula, and antibiotic classes; and differences in the timing of antibiotic administration.
An anti-TLR4 treatment strategy also is supported by recent data obtained with eritoran (E5564), a synthetic LPS antagonist that binds to MD-2 (12, 31), and TAK-242, a cyclohexene derivative that inhibits TLR4-mediated signal transduction, which prevented lethality in experimental models of LPS shock or bacterial sepsis in rodents (32, 33). At a time when most antisepsis clinical trials have yielded frustratingly negative results (2, 34), our experimental data lend strong support to TLR4-targeted therapy (i.e., eritoran and TAK-242) currently under development in patients with Gram-negative sepsis.
Materials and Methods
Mice.
Eight- to 10-week-old female OF1, BALB/c, and C57BL/6 mice were purchased from Charles River Laboratories. MyD88−/−, TLR2−/−, and TLR4−/− C57BL/6 mice have been described previously (4, 17, 35). Mice were bred and housed in specific pathogen-free conditions in groups of 5–10 mice per cage with free access to food and water. All animal procedures were approved by the Office Vétérinaire du Canton de Vaud (authorization numbers 876.5, 877.5, and 1009.4) and performed in accordance with the institutional guidelines for animal experiments.
Cells and Reagents.
HEK 293T cells were cultured in OptiMEM medium. RAW 264.7 murine macrophages were grown in RPMI medium 1640 containing 2 mM glutamine. Mouse bone marrow–derived macrophages (BMDMs) were obtained as described previously (36) and cultured in Iscove's modified Dulbecco's medium containing 2-mercaptoethanol. All media were supplemented with 10% heat-inactivated FCS (Seromed) and antibiotics. Thioglycollate-elicited peritoneal macrophages were harvested from mice 3 days after i.p. injection of 2 mL of 3% thioglycollate solution (BD Biosciences). Heparinized blood was collected from OF1 mice. Where indicated, cells, or blood were incubated with 1–100 ng/mL of Salmonella minnesota Ultra Pure LPS (List Biologicals Laboratories), 10 μg/mL of Staphylococcus aureus peptidoglycan (PGN; Sigma), 1 μg/mL of Pam3CSK4 (EMC microcollections), or 0.1 μM CpG ODN (Coley Pharmaceutical Group).
Soluble Chimeric mTLR4-Fc.
A DNA fragment encoding for amino acids 1–334 of mouse TLR4 (mTLR4) was amplified by PCR using the Expand High-Fidelity PCR system (Roche Applied Science) and mT4Fc sense (TCCGTCGACGCCACCATGATGCCTCCCTGGCTC) and mT4Fc antisense (GGGTCGACTGATAAGGATTGCCATTTGAA) oligonucleotides containing a SalI site (indicated in bold). The amplicon was cloned into the pGEM-T Easy vector (Promega), sequenced, excised with SalI, and subcloned upstream of the sequence encoding for the human IgG1 Fc segment into the pFc plasmid (Apotech). Recombinant mTLR4pFc-expressing vector was transfected into HEK 293T cells using the calcium-precipitation method. The transfected HEK 293T cells were incubated for 3 days in OptiMEM medium (Invitrogen). Supernatant was collected and centrifuged, and soluble recombinant mTLR4-Fc fusion protein was purified by protein A (APBiotech) immunoaffinity chromatography. The molecular weight of the recombinant protein was verified by SDS/PAGE analysis, and the presence of the Fc fragment of human IgG was confirmed by Western blot analysis using the mouse GG-7 Fc-specific anti-human IgG antibody (Sigma).
Anti-TLR4 Antibodies.
Anti-TLR4 antibodies were produced in New Zealand White rabbits by repeated immunization with 100 μg of purified mTLR4-Fc fusion protein in Specol. Anti-TLR4 antibody titers were measured by ELISA as described below. Rabbits were bled when anti-TLR4 antibody titers reached a plateau. Nonimmune and anti–mTLR4-Fc antibodies were isolated from rabbit serum by protein A affinity chromatography following the manufacturer's recommendations (GE Healthcare). Affinity-purified anti–mTLR4 antibodies used in some experiments were isolated from anti–mTLR4-Fc sera using a 3-step procedure that included IgG purification using protein A chromatography, followed by anti-Fc antibody depletion using an mGITR-Fc–coupled affinity column and a final step of mTLR4-specific antibody purification using a mTLR4-Fc–coupled affinity Hi-trap NHS-activated column (APBiotech). The endotoxin content of the purified antibodies was 100 pg per mg of antibodies as measured by the limulus amebocyte lysate assay (Charles River Laboratories).
ELISA for Measurement of Anti-TLR4 Antibodies.
First, 96-well plates were coated overnight at 4 °C with 1 μg/mL of mTLR4-Fc or mGITR-Fc fusion protein as a negative control. After washing, the plates were incubated for 1 h at 37 °C with PBS containing 5% FCS and then with serial dilutions of preimmune or immune rabbit serum, before a final incubation step with HRP-conjugated goat anti-rabbit IgG (Pierce). Peroxidase activity was assessed with the TMB (3,3′, 5,5′-tetramethylbenzidine) substrate kit (Pierce), with optical density measured at 450 nm.
Flow Cytometric Analysis.
After Fc receptors were blocked with 2.4G2 hybridoma supernatant, expression of TLR4 was evaluated by first incubating thioglycollate-elicited peritoneal macrophages with affinity-purified anti-TLR4 or control antibodies and then with phycoerythrin-conjugated sheep anti-rabbit IgG (Serotec). Acquisition and analysis were performed with a FACSCalibur flow cytometer (BD Biosciences) and FlowJo 8.5.3 software (FlowJow).
Cytokine Measurements.
RAW 264.7 murine macrophages were plated at a density of 2 × 104 cells per well in 96-well culture plates (Costar). A whole-blood stimulation assay was performed in the 96-well culture plates in a total volume of 200 μL (90 μL of blood and 110 μL of RPMI medium 1640). Cells and whole blood were stimulated for 4 h with LPS, PGN, Pam3CSK4, or CpG ODN with or without a 30-min preincubation with anti-TLR4 or control antibodies. The concentrations of TNF and IL-6 in cell culture supernatants were measured as described previously (37).
Transient Transfection.
RAW 264.7 macrophages grown at 60% confluency in 24-well plates (Costar) were transiently transfected with 500 ng of a trimeric κB site–pGL2 luciferase vector and 100 ng of the Renilla pRL-TK vector (Promega) as described previously (38). At 8 h after transfection, cells were preincubated for 30 min with 100 μg/mL of either anti-TLR4 or control antibodies and then stimulated for 18 h with 10 ng/mL of LPS or 2 μg/mL of Pam3CSK4. Luciferase and Renilla luciferase activities were measured using the Dual-Luciferase Reporter Assay System (Promega). Results are expressed as relative luciferase activity (ratio of luciferase activity to Renilla luciferase activity).
Western Blot Analyses.
BMDMs were plated at a density of 2 × 106 cells per well in 6-well culture plates and incubated as described in Fig. 2D. Cell lysates were fractioned through 12% SDS/PAGE gels and then transferred onto nitrocellulose membranes. Membranes were incubated overnight at 4 °C with antibodies specific for ERK1/2 (Cell Signaling Technology). After washing, membranes were incubated for 1 h with secondary HRP-conjugated goat anti-rabbit IgG. Signals were measured using the ECL Western blot analysis system (GE Healthcare). Membranes were then stripped and reprobed with anti-ERK1/2 antibodies (Cell Signaling Technology).
Endotoxic Shock and Bacterial Sepsis Models.
Two models of endotoxic shock were used. In the low-dose LPS model, mice were sensitized with an i.p. injection of 40 mg of D-galactosamine (Sigma) administered 15 min before an i.v. injection of 50 ng of E. coli ultra Pure O111:B4 LPS (List Biological Laboratories). In the high-dose model, mice were injected i.p. with 1 mg of E. coli O111:B4 LPS.
In the bacterial sepsis models, bacterial peritonitis was induced by an i.p. injection of either 2 × 105 or 2 × 109 cfu of E. coli O18. Mice were treated with ceftriaxone (100 mg/kg i.p.) plus gentamicin (20 mg/kg i.p.) given at + 15 min (ceftriaxone), +4 h (ceftriaxone and gentamicin) and then every 12 h in mice inoculated with 2 × 109 cfu of E. coli O18 and at + 12 h (ceftriaxone) and + 24 h (ceftriaxone and gentamicin) and then every 12 h in mice inoculated with 2 × 105 cfu of E. coli O18. Anti-TLR4 and control antibodies were administered either prophylactically or therapeutically, as described in Fig. 4. Mice were monitored at least twice daily until death or complete recovery occurred. Blood samples were harvested from the tail vein for quantification of circulating bacteria and measurements of serum TNF and IL-6 concentrations.
Endotoxin Measurements.
Endotoxin was measured in heparinized mouse plasma using Limulus Amebocyte Lysate Test Cartridges and the Endosafe PTS Portable Test System (Charles River Laboratories). The detection limit of the assay was 5 pg/mL.
Statistical Analyses.
Comparisons among treatment groups were performed using Fisher's exact test for categorical data and the Mann-Whitney test for continuous variables. The Kaplan-Meier method was used for survival, and differences were analyzed by the log-rank sum test. All analyses were performed using GraphPad PRISM. All reported P values are 2-sided, and values .05 are considered to indicate statistical significance.
Supplementary Material
Acknowledgments.
This work was supported by grants from the Swiss National Science Foundation (3100–066972.01 and 3100–118266.07), the Bristol-Myers Squibb Foundation, the Leenaards Foundation, and the Santos-Suarez Foundation for Medical Research (to T.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808146106/DCSupplemental.
References
- 1.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 2.Russell JA. Management of sepsis. N Engl J Med. 2006;355:1699–1713. doi: 10.1056/NEJMra043632. [DOI] [PubMed] [Google Scholar]
- 3.Beutler B, Rietschel ET. Innate immune sensing and its roots: The story of endotoxin. Nat Rev Immunol. 2003;3:169–176. doi: 10.1038/nri1004. [DOI] [PubMed] [Google Scholar]
- 4.Hoshino KO, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 5.Poltorak A, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 6.Qureshi ST, et al. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J Exp Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schumann RR, et al. Structure and function of lipopolysaccharide binding protein. Science. 1990;249:1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- 8.Shimazu R, et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tobias PS, Soldau K, Ulevitch RJ. Isolation of a lipopolysaccharide-binding acute-phase reactant from rabbit serum. J Exp Med. 1986;164:777–793. doi: 10.1084/jem.164.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS-binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 11.Kim JI, et al. Crystal structure of CD14 and its implications for lipopolysaccharide signaling. J Biol Chem. 2005;280:11347–11351. doi: 10.1074/jbc.M414607200. [DOI] [PubMed] [Google Scholar]
- 12.Kim HM, et al. Crystal structure of the TLR4–MD-2 complex with bound endotoxin antagonist Eritoran. Cell. 2007;130:906–917. doi: 10.1016/j.cell.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Ohto U, Fukase K, Miyake K, Satow Y. Crystal structures of human MD-2 and its complex with antiendotoxic lipid IVa. Science. 2007;316:1632–1634. doi: 10.1126/science.1139111. [DOI] [PubMed] [Google Scholar]
- 14.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;365:63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- 16.Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol. 2000;165:5392–5396. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- 17.Takeuchi O, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 18.Brodsky I, Medzhitov R. Two modes of ligand recognition by TLRs. Cell. 2007;130:979–981. doi: 10.1016/j.cell.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Brandtzaeg P, Ovsteboo R, Kierulf P. Compartmentalization of lipopolysaccharide production correlates with clinical presentation in meningococcal disease. J Infect Dis. 1992;166:650–652. doi: 10.1093/infdis/166.3.650. [DOI] [PubMed] [Google Scholar]
- 20.Nagai Y, et al. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3:667–672. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- 21.Frevert CW, et al. Effect of CD14 blockade in rabbits with Escherichia coli pneumonia and sepsis. J Immunol. 2000;164:5439–5445. doi: 10.4049/jimmunol.164.10.5439. [DOI] [PubMed] [Google Scholar]
- 22.Gallay P, Heumann D, Le Roy D, Barras C, Glauser MP. Lipopolysaccharide-binding protein as a major plasma protein responsible for endotoxemic shock. Proc Natl Acad Sci U S A. 1993;90:9935–9938. doi: 10.1073/pnas.90.21.9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Roy D, et al. Critical role of lipopolysaccharide-binding protein and CD14 in immune responses against gram-negative bacteria. J Immunol. 2001;167:2759–2765. doi: 10.4049/jimmunol.167.5.2759. [DOI] [PubMed] [Google Scholar]
- 24.Levin M, et al. Recombinant bactericidal/permeability-increasing protein (rBPI21) as adjunctive treatment for children with severe meningococcal sepsis: A randomised trial. rBPI21 Meningococcal Sepsis Study Group. Lancet. 2000;356:961–967. doi: 10.1016/s0140-6736(00)02712-4. [DOI] [PubMed] [Google Scholar]
- 25.Nadel S, et al. Drotrecogin alfa (activated) in children with severe sepsis: A multicentre phase III randomised controlled trial. Lancet. 2007;369:836–843. doi: 10.1016/S0140-6736(07)60411-5. [DOI] [PubMed] [Google Scholar]
- 26.Daubeuf B, et al. TLR4/MD-2 monoclonal antibody therapy affords protection in experimental models of septic shock. J Immunol. 2007;179:6107–6114. doi: 10.4049/jimmunol.179.9.6107. [DOI] [PubMed] [Google Scholar]
- 27.Spiller S, et al. TLR4-induced IFN-{gamma} production increases TLR2 sensitivity and drives gram-negative sepsis in mice. J Exp Med. 2008;205:1747–1754. doi: 10.1084/jem.20071990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 29.Elson G, Dunn-Siegrist I, Daubeuf B, Pugin J. Contribution of Toll-like receptors to the innate immune response to gram-negative and gram-positive bacteria. Blood. 2007;109:1574–1583. doi: 10.1182/blood-2006-06-032961. [DOI] [PubMed] [Google Scholar]
- 30.Werts C, et al. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat Immunol. 2001;2:346–352. doi: 10.1038/86354. [DOI] [PubMed] [Google Scholar]
- 31.Lynn M, et al. Blocking of responses to endotoxin by E5564 in healthy volunteers with experimental endotoxemia. J Infect Dis. 2003;187:631–639. doi: 10.1086/367990. [DOI] [PubMed] [Google Scholar]
- 32.Mullarkey M, et al. Inhibition of endotoxin response by e5564, a novel Toll-like receptor 4–directed endotoxin antagonist. J Pharmacol Exp Ther. 2003;304:1093–1102. doi: 10.1124/jpet.102.044487. [DOI] [PubMed] [Google Scholar]
- 33.Solomon SB, et al. Effective dosing of lipid A analogue E5564 in rats depends on the timing of treatment and the route of Escherichia coli infection. J Infect Dis. 2006;193:634–644. doi: 10.1086/500147. [DOI] [PubMed] [Google Scholar]
- 34.Marshall JC. Sepsis: Rethinking the approach to clinical research. J Leukoc Biol. 2008;83:471–482. doi: 10.1189/jlb.0607380. [DOI] [PubMed] [Google Scholar]
- 35.Adachi O, et al. Targeted disruption of the MyD88 gene results in loss of IL-1– and IL-18–mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 36.Roger T, Ding X, Chanson AL, Renner P, Calandra T. Regulation of constitutive and microbial pathogen-induced human macrophage migration inhibitory factor (MIF) gene expression. Eur J Immunol. 2007;37:3509–3521. doi: 10.1002/eji.200737357. [DOI] [PubMed] [Google Scholar]
- 37.Roger T, David J, Glauser MP, Calandra T. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature. 2001;414:920–924. doi: 10.1038/414920a. [DOI] [PubMed] [Google Scholar]
- 38.Roger T, Chanson AL, Knaup-Reymond M, Calandra T. Macrophage migration inhibitory factor promotes innate immune responses by suppressing glucocorticoid-induced expression of mitogen-activated protein kinase phosphatase-1. Eur J Immunol. 2005;35:3405–3413. doi: 10.1002/eji.200535413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.