Abstract
Retinal ganglion cells form orderly topographic connections with the tectum, establishing a continuous neural representation of visual space. Mapping along the dorsal–ventral axis requires interactions between EphB and ephrin-B cell-surface molecules expressed as countergradients in both retina and tectum. We have discovered that the diffusible TGFß-related factor Radar (Gdf6a) is necessary and sufficient for activation of dorsal markers, such as Bmp4, Tbx5, Tbx2b, and Ephrin-B2, and suppression of the ventral marker Vax2 in the zebrafish retina. Radar mutant axons innervate only the dorsal half of the tectum, where they form a compressed retinotectal map. Wild-type cells transplanted into the dorsal retina are able to rescue the dorsal identity of nearby mutant cells. Moreover, Radar overexpression “dorsalizes” retinal ganglion cell identity in the ventral retina. We conclude that Radar is near the top of a signaling cascade that establishes dorsal–ventral positional information in the retina and controls the formation of the retinotectal map.
Keywords: patterning, eye development, ocular coloboma, bone morphogenetic protein, tectum
The dorsal–ventral axis of the retina is specified during embryonic development by signaling mechanisms involving locally secreted factors and spatial gradients of transcription factors (1, 2). These patterning mechanisms eventually provide positional information to retinal ganglion cells (RGCs), which enable their axons to project to the topographically correct target regions in the optic tectum. Axons from dorsally located RGCs project to ventral positions in the tectum, whereas axons of ventral RGCs project to the dorsal half of the tectum (1, 2). This selectivity is achieved by signaling between RGC axons and tectal neurons. Along the dorsal–ventral axes of both the retina and the tectum, expression gradients of EphB and ephrin-B molecules are responsible for translating graded positional information into a smooth retinotectal map (3, 4).
Previous studies have emphasized the importance of bone morphogenetic proteins (Bmps), particularly Bmp4, in dorsalizing retinal tissue through activation of the T-box transcription factor Tbx5. Bmps belong to a family of secreted factors related to TGFß. Bmp4 has been assigned a central role in dorsal–ventral patterning of the eye, largely based on its expression in the dorsal retina and on results from overexpression in chick, mouse, and Xenopus (5–8). Despite compelling gain-of-function effects, however, loss-of-function analysis has yet to support a role for Bmp4 in dorsal–ventral patterning of the eye. Mouse and zebrafish Bmp4 mutants die around gastrulation, or are severely malformed, hampering investigations of their eye phenotypes (9, 10). Heterozygous Bmp4+/− mouse mutants show ocular malformations that appear unrelated to dorsal–ventral patterning (9). A related factor, Bmp2 (Bmp2b in zebrafish) (11) has also been implicated in dorsal patterning of the retina by virtue of its restricted expression in the dorsal retina and its gain-of-function phenotype (12). However, similar to Bmp4, Bmp2 may not be necessary, or even sufficient under physiological conditions, to induce dorsal retinal cell fate. Considering the similarity among Bmp molecules and the promiscuity of their receptors, it is possible that Bmp4 and/or Bmp2 overexpression adds to a function normally carried out by a different Bmp family member.
In a large-scale chemical mutagenesis screen for disruptions of visual behavior (13), we have recently discovered a zebrafish mutant (initially named dark half s327) with small eyes, but otherwise normal external morphology. The mutation is lethal at late-larval or early juvenile stages, with some mutant animals surviving for 21 days postfertilization (dpf). The mutants exhibit a deficit in a subset of behavioral responses to visual stimulation. Injection of axon tracer dyes into the eye of the mutant revealed that only the dorsal half of the tectum was innervated by retinal axons (13). This suggests strongly that the mutation disrupts a genetic locus required for differentiation of dorsal RGCs or for projection of their axons to the ventral tectum.
Here we report that the gene mutated in dark half s327 encodes the TGFß-related factor Radar, which belongs to the growth differentiation factor (Gdf) branch of the Bmp family. We show that this secreted molecule is necessary and sufficient to induce dorsal fate in the retina. Loss of this factor prevents specification of RGCs with dorsal identity and thus prevents innervation of the ventral tectum. Conversely, misexpression of Radar is able to override ventralizing signals. Radar activates known dorsal marker expression, notably bmp4, tbx5, tbx2b, and efnb2, and represses expression of the ventral fate determinant vax2. Overexpression of Bmp4 expands the tbx5 domain in the zebrafish retina, as it does in chick and mouse, but requires functional Radar for this effect. We arrive at a model in which a gradient of Radar acts in concert with ventral Sonic Hedgehog (Shh) to organize the dorsal–ventral axis of the retina and, thus, one of the axes of the retinotectal map.
Results and Discussion
radar Mutants Have Small Eyes and Retinotectal Mapping Defects.
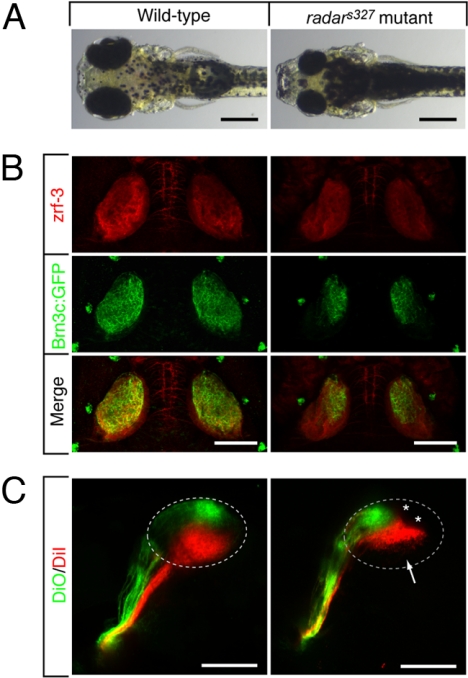
The external morphology of radars327 mutants is inconspicuous, with the exception of their smaller eyes (Fig. 1A). Mutants seem to develop on a normal schedule and inflate their swimbladders after hatching similar to WT. We observed a transient increase in cell death in the embryonic retina of the mutant, which could explain the reduced eye size [supporting information (SI) Fig. S1]. The radars327 retina is laminated normally (L. Nevin and H. B., unpublished work) and supports most visual responses (13). The mutants appear darker overall than their WT siblings, which is a result of their failure to contract melanin granules intheir pigment cells in response to light. This neuroendocrine response, termed visual background adaptation (VBA) (14, 15), depends on retinal light perception and transmission of visual signals to the hypothalamus, which in turn control the release of melanin-concentrating hormone from the pituitary. This pathway appears disrupted in the radar mutant.
Fig. 1.
Morphological and retinotectal phenotypes of radars327. (A) Zebrafish radars327 mutants have small eyes and appear dark, because of a VBA defect (see text). Dorsal brightfield views of 7 dpf WT sibling and homozygous radars327 mutant larvae. (B) radars327 mutants lack ventral innervation of the optic tectum. Dorsal confocal projections of 7 dpf larvae show that innervating RGC axons (expressing Brn3c:mGFP) are confined to the dorsal tectum in the mutant. Costaining with a neuropil marker (zrf-3 antibody) reveals that the size of the tectum is similar in WT and mutant. (C) radars327 mutants have a compressed dorsal–ventral retinotectal map. Fixed WT and radars327 eyes (7 dpf) were injected with DiO (ventrally) and DiI (dorsally). Lateral confocal projections are shown. Arrow highlights ventral tectal region not innervated by RGCs in radars327; asterisks show positions of skin melanophores. (Scale bars: 300 μm in A, 100 μm in B and C.)
Injection of axon tracer dyes into the eye revealed an abnormal retinotectal projection in radars327 mutants (Fig. 1B). In WT larvae, the projection zone of RGC axons, as visualized by the Brn3c:mGFP transgene (16), fills the entire dorsal–ventral extent of the tectal neuropil. In radars327mutants, by contrast, retinal axons project to only the dorsal half the tectum. The ventral half of the tectum, although of normal size and apparently fully differentiated, is devoid of retinal afferents. Axon tracing with the lipophilic dyes DiI and DiO injected into the dorsal and ventral retina, respectively, showed that, in the half-innervated radars327 tectum, retinotopic order persists (Fig. 1C). Thus, some dorsal–ventral positional information is retained among RGC axons in radars327 mutants.
The s327 Mutation Introduces a Stop Codon in radar and Abolishes Its Expression in the Dorsal Retina.
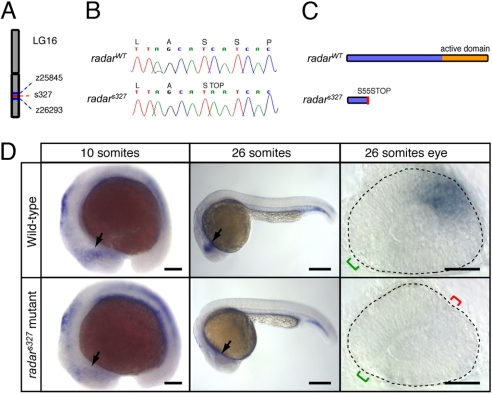
To identify the genetic lesion responsible for the phenotype, we mapped s327 to chromosome 16 near marker z26293 by using a panel of 874 recombinant embryos (17, 18) (Fig. 2A). The radar gene, encoding the secreted Bmp-related factor Radar/Gdf6a, represented an excellent candidate for s327 based on its known expression in the dorsal retina during embryogenesis (19–21) and recent implication as a regulator of retinal marker expression (20, 22, 23). PCR amplification and sequencing of the radar cDNA in radars327 mutants and WT siblings revealed a single C-to-A transversion, which introduces a stop codon early in the ORF (Fig. 2B). The mutant allele is predicted to encode a truncated pro-protein of 54 aa, which lacks the putative C-terminal mature signaling peptide characteristic of many TGFß proteins (19) (Fig. 2C).
Fig. 2.
Positional cloning and expression pattern of radar. (A) s327 maps to chromosome 16 between z25845 (2.3 cM) and z26293 (0.7 cM). (B) Sequencing of WT and s327 cDNA reveals a single C-to-A substitution in position 164 of the radar ORF, resulting in a premature stop codon. (C) Predicted translated peptides arising from radarWT and radars327. The mutation is predicted to result in a truncated protein, lacking the mature signaling domain. (D) Whole-mount in situ hybridization shows a restricted pattern of radar expression in WT embryos. radar mRNA is largely absent from the retina of radars327 mutants at all stages. In WT, expression is evident in the distal optic vesicle of WT embryos at 10 somites (arrow). At 26 somites, radar is expressed dorsally, opposite of the optic fissure (green bracket). Note ectopic fissures (red bracket) in radars327 mutants. (Scale bars: 150 μm for 10 somites, 250 μm for 26 somites, 50 μm for dissected 26 somite eyes.)
Consistent with a role in dorsal–ventral patterning of the embryonic retina, we found radar to be expressed in the distal optic vesicle of WT zebrafish embryos as early as the 10-somite stage, with expression maintained in the dorsal retina beyond the 26-somite stage (Fig. 2D). In radars327 mutant embryos, radar expression is reduced in the optic vesicle at the 10-somite stage and absent in the retina by the 26-somite stage (Fig. 2D). The early loss of mRNA is likely because of nonsense-mediated decay of the radars327 mutant transcript and further suggests a complete loss of zygotic Radar function in radars327 mutants. In addition, absence of mRNA may suggest a possible autoregulatory role for radar, which is interrupted in the mutant.
Conditional Overxpression of radar Rescues Visual Background Adaptation.
To confirm that s327 is a loss-of-function allele of radar, we sought to rescue the VBA by reintroducing WT radar. Overexpression of radar disrupts early gastrulation and axial patterning events (24). We therefore devised a conditional gene expression approach by creating a heatshock-inducible radar construct (hsp70:radarWT). We confirmed by in situ hybridization that this conditional system was highly effective for misexpression of radar in a broadly scattered and variable subset of cells; this mosaicism is likely because of unequal inheritance of the injected plasmid (Fig. S2). To identify the appropriate stage of development, in which radar is required for proper eye patterning, we used hs:dnBMPR transgenic embryos, in which all Bmp/Gdf signaling is blocked following expression of a dominant-negative receptor (25). Heatshock expression of dnBMPR as early as the 12- to 14-somite stage disrupted dorsal retinal fate (Fig. S3). We reasoned that Radar was likely required in the eye from this stage onwards.
In control clutches from a cross of two heterozygous carriers, VBA-negative (dark) larvae were found near the expected Mendelian frequency (uninjected with heat shock: 26.7%, n = 105; injected without heat shock: 20.3%, n = 69). Progeny from the same cross injected with 10 ng/μL hsp70:radarWT and heat-shocked at the 12-somite stage showed a significant reduction in the fraction of VBA-negative larvae (2.2%, n = 92). Injection and induced expression of the mutant allele found in radars327 mutants (hsp70:radars327) failed to reduce the number of dark larvae (25.4%, n = 59). Thus, reintroduction of WT radar rescues the VBA.
Radar Overexpression Is Sufficient to Induce Dorsal Retinal Fate.
We asked whether radar gain of function is sufficient to dorsalize WT cells and to rescue ventral tectum innervation in radar mutants. Heterozygous radars327 adults carrying Brn3c:mGFP were mated and their offspring injected with 25 ng/μL hsp70:radarWT DNA at the one or two-cell stage. Embryos were then heat-shocked at the 12-somite stage. Injected embryos were raised to 7 dpf and their retinotectal projections investigated. In WT, overexpression of radarWT often resulted in embryos with small eyes lacking ventral characteristics (35%; n = 50 injected embryos). In these eyes, the optic fissure failed to close, consistent with a loss of ventral retinal identity (26). This is opposite to uninjected radars327 mutants, in which an extra fissure could often be detected in the dorsal retina (see Fig. 2D).
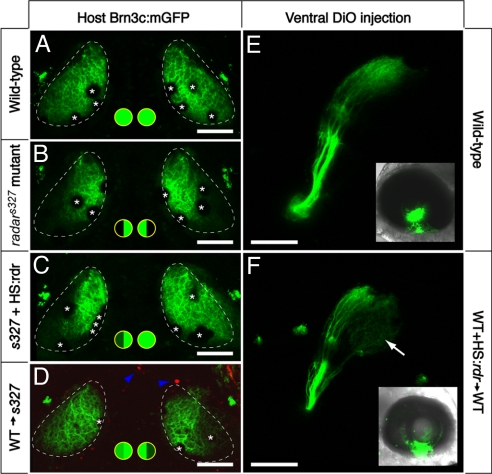
In radars327 mutants, heatshock-induced expression of radarWT restored innervation of the ventral tectum in 60% of the larvae analyzed (n = 20; Fig. 3 A–C). The stochastic expression pattern of radar in these experiments does not allow us to draw strong conclusions about the source of Radar protein necessary for successful rescue of the mutant, but we interpret the failure rate (40%) to cases in which the plasmid was not present in sufficient quantities in the eye (Fig. 3C shows an example of a unilateral retinotectal rescue; see Fig. S2 for evidence of mosaicism). Overexpression of radar carrying the point mutation (hsp70:radars327) was not able to rescue the mutant phenotype in n = 59 embryos. Heatshock alone (n = 105) or injection of radarWT alone (n = 69) did not change the retinotectal mapping phenotype in either mutant or WT. Together, these experiments demonstrate that Radar is sufficient, when overexpressed, to specify the retinotectal specificity of dorsal RGCs.
Fig. 3.
Cell–cell signaling through Radar is sufficient for ventral tectum innervation. (A–D) Rescue experiment. Brn3c:mGFP-labeled retinotectal projections were imaged in vivo at 7 dpf. The tectal neuropil is outlined with a dashed line. Green-filled circles summarize dorsal and ventral tectum innervation results. (A) WT tecta show full innervation. (B) radars327 mutants lack ventral innervation. (C) radarWT expression from heatshock-promoter rescues the retinotectal phenotype of the mutant. In the case shown, only one side was rescued. PCR-based genotyping confirmed that only the rescued eye contained hsp70:radarWT; the other eye had likely not received the injected plasmid because of the mosaicism inherent in transient transgenesis. (D) The radar gene acts cell-nonautonomously in the retina. WT cells transplanted into radars327 host embryos are sufficient to rescue ventral innervation. Only the host carried the Brn3c:mGFP transgene. Donor-derived cells (blue arrowheads) were labeled with rhodamine dextran, and do not contribute to the tectum. (E and F) Gain-of-function experiment. DiO was injected into the ventral retina, and its labeling pattern was imaged from a lateral view. Insets show injected eye. In normal WT larvae (E), ventral RGCs project exclusively to the dorsal tectum. In chimeric WT larvae (F) that have received a transplant of WT cells carrying the hsp70:radarWT construct, some ventral axons ectopically innervate the ventral tectum (arrow). Asterisks (in A–D) show positions of skin melanophores. (Scale bars, 100 μm.)
Radar Acts on Neighboring Cells in a Position-Dependent Manner.
As Gdf6 has previously been shown to be secreted in Xenopus (21), we expected that zebrafish Radar could act on neighboring cells in a cell-nonautonomous fashion. We therefore tested whether WT retinal cells could rescue the mutant retinotectal projection by transplanting cells at the blastula stage from WT donors to radars327 mutant hosts (the latter labeled with Brn3c:mGFP). From a large number of transplantations, 41 chimeras were selected for the presence of small clones of WT cells in the mutant eye. In a majority of them, we observed the rescue of GFP-expressing (i.e., genotypically mutant) axons innervating the ventral tectum (Fig. 3D). This result demonstrated that Radar acts nonautonomously and at physiological concentrations to instruct a dorsal fate.
The positions and clonal sizes of the transplanted cells in the retina were highly variable and difficult to image, making exact quantifications difficult. We nevertheless grouped the chimeras into those with dorsal, ventral, or mixed clone locations. This analysis revealed that the retinotectal projection rescue was limited to cases with WT cells in the dorsal retina, including mixed locations (Fig. S4 A and B). Chimeras in which the WT cells were only detectable in the ventral retina were not rescued; they showed a mutant retinotectal phenotype (Fig. S4 C and D). Moreover, in rare instances, we encountered chimeras in which the donor cells populated the tissue along the axon tract and in the tectum, but were apparently completely excluded from the retina. The retinotectal projection of these chimeras was also not rescued (Fig. S4 E and F). These observations are consistent with a retina-intrinsic and position-dependent role of Radar in patterning the retinotectal map.
We next asked whether Radar misexpression was sufficient to “reprogram” the fate of ventrally located RGCs in WT. For this experiment, we transplanted blastomeres from hsp70:radarWT mosaic embryo into a WT host. The resulting chimeras were heat-shocked at the 12-somite stage to induce radar expression, sorted for the presence of donor-derived cells in the retina, and allowed to develop. At 7 dpf, DiO was injected at the ventral margin of the retina to label the retinotectal projection. Whereas in all nonheatshocked controls (n = 10), DiO-labeled axons projected exclusively to the dorsal tectum (Fig. 3E), in heatshocked chimeras (n = 3), axons were seen terminating in the ventral tectum (Fig. 3F). These data suggest that Radar can override ventral-fate determining factors.
Expression of Bmp4 and Other Dorsal Markers Is Dependent on Radar.
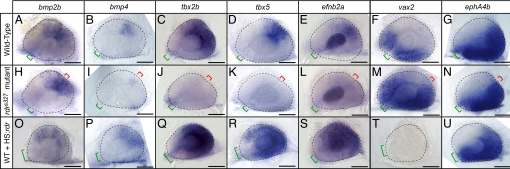
A number of studies have shown previously that overexpression of Bmp4 and Bmp2 in the developing optic vesicle stimulates expression of Tbx5 and Tbx2, which in turn activate efnb2, the gene encoding Ephrin-B2 (5, 12), and repress the ventral transcription factor Vax2 (27, 28). Altered expression of each of these factors leads to abnormal retinotectal mapping (2, 5, 29). To determine whether these same patterning components are dependent on Radar function, we performed RNA in situ hybridizations. In WT retinas at the 24–26 somite stage, bmp2b and bmp4, two genes encoding members of the Bmp family known to be expressed in the embryonic retina, are restricted to the dorsal retina (Fig. 4 A and B). Similar patterns are seen for tbx2b (30), tbx5, and efnb2a (Fig. 4 C–E), whereas vax2 is restricted to the ventral retina (Fig. 4F). These patterns recapitulate the situation reported for other vertebrate species.
Fig. 4.
Radar signaling affects known determinants of dorsal–ventral retinal patterning. Expression patterns are visualized by whole-mount in situ hybridization of eyes from 26-somite embryos. Retinal markers in WT (A–G), radars327 mutants (H--N), and WT overexpressing hsp70:radarWT (O--U). In radars327 mutants, retinal bmp2b expression remained normal, bmp4 expression was severely reduced, tbx2b, tbx5, and efnb2a expression was absent, and vax2 expression was expanded dorsally. In radar-overexpressing embryos, bmp2b, bmp4, tbx2b, tbx5 and efnb2a expression were expanded ventrally, and vax2 was lost. ephA4b was unaffected by either loss or gain of radar function. Eyes overexpressing radar were often small and failed to close at the optic fissure. Neural retina is outlined with dashed line; green bracket identifies optic fissure location and size; red bracket indicates location of ectopic fissures in mutants. (Scale bars, 50 μm.)
Dorsal–ventral patterning is dramatically altered by loss of radar function. In radars327 mutants, bmp2b expression is largely unaffected, bmp4 expression is substantially reduced, and tbx2b, tbx5, and efnb2a are completely absent (n ≥ 10 embryos for each marker; Fig. 4 H–L). Additionally, the vax2 expression domain is expanded dorsally (Fig. 4M). Gain of radar function has largely opposite effects. Overexpression of radar with hsp70:radarWT induces expansion of bmp2b, bmp4, tbx2b, tbx5, and efnb2a and completely abolishes retinal vax2 in a majority of the injected embryos (n ≥ 25 for each marker; Fig. 4 O--T). Radar induction of bmp2b is more subtle than that of bmp4 but consistently detectable (n = 27).
Although recent studies have speculated on a role for Radar in nasotemporal specification based on morpholino-mediated knockdown (20), we did not observe significant differences between expression patterns of the temporal marker ephA4b in WT, radars327 mutant, and hsp70:radarWT overexpressing embryos (Fig. 4 G, N, and U).
Interestingly, radar appeared resilient to manipulations of bmp4 and bmp2b; its expression was unaltered following either overexpression or morpholino-mediated knockdown of these factors (Fig. 5A; Fig. S5E; and data not shown). In summary, these studies place Radar, perhaps together with Bmp2b, upstream of bmp4 expression and near the top of a genetic pathway that determines dorsal retinal fate.
Fig. 5.
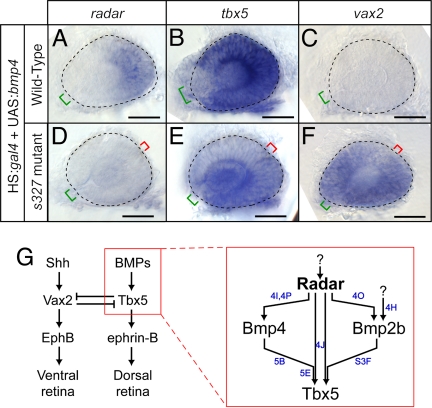
Overexpression of bmp4 requires radar to alter dorsal–ventral patterning. (A--F) Expression patterns visualized by whole-mount in situ hybridization of eyes from 26-somite embryos. Neural retina is outlined with dashed line; green bracket identifies optic fissure location and size; red bracket indicates location of ectopic fissures in mutants. (Scale bars, 50 μm.) radar expression is mildly expanded (A), tbx5 expression dramatically increases (B), and vax2 expression is eliminated (C) in WT; hsp70:gal4; UAS:bmp4 embryos. radar expression remains absent (D), tbx5 is expressed at low levels in ectopic locations (E), and vax2 expression is dorsally expanded (F) in radars327 mutant; hsp70:gal4; UAS:bmp4 embryos. (G) Summary model for retinal patterning, synthesizing roles for Radar with known dorsal–ventral patterning genes. Radar is the critical determinant of dorsal identity and is required for normal expression of Bmp4, Tbx transcription factors, and ephrin-B. Blue letters indicate which panels in this figure and in Fig. 4 and Fig. S3F provide evidence for the relationships shown.
Bmp4 Signaling in the Dorsal Retina Requires the Presence of Radar.
We asked whether Bmp4 overexpression induces dorsal retinal markers in the zebrafish retina, as it does in chick and mouse. Indeed, bmp4 overexpression from the heatshock promoter expanded the domains of tbx2b and tbx5 and suppressed vax2. However, this effect was absent in radar mutants, i.e., depended on a functional radar gene (Fig. 5 B–F; Fig. S5 F and G). The latter finding is significant, because TGFß ligands are known to interact as dimers with their receptor, and several studies have demonstrated that Bmp heterodimers are more potent than homodimers in activating downstream targets (21, 31). The genetic evidence provided here suggests that Radar not only induces bmp4, but may also be a necessary component of active Bmp dimers in the dorsal retina (Fig. 5G). Alternatively, Radar may be required for up-regulation of another component without which Bmp4 cannot exert its dorsalizing effect.
A Network of Bmp Factors Patterns Both Embryo and Retina.
The central role of Radar shown here is reminiscent of its function during dorsal–ventral axial patterning of the zebrafish embryo (24, 32). Indeed, we found that embryonic and retinal patterning mechanisms share key elements of the underlying signaling pathway. Maternally deposited Radar acts through the type I Bmp receptor Alk8 to induce bmp2b (swirl) and bmp4, which in turn signal through Alk8 to activate ventral factors (10, 24, 32). Another Bmp-encoding gene, bmp7 (snailhouse), is not dependent on induction by Radar (33). We found that Alk8, which is broadly expressed in the retina, appears to serve as a receptor for Radar, as evidenced by reduction of tbx5 expression in zygotic lost-a-fin mutants, in which Alk8 is disrupted (34) (Fig. S5 A and B). Another similarity between retina and early embryo is the independence of radar expression from other Bmps; its dorsal-ventral gradient is not detectably altered by manipulations of either bmp4 or bmp2b.
However, the details of how the Bmp ligands interact in the dorsal retina are different from the situation in the pregastrulation embryo. First, Bmp7's role (if any) in the retina is presently unclear. Second, bmp4 overexpression overrides normal specification, but with differing dependencies on radar: ventralization of the early embryo occurs independent of radar, while dorsalization of the retina requires radar (24). Third, in the retina, Radar is necessary and sufficient for bmp4 induction. Our findings reveal that there are specific molecular interdependencies of the BMP ligands, which differ between early embryo and retina. The genetic network acting in the retina is summarized in Fig. 5G.
Shh Only Slightly Affects Radar Expression.
Although a network of Bmp-related genes specify dorsal retinal fate, hedgehog factors, such as Shh, act in a countergradient to instruct ventral characteristics (26) (see Fig. 5G). Shh, although not expressed in the eye before 28 hpf, is secreted from the midline of the neural tube and promotes ventral expression of vax genes (26) and also inhibits dorsal bmp4 expression (35). We found that radar expression is slightly, expanded in the retinas of zebrafish syu mutants, which lack Shh (36) (Fig. S5 C and D). Direct interactions between these two signals therefore appear very weak. Residual dorsal–ventral information seen in the retinotectal map of radars327 mutants (see Fig. 1C) may thus be conveyed either by genes downstream of Shh (26, 35) or by Radar-independent Bmp signaling (6).
Materials and Methods
Strains and Maintenance.
Fish were maintained as previously described (13). Mutant alleles used were radar/gdf6as327, lost a fin/alk8/acvr1m100, and syu/shh/shhat4. Transgenic lines used were Brn3c:mGFP/Tg(pou4f3:gap43-GFP)s356t, hs:dnBMPR/Tg(hsp70l:dnBmpr-GFP)w30, hs:gal4/Tg(hsp70l:Gal4)1.5kca4, and hs:bmp2b/Tg(hsp70l:bmp2b)fr13.
Immunohistochemistry.
Larvae were raised in with 0.003% (wt/vol) 1-phenyl-2-thiourea (PTU) to inhibit melanin synthesis. Whole-mount TUNEL staining and immunohistochemistry were performed as described elsewhere (37), by using antibodies zrf-3 (Oregon Monoclonal Bank) diluted 1:250, and anti-GFP (Molecular Probes) diluted 1:1000.
Fluorescent Axon Tracing.
Dye injections were performed as previously described (13).
Confocal Microscopy.
Live larvae were mounted in 1% low melting point agarose in E3 medium and treated with 0.8% norepinephrine to aggregate melanin pigment granules and anesthetized with 0.016% tricaine. Fixed larvae were mounted in 1.6% low melting point agarose in PBS. Confocal imaging was performed with long-working distance lenses (20×, NA 0.5; 40×, NA 0.8) on a Zeiss Pascal confocal microscope. Images were analyzed and processed with ImageJ and Adobe Photoshop.
Positional Cloning and RFLP Analysis.
Linkage mapping was performed as described (13). First strand cDNA was synthesized from 8 dpf homozygous radars327 mutants, WT siblings, and homozygous Tüpfel Longfin (WT strain) larval zebrafish. The radar ORF was amplified by PCR using specific primer sequences (forward 5′-ATGGATGCCTTGAGAGCAGTC-3′ and reverse 5′-CTACCTGCAGCCACACTGTTC-3′). The s327 mutation destroys an SfaNI site, allowing identification of carriers by restriction fragment length polymorphism (RFLP) analysis. Amplification from genomic DNA by PCR with the forward primer and the RFLP reverse primer (5′-TTGAAGAGCGGAAAAAGCTC-3′), followed by digestion with SfaNI resulted in products of 170 and 110 bp in WT and a single band of 280 bp from radars327 mutants.
In situ Hybridization.
Dig-labeled riboprobes for full-length radar, tbx2b, tbx5, bmp2b and vax2 were transcribed in vitro. radars327 mutants and WT siblings were stained as a clutch, with expected mutant frequencies of 25%. laf and syu mutants were sorted before staining, then treated with identical conditions. Whole-mount in situ hybridizations were carried out as previously described (38) and stored in 87% glycerol. Eyes were dissected using tungsten needles and mounted in a similar orientation. For bmp4-, tbx5-, and vax2-stained embryos lacking staining in the retina, eyes were dissected from animals that had comparable staining in other tissues. All images were collected with a Leica dissection microscope or a Zeiss compound microscope equipped with a Spot CCD camera (Diagnostic Instruments) or AxioCam MRC (Zeiss), and prepared by using Adobe Photoshop.
radars327 mutant and WT sibling embryos were genotyped by RFLP analysis (see above), with DNA isolated before imaging by clipping the trunk, or following imaging using the entire embryo. DNA was isolated by methanol dehydration, tissue maceration, and overnight incubation in 1.7 mg/μL proteinase-K (Roche) in 10 mM Tris, pH 8.0.
Injection and Heatshock Induction.
The radar ORF was amplified with modified forward and reverse cloning primers (see above) containing SalI restriction sites, and subcloned downstream of the hsp70 promoter in a construct flanked by Tol2 transposase recognition sites (39). For all rescue experiments, 10 ng/μL DNA was coinjected with 25 ng/μL Tol2 transposase mRNA, and 25 ng/μL GFP mRNA generated with the mMessage mMachine kit (Ambion) at the 1–2 cell stage. Poorly injected (identified by a lack of strong GFP fluorescence) and malformed embryos were excluded from the experiment before heatshock. Heatshock induction was carried out as described (25).
The bmp4 ORF was amplified by PCR and Xi cloned (Gene Technology Systems) into a vector containing 14 repeats of the upstream activating sequence (UAS), and flanked by Tol2 sequences (39). 10 ng/μL DNA was injected into an in-cross of carriers of the s327 mutation and the hsp70:gal4 transgene. Heatshock induction was carried out at 12 somites to allow sufficient time for Gal4-mediated transactivation. Similar injection controls were done, and only animals with increased trunk and tail thickness indicative of BMP overexpression were kept for staining. Heatshock-induced overexpression of bmp2b was similarly achieved by using transgenic embryos (40).
Transplantation Experiments.
Donor embryos were injected with a solution of 1–5% tetramethyl-rhodamine dextran amine (Molecular Probes). Blastula-stage transplants were performed as described (38). Chimeras were sorted at 30–36 hours postfertilization (hpf) for the presence of rhodamine-positive cells in the neural retina and treated with PTU. For overexpressing chimera experiments, donors were also coinjected with 25 ng/μL hsp70:radarWT, 25 ng/μL Tol2 transposase mRNA, and 25 ng/μL GFP mRNA. Following heatshock at 12 somites, chimeras were sorted and treated as above.
Morpholino Knockdown.
Morpholino target sequences for bmp4 (40) and bmp2b knockdown (41) were published elsewhere.
Supplementary Material
Acknowledgments.
We thank D. Stainier (University of California, San Francisco), A. Picker (Max-Planck-Institut of Molecular Cell Biology and Genetics), M. Mullins (University of Pennsylvania), and the Zebrafish International Resource Center for reagents and fish strains, and J. Pinkston-Gosse and members of the Baier lab for review of the manuscript. This work was funded by the March of Dimes Foundation and the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803202106/DCSupplemental.
References
- 1.Harada T, Harada C, Parada LF. Molecular regulation of visual system development: More than meets the eye. Gene Dev. 2007;21:367–378. doi: 10.1101/gad.1504307. [DOI] [PubMed] [Google Scholar]
- 2.McLaughlin T, Hindges R, O'Leary DD. Regulation of axial patterning of the retina and its topographic mapping in the brain. Curr Opin Neurobiol. 2003;13:57–69. doi: 10.1016/s0959-4388(03)00014-x. [DOI] [PubMed] [Google Scholar]
- 3.Hindges R, McLaughlin T, Genoud N, Henkemeyer M, O'Leary DD. EphB forward signaling controls directional branch extension and arborization required for dorsal-ventral retinotopic mapping. Neuron. 2002;35:475–487. doi: 10.1016/s0896-6273(02)00799-7. [DOI] [PubMed] [Google Scholar]
- 4.Mann F, Ray S, Harris W, Holt C. Topographic mapping in dorsoventral axis of the Xenopus retinotectal system depends on signaling through ephrin-B ligands. Neuron. 2002;35:461–473. doi: 10.1016/s0896-6273(02)00786-9. [DOI] [PubMed] [Google Scholar]
- 5.Koshiba-Takeuchi K, et al. Tbx5 and the retinotectum projection. Science. 2000;287:134–137. doi: 10.1126/science.287.5450.134. [DOI] [PubMed] [Google Scholar]
- 6.Behesti H, Holt JK, Sowden JC. The level of BMP4 signaling is critical for the regulation of distinct T-box gene expression domains and growth along the dorso-ventral axis of the optic cup. BMC Dev Biol. 2006;6:62. doi: 10.1186/1471-213X-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sasagawa S, Takabatake T, Takabatake Y, Muramatsu T, Takeshima K. Axes establishment during eye morphogenesis in Xenopus by coordinate and antagonistic actions of BMP4, Shh, and RA. Genesis. 2002;33:86–96. doi: 10.1002/gene.10095. [DOI] [PubMed] [Google Scholar]
- 8.Trousse F, Esteve P, Bovolenta P. Bmp4 mediates apoptotic cell death in the developing chick eye. J Neurosci. 2001;21:1292–1301. doi: 10.1523/JNEUROSCI.21-04-01292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang B, et al. Haploinsufficient Bmp4 ocular phenotypes include anterior segment dysgenesis with elevated intraocular pressure. BMC Genet. 2001;2:18. doi: 10.1186/1471-2156-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stickney HL, Imai Y, Draper B, Moens C, Talbot WS. Zebrafish bmp4 functions during late gastrulation to specify ventroposterior cell fates. Dev Biol. 2007;310:71–84. doi: 10.1016/j.ydbio.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kishimoto Y, Lee KH, Zon L, Hammerschmidt M, Schulte-Merker S. The molecular nature of zebrafish swirl: BMP2 function is essential during early dorsoventral patterning. Development. 1997;124:4457–4466. doi: 10.1242/dev.124.22.4457. [DOI] [PubMed] [Google Scholar]
- 12.Sakuta H, et al. Role of bone morphogenic protein 2 in retinal patterning and retinotectal projection. J Neurosci. 2006;26:10868–10878. doi: 10.1523/JNEUROSCI.3027-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muto A, et al. Forward genetic analysis of visual behavior in zebrafish. PLoS Genet. 2005;1:e66. doi: 10.1371/journal.pgen.0010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neuhauss SC, et al. Genetic disorders of vision revealed by a behavioral screen of 400 essential loci in zebrafish. J Neurosci. 1999;19:8603–8615. doi: 10.1523/JNEUROSCI.19-19-08603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kay JN, Finger-Baier KC, Roeser T, Staub W, Baier H. Retinal ganglion cell genesis requires lakritz, a Zebrafish atonal Homolog. Neuron. 2001;30:725–736. doi: 10.1016/s0896-6273(01)00312-9. [DOI] [PubMed] [Google Scholar]
- 16.Xiao T, Roeser T, Staub W, Baier H. A GFP-based genetic screen reveals mutations that disrupt the architecture of the zebrafish retinotectal projection. Development. 2005;132:2955–2967. doi: 10.1242/dev.01861. [DOI] [PubMed] [Google Scholar]
- 17.Shimoda N, et al. Zebrafish genetic map with 2000 microsatellite markers. Genomics. 1999;58:219–232. doi: 10.1006/geno.1999.5824. [DOI] [PubMed] [Google Scholar]
- 18.Knapik EW, et al. A microsatellite genetic linkage map for zebrafish (Danio rerio) Nat Genet. 1998;18:338–343. doi: 10.1038/ng0498-338. [DOI] [PubMed] [Google Scholar]
- 19.Rissi M, Wittbrodt J, Delot E, Naegeli M, Rosa FM. Zebrafish Radar: A new member of the TGF-beta superfamily defines dorsal regions of the neural plate and the embryonic retina. Mech Dev. 1995;49:223–234. doi: 10.1016/0925-4773(94)00320-m. [DOI] [PubMed] [Google Scholar]
- 20.Asai-Coakwell M, et al. GDF6, a novel locus for a spectrum of ocular developmental anomalies. Am J Hum Genet. 2007;80:306–315. doi: 10.1086/511280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang C, Hemmati-Brivanlou A. Xenopus GDF6, a new antagonist of noggin and a partner of BMPs. Development. 1999;126:3347–3357. doi: 10.1242/dev.126.15.3347. [DOI] [PubMed] [Google Scholar]
- 22.Hanel ML, Hensey C. Eye and neural defects associated with loss of GDF6. BMC Dev Biol. 2006;6:43. doi: 10.1186/1471-213X-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.French CR, et al. Pbx homeodomain proteins pattern both the zebrafish retina and tectum. BMC Dev Biol. 2007;7:85. doi: 10.1186/1471-213X-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sidi S, Goutel C, Peyrieras N, Rosa FM. Maternal induction of ventral fate by zebrafish radar. Proc Natl Acad Sci USA. 2003;100(6):3315–3320. doi: 10.1073/pnas.0530115100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pyati UJ, Webb AE, Kimelman D. Transgenic zebrafish reveal stage-specific roles for Bmp signaling in ventral and posterior mesoderm development. Development. 2005;132:2333–2343. doi: 10.1242/dev.01806. [DOI] [PubMed] [Google Scholar]
- 26.Take-uchi M, Clarke JD, Wilson SW. Hedgehog signalling maintains the optic stalk-retinal interface through the regulation of Vax gene activity. Development. 2003;130:955–968. doi: 10.1242/dev.00305. [DOI] [PubMed] [Google Scholar]
- 27.Barbieri AM, et al. A homeobox gene, vax2, controls the patterning of the eye dorsoventral axis. Proc Natl Acad Sci USA. 1999;96(19):19–10729. doi: 10.1073/pnas.96.19.10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mui SH, Hindges R, O'Leary DD, Lemke G, Bertuzzi S. The homeodomain protein Vax2 patterns the dorsoventral and nasotemporal axes of the eye. Development. 2002;129:797–804. doi: 10.1242/dev.129.3.797. [DOI] [PubMed] [Google Scholar]
- 29.Schulte D, Furukawa T, Peters MA, Kozak CA, Cepko CL. Misexpression of the Emx-related homeobox genes cVax and mVax2 ventralizes the retina and perturbs the retinotectal map. Neuron. 1999;24:541–553. doi: 10.1016/s0896-6273(00)81111-3. [DOI] [PubMed] [Google Scholar]
- 30.Gross JM, Dowling JE. Tbx2b is essential for neuronal differentiation along the dorsal/ventral axis of the zebrafish retina. Proc Natl Acad Sci USA. 2005;102:4371–4376. doi: 10.1073/pnas.0501061102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butler SJ, Dodd J. A role for BMP heterodimers in roof plate-mediated repulsion of commissural axons. Neuron. 2003;38:389–401. doi: 10.1016/s0896-6273(03)00254-x. [DOI] [PubMed] [Google Scholar]
- 32.Goutel C, Kishimoto Y, Schulte-Merker S, Rosa F. The ventralizing activity of Radar, a maternally expressed bone morphogenetic protein, reveals complex bone morphogenetic protein interactions controlling dorso-ventral patterning in zebrafish. Mech Dev. 2000;99:15–27. doi: 10.1016/s0925-4773(00)00470-6. [DOI] [PubMed] [Google Scholar]
- 33.Wilm TP, Solnica-Krezel L. Radar breaks the fog: Insights into dorsoventral patterning in zebrafish. Proc Natl Acad Sci USA. 2003;100:4363–4365. doi: 10.1073/pnas.0931010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mintzer KA, et al. Lost-a-fin encodes a type I BMP receptor, Alk8, acting maternally and zygotically in dorsoventral pattern formation. Development. 2001;128:859–869. doi: 10.1242/dev.128.6.859. [DOI] [PubMed] [Google Scholar]
- 35.Zhang XM, Yang XJ. Temporal and spatial effects of Sonic hedgehog signaling in chick eye morphogenesis. Dev Biol. 2001;233:271–290. doi: 10.1006/dbio.2000.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schauerte HE, et al. Sonic hedgehog is not required for the induction of medial floor plate cells in the zebrafish. Development. 1998;125:2983–2993. doi: 10.1242/dev.125.15.2983. [DOI] [PubMed] [Google Scholar]
- 37.Wehman AM, Staub W, Meyers JR, Raymond PA, Baier H. Genetic dissection of the zebrafish retinal stem-cell compartment. Dev Biol. 2005;281:53–65. doi: 10.1016/j.ydbio.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 38.Kay JN, Link BA, Baier H. Staggered cell-intrinsic timing of ath5 expression underlies the wave of ganglion cell neurogenesis in the zebrafish retina. Development. 2005;132:2573–2585. doi: 10.1242/dev.01831. [DOI] [PubMed] [Google Scholar]
- 39.Scott EK, et al. Targeting neural circuitry in zebrafish using GAL4 enhancer trapping. Nat Methods. 2007;4:323–326. doi: 10.1038/nmeth1033. [DOI] [PubMed] [Google Scholar]
- 40.Shin D, et al. Bmp and Fgf signaling are essential for liver specification in zebrafish. Development. 2007;134:2041–2050. doi: 10.1242/dev.000281. [DOI] [PubMed] [Google Scholar]
- 41.Imai Y, Talbot WS. Morpholino phenocopies of the bmp2b/swirl and bmp7/snailhouse mutations. Genesis. 2001;30:160–163. doi: 10.1002/gene.1055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.