Abstract
The Epstein–Barr virus (EBV) nuclear antigen (EBNA)-1 is the only viral protein expressed in all EBV-carrying malignancies, but its contribution to oncogenesis has remained enigmatic. We show that EBNA-1 induces chromosomal aberrations, DNA double-strand breaks, and engagement of the DNA damage response (DDR). These signs of genomic instability are associated with the production of reactive oxygen species (ROS) and are reversed by antioxidants. The catalytic subunit of the leukocyte NADPH oxidase, NOX2/gp91phox, is transcriptionally activated in EBNA-1–expressing cells, whereas inactivation of the enzyme by chemical inhibitors or RNAi halts ROS production and DDR. These findings highlight a novel function of EBNA-1 and a possible mechanism by which expression of this viral protein could contribute to malignant transformation and tumor progression.
Keywords: EBNA-1, ROS, EBV, DNA damage
Epstein–Barr virus (EBV) is a human gamma-herpesvirus that establishes latent infections in B lymphocytes, where only a subset of viral genes is expressed and virus replication is suppressed (1). The proteins encoded by the latency genes, including 6 EBV-encoded nuclear antigens (EBNA-1, -2, -3A, -3B, -3C, and -5) and 3 latent membrane proteins (LMP1, -2A, and -2B), induce growth transformation by capturing multiple signaling pathways that control B cell proliferation and apoptosis. It is generally assumed that the continuous expression of viral genes underlies the association of EBV with a variety of human malignancies, including Burkitt's lymphoma (BL), Hodgkin's disease (HD), nasopharyngeal carcinoma (NPC), and posttransplant lymphoproliferative disease (PTLD) (2). Some EBV-positive tumors do not express all of the latency proteins, leading to restricted forms of latency in which EBNA-1 is detected either alone (latency I, found in BL) or together with the LMPs (latency II, found in HD and NPC). Thus, EBNA-1 is the only viral protein regularly expressed in all EBV-carrying malignancies.
EBNA-1 binds to the viral origin of replication (oriP) and is required for the correct partitioning of the viral episomes in proliferating cells (3). It may confer a growth advantage to BL cells (4) and protect them from apoptosis (5) but does not act as an autonomous oncogene (6) and seems to be dispensable for B cell immortalization in vitro (7). Hence, the mechanism by which EBNA-1 may contribute to malignant transformation is not understood.
Genomic instability is common in malignant cells and was observed in EBV-carrying tumors (8–10). EBNA-3C (11) and LMP-1 (12) may promote this phenotype through inhibition of DNA repair or inactivation of cell cycle checkpoints, which allow the propagation of DNA damage. However, these viral proteins are not expressed in EBV-carrying BLs, and only half of HDs and NPCs express detectable levels of LMP1, suggesting a limited role in EBV oncogenesis. A possible involvement of EBNA-1 in the induction of genomic instability is suggested by a significant increase of transient chromosomal aberrations, such as dicentric chromosomes, chromosome fragments, and gaps, in EBV-positive BLs expressing latency I compared with EBV-negative tumors (13). We have now investigated this finding in a panel of EBV-positive and EBV-negative BL cell lines and sublines of EBV-negative cell lines with stable or inducible expression of EBNA-1. We show that EBNA-1 induces chromosomal aberrations, DNA double-strand breaks, and engagement of the DNA damage response (DDR) in malignant B cells. These effects are mediated by the production of reactive oxygen species (ROS) via transcriptional activation of the catalytic subunit of the leukocyte NADPH oxidase, NOX2/gp91phox.
Results
EBNA-1 Induces Chromosomal Instability and DNA Damage.
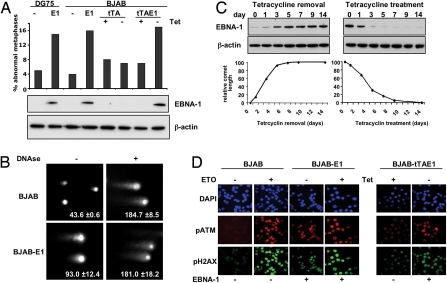
To address the role of EBNA-1 in oncogenesis we searched for signs of genomic instability in B cell lines that express either constitutive or tetracycline-regulated EBNA-1. A 3- to 4-fold increase of metaphases with dicentric chromosomes, chromosome fragments, and gaps was observed in stable EBNA-1–expressing sublines of the EBV-negative DG75 and BJAB (DG75-E1 and BJAB-E1). A similar increase was induced upon removal of tetracycline in BJAB cells carrying a Tet-off–regulated EBNA-1 (BJAB-tTAE1; Fig. 1A). These chromosomal aberrations are generated by the improper rejoining of DNA breaks (14). We therefore investigated whether EBNA-1 expression is associated with DNA damage. A reproducible increase of DNA double-strand breaks was detected in EBNA-1–expressing cells by comet assays performed under neutral conditions (i.e., in the absence of exogenously induced damage) (Fig. 1B). Analysis of comet length in BJAB-tTAE1 after up- or down-regulation of EBNA-1 by removal or addition of tetracycline (Fig. 1C) confirmed that the DNA fragmentation is directly proportional to the amount of EBNA-1. Expression of EBNA-1 was also associated with a subtle but reproducible slow-down of cell proliferation [supporting information (SI) Fig. S1], which may reflect a lengthening of the cell cycle because of activation of DNA damage checkpoints. In line with this possibility, the phosphorylation-dependent activation of 2 proteins involved in DDR, the kinase ataxia telangiectasia mutated (ATMpS1981) and its target histone H2AX (H2AXpSer139), was increased in EBNA-1 positive cells (Fig. 1D).
Fig. 1.
EBNA-1 induces genomic instability in B cells. (A) Metaphase chromosomes were analyzed in DG75 and BJAB (P), their sublines expressing stable EBNA-1 (E1), and BJAB transfectants expressing the tetracycline regulator alone (tTA) or tetracycline-regulated EBNA-1 (tTAE1) with and without treatment with 1 μg/ml tetracycline (Tet). EBNA-1 was detected in Western blots probed with the OT1X antibody, and β-actin was used as a loading control. (B) Comet assays were performed under neutral condition, and treatment with 1 μg of DNase was used as positive control. Representative micrographs illustrating the increased comet length in BJAB-E1 are shown. The mean ± SD comet length in 3 experiments is indicated in each panel. (C) Double-strand DNA breaks were quantified in BJAB-tTAE1 cells after EBNA-1 induction by removal of tetracycline (Left) or suppression by addition of tetracycline (Right). Minimal and maximal comet lengths were determined in BJAB-tTAE1 kept with or without tetracycline for at least 3 weeks. (D) pATM (red) and pH2AX (green) were visualized by immunofluorescence in cells untreated (Right) or pretreated for 18 h with 2 μg/ml etoposide (ETO) (Left). The nuclei were stained with DAPI (blue).
EBNA-1 Induces the Production of ROS.
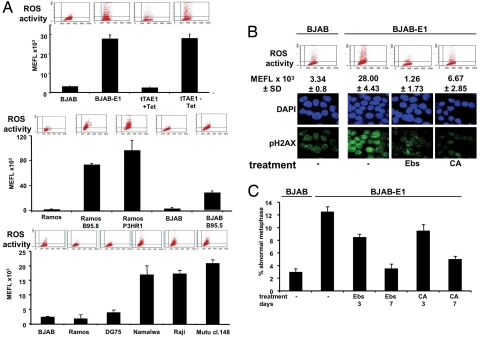
DNA damage is induced by DNA replication stress, as observed upon activation of oncogenes such as myc (15) and ras (16), or by a variety of exogenous or endogenous insults that converge on the production of ROS (17). Because EBNA-1 does not promote cellular DNA replication, we surmised that production of ROS might be involved in the induction of DNA damage. To investigate this possibility, control and EBNA-1–expressing cells were labeled with the membrane-permeable indicator 2,7-dichlorofluorescin diacetate (DCFDA), which becomes fluorescent upon oxidation. A >10-fold increase in ROS was observed in stable or inducible EBNA-1-expressing sublines of BJAB, and a similar increase was detected in EBV converted sublines of the EBV-negative BJAB and Ramos and in a panel of cell lines derived from EBV-carrying BLs (Fig. 2A). These findings confirm previous observations of elevated ROS levels in EBV-carrying B cells (18, 19) and demonstrate that EBNA-1 alone is sufficient for this effect. In line with the involvement of ROS in the induction of genomic instability, the intensity of pH2AX staining was decreased by treatment of BJAB-E1 and BJAB-tTAE1 with ROS scavengers, such as the glutathione peroxidase mimetic ebselen and citric acid (Fig. 2B). Furthermore, prolonged treatment reduced the number of aberrant chromosomes that returned to the levels observed in EBV-negative cells within ≈7 days of treatment (Fig. 2C), thus establishing a direct link between the induction of ROS, DNA damage, and chromosomal aberrations in EBNA-1-positive cells.
Fig. 2.
EBNA1 induces DNA damage and chromosomal aberrations via production of ROS. (A) The endogenous levels of ROS were determined by DCFDA staining in EBNA-1–positive and -negative BJAB (Top), in vitro EBV-converted BJAB and Ramos (Middle), and a panel of originally EBV-negative or -positive BL lines (Bottom). Representative FACS plots are shown for each cell line. The mean ± SD molecule equivalent of fluorescence (MEFL) of 3 experiments is shown. (B) Induction of DNA damage is abrogated by treatment with antioxidants. The cells were treated for 18 h with 3.5 μM ebselen (Ebs) or 1 mM citric acid (CA). Top:representative FACS plots and the mean ± SD MEFL of 3 independent experiments; Bottom: representative immunostaining for pH2AX. (C) Metaphase plates were analyzed in untreated cells and cells treated for the indicated time with 1.7 μM ebselen or 500 μM citric acid. Mean ± SD of 3 experiments.
EBNA-1 Activated the NADPH Oxides via Transcriptional Activation of NOX2.
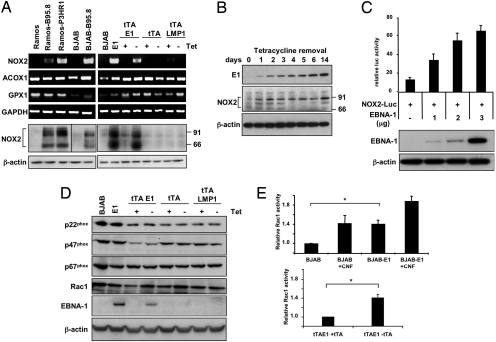
Accumulation of ROS may be caused by enhanced oxidative metabolism or by deregulation of enzymes involved in their production and conversion to nontoxic compounds (20). Because the increase of ROS was not abrogated by inhibition of the respiratory chain with natrium fluoride (Fig. S2 a and b), we took advantage of gene expression profiles available in public databases to look for possible mechanisms by which EBNA-1 could induce ROS. The expression levels of genes involved in ROS metabolism (Table S1) were extracted from a collection of 24 gene expression datasets, derived from 18 EBV-positive and -negative B cell lines (Table S2), and significance analysis of microarray was used to identify genes that are differentially regulated in EBV-carrying cells. Two genes involved in ROS production, Acyl-CoA oxigenase-1 (ACOX1) and cytochrome b-245 heavy chain (CYBB, NOX2), and the ROS scavenger glutathione peroxidase-1 (GPX1) were significantly up-regulated, whereas lysyl oxidase like-2 (LOXL2) was down-regulated in EBV-positive cells (Fig. S3 a and b). However, only NOX2 mRNA was strongly up-regulated in EBV-carrying Ramos and BJAB (Fig. 3A), whereas minor or no differences were observed for ACOX1, GPX1, and LOXL2 (data not shown). The up-regulation of NOX2 was confirmed by detection of the protein only in EBV-carrying cell lines. Because we did not observe a consistent correlation between the levels of NOX2 mRNA and the EBV latency type of the cell lines included in the analysis (Table S1, Fig. S3c), we surmised that EBNA-1 might be sufficient for NOX2 up-regulation. Confirming this possibility, higher levels of NOX2 mRNA and protein were detected in BJAB expressing stable or inducible EBNA-1, whereas LMP-1, a diagnostic marker of EBV latency III, had no effect (Fig. 3a). To explore the mechanism by which EBNA-1 may regulate NOX2, we took advantage of the virtually complete switch-off of EBNA-1 in BJAB-tTAE1 cultured in the presence of tetracycline. EBNA-1 is rapidly up-regulated in these cells upon removal of tetracycline, is readily detected after 24 h, and reaches maximal levels within 1 to 2 weeks. Up-regulation of EBNA-1 was accompanied by an equally rapid up-regulation of NOX2. The levels of NOX2 seemed to be maximal after only 24 h (Fig. 3B), suggesting that EBNA-1 may be directly involved in the regulation. To test this possibility, we constructed a reporter plasmid in which a fragment of the NOX2 promoter that contains several regulatory elements (21) drives expression of the firefly luciferase gene (Fig. S4). Because it is difficult to consistently achieve high levels of transfection in B lymphoma lines, the NOX2-positive promyelocytic leukemia line HL60 was used to assess the effect of EBNA-1 on transcription. Coexpression of NOX2-Luc with EBNA-1 resulted in a dose-dependent increase of luciferase activity (Fig. 3C). In keeping with the notion that NOX2 is preferentially expressed in the hematopoietic lineage, and supporting our failure to induce the production of ROS and expression of NOX2 by transfecting EBNA-1 in epithelial cells (Fig. S5 a and b), the NOX2-Luc reporter was inactive in HEK293 (Fig. S5c), HeLa, and TWO3 (data not shown) and was not induced by EBNA-1. Thus, EBNA-1 and transcription factors expressed in B cells seem to cooperate in regulating the NOX2 promoter.
Fig. 3.
EBNA-1 regulates the transcription of NOX2 and activation of the NADPH oxidase in EBV-positive cells. (A) The transcription levels of ACOX1, GPX1, NOX2, and GAPDH (control) were assayed by RT-PCR. NOX2 protein expression was visualized by Western blot. Beta-actin was used as loading control (Bottom). One representative experiment out of 3 performed with each cell line. (B) NOX2 was detected by Western blot in BJAB-tTAE1 after induction of EBNA-1 by removal of tetracycline. One representative experiment out of 3. (C) HL60 cells were cotransfected with the NOX2-Luc reporter and increasing amounts of the pCDNA3-FlagEBNA-1 plasmid. Relative luciferase activity was calculated as the ratio between the activity of NOX2-Luc and the maximal activity of a SV40-Luc reporter. All values were normalized to the activity of a cotransfected SV40-Renilla reporter. (D) EBNA-1 does not affect the expression of the NADPH oxidase subunits p22phox, p47phox, p67phox, and Rac1. Western blots were probed with the indicated specific antibodies. One representative experiment out of 3. (E) Rac1GTP was detected by the G-Lisa Rac Activation Assay (Cytoskeleton). Where indicated, the cells were treated for 4 h with 1 μg/ml cytotoxic necrotizing factor (CNF). The mean ± SD of 3 experiments is shown. *, BJAB/BJAB-E1, P < 0.04; BJAB-tTAE1 ± tetracycline, P < 0.05.
NOX2 (gp91phox) is the catalytic subunit of the NADPH oxidase expressed in leukocytes, which also contains the p22phox, p47phox, p40phox, and p67phox subunits (22). NOX2/p22phox heterodimers form the inactive flavinocytochrome b558 in the plasma membrane. Activation of the enzyme requires the phosphorylation-dependent binding of cytosolic p47phox to p22phox and subsequent recruitment of p40phox, p67phox, and activated Rac1GTP. We therefore asked whether the protein levels of p22phox, p47phox, p67phox, and Rac1 and Rac1 activation are also affected by EBNA-1. Western blot analysis of BJAB-E1 or BJAB carrying Tet-regulated EBNA-1 or LMP-1 did not reveal significant changes in the steady-state levels of p22phox, p47phox, p67phox, and Rac1 compared with the parental BJAB (Fig. 3d), whereas the levels of activated Rac1GTP were significantly increased in EBNA-1–positive cells (Fig. 3e). Thus, EBNA-1 expression is associated with selective up-regulation of NOX2, which seems to be sufficient for functional activation of the NADPH oxidase in B cells.
Activation of the NADPH Oxidase Induces DNA Damage.
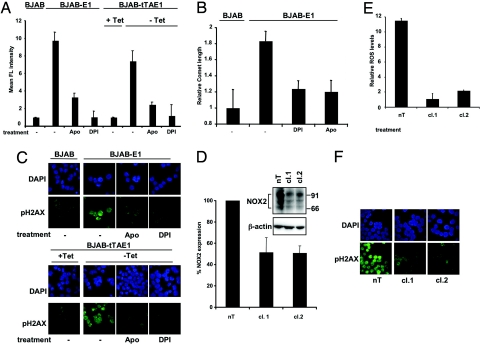
In the final set of experiments we asked whether activation of the NADPH oxidase is directly involved in the increase of ROS and induction of genomic instability in EBNA-1-expressing cells. BJAB-E1 and BJAB-tTAE1 were treated with the NADPH oxidase inhibitors diphenylene iodonium (DPI) and apocynin (Apo) or transduced with recombinant lentiviruses expressing NOX2-specific shRNAs. Treatment with DPI or Apo reduced the levels of ROS to those observed in EBNA-1-negative cells (Fig. 4A). DNA double-strand breaks detected by comet assay were also diminished by both treatments (Fig. 4B), and this correlated with a significant decrease of pH2AX (Fig. 4C). Transduction of BJAB-E1 or BJAB-tTAE1 (data not shown) with 2 lentiviruses expressing NOX2-specific shRNAs (cl.1, cl.2) resulted in ≈50% reduction of NOX2 expression (Fig. 4D), which correlated with a dramatic decrease in ROS levels (Fig. 4E) and with decreased DNA damage and DDR as assessed by pH2AX staining (Fig. 4F). Collectively, these findings demonstrate that NOX2 is responsible for the production of ROS and consequent induction of genomic instability in EBNA-1-expressing B cells.
Fig. 4.
Inhibition of the NADPH oxidase reverses the effect of EBNA-1. (A) Treatment with chemical inhibitors of the NADPH oxidase decreases the levels of ROS. The cells were pretreated with 100 μM Apo and/or 40 μM DPI for 18 h, and ROS activity was detected by DCFDA. Mean ± SD MEFL in 3 experiments. (B) Treatment with NADPH oxidase inhibitors decreases DNA damage. Neutral comet assays were performed in cells untreated or treated with Apo and DPI. Mean ± SD of comet length in 3 experiments. (C) Representative micrographs illustrating the decrease of pH2AX in cells treated with Apo and DPI. (D) BJAB-E1 cells were infected for 48 h with lentiviruses expressing nontargeting (nT) or NOX2-specific (cl.1, cl.2) shRNAs. NOX2 expression was detected by Western blots, and the intensity of the specific bands was quantified by densitometry. The mean ± SD of NOX2 levels in 3 experiments is shown. (E) NOX2 knockdown decreases the endogenous levels of ROS. ROS activity was detected by labeling with DCFDA. Mean ± SD fluorescence intensity in 3 experiments. (F) Representative micrographs illustrating the decreased pH2AX fluorescence after NOX2 knockdown are shown.
Discussion
This study addresses a longstanding question of EBV oncogenesis by linking EBNA-1 to the induction of genomic instability, a key feature of malignant cells that contributes to the progressive selection of clones with enhanced growth and metastatic potential. Several latency-associated EBV proteins have been proposed to promote genomic instability by suppressing the checkpoint machineries that safeguard genome integrity (11, 12, 23). However, their overall contribution to the tumor-promoting effect of EBV is likely to be limited, given that the viral proteins associated with these effects are either not expressed or expressed only in a subset of the tumors.
Only EBNA-1 is regularly detected in all EBV-associated malignancies. Our findings demonstrate that expression of EBNA-1 alone is sufficient to initiate a cascade of events that will eventually result in the generation of chromosomal aberrations and double-strand DNA breaks. This type of DNA damage is induced by cellular oncoproteins, such as Ras (16) and Myc (15), that promote aberrant DNA replication by augmenting the number of active replicons and/or altering the progression of DNA replication forks. EBNA-1 does not interfere with cellular DNA replication but could nevertheless synergize with cellular oncogenes by inducting S-phase-independent DNA damage, thus providing a rational explanation for the increased incidence of EBV-carrying variants of tumors, such as BL, in which oncogene activation is believed to be the primary initiating event. The capacity of EBNA-1 to induce a mutator phenotype is substantiated by the increased frequency of ongoing somatic hypermutations in EBV-carrying BL lines (24) and in biopsies from EBV-positive endemic and HIV-associated BLs compared with EBV-negative tumors (25).
The increased levels of ROS, together with the reversal of chromosomal aberrations, DNA damage, and DDR by treatment with antioxidants, demonstrate that EBNA-1 promotes genomic instability via induction of ROS. Earlier studies have reported increased levels of ROS during primary EBV infection (26) and in EBV-carrying tumor cells (18, 19), attributing this effect to virion-mediated triggering of the CR2 receptor (27), induction of IL-10 by the viral EBV-encoded, untranslated RNAs (EBERs) (18), or induction of lipoxygenases by unknown viral genes (19). Although it is possible that EBV might have evolved different strategies for inducing ROS during different phases of the infection, the selective up-regulation of NOX2 and activation of the NADPH oxidase in EBNA-1-positive B cells suggest a pivotal role of ROS in growth transformation. Together with reactive nitrogen species, oxygen-free radicals, including O2−, H2O2, and OH−, play a dual role in cell physiology and pathology (20). In addition to inducing oxidative DNA lesions, they oxidize proteins and lipids and interfere thereby with a plethora of signaling cascades that regulate cell growth, differentiation, and apoptosis. Growth transformation of B lymphocytes is essential for the establishment of persistent EBV infection and is critically required for entry of the virus into the latent reservoir in memory B cells. Through the induction of ROS, EBNA-1 may promote transformation by initiating signaling cascades that are further activated by other viral genes expressed in latently infected cells, exemplified by the activation of NF-κB by LMP-1. This scenario is supported by the observation that the establishment of EBV-immortalized lymphoblastoid cell lines is promoted by oxidative stress (28) and inhibited by antioxidants (29). Interestingly, the transport of extracellular cystine, a rate-limiting substrate for the synthesis of the endogenous antioxidant glutathione, is deficient in B cells that may therefore be primed to respond to small changes of intracellular ROS (30). Collectively, these findings suggest that the induction of genomic instability may be an accident of the strategy used by EBV to colonize the B cell compartment. The potentially dangerous consequences of this strategy are kept under strict control in healthy virus carriers by potent virus-specific immune responses that restrict the proliferation of virus-infected cells.
Our findings have interesting implications for the pathogenesis of EBV-associated PTLD. The incidence of PTLD has increased since cyclosporin A (CsA) became a treatment of choice for transplant immunosuppression (31). In addition to its potent immunosuppressive activity, CsA also induces oxidative stress (28) and could thereby enhance the effect of EBNA-1. The observation that PTLD lymphomas are almost invariably oligo- or monoclonal (32) implies that clones with enhanced growth potential are selected in vivo. EBNA-1 could promote the rapid generation of these variants by driving genomic instability via sustained production of ROS. This effect may be enhanced by the expression of other viral proteins (e.g., EBNA3C and LMP-1) that interfere with DNA repair. A better understanding of the cellular and molecular mechanisms that regulate these events has a clear potential to benefit the management of symptomatic EBV infections and the clinical practice of EBV associated malignancies.
Materials and Methods
Cell Lines.
Details of the cell lines used in this study are shown in Table S3. A BJAB subline expressing a tetracycline-regulated EBNA-1 (BJAB-tTAE1) was produced by transfecting the pTRE2pur-FlagEBNA-1 plasmid into BJAB-tTA cells that carry a tet-off-regulated transactivator (detailed in SI Materials and Methods).
Scoring of Chromosomal Abnormalities.
Metaphase arrest was induced in rapidly growing cells by treatment with colcemide (KaryoMAX; Invitrogen), and chromosome spreads were mounted in DAPI containing Vectashield (Vector Laboratories). Digital images were captured with a LEITZ-BMRB fluorescence microscope (Leica) equipped with a CCD camera (Hamamatsu Photonics). At least 50 metaphases were examined for the presence of dicentric chromosomes, chromosome fragments, rings, gaps, and double minutes. Metaphases containing one aberration or more were scored as abnormal.
Detection of DNA Double-Strand Breaks and DNA Damage Response.
Comet assays were performed as described by Blasiak et al. (33). Phosphorylated ATM and H2AX were detected by immunofluorescence using specific antibodies (Upstate and Novus Biologicals, respectively) and goat antirabbit or goat antimouse IgG Alexa Fluor 488 (Molecular Probes, Invitrogen).
Analysis of EBV-Regulated Genes.
Gene expression datasets from 18 EBV-negative and EBV-carrying BL lines and EBV-transformed lymphoblastoid cell lines (LCLs) (Table S2) were extracted from the gene expression profiles of 336 human B cell phenotypes representative of normal, transformed, and experimentally manipulated B cells obtained using the Affymetrix GeneChip HG-U95Av2 array that contains ≈10,000 human genes (PubMed ID: 15778709; National Center for Biotechnology Information Gene Expression Omnibus: GSE2350). Genes involved in ROS metabolism (Table S1) were identified on the basis of their annotation in the Gene Ontology database. One hundred twenty-one probe sets corresponding to 103 of the 134 genes were present in the HG-U95Av2 array. Significance analysis of microarray was used to identify genes that are differentially regulated in EBV-positive compared with EBV-negative cells. The expression of 4 genes showing a cut-off δ value ≥2 was examined by RT-PCR, and NOX2 protein expression was detected by Western blots.
NOX2 Promoter Activity.
A firefly luciferase reporter plasmid was constructed by cloning a fragment corresponding to nucleotide −533 to +6 of the human NOX2 gene into the pGL3-Enhancer vector (Promega). HL60 cells were transfected with the NOX2-Luc reporter plasmid either alone or with increasing amount of the EBNA-1-expressing plasmid pCDNA3-FlagEBNA-1. A Renilla luciferase plasmid was cotransfected in all samples for normalization of transfection efficiency.
Inhibition of NOX2.
For chemical inhibition of NOX2 activity, the cells were treated overnight with 0.1 mM Apo or 40 μM DPI (both from Sigma–Aldrich). Lentiviruses expressing NOX2-specific shRNAs (TRC0000064588, cl.1, TRC0000064590, cl.2; Mission shRNA NOX2 transduction particles; Invitrogen) and nontarget shRNA (Mission nontarget shRNA control transduction particles; Invitrogen) were used for transduction of BJAB-E1 cells at 2.7, 2.9, and 9.6 transfection units per milliliter, respectively.
Supplementary Material
Acknowledgments.
We thank Martin Rowe (University of Birmingham, United Kingdom) for the BJAB-tTA cell line and many colleagues for helpful discussions. This work was supported by grants awarded by the Swedish Cancer Society, the Swedish Medical Research Council, and Karolinska Institutet; and by the European Community Integrated Project on Infection and Cancer (INCA) project LSHC-CT-2005-018704.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 2091.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810619106/DCSupplemental.
References
- 1.Kieff E, Liebowitz D. In: Virology. 2nd Ed. Fields B, Knipe D, editors. New York: Raven Press; 1990. pp. 1889–1920. [Google Scholar]
- 2.Young LS, Rickinson AB. Epstein–Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 3.Leight ER, Sugden B. EBNA-1: A protein pivotal to latent infection by Epstein–Barr virus. Rev Med Virol. 2000;10:83–100. doi: 10.1002/(sici)1099-1654(200003/04)10:2<83::aid-rmv262>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 4.Hong M, et al. Suppression of Epstein–Barr nuclear antigen 1 (EBNA1) by RNA interference inhibits proliferation of EBV-positive Burkitt's lymphoma cells. J Cancer Res Clin Oncol. 2006;132:1–8. doi: 10.1007/s00432-005-0036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy G, Komano J, Sugden B. Epstein–Barr virus provides a survival factor to Burkitt's lymphomas. Proc Natl Acad Sci USA. 2003;100:14269–14274. doi: 10.1073/pnas.2336099100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang MS, et al. Epstein–Barr virus nuclear antigen 1 does not induce lymphoma in transgenic FVB mice. Proc Natl Acad Sci USA. 2005;102:820–825. doi: 10.1073/pnas.0408774102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Humme S, et al. The EBV nuclear antigen 1 (EBNA1) enhances B cell immortalization several thousandfold. Proc Natl Acad Sci USA. 2003;100:10989–10994. doi: 10.1073/pnas.1832776100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zattara-Cannoni H, et al. Unusual chromosome abnormalities in primary central nervous system lymphoma. Leuk Lymphoma. 1996;21:515–517. doi: 10.3109/10428199609093453. [DOI] [PubMed] [Google Scholar]
- 9.Stollmann B, Fonatsch C, Havers W. Persistent Epstein–Barr virus infection associated with monosomy 7 or chromosome 3 abnormality in childhood myeloproliferative disorders. Br J Haematol. 1985;60:183–196. doi: 10.1111/j.1365-2141.1985.tb07399.x. [DOI] [PubMed] [Google Scholar]
- 10.Chan WY, et al. Recurrent genomic aberrations in gastric carcinomas associated with Helicobacter pylori and Epstein–Barr virus. Diagn Mol Pathol. 2002;11:127–134. doi: 10.1097/00019606-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Parker GA, Touitou R, Allday MJ. Epstein–Barr virus EBNA3C can disrupt multiple cell cycle checkpoints and induce nuclear division divorced from cytokinesis. Oncogene. 2000;19:700–709. doi: 10.1038/sj.onc.1203327. [DOI] [PubMed] [Google Scholar]
- 12.Chen YR, et al. Epstein–Barr virus latent membrane protein 1 represses DNA repair through the PI3K/Akt/FOXO3a pathway in human epithelial cells. J Virol. 2008;82:8124–8137. doi: 10.1128/JVI.00430-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamranvar SA, Gruhne B, Szeles A, Masucci MG. Epstein–Barr virus promotes genomic instability in Burkitt's lymphoma. Oncogene. 2007;26:5115–5123. doi: 10.1038/sj.onc.1210324. [DOI] [PubMed] [Google Scholar]
- 14.Raptis S, Bapat B. Genetic instability in human tumors. Exs. 2006;96:303–320. doi: 10.1007/3-7643-7378-4_13. [DOI] [PubMed] [Google Scholar]
- 15.Dominguez-Sola D, et al. Non-transcriptional control of DNA replication by c-Myc. Nature. 2007;448:445–451. doi: 10.1038/nature05953. [DOI] [PubMed] [Google Scholar]
- 16.Di Micco R, et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- 17.Slupphaug G, Kavli B, Krokan HE. The interacting pathways for prevention and repair of oxidative DNA damage. Mutat Res. 2003;531:231–251. doi: 10.1016/j.mrfmmm.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Cerimele F, et al. Reactive oxygen signaling and MAPK activation distinguish Epstein–Barr Virus (EBV)-positive versus EBV-negative Burkitt's lymphoma. Proc Natl Acad Sci USA. 2005;102:175–179. doi: 10.1073/pnas.0408381102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belfiore MC, et al. Involvement of 5-lipoxygenase in survival of Epstein–Barr virus (EBV)-converted B lymphoma cells. Cancer Lett. 2007;254:236–243. doi: 10.1016/j.canlet.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 20.D'Autreaux B, Toledano MB. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 21.Kumatori A, Yang D, Suzuki S, Nakamura M. Cooperation of STAT-1 and IRF-1 in interferon-gamma-induced transcription of the gp91(phox) gene. J Biol Chem. 2002;277:9103–9111. doi: 10.1074/jbc.M109803200. [DOI] [PubMed] [Google Scholar]
- 22.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 23.Liu MT, et al. Epstein–Barr virus latent membrane protein 1 induces micronucleus formation, represses DNA repair and enhances sensitivity to DNA-damaging agents in human epithelial cells. Oncogene. 2004;23:2531–2539. doi: 10.1038/sj.onc.1207375. [DOI] [PubMed] [Google Scholar]
- 24.Chapman CJ, Zhou JX, Gregory C, Rickinson AB, Stevenson FK. VH and VL gene analysis in sporadic Burkitt's lymphoma shows somatic hypermutation, intraclonal heterogeneity, and a role for antigen selection. Blood. 1996;88:3562–3568. [PubMed] [Google Scholar]
- 25.Bellan C, et al. Immunoglobulin gene analysis reveals 2 distinct cells of origin for EBV-positive and EBV-negative Burkitt lymphomas. Blood. 2005;106:1031–1036. doi: 10.1182/blood-2005-01-0168. [DOI] [PubMed] [Google Scholar]
- 26.Lassoued S, et al. Epstein–Barr virus induces an oxidative stress during the early stages of infection in B lymphocytes, epithelial, and lymphoblastoid cell lines. Mol Cell Biochem. 2008;313:179–186. doi: 10.1007/s11010-008-9755-z. [DOI] [PubMed] [Google Scholar]
- 27.Kim YS, et al. Ligation of centrocyte/centroblast marker 1 on Epstein–Barr virus–transformed B lymphocytes induces cell death in a reactive oxygen species–dependent manner. Hum Immunol. 2006;67:795–807. doi: 10.1016/j.humimm.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Ranjan D, Siquijor A, Johnston TD, Wu G, Nagabhuskahn M. The effect of curcumin on human B-cell immortalization by Epstein–Barr virus. Am Surg. 1998;64:47–51. discussion 51–42. [PubMed] [Google Scholar]
- 29.Chen C, et al. Cyclosporin A-induced lipid and protein oxidation in human B-cells and in Epstein–Barr virus-infected B-cells is prevented by antioxidants. J Invest Surg. 2008;21:201–208. doi: 10.1080/08941930802262223. [DOI] [PubMed] [Google Scholar]
- 30.Banjac A, et al. The cystine/cysteine cycle: A redox cycle regulating susceptibility versus resistance to cell death. Oncogene. 2008;27:1618–1628. doi: 10.1038/sj.onc.1210796. [DOI] [PubMed] [Google Scholar]
- 31.Penn I. Incidence and treatment of neoplasia after transplantation. J Heart Lung Transplant. 1993;12:S328–S336. [PubMed] [Google Scholar]
- 32.Thomas JA, Allday MJ, Crawford DH. Epstein–Barr virus-associated lymphoproliferative disorders in immunocompromised individuals. Adv Cancer Res. 1991;57:329–380. doi: 10.1016/s0065-230x(08)61003-9. [DOI] [PubMed] [Google Scholar]
- 33.Blasiak J, Kowalik J, Malecka-Panas E, Drzewoski J, Wojewodzka M. DNA damage and repair in human lymphocytes exposed to three anticancer platinum drugs. Teratog Carcinog Mutagen. 2000;20:119–131. doi: 10.1002/(sici)1520-6866(2000)20:3<119::aid-tcm3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.