Abstract
R-Spondin1 (RSpo1) is a novel secreted protein that augments canonical Wnt/β-catenin signaling. We injected recombinant RSpo1 protein into transgenic Wnt reporter TOPGAL mice and have identified the oral mucosa as a target tissue for RSpo1. Administration of RSpo1 into normal mice triggered nuclear translocation of β-catenin and resulted in increased basal layer cellularity, thickened mucosa, and elevated epithelial cell proliferation in tongue. We herein evaluated the therapeutic potential of RSpo1 in treating chemotherapy or radiotherapy-induced oral mucositis in several mouse models. Prophylactic treatment with RSpo1 dose-dependently overcame the reduction of basal layer epithelial cellularity, mucosal thickness, and epithelial cell proliferation in tongues of mice exposed to whole-body irradiation. RSpo1 administration also substantially alleviated tongue mucositis in the oral cavity of mice receiving concomitant 5-fluorouracil and x-ray radiation. Furthermore, RSpo1 significantly reduced the extent of tongue ulceration in mice receiving a single fraction, high dose head-only radiation in a dose-dependent manner. Moreover, combined therapy of RSpo1 and keratinocyte growth factor resulted in complete healing of tongue ulcers in mice subjected to snout-only irradiation. In conclusion, our results demonstrate RSpo1 to be a potent therapeutic agent for oral mucositis by enhancing basal layer epithelial regeneration and accelerating mucosal repair through up-regulation of Wnt/β-catenin pathway.
Keywords: RSpo1, tongue ulceration, head-only radiation, TOPGAL, 5-FU
The R-Spondin (RSpo) family of secreted proteins is implicated in the amplification of canonical Wnt/β-catenin signaling pathway both in vitro and in vivo (1–4). All four RSpo family members contain an N-terminal signal peptide, two furin-like domains, followed by a thrombospondin type1 domain, and a C-terminal region rich with positively charged amino acids (5, 6). The Wnt/β-catenin signaling plays a pivotal role in both embryonic development and homeostatic self-renewal of adult tissues, including gastrointestinal (GI) and oral mucosa (7). Abdominal distension along the entire intestinal tract with a correlation of nuclear translocation of β-catenin in mucosal epithelial cells was seen in both RSpo1 transgenic mice and normal mice injected with recombinant RSpo1 protein (1, 8, 9). Additionally, mucosal injury in mouse models of enterocolitis could be ameliorated through RSpo1-mediated stimulation of epithelial cell growth in GI mucosa (8).
Oral mucositis characterized by mucosal damage and inflammation in the oral cavity is a common complication of chemotherapy and/or radiotherapy for cancer patients with head and neck tumors and hematological malignancies (10). Impairment of the regenerative capacity of the oral mucosal epithelium leads to atrophy, ulceration, and loss of barrier function during pathogenesis and progression of oral mucositis (11). As a consequence, oral mucositis is frequently associated with pain, increased risk of infection, and can lead to impaired quality of life in affected individuals (12). Oral mucositis also limits the ability of patients to tolerate optimal anti-tumor treatment regimens, thus compromising cancer therapy outcomes and overall patient survival (13).
Mucosal epithelial growth factors such as keratinocyte growth factor (KGF) have been shown to be efficacious in treating experimental animal models of chemotherapy or radiotherapy-induced oral mucositis, and is the first approved biologic therapy for human cancer patients with oral mucositis (14–20). However, KGF has been shown to enhance the growth of human epithelial tumor cell lines in vitro and to increase the rate of tumor cell growth in human carcinoma xenograft models, probably by binding to its receptor present on the surface of these cells (21). In comparison, we have recently demonstrated that RSpo1 acts as a GI mucosal proliferative agent by triggering the physiological regenerative mechanism through amplification of the Wnt/β-catenin pathway (4, 8, 9). While the biological outcome is the growth of the GI mucosal epithelium, RSpo1 activity appears to be dependent on Wnt ligands (4, 8, 9).
To explore the in vivo role of RSpo1 in the augmentation of the canonical Wnt/β-catenin pathway, we chose the Wnt reporter TOPGAL mice (22) and identified oral mucosa as a target tissue for RSpo1 responsiveness. Here, we show that RSpo1 stimulates epithelial cell growth in the tongue mucosa and significantly reduces oral mucositis induced by either chemotherapy or radiotherapy in mice. RSpo1 regenerates mouse tongue mucosa through augmentation of Wnt/β-catenin pathway in squamous epithelium. Moreover, RSpo1 attenuates head-only irradiation-induced overt tongue ulceration in mice both alone and in combination with KGF. Taken together, our results support RSpo1 as a novel approach for potential treatment of oral mucosal damage in human cancer patients receiving intensive chemotherapy and/or radiotherapy.
Results
RSpo1 Augments the Canonical Wnt/β-Catenin Signaling in TOPGAL Mouse Tongue.
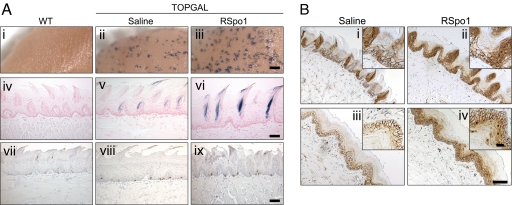
To explore the in vivo role of RSpo1 in the augmentation of canonical Wnt/β-catenin signaling in target tissues, we used Wnt reporter TOPGAL mice. Recombinant RSpo1 protein was injected into TOPGAL mice and multiple tissues were analyzed including tongue, stomach, lung, liver, small intestine, colon, skin, and reproductive organs. While β-galactosidase activity was broadly detected in dorsal tongue with whole-mount Bluo-Gal staining (Fig. 1Aii), an increase in β-galactosidase staining was also detected in TOPGAL mice injected with RSpo1 (Fig. 1Aiii). To further identify RSpo1 responsive cells, mice tongues were sectioned and examined for β-galactosidase activity. Elevation of LacZ gene expression was detected in the dorsal mucosal epithelium of TOPGAL mice injected with RSpo1, especially at sites underneath filliform papillae (Fig. 1A e and f). No β-galactosidase activity was found on the TOPGAL mouse tongue besides dorsal mucosa. There was no detectable β-galactosidase activity in wild-type control mice (Fig. 1 Ai and Aiv).
Fig. 1.
Effect of RSpo1 on tongue mucosa in TOPGAL mice. (A) (i–iii) Whole-mount Bluo-Gal-stained mouse dorsal tongues. (Scale bar, 0.5 mm.) (iv–vi) Coronal sections of Bluo-Gal-stained mouse dorsal tongues with nuclear fast red counterstain. (Scale bar, 100 μm.) (vii–ix) BrdU immunocytochemistry of mouse dorsal tongue mucosa. (Scale bar, 100 μm.) (i, iv, and vii) WT, (ii, v, and viii) TOPGAL treated with saline, (iii, vi, and ix) TOPGAL treated with RSpo1. (B) β-catenin immunohistochemical study in dorsal (i and ii) and ventral (iii and iv) tongues of TOPGAL mice. (i and iii) Saline; (ii and iv) RSpo1. (Scale bar, 100 μm; Insets, 25 μm.)
Administration of RSpo1 protein also enhanced basal layer epithelial cell proliferation, as revealed by bromodeoxyuridine (BrdU) immunohistochemistry, in the dorsal mucosa in TOPGAL mouse tongue (Fig. 1Avii–ix). A similar effect was also found in the mucosal basal layer of the ventral tongue in TOPGAL mice, although β-galactosidase activity was not detected in the ventral tongue (data not shown).
To confirm the augmentation of canonical Wnt/β-catenin pathway by RSpo1, we performed β-catenin immunohistochemical staining on tongue sections from TOPGAL mice. In control TOPGAL mice, β-catenin reactivity was detected predominately on the cell surface of the mucosal epithelium in both dorsal and ventral tongue (Fig. 1 Bi and Biii). In comparison, activation of the Wnt pathway was evident in RSpo1-treated TOPGAL mice as indicated by nuclear β-catenin staining in mucosal epithelium in both dorsal and ventral tongue (Fig. 1 Bii, Biv, and Biv).
Taken together, our data indicate that augmentation of the Wnt pathway by RSpo1 in mouse tongue is associated with proliferation of the tongue mucosa.
RSpo1 Induces Mucosal Hyperplasia in Mouse Tongue by Antagonizing Dickkopf-1 (Dkk1).
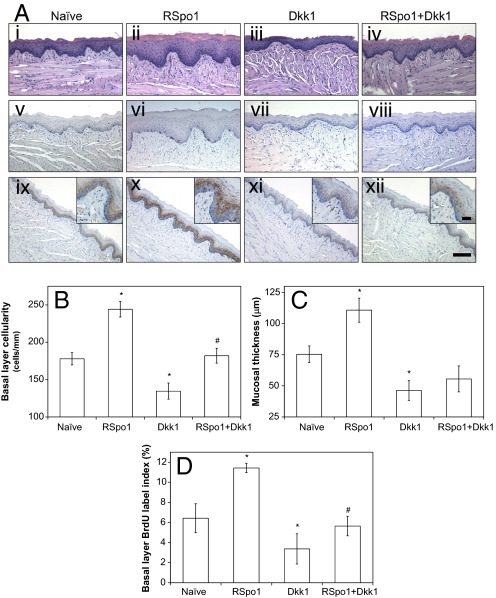
We have previously shown that RSpo1 modulates the canonical Wnt/β-catenin signaling by relieving the Dkk1 inhibition imposed on the Wnt pathway, and subsequently increasing cell surface levels of the Wnt pathway coreceptor LRP6 (4). To further define the physiological mechanism of RSpo1 in regulating mouse tongue mucosal homeostasis, we treated mice with RSpo1 alone or in combination with an adenoviral administration of Dkk1. While mucosal hyperplasia was detected in RSpo1 injected animals (Fig. 2Aii), administration of adenoviral Dkk1 to mice resulted in mucosal hypoplasia and reduction of mucosal thickness in the tongue (Fig. 2Aiii), suggesting that Wnt signaling plays a role in regulating the growth of the tongue mucosa. Furthermore, RSpo1 treatment antagonized Dkk1-mediated mucosal damage, thus partially restoring the integrity of the tongue mucosa (Fig. 2Aiv).
Fig. 2.
Effect of RSpo1 and/or Dkk1 on mouse tongue mucosa. (A) H&E staining (i–iv) BrdU immunohistochemistry, (v–viii) and β-catenin immunohistochemistry (ix–xii) of mouse ventral tongue mucosa. i, v, and ix, naïve; ii, vi, and x, RSpo1; iii, vii, and xi, Dkk1; viii, and iv, RSpo1+Dkk1. (Scale bar, 100 μm; Insets, 25 μm .) (B) Basal layer epithelial cellularity in mouse ventral tongue mucosa. (C) Mucosal thickness in mouse ventral tongue. (D) Basal epithelial layer BrdU labeling index in mouse ventral tongue. *P < 0.05 (RSpo1 vs. naïve, Dkk1 vs. naïve); #P < 0.05 (RSpo1+Dkk1 vs. Dkk1).
Administration of RSpo1 significantly increased basal layer epithelial cell density (Fig. 2B, P < 0.05), mucosal thickness (Fig. 2C, P < 0.05), and mitotic cell number in the basal layer epithelium (Fig. 2A v–viii, and D, P < 0.05) of the mouse ventral tongue mucosa. In contrast, adenoviral Dkk1 administration significantly reduced basal layer epithelial cellularity (Fig. 2B, P < 0.05), mucosal thickness (Fig. 2C, P < 0.05), and basal layer cell proliferative index (Fig. 2D, P < 0.05) in the mucosa of mouse ventral tongue. In comparison, addition of RSpo1 partially rescued Dkk1-induced inhibition of basal layer cellularity (Fig. 2B, P < 0.05), mucosal thickness (Fig. 2C, P = 0.12), and basal layer cell proliferation (Fig. 2D, P < 0.05) in mouse ventral tongue mucosa.
Immunohistochemistry of β-catenin in mouse ventral tongue mucosa (Fig. 2A ix–xii) also demonstrated that RSpo1 induced nuclear localization of β-catenin (Fig. 2Ax) in mice that received adenoviral Dkk1 (Fig. 2Axii). These results confirmed our previous findings that RSpo1 acts as an antagonist of the Wnt pathway inhibitor Dkk1 and suggest that RSpo1-mediated tongue mucosal proliferation is achieved through up-regulation of the canonical Wnt/β-catenin pathway.
The hyperplastic effect of RSpo1 in the oral mucosa is not restricted to a single mouse strain. Similar biological activity was also seen in BALB/c [supporting information (SI) Fig. S1], BDF-1, and CD-1 mice (data not shown). As a Wnt/β-catenin pathway modulator, RSpo1 appears to play a key role in regulating epithelial cell proliferation and regeneration in mouse tongue mucosa.
RSpo1 potentially plays a central role in enhancing the endogenous canonical Wnt signaling pathway required for tissue homeostasis and repair. Therefore, RSpo1 could be used for treating tongue mucosal hypoplasia, a hallmark of oral mucositis. To evaluate the therapeutic potential of RSpo1, we tested its ability to reduce experimental oral mucositis in several commonly-used mouse models.
RSpo1 Treatment Inhibits Whole-Body Irradiation-Induced Oral Mucositis in Mice.
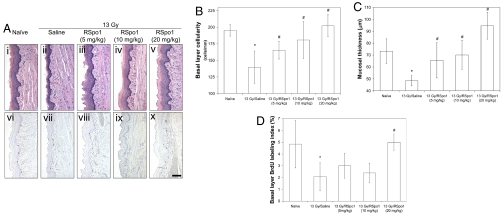
To test the protective effect of RSpo1 treatment against ionizing radiation-induced oral mucositis, we induced oral mucositis by exposing BDF-1 mice to whole-body radiation at a single fraction of 13 Gy. Mice were pretreated with 3 daily injections of RSpo1 (5 to 20 mg/kg) before irradiation, and mouse tongue tissue was harvested 4 days after radiation and examined for extent of mucosal damage. As shown in Fig. 3A, while radiation caused extensive mucosal injury characterized by reduced mucosal basal layer epithelial cellularity and mucosal thickness in ventral tongue of saline-treated mice (Fig. 3Ai and Aii), RSpo1 treatment protected mice from whole-body radiation-induced ventral tongue mucosal erosion in a dose-dependent manner (Fig. 3A iii–v). Likewise, BrdU immunostaining showed that RSpo1 administration overrode irradiation-induced inhibition of ventral tongue mucosal basal layer epithelial cell proliferation in a dose-dependent fashion (Fig. 3A vi–x).
Fig. 3.
Effect of RSpo1 on tongue mucosa in whole-body irradiated mice. (A) Microscopic images of H&E staining (i–v) and BrdU immunohistochemistry (vi–x) of ventral tongue mucosa in mice. i and vi, naïve; ii and vii, 13 Gy/saline; iii and viii, 13 Gy/RSpo1 (5 mg/kg); iv and ix, 13 Gy/RSpo1 (10 mg/kg); v and x, 13 Gy/RSpo1 (20 mg/kg). (Scale bar, 100 μm.) (B) Basal layer epithelial cellularity in ventral tongue mucosa in mice. (C) Mucosal thickness in ventral tongue in mice. (D) BrdU labeling index in basal epithelial layer of mouse ventral tongue. *P < 0.05 (13 Gy/saline vs. naïve); #P < 0.05 (13 Gy/RSpo1 vs. 13 Gy/saline).
Morphometric analysis of hematoxylin and eosin (H&E) or BrdU-stained ventral tongue sections confirmed the protective effect of RSpo1 in treating whole-body radiation-induced oral mucositis in mice. While 13 Gy radiation reduced mucosal basal layer epithelial cellularity in ventral tongue from 195 cells/mm in naïve mice to 140 cells/mm (P < 0.05), RSpo1 treatment significantly increased basal layer epithelial cellularity in ventral tongue mucosa to 166 (P < 0.05), 181 (P < 0.05), and 203 cells/mm (P < 0.05) at respective doses of 5, 10, and 20 mg/kg in mice receiving 13 Gy whole-body radiation (Fig. 3B). Likewise, while whole-body radiation thinned the ventral tongue mucosa in mice from 73.3 μm in naïve to 48.4 μm (P < 0.05), administration of RSpo1 (5, 10, and 20 mg/kg) dose-dependently restored mucosal thickness to 65.6 (P < 0.05), 70.1 (P < 0.05), and 94.6 μm, respectively (P < 0.05) (Fig. 3C). Consistent with the above observations, RSpo1 also reversed the radiation-induced inhibition of basal layer epithelial cell mitosis in mouse ventral tongue mucosa (Fig. 3D).
A similar protective effect of RSpo1 was also seen in dorsal tongue mucosa and cheek mucosa in the oral cavity of irradiated mice (data not shown). These results demonstrate that RSpo1 acts as a potent epithelial regenerative protein and is an effective therapeutic agent in reducing irradiation-induced mucosal injury in mouse oral cavity.
To further assess the therapeutic effects of RSpo1 during oral mucosal injury, we also established an oral mucositis model using the chemotherapeutic agent CPT-11. Similar to the irradiation-induced oral mucositis model, injection of RSpo1 alleviated CTP-11-induced mucosal damage to the mouse tongue (Fig. S2). We also found that RSpo1 reduced the severity of oral mucositis in mice receiving 5-fluorouracil (5-FU), another cytoablative agent (data not shown). Together, our data indicate that augmentation of the canonical Wnt/β-catenin pathway by RSpo1 triggers epithelial proliferation and subsequently restores the mucosal barrier in mice exposed to either radiotherapy or chemotherapy.
Administration of RSpo1 Suppresses Concomitant Chemotherapy and Radiation-Induced Oral Mucositis in Mice.
To further study the therapeutic potential of RSpo1 in healing tongue mucosa, we next developed an oral mucositis mouse model that is induced by concomitant chemotherapy and radiotherapy, a preclinical setup that is more representative of human cancer patients. Oral mucositis was induced in mice using 5-FU (50 mg/kg, qdx2) followed by a relatively low dose of whole-body radiation (10 Gy, single fraction).
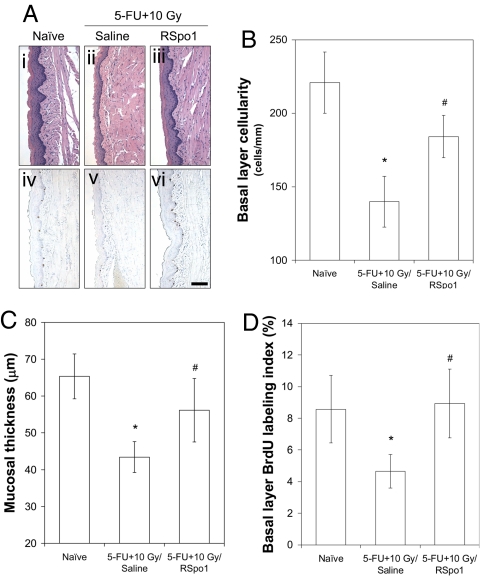
Saline control mice with 5-FU and 10 Gy radiation developed severe oral mucositis in the ventral tongue characterized by reduced mucosal basal layer cellularity, thinned mucosal layer, and inhibited mucosal basal layer epithelial cell proliferation (Fig. 4A). Concomitant 5-FU and 10 Gy radiation significantly reduced the mucosal basal layer cellularity in mouse ventral tongue from 221 cells/mm in naïve mice to 140 (P < 0.05) (Fig. 4B). However, RSpo1 partially increased mucosal basal layer cellularity in ventral tongue up to 184 cells/mm (P < 0.05) in mice treated with both 5-FU and 10 Gy irradiation (Fig. 4B). While combined 5-FU and 10 Gy radiation significantly eroded mucosal thickness to 43.4 μm (P < 0.05) in comparison to 65.3 μm in naïve mice, RSpo1 treatment markedly increased mucosal thickness in ventral tongue to 56.1 μm in mice receiving concomitant 5-FU and 10 Gy radiation (Fig. 4C). BrdU morphometry also revealed that RSpo1 stimulated the basal layer epithelial cell proliferation in the ventral tongue of mice exposed to both 5-FU and 10 Gy irradiation (Fig. 4D). These results demonstrate that RSpo1 ameliorates oral mucositis in mice induced by concomitant chemotherapy and radiotherapy, by repopulating epithelial cells within the tongue mucosa.
Fig. 4.
Characterization of RSpo1 on tongue mucosa in mice receiving combined treatment of 5-FU and whole-body radiation. (A) Representative microscopic images of H&E staining (i–iii) and BrdU immunohistochemistry (iv–vi) from mouse ventral tongue mucosa. (i and iv) Naïve, (ii and v) (5-FU + 10 Gy)/saline, (iii and vi) (5-FU + 10 Gy)/RSpo1. (Scale bar, 100 μm.) (B) Epithelial cellularity of ventral tongue mucosal basal layer in mice. (C) Ventral tongue mucosal thickness in mice. (D) Epithelial cell BrdU labeling index in mouse ventral tongue mucosal basal layer. *P < 0.05 [(5-FU + 10 Gy)/saline vs. naïve]; #P < 0.05 [(5-FU + 10 Gy)/RSpo1 vs. (5-FU + 10 Gy)/saline].
RSpo1 Reduces Overt Tongue Ulceration in Mice Induced by High Dose, Head (Snout)-Only Radiation.
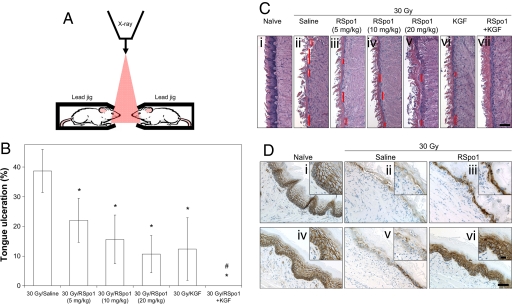
Tongue ulceration represents the mucosal damage seen in high grade oral mucositis in cancer patients. To evaluate the therapeutic potential of RSpo1 during ulcerative oral mucositis, we have used a mouse model that recapitulates the clinical features of severe oral mucositis. Herein, we designed custom-made mouse jigs with lead shields (1 inch thick) so that the radiation could be limited to the snout region of mouse heads (see Fig. 5A for illustration). In preliminary studies, we demonstrated the correlation of radiation dose to degree of tongue mucosal damage and determined that a single fraction of high level radiation at 30 Gy directly delivered to the mouse heads (snout area) reliably resulted in overt tongue mucosal ulceration which peaked at 9 days after irradiation (data not shown). Very low mortality rates (<10%) of mice were seen during the experimental period following 30 Gy irradiation restricted to mouse heads (data not shown). In the current study, a single fraction 30 Gy snout-only irradiation resulted in 38.7% tongue ulceration with completely denuded mucosal epithelium in saline control mice (Fig. 5B). In contrast, administration of RSpo1 to mice significantly reduced tongue ulceration in a dose-dependent fashion to 22.1% (P < 0.05), 15.6% (P < 0.05), and 10.7% (P < 0.05) at 5, 10, and 20 mg/kg, respectively (Fig. 5B). Furthermore, while KGF (6.25 mg/kg) alone significantly reduced tongue ulceration to 12.4% (P < 0.05), combined use of RSpo1 (10 mg/kg) and KGF resulted in complete closure of tongue ulceration (0%, P < 0.05) (Fig. 5B). Besides reduction of overt tongue ulceration, administration of RSpo1 also increased the mucosal thickness and basal layer cellularity in non-ulcerated areas of mouse tongue (Fig. 5C).
Fig. 5.
Activity of RSpo1 on tongue ulceration in mice treated with high dose head-only irradiation. (A) Schematic illustration of setup of head-only radiation for mice. Red triangle represents passage of x-ray irradiation. (B) Overt ulceration in mouse tongue mucosa. *P < 0.05 (30 Gy/RSpo1 or 30 Gy/KGF vs. 30 Gy/saline); #P < 0.05 [30 Gy/(RSpo1+KGF) vs. 30 Gy/saline, 30 Gy/RSpo1, or 30 Gy/KGF]. (C) Histological representations of mouse dorsal tongue (H&E staining). Red lines donate tongue ulceration with complete mucosal denudation. (i) Naïve, (ii) 30 Gy/saline, (iii) 30 Gy/RSpo1 (5 mg/kg), (iv) 30 Gy/RSpo1 (10 mg/kg), (v) 30 Gy/RSpo1 (20 mg/kg), (vi) 30 Gy/KGF, (vii) 30 Gy/(RSpo1+KGF). (Scale bar, 100 μm.) (D) β-catenin immunohistochemical staining in mouse dorsal (i–iii) and ventral (v and vi) tongues. (i and iv) Naïve, (ii and v) 30 Gy/saline, (iii and vi) 30 Gy/RSpo1. (Scale bar, 100 μm; Insets, 25 μm.)
To further evaluate the implication of RSpo1 in the Wnt/β-catenin signaling process during repair of oral mucositis, we next examined the RSpo1 effect on β-catenin localization in mouse tongue following head-only irradiation (Fig. 5D). While β-catenin was detected mainly on the cell surface in normal tongue mucosa, reduced β-catenin immunostaining was found in both dorsal and ventral tongue mucosa of mice that received 30 Gy head-only irradiation (Fig. 5D i, ii, iv, and v). In contrast, RSpo1 treatment led to an increase in both immunostaining intensity and nuclear localization of β-catenin in stratified mucosal epithelial cells in irradiated mice (Fig. 5D iii and vi).
Taken together, RSpo1 treatment significantly suppresses high dose, head-only irradiation-induced overt tongue ulceration in mice by amplifying the Wnt/β-catenin signaling pathway and stimulates the proliferative response required for the regeneration of mucosal barrier function. We also show that RSpo1 may potentiate the effect of KGF in inhibiting oral mucosal damage and ulceration in mice receiving irradiation.
Discussion
RSpo family proteins play a key role in modulating the Wnt pathway (1, 4). We have previously demonstrated that the implication of RSpo1 in GI epithelial proliferation and its potential therapeutic application for repairing damaged GI mucosa (8, 9). Here we have extended this observation and have shown that RSpo1 also stimulates epithelial proliferation in the oral mucosa via augmentation of the Wnt/β-catenin signaling, thereby amplifying the physiological regenerative mechanism to restore the damaged oral mucosa. Since Wnt ligands are indispensable for RSpo function, RSpo proteins act as Wnt modulators not as bona fide growth factors (4).
In the current study, we initiated a comprehensive approach to explore the in vivo role of RSpo1 in regulating the canonical Wnt pathway using TOPGAL mice. TOPGAL mice were previously developed to monitor canonical Wnt/β-catenin activity by engineering the transgene containing the LacZ gene under the control of a regulatory sequence consisting of three consensus LEF/TCF-binding motifs upstream of a minimal c-fos promoter (22). The TOPGAL transgenic mouse model was extensively used to analyze the canonical Wnt signaling in vivo (23–26).
We injected TOPGAL mice with RSpo1 protein and examined multiple tissues for in vivo augmentation of the Wnt/β-catenin pathway as detected by the LacZ reporter gene. Among harvested tissues, tongue was found to have substantially enhanced β-galactosidase signal in mucosal epithelium. We also observed β-galactosidase activity in various other tissues in both saline and RSpo1-treated TOPGAL mice including lung, liver, hair follicles, and stomach, suggesting constitutive activity of the Wnt signaling pathway in these tissues (data not shown). RSpo1-mediated signal was seen in the stratified epithelium of the oral mucosa in TOPGAL mice. In agreement with this result, Wnt/β-catenin signaling has recently been shown to be critical for fungiform papilla and taste bud formation during tongue development in mice (25, 27). Our data indicate that RSpo1 may play an essential role in regulating tongue mucosa during homeostasis and pathogenesis.
The TOPGAL mouse system has its own limitations. Multimerized TCF reporter is necessary but may not be sufficient to give a complete and definitive readout of Wnt signaling in vivo (22). The discrepancies between TOPGAL reporter activity and Wnt signaling have been described previously (28, 29) and may be due to differences in the regulation of transgene expression in specific tissue contexts. We observed that the Wnt response cells, via either TOPGAL reporter activity or nuclear localization of β-catenin, do not completely correlate with mitotic cells. Therefore, we cannot exclude the possibility that the induction of cell proliferation in tongue mucosa may be an indirect result of enactment of the canonical Wnt pathway in distal cells.
To further investigate the role of the Wnt pathway during oral mucosal homeostasis, we examined the effect of the Wnt inhibitor Dkk1. We demonstrated that overexpression of Dkk1 resulted in a mucositis-like hypoplastic phenotype in mouse tongue, indicating a pivotal role of Wnt signaling in normal homeostasis of tongue mucosa. In addition, Dkk1-induced mucosal damage and reduction of β-catenin expression was efficiently reversed by RSpo1 treatment, suggesting that RSpo1-mediated mucosal hyperplasia in the oral cavity is an outcome of the up-regulation of the Wnt/β-catenin pathway. These observations are consistent with our previously proposed mechanism that RSpo1 modulates the Wnt signaling pathway by interfering with Dkk1 function (4). As we recently demonstrated, RSpo1 plays a central role in regulating cell surface levels of the coreceptor LRP6, by interfering with Dkk1/Kremen-mediated LRP6 internalization (4). Based on the molecular mechanism of action of RSpo1, we proposed that RSpo1 could be an effective therapeutic agent in treating diseases that are dependent on Wnt signaling for tissue repair.
Using experimental colitis models, we have previously shown that RSpo1 can effectively regenerate the GI mucosa, a tissue that is fully dependent on canonical Wnt/β-catenin signaling for its self-renewal (8, 30). We herein provided further evidence that demonstrates the therapeutic potential of RSpo1 in tissue regeneration. We used several mouse models of experimental oral mucosal damage induced by chemotherapy and/or radiation to demonstrate that enhancement of the Wnt/β-catenin pathway by RSpo1 accelerates the oral mucosal epithelial cell regeneration. Furthermore, RSpo1, either alone or together with the growth factor KGF, significantly inhibits overt tongue ulceration in mice that received high dose, snout-only irradiation.
The mucosal epithelium lining the oral cavity undergoes rapid self-renewal and is thus susceptible to the non-specific toxicity that occurs during radiation therapy and chemotherapy (13, 31). Oral mucositis is a common side-effect among patients who receive aggressive myeloablative chemotherapy for hematologic malignancies and in patients who get radiotherapy as a treatment for cancers of the oral cavity, oropharynx, and nasopharynx (11). In its mild form, oral mucositis presents an erythematous, atrophic lesion in which the mucosal lining still remains intact. In contrast, patients with more severe oral mucositis develop visible ulcerations that penetrate into the submucosal lamina propria and cause impairment on the quality of life (32). Oral mucositis also markedly increases the risk of infection and compromises the efficacy of treatment plans, by necessitating breaks and reductions in doses used in chemotherapy and radiotherapy for affected patients (12).
Pathogenesis of oral mucositis, induced by chemotherapy and radiotherapy, in mice is similar to that seen in humans and have thus been widely used in proof-of-concept studies for preclinical testing of therapeutic candidate agents (33, 34). As a Wnt modulator, RSpo1 significantly reduces both chemotherapy and radiotherapy-induced damage to the oral mucosa by amplifying the Wnt/β-catenin signaling and subsequently triggering epithelial cell growth to accelerate mucosal healing in mice. Moreover, RSpo1 decreases the extent of overt tongue ulceration by repairing disintegrated mucosa in mice exposed to high dose radiation delivered to head region only. As we have shown in several animal models, RSpo1 appears to be an effective mucosal repair agent for regenerating tongue mucosa damaged by intensive chemotherapy or radiotherapy.
Oral mucositis closely follows the paradigm of an acute mucosal damage phase characterized by inflammation, epithelial cell apoptosis, and ulcerative lesions, followed by a self-healing phase with restoration of mucosal epithelium and barrier function (10). We thus assessed the time-dependent effect of RSpo1 in reducing oral mucositis in irradiated mice (Fig. S3). We found that RSpo1 is efficacious in both injury and healing phases of oral mucositis in mice by increasing the basal layer epithelial cell density in tongue (Fig. S3). Moreover, RSpo1 treatment is also effective in reducing oral mucositis activity in mice during courses of both non-ulcerated and ulcerative mucosal damages in mice receiving high dose, head-only irradiation (Fig. S4). Therefore, use of RSpo1 appears to be efficacious during various stages of experimental oral mucositis in mice.
Unlike KGF (21), high dose and long-term treatment with RSpo1 did not promote tumor xenograft growth in immunodeficient mice inoculated with various human colorectal tumor cell lines including COLO 205, HT29, and SW620 (data not shown), indicating that RSpo1 does not have an effect on tumorigenesis and tumor growth. Moreover, RSpo1 did not further increase the steady state level of cytosolic β-catenin in HCT116 or SW620 human colorectal carcinoma cells in culture (data not shown). Furthermore, our recent studies show that RSpo2 administration did not increase the intestinal tumorigenesis and progression that occur spontaneously in ApcMin/+ mice (data not shown). These data are consistent with our molecular signaling model that RSpo1 amplifies Wnt signaling at the level of the LRP6 receptor (4), and may not further enhance Wnt activation in colorectal cancers with downstream mutations of APC or β-catenin.
In summary, we have provided extensive evidences that the modulation of canonical Wnt/β-catenin signaling by RSpo1 alleviates oral mucosal damage induced by chemotherapy and/or radiation in mice. Administration of RSpo1 significantly improves tongue mucosal integrity by repopulating stratified epithelium through activation of canonical Wnt/β-catenin signaling. In addition, simultaneous administration of RSpo1 and KGF completely heals tongue ulcers by repairing damaged mucosa after the exposure to a high dose head-only irradiation in mice. Together with our early report that RSpo1 ameliorates experimental colitis in mice (8), our current results extend our understanding of RSpo1 biology and suggest that RSpo1 may be a potent epithelial repair agent suitable for therapy in diseases involving both oral and GI mucosal damages such as gastric ulceration, short bowel syndrome, esophagitis, and oral and intestinal mucositis. The ongoing preclinical research of RSpo1 as a novel biologic agent will undoubtedly advance our knowledge regarding the specific molecular mechanisms governing the pathogenesis of oral mucosal injury and subsequent regeneration.
Materials and Methods
Chemotherapy-Induced Oral Mucositis.
Oral mucositis in mice were induced by i.p. administration of either 5-FU (50 mg/kg, qdx4) or CPT-11 (250 mg/kg, qdx3). Mice were humanely killed 2 days after last injection of either 5-FU or CPT-11 and tongue and buccal tissues were collected for further analysis. Harvested tongue and buccal tissues were rinsed with ice-cold saline buffer and fixed immediately in 10% neutral buffered formalin (Sigma) for histological processing.
Radiation-Induced Oral Mucositis.
Young adult male BDF-1 mice were immobilized for irradiation with ketamine. Whole-body radiation was performed in an x-ray irradiator (250 KVp, 12 mA, SRI International) using a single fraction dose of 13 Gy or 10 Gy (when used in combination with 5-FU) delivered to anesthetized mice in a supine position. The dose rate (2.6 Gy/min) was checked regularly using a dosimeter during the radiation procedure and was constant. Hence, the single fraction dose was achieved by adjustment of the irradiation time. Postirradiation mice were allowed to recover on heated pad before return to vivarium. Mouse necropsy was done 4 days after whole-body radiation.
The technique and set-up for head-only radiation treatment in mice was modified based on previously published studies (14, 35, 36). Custom-made lead shields were used for mice to limit the radiation to the heads. Mice received a high dose, single fractionated 30 Gy x-ray radiation directly to their head region at rate of 2.5 Gy/min. Mice were harvested 9 days after head-only radiation.
Evaluation of Oral Mucositis.
Mice were humanely killed and the whole tongue was then removed from oral cavity for fixation in 10% neutral buffered formalin. H&E-stained microscopic sections from the mid-sagittal plane of each mouse tongue were used for assessment of oral mucositis (36). Tongue histology was recorded using a Leica DM4000B digital microscope equipped with image capturing software. Histometric analysis including percentage of ulcerated tongue, mucosal basal epithelial layer cellularity, and mucosal thickness was determined using Image-Pro Discovery software (Media Cybernetics Inc.). Ulceration is defined as tongue surface with completely denuded mucosa where the underlying lamina propria is exposed (35, 36). At least 10 independent measurements from 3 different tongue sections were made for each parameter of oral mucositis severity. The scorer was blinded and was not aware of the specimen conditions.
Supplementary Material
Acknowledgments.
We thank Dr. James Bakke (SRI International) for performing radiation on mice, Drs. Calvin Kuo and Akifumi Ootani (Stanford University, Palo Alto, CA) for supplying TOPGAL mice. We also thank Eric Beck, Karolyn Tran, and Gerard Aguilar for technical assistances, Luke Garcia for mouse colony expansion, and Jessica Bright for the critical reading of the manuscript.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805159106/DCSupplemental.
References
- 1.Kim KA, et al. R-Spondin proteins: A novel link to beta-catenin activation. Cell Cycle. 2006;5:23–26. doi: 10.4161/cc.5.1.2305. [DOI] [PubMed] [Google Scholar]
- 2.Nam JS, Turcotte TJ, Smith PF, Choi S, Yoon JK. Mouse cristin/R-spondin family proteins are novel ligands for the Frizzled 8 and LRP6 receptors and activate beta-catenin-dependent gene expression. J Biol Chem. 2006;281:13247–13257. doi: 10.1074/jbc.M508324200. [DOI] [PubMed] [Google Scholar]
- 3.Wei Q, et al. R-spondin1 is a high affinity ligand for LRP6 and induces LRP6 phosphorylation and beta-catenin signaling. J Biol Chem. 2007;282:15903–15911. doi: 10.1074/jbc.M701927200. [DOI] [PubMed] [Google Scholar]
- 4.Binnerts ME, et al. R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proc Natl Acad Sci USA. 2007;104:14700–14705. doi: 10.1073/pnas.0702305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamata T, et al. R-spondin, a novel gene with thrombospondin type 1 domain, was expressed in the dorsal neural tube and affected in Wnts mutants. Biochim Biophys Acta. 2004;1676:51–62. doi: 10.1016/j.bbaexp.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Kazanskaya O, et al. R-Spondin2 is a secreted activator of Wnt/beta-catenin signaling and is required for Xenopus myogenesis. Dev Cell. 2004;7:525–534. doi: 10.1016/j.devcel.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 7.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Zhao J, et al. R-spondin1, a novel intestinotrophic mitogen, ameliorates experimental colitis in mice. Gastroenterology. 2007;132:1331–1343. doi: 10.1053/j.gastro.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Kim KA, et al. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science. 2005;309:1256–1259. doi: 10.1126/science.1112521. [DOI] [PubMed] [Google Scholar]
- 10.Sonis ST. Oral mucositis in cancer therapy. J Support Oncol. 2004;2:3–8. [PubMed] [Google Scholar]
- 11.Treister N, Sonis S. Mucositis: Biology and management. Curr Opin Otolaryngol Head Neck Surg. 2007;15:123–129. doi: 10.1097/MOO.0b013e3280523ad6. [DOI] [PubMed] [Google Scholar]
- 12.Peterson DE. New strategies for management of oral mucositis in cancer patients. J Support Oncol. 2006;4:9–13. [PubMed] [Google Scholar]
- 13.Sonis ST. The pathobiology of mucositis. Nat Rev Cancer. 2004;4:277–284. doi: 10.1038/nrc1318. [DOI] [PubMed] [Google Scholar]
- 14.Dorr W, Kummermehr J. Accelerated repopulation of mouse tongue epithelium during fractionated irradiations or following single doses. Radiother Oncol. 1990;17:249–259. doi: 10.1016/0167-8140(90)90209-f. [DOI] [PubMed] [Google Scholar]
- 15.Dorr W, Noack R, Spekl K, Farrell CL. Modification of oral mucositis by keratinocyte growth factor: single radiation exposure. Int J Radiat Biol. 2001;77:341–347. doi: 10.1080/09553000010018873. [DOI] [PubMed] [Google Scholar]
- 16.Farrell CL, et al. Keratinocyte growth factor protects mice from chemotherapy and radiation-induced gastrointestinal injury and mortality. Cancer Res. 1998;58:933–939. [PubMed] [Google Scholar]
- 17.Farrell CL, et al. The effects of keratinocyte growth factor in preclinical models of mucositis. Cell Prolif. 2002;35:78–85. doi: 10.1046/j.1365-2184.35.s1.8.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farrell CL, et al. Effects of keratinocyte growth factor in the squamous epithelium of the upper aerodigestive tract of normal and irradiated mice. Int J Radiat Biol. 1999;75:609–620. doi: 10.1080/095530099140258. [DOI] [PubMed] [Google Scholar]
- 19.Spielberger R, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351:2590–2598. doi: 10.1056/NEJMoa040125. [DOI] [PubMed] [Google Scholar]
- 20.Braun S, et al. Keratinocyte growth factor protects epidermis and hair follicles from cell death induced by UV irradiation, chemotherapeutic or cytotoxic agents. J Cell Sci. 2006;119:4841–4849. doi: 10.1242/jcs.03259. [DOI] [PubMed] [Google Scholar]
- 21.Danilenko DM. Preclinical and early clinical development of keratinocyte growth factor, an epithelial-specific tissue growth factor. Toxicol Pathol. 1999;27:64–71. doi: 10.1177/019262339902700113. [DOI] [PubMed] [Google Scholar]
- 22.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- 23.Driskell RR, et al. Wnt3a regulates Lef-1 expression during airway submucosal gland morphogenesis. Dev Biol. 2007;305:90–102. doi: 10.1016/j.ydbio.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwatsuki K, et al. Wnt signaling interacts with Shh to regulate taste papilla development. Proc Natl Acad Sci USA. 2007;104:2253–2258. doi: 10.1073/pnas.0607399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu F, et al. Wnt-beta-catenin signaling initiates taste papilla development. Nat Genet. 2007;39:106–112. doi: 10.1038/ng1932. [DOI] [PubMed] [Google Scholar]
- 26.Shao JS, et al. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest. 2005;115:1210–1220. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okubo T, Pevny LH, Hogan BL. Sox2 is required for development of taste bud sensory cells. Genes Dev. 2006;20:2654–2659. doi: 10.1101/gad.1457106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barolo S. Transgenic Wnt/TCF pathway reporters: All you need is Lef? Oncogene. 2006;25:7505–7511. doi: 10.1038/sj.onc.1210057. [DOI] [PubMed] [Google Scholar]
- 29.Maretto S, et al. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci USA. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radtke F, Clevers H. Self-renewal and cancer of the gut: Two sides of a coin. Science. 2005;307:1904–1909. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- 31.Alterio D, et al. Cancer treatment-induced oral mucositis. Anticancer Res. 2007;27:1105–1125. [PubMed] [Google Scholar]
- 32.Silverman S., Jr Diagnosis and management of oral mucositis. J Support Oncol. 2007;5:13–21. [PubMed] [Google Scholar]
- 33.Wardley AM, Booth D, Roberts SA, Scarffe JH, Potten CS. A quantitative histometric murine in vivo model of radiation-induced oral mucositis. Arch Oral Biol. 1998;43:567–577. doi: 10.1016/s0003-9969(98)00031-4. [DOI] [PubMed] [Google Scholar]
- 34.Dorr W, Emmendorfer H, Haide E, Kummermehr J. Proliferation equivalent of “accelerated repopulation” in mouse oral mucosa. Int J Radiat Biol. 1994;66:157–167. doi: 10.1080/09553009414551061. [DOI] [PubMed] [Google Scholar]
- 35.Muanza TM, et al. Evaluation of radiation-induced oral mucositis by optical coherence tomography. Clin Cancer Res. 2005;11:5121–5127. doi: 10.1158/1078-0432.CCR-05-0403. [DOI] [PubMed] [Google Scholar]
- 36.Guo H, et al. Prevention of radiation-induced oral cavity mucositis by plasmid/liposome delivery of the human manganese superoxide dismutase (SOD2) transgene. Radiat Res. 2003;159:361–370. doi: 10.1667/0033-7587(2003)159[0361:porioc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.