Abstract
Acute and chronic injuries to the heart result in perturbation of intracellular calcium signaling, which leads to pathological cardiac hypertrophy and remodeling. Calcium/calmodulin-dependent protein kinase II (CaMKII) has been implicated in the transduction of calcium signals in the heart, but the specific isoforms of CaMKII that mediate pathological cardiac signaling have not been fully defined. To investigate the potential involvement in heart disease of CaMKIIδ, the major CaMKII isoform expressed in the heart, we generated CaMKIIδ-null mice. These mice are viable and display no overt abnormalities in cardiac structure or function in the absence of stress. However, pathological cardiac hypertrophy and remodeling are attenuated in response to pressure overload in these animals. Cardiac extracts from CaMKIIδ-null mice showed diminished kinase activity toward histone deacetylase 4 (HDAC4), a substrate of stress-responsive protein kinases and suppressor of stress-dependent cardiac remodeling. In contrast, phosphorylation of the closely related HDAC5 was unaffected in hearts of CaMKIIδ-null mice, underscoring the specificity of the CaMKIIδ signaling pathway for HDAC4 phosphorylation. We conclude that CaMKIIδ functions as an important transducer of stress stimuli involved in pathological cardiac remodeling in vivo, which is mediated, at least in part, by the phosphorylation of HDAC4. These findings point to CaMKIIδ as a potential therapeutic target for the maintenance of cardiac function in the setting of pressure overload.
Keywords: histone deacetylase 4 (HDAC4), calcium signaling, excitation contraction coupling (EC coupling), thoracic aortic constriction (TAC), CaM Kinase II inhibitory peptide (AC3-I)
The heart responds to a variety of chronic and acute stresses by hypertrophic growth, which is accompanied by activation of a fetal cardiac gene program (1, 2). Prolonged stress often leads to cardiomyopathy, fibrosis, and eventually to heart failure or sudden death from arrhythmias (3). Aberrant Ca2+ signaling has been implicated in the transmission of stress signals leading to pathological cardiac remodeling (4). Multiple Ca2+-dependent signaling molecules, including the Ca2+/calmodulin-dependent phosphatase calcineurin, protein kinase D (PKD) and Ca2+/calmodulin-dependent protein kinase II (CaMKII) have been shown to transduce pathological Ca2+ signals in the heart, and inhibitors directed against these enzymes sustain cardiac function in response to stress (5–9). However, the relative roles of these signal transducers and the extent to which their functions are distinct or redundant have not been fully elucidated.
CaMKII is a serine/threonine protein kinase with a broad spectrum of substrates (10, 11). The four isoforms of CaMKII (α, β, δ, and γ) are encoded by different genes, which display distinct but overlapping expression patterns (12). The α and β isoforms are almost exclusively expressed in the brain, whereas the δ and γ isoforms are expressed more ubiquitously. CaMKIIδ and γ are the predominant CaMKII isoforms in the heart with CaMKIIδ displaying the highest level of expression. Each isoform contains an N-terminal kinase domain, a regulatory Ca2+/calmodulin-binding region, and a C-terminal association domain, which is necessary for the formation of homo- and heteromultimeric holoenzymes composed of 12 subunits (13). The four different CaMKII isoforms possess similar catalytic and regulatory properties.
CaMKII modulates numerous biological processes, including Ca2+ homoeostasis, membrane excitability, cell cycle progression, protein secretion, cytoskeletal organization, learning and memory, fertilization, and gene expression (14–16). In the heart, CaMKII has been implicated in excitation–contraction (EC) coupling, gene transcription, and apoptosis (5, 17). Many substrates involved in EC coupling such as phospholamban (PLB), the ryanodine receptor (RyR) and the L-type Ca2+ channel (LTCC) have been reported (17–22). We have also demonstrated that CaMKII isoforms associate specifically with histone deacetylase 4 (HDAC4), a transcriptional repressor (23) that, when phosphorylated, dissociates from the MEF2 transcription factor and translocates from the nucleus to the cytoplasm through its association with the 14-3-3 chaperone protein (18). Activation of MEF2D is sufficient and necessary for pathological remodeling of the heart (24). Thus, the signal-dependent association of HDAC4 with MEF2D provides a mechanism for coupling pathological Ca2+ signaling with the cardiac genome and the downstream gene programs leading to cardiac dysfunction.
Several lines of evidence indicate an important role for CaMKIIδ in pathological cardiac remodeling. CaMKIIδ expression and activity are up-regulated in structural heart disease (25, 26), and transgenic overexpression of the nuclear splice variant CaMKIIδB (27) or the cytosolic splice variant CaMKIIδC (28) promotes cardiac hypertrophy. Conversely, inhibition of CaMKII activity with a peptide (AC3-I) diminishes pathological cardiac remodeling in response to stress (7). However, whether the involvement of CaMKII in heart disease reflects the actions of a specific isoform or multiple isoforms of the kinase remains to be determined.
To date, CaMKIIα is the only isoform of CaMKII to have been deleted through homologous recombination in mice. The knockout (KO) of CaMKIIα launched a new subfield in neuroscience, in which mouse mutants were used as a tool to gain insight into the molecular basis of memory and learning (16, 29). No gene targeting models have been described for the other CaMKII isoforms, β, δ, and γ, leaving numerous open questions about their possible unique or redundant functions.
In this work, we report the generation and initial characterization of mice lacking the δ isoform of CaMKII. These mice develop normally until adulthood. Under unstressed conditions, cardiac structure and function appear normal in CaMKIIδ-KO mice, but upon pressure overload, cardiac remodeling is attenuated, and cardiac phosphorylation of HDAC4 is markedly reduced. We conclude that CaMKIIδ plays a key role in transmission of pathological Ca2+ signals involved in stress-dependent remodeling of the heart.
Results
AC3-I Inhibits CaMKII and PKD.
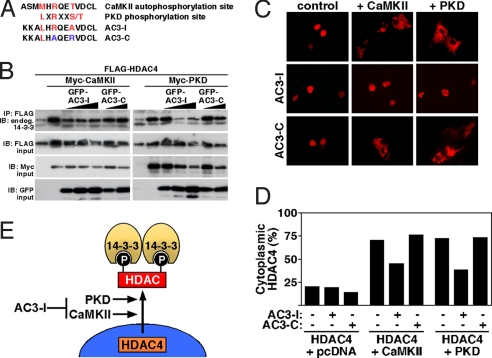
Transgenic overexpression of a CaMKII inhibitory peptide (AC3-I) in mice protects the heart against pathological cardiac remodeling in response to myocardial infarction and β-adrenergic stimulation (7). AC3-I was derived from the regulatory domain of CaMKII around the autophosphorylation site Thr-287 (Met-X-Arg-X-X-Thr) (Fig. 1A). Mutation of this threonine to an alanine (Met-X-Arg-X-X-Ala) converts this peptide into a CaMKII pseudosubstrate. However, the AC3-I peptide also contains a methionine-to-leucine substitution at the −5 position, which creates an ideal pseudosubstrate (Leu-X-Arg-X-X-Ala) for PKD (Fig. 1A), another kinase from the CaMK superfamily (5). Like CaMKII, PKD is a class IIa HDAC kinase that triggers the nuclear export of HDAC4 and other class IIa HDACs by creating phospho-docking sites for the chaperone 14-3-3, resulting in nuclear export of HDAC4 (5). PKD1 activation has been shown to be sufficient and necessary for pathological cardiac remodeling (6). Thus, we hypothesized that AC3-I might inhibit PKD and CaMKII. Indeed, when we coexpressed GFP-AC3-I with PKD and HDAC4 in COS cells, the induction of 14-3-3 binding to HDAC4, a readout of phosphorylation and nuclear export of class II HDACs, was inhibited as effectively as when we coexpressed GFP-AC3-I with CaMKII and HDAC4 (Fig. 1B). The control peptide AC3-C (Leu-X-Ala-X-X-Arg), which is not a pseudosubstrate for either CaMKII or PKD, did not block either the CaMKII or the PKD effect. Consistently, by using immunocytochemistry, AC3-I was found to block not only CaMKII-induced nuclear export of HDAC4 by 35% but also PKD-induced nuclear export of HDAC4 to a similar degree (Fig. 1 C and D). We conclude that AC3-I inhibits not only CaMKII but also PKD (Fig. 1E). Thus, inhibition of PKD should be taken in account when interpreting data from studies with this inhibitor. Therefore, it was our goal to develop a genetic CaMKII loss-of-function model to evaluate the specific role of CaMKII in cardiac disease.
Fig. 1.
AC3-I inhibits CaMKII and PKD. (A) Comparison of amino acid sequences of the CaMKII autophosphorylation site, the ideal PKD phosphorylation site, the so-called CaMKII inhibitory peptide (AC3-I), and its control peptide (AC3–3). (B and C). COS cells were transfected with FLAG-HDAC4, Myc-CaMKII, Myc-PKD1, and GFP-AC3-I or GFP-AC3-C as indicated. (B) Coimmunoprecipitation assays with COS cell lysates expressing the indicated proteins. Phosphorylation of HDAC4 was monitored by immunoprecipitation (IP) with anti-FLAG followed by immunoblotting (IB) with antibody against endogenous 14-3-3. Input proteins were detected by immunoblotting with antibodies against the indicated epitopes. (C) Representative immunocytochemistry showing the cellular localization of HDAC4 in cells transfected with FLAG-HDAC4, Myc-CaMKII, Myc-PKD1, and GFP-AC3-I or GFP-AC3-C as indicated. (D) Quantitative analysis of immunocytochemistry. (E) Illustration showing that HDAC4 is a common substrate of CaMKII and PKD, resulting in 14-3-3-mediated nuclear export. AC3-I inhibits both kinases.
Genetic Deletion of CaMKIIδ.
Because CaMKIIδ is the most abundant CaMK isoform expressed in the heart, we chose to delete the CaMKIIδ gene in mice and determine the consequences on cardiac growth, development, and stress responsiveness. We generated a conditional CaMKIIδ-null allele by using the Cre-loxP recombination system because we were uncertain whether global deletion of the gene might cause lethality because of functions in tissues other than the heart. LoxP sites were inserted into the CaMKIIδ locus to flank exons 1 and 2, which encode part of the catalytic domain of the enzyme, including the ATP-binding motif that is essential for kinase function (Fig. 2A). Correct targeting was confirmed by Southern blot hybridization by using probes that hybridized to genomic sequences 5′ (Fig. 2B) and 3′ of the targeted region of the gene. Expression of Cre recombinase results in deletion of the region between the loxP sites, eliminating the function of CaMKIIδ as a kinase. We initially deleted the gene using a CAG-Cre transgene (30), which expresses Cre recombinase in the embryo at the zygote stage. Correct deletion of exons 1 and 2 by the CAG-Cre transgene was confirmed by Southern blot hybridization.
Fig. 2.
Targeting of the mouse CaMKIIδ gene. (A) CaMKIIδ protein structure, intron–exon structure of the CaMKIIδ gene, and gene targeting strategy. LoxP sites were inserted in the introns flanking exons 1 and 2. Exon 2 encodes the ATP-binding motif required for kinase function. The neomycin resistance cassette (neo) was removed in the mouse germ line by breeding heterozygous mice to hACTB::FLPe transgenic mice, and deletion of exons 1 and 2 was achieved by breeding CaMKIIδloxP/loxP mice to CAG-Cre transgenic mice. (B) Representative Southern blot of genomic DNA from gene-targeted ES cells digested with PstI (P) using a probe hybridizing to a genomic region upstream (5′) of the long arm of the targeted region. The expected fragments were generated. (C) RT-PCR to detect WT and mutant CaMKIIδ (mut) transcripts, confirming that exons 1–4 are not transcribed in CaMKIIδ-KO mice. The reverse primer lies in exon 18, the numbers of the forward primers correspond to the exons containing their sequence.
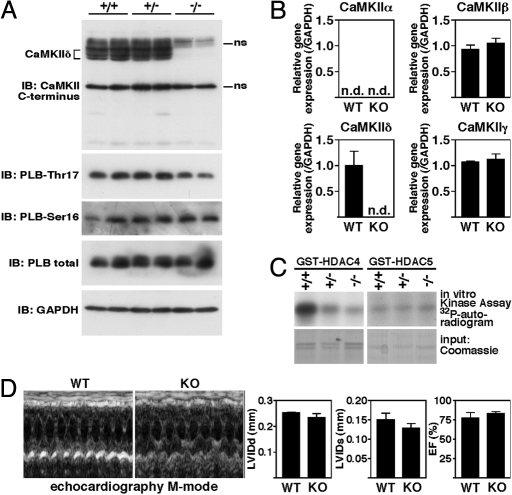
By using RT-PCR, we confirmed that no CaMKIIδ transcript from exon 1–4 was produced in ventricular RNA samples from CaMKIIδ-KO mice (Fig. 2C). An alternative transcript with transcription starting at exon 5 was observed in the mutant mice. However, sequence analysis revealed that the next in-frame ATG does not occur until exon 8. Thus, if the alternative transcript were translated, it would not encode the major part of the catalytic domain (Fig. 2A) and therefore would be nonfunctional. Moreover, by Western blot analysis using an antibody recognizing the C terminus of all CaMKII isoforms, we confirmed the loss of CaMKIIδ protein in the mutant mice (Fig. 3A). Because we did not detect an additional smaller peptide in the CaMKIIδ-KO extracts, it is unlikely that an alternative protein is synthesized from the mutant allele.
Fig. 3.
Baseline characterization of CaMKIIδ-KO mice. (A) (Upper) Western blot analysis of endogenous CaMKII in cardiac extracts from CaMKIIδ+/+ (WT), CaMKIIδ+/− (HET), and CaMKIIδ−/− (KO) mice by using an antibody directed against the C terminus of all four CaMKII isoforms. (Lower) Western blot analysis of PLB at the CaMKII phosphorylation site Thr-17 and at the PKA phosphorylation site Ser-16 as well as total amounts of PLB and GAPDH as loading control; ns, nonspecific. (B) Transcripts for CaMKIIα, β, δ, and γ in hearts from WT and CaMKIIδ-KO mice were detected by quantitative PCR (n = 3 per group). Values indicate relative expression level to WT (±SEM). n.d., not detectable. (C) HDAC4 and HDAC5 kinase activity assays were performed in ventricular extracts from CaMKIIδ-KO mice and their WT littermates by using GST-HDAC4 and GST-HDAC5 substrates. HDAC4 but not HDAC5 phosphorylation was significantly decreased in ventricular extracts from CaMKIIδ-KO mice. Coomassie staining was used to demonstrate equivalent GST-HDAC4/HDAC5 input. (D) Transthoracic echocardiography revealed normal dimensions and function of CaMKIIδ-KO hearts. Shown are two representative M mode images and the quantitative analysis of the diastolic (LVIDd) and systolic (LVIDs) left ventricular internal diameter and ejection fraction (EF).
Phosphorylation of Cardiac PLB in CaMKIIδ-KO Mice.
Among the typical CaMKII substrates are proteins that modulate cardiac EC coupling, including PLB, RyR, and LTCC (17). Because the CaMKII phosphorylation sites in PLB have been well defined (31), we investigated the phosphorylation status of PLB in WT and CaMKIIδ-KO mice at the CaMKII and the protein kinase A (PKA) phosphorylation site. We observed a slight decrease at Thr-17 but not at Ser-16 of PLB, indicating that Thr-17 of PLB is indeed an endogenous substrate of CaMKIIδ but also that other kinases probably compensate for phosphorylation at this site to a significant degree (Fig. 3A). To evaluate whether one of the other CaMKII isoforms is up-regulated to compensate for the absence of the δ isoform, we quantified the mRNA levels of CaMKIIα, β, and γ. Whereas CaMKIIα was not detectable, CaMKIIβ and γ were not increased in cardiac RNA samples from CaMKIIδ-KO mice (Fig. 3B).
Reduced Cardiac HDAC4 Kinase Activity in CaMKIIδ-KO Mice.
We next tested the kinase activity of WT, CaMKIIδ+/− (HET), and CaMKIIδ−/− (KO) cardiac extracts against two HDAC substrates, HDAC4 and HDAC5, which are involved in the transmission of Ca2+-dependent signals to the MEF2 transcription factor (32) and have been implicated in cardiomyocyte hypertrophy (18). Consistent with our previous findings that CaMKII selectively phosphorylates HDAC4 (18), we observed a clear reduction in phosphorylation of HDAC4 but not HDAC5 in ventricular lysates from CaMKIIδ-KO mice (Fig. 3C), consistent with CaMKIIδ being an endogenous HDAC4 kinase. This raised the possibility that CaMKIIδ-KO mice might be protected against pathological cardiac remodeling and activation of fetal genes in response to stress signaling.
CaMKIIδ-KO Mice Are Protected Against Hypertrophy and Fibrosis in Response to Pressure Overload.
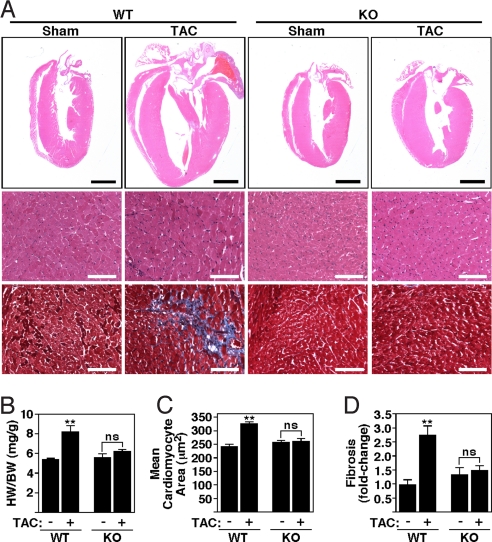
CaMKIIδ-KO mice were viable and fertile and developed normally until early adulthood. Cardiac function based on echocardiographic studies appeared normal under unstressed conditions (Fig. 3D). Thus, we chose to challenge CaMKIIδ-KO mice by pressure overload induced by thoracic aortic constriction (TAC). The hearts of wild-type (WT) and CaMKIIδ-KO mice were comparable in size and structure in the absence of stress (Fig. 4 A–D). Twenty-one days after TAC, WT mice showed a 52% increase in heart weight/body weight (HW/BW: 5.39 ± 0.15 vs. 8.17 ± 0.63 mg/kg) with thickening of the left ventricular free wall and interventricular septum (Fig. 4 A and B). In contrast, CaMKIIδ-KO mice showed only a 12% increase in HW/BW (5.56 ± 0.40 vs. 6.24 ± 0.20 mg/kg) with a minimal increase in wall thickness. Analysis of the cross-sectional cardiomyocyte areas also revealed an increase in cardiomyocyte size after TAC in WT but not KO mice (Fig. 4 A and C). Pressure overload hypertrophy in WT mice is accompanied by extensive fibrosis of the ventricular wall, as detected by Masson's trichrome staining (Fig. 4 A and D). No significant levels of fibrosis were observed in CaMKIIδ-KO mice after TAC.
Fig. 4.
Diminished cardiac hypertrophy of CaMKIIδ-KO mice after TAC. CaMKIIδ-KO mice were subjected to either a sham operation (WT and KO mice, n = 4) or TAC (WT and KO, n = 6). Hearts for analysis were taken 21 days after sham or TAC. (A) Histological sections stained with H&E and Masson's trichrome to detect fibrosis. [Scale bars, 2 mm (Upper) and 100 μm (Lower).] (B) HW/BW ratios (±SEM) of WT and CaMKIIδ-KO mice. (C) Mean cross-sectional area of cardiomyocytes (±SEM). (D) Quantification of fibrosis. Values indicate fold changes of fibrosis in each group compared with a group of sham-operated WT mice (±SEM); ns (nonsignificant), P > 0.05; **, P < 0.001.
To characterize further the size and function of CaMKIIδ-KO cardiomyocytes, we isolated single adult mouse ventricular myocytes from WT and CaMKIIδ-KO mice 6 weeks after TAC. As assessed by quantification of cell area, myocyte size was similar between WT and CaMKIIδ-KO myocytes under basal conditions (Fig. 5 A and B). However, after TAC, WT myocytes became more hypertrophic (55.7 ± 2.8 pL) than CaMKIIδ-KO myocytes (47.9 ± 1.9 pL), again suggesting that the diminished cardiac growth upon pressure overload reflects reduction of cardiomyocyte hypertrophy.
Fig. 5.
Single-cell experiments. (A) Typical cells from WT and CaMKIIδ-KO mice under basal conditions and 6 weeks after TAC. (B) Under basal conditions, cell volume is not different between WT and KO mice. After TAC, both WT and KO myocytes show cellular hypertrophy. However, this hypertrophy is significantly diminished in KO-TAC compared with WT-TAC. (C) Representative Ca2+ transients from WT and KO animals both under basal conditions and after TAC. (D) Ca2+ transient amplitude (ΔF/F0) is slightly increased in KO myocytes under basal conditions but is not different between WT and KO after TAC. (E) SR Ca2+ load as measured by rapid application of caffeine is not different between KO and WT under basal conditions or after TAC; n.s. (nonsignificant), P > 0.05; *, P < 0.05.
Ca2+ Handling.
During EC coupling, an increase in cytosolic Ca2+, referred to as a Ca2+ transient, typically occurs. CaMKII overexpression dramatically decreases cytosolic Ca2+ transients, probably because of a reduced Ca2+ content of the sarcoplasmic reticulum (SR), leading to contractile dysfunction (33). Thus, we asked whether the deletion of CaMKIIδ results in an opposite effect. However, cardiomyocytes from CaMKIIδ-KO mice did not show disturbed intracellular Ca2+ handling under either unstressed or TAC conditions with respect to intracellular Ca2+ transients (Fig. 5 C and D) or SR Ca2+ content (Fig. 5E).
Fetal Gene Activation Is Blunted in CaMKIIδ-KO Hearts in Response to TAC.
CaMKIIδ was essential for maximal fetal gene activation in response to TAC. Up-regulation of the hypertrophic gene markers, atrial natriuretic peptide (ANP, 5.1- in WT vs. 3.2-fold in KO), brain natriuretic peptide (BNP, 2.7-fold in WT vs. 1.3-fold in KO), and myosin heavy polypeptide 7 (Myh7, βMHC, 4.8- in WT vs. 2.2-fold in KO) was attenuated in mutant mice (Fig. 6). Baseline expression of fetal cardiac genes was unaltered in CaMKIIδ-KO mice, suggesting that deletion of CaMKIIδ does not itself impose a stress on the heart.
Fig. 6.
Activation of fetal genes in CaMKIIδ-KO mice after TAC. Transcripts for markers of hypertrophy in hearts from WT and CaMKIIδ-KO mice were detected by quantitative PCR 21 days after TAC (n = 3 per group). Values indicate relative expression level to a WT sham-operated group (±SEM). ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; Myh7, βMHC, myosin heavy chain; n.s. (nonsignificant), P > 0.05; *, P < 0.05; **, P < 0.001.
Discussion
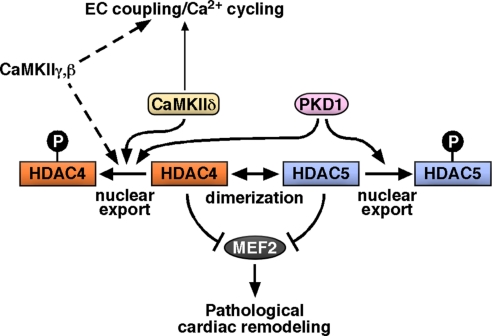
The results of this study demonstrate that CaMKIIδ, the major CaMKII isoform in the heart (25), is a critical component of the signaling pathways through which pressure overload drives pathological cardiac remodeling (Fig. 7). Numerous approaches for inhibition of CaMKII function have been described. However, chemical inhibitors such as KN63 or KN92 also exert off-target effects and general toxicity (11, 13). Recently, inhibitory peptides, including AC3-I, were also used as a means of inhibiting CaMKII activity in vivo (7). Our results indicate that this peptide inhibits other kinases from the CaMK superfamily, namely PKD, which also functions as a potent class II HDAC kinase. CaMKIIδ-KO mice thus represent the only mouse model, to date, in which the major cardiac CaMKII isoform is specifically deleted.
Fig. 7.
Model of molecular mechanism. CaMKIIδ is required for pathological cardiac remodeling at least in part by phosphorylating HDAC4. Regarding EC coupling, redundant roles of other CaMKII isoforms expressed in the heart need to be evaluated. PKD1 also phosphorylates class IIa HDACs and induces pathological cardiac remodeling. Target-specific inhibition (e.g., disrupting the CaMKII–HDAC4 interaction) might be a way to inhibit the CaMKII effects on pathological cardiac remodeling without affecting possible essential CaMKII functions.
Signal-Dependent Control of CaMKII.
The canonical pathway for activation of CaMKII involves elevation of intracellular Ca2+ levels and calmodulin binding (13). Recent evidence has also revealed that CaMKII can be activated by direct oxidation (34). CaMKII phosphorylates a variety of substrates involved in EC coupling, gene transcription, and apoptosis (17). Our results show that the loss of CaMKIIδ attenuates fetal gene activation and pathological cardiac remodeling in response to pressure overload without affecting general Ca2+ handling. Prior studies showed that CaMKIIδ and CaMKIIγ are activated in response to pressure overload (26). CaMKIIβ is also expressed at a low level in the heart (35). Thus, the lack of an effect of CaMKIIδ deletion on EC coupling might reflect redundant functions of CaMKIIγ and β. Although we did not find an up-regulation of these isoforms at the RNA or protein level, this does not rule out a possible compensatory role of CaMKIIγ or β activity. To address this issue, we are currently characterizing mice with deletion of CaMKIIγ. In addition, in this work we used a pressure overload model involving a degree of aortic stenosis (27 g) that results in hypertrophy and pathological cardiac remodeling rather than heart failure (36). It remains to be determined whether CaMKIIδ, in addition to its role in the control of fetal gene activation and hypertrophy, might also contribute to changes in EC coupling under heart failure conditions.
CaMKIIδ Regulates the HDAC4-MEF2 Axis.
Previously, we reported that CaMKII interacted with a specific docking site on HDAC4, resulting in the phosphorylation of HDAC4 and its cytosolic accumulation with consequent activation of fetal cardiac genes and cardiomyocyte hypertrophy in response to stress signaling (18). In our hands, HDAC5, a closely related HDAC, failed to respond directly to CaMKII signaling, which presumably reflects the absence of the CaMKII docking site (18, 20). The results of the present work further substantiate HDAC4 as a specific direct substrate of CaMKIIδ, based on the finding that HDAC4 phosphorylation was abolished in cardiac extracts from CaMKIIδ-KO mice, whereas HDAC5 phosphorylation was unaffected. Others have reported that CaMKII induces cytosolic accumulation of both HDAC4 and HDAC5 (19, 37, 38). Because HDAC5 can acquire CaMKII responsiveness by oligomerization with HDAC4 (20), the nuclear export of HDAC5 in response to CaMKII signaling may reflect, at least in part, this type of indirect mechanism of HDAC5 regulation. We conclude that phosphorylation of HDAC4 serves to connect extracellular stimuli with the genome by governing the expression of HDAC target genes.
Deletion of PKD1, another class IIa HDAC kinase that regulates MEF2 activity, results in a phenotype similar to that of CaMKIIδ-KO mice with attenuation of cardiac remodeling after TAC (6). This raises the question of why PKD1 cannot compensate in CaMKIIδ-KO mice to promote overt cardiac hypertrophy after TAC. We speculate that several signaling pathways must act in concert to activate MEF2 fully. However, the deletion of one class IIa HDAC kinase is sufficient to attenuate pathological cardiac remodeling, pointing to both convergent arms of MEF2 activation, CaMKII and PKD signaling, as attractive drug targets (Fig. 7).
The pivotal role of MEF2 in hypertrophy in pressure overload was recently substantiated further by the deletion of the MEF2D gene, which encodes the major MEF2 isoform in the adult heart (24). MEF2D-KO mice also showed attenuation of pathological cardiac remodeling after TAC, providing support for the involvement of MEF2D as an endogenous downstream effector of CaMKIIδ and PKD1 in the adult heart.
Issues for the Future.
The results of this work validate CaMKIIδ as a promising drug target to prevent pathological cardiac hypertrophy. However, the most effective approach to inhibit CaMKII in the setting of heart disease remains to be determined. Obviously, one approach would be to develop pharmacologic inhibitors of CaMKII. However, global inhibition would affect CaMKII isoforms in addition to CaMKIIδ, which could cause toxicity. CaMKIIα, for example, is involved in learning and memory (16), and our unpublished data show that CaMKIIγ is required for fertilization. From this work, one may also conclude that specific inhibition of the interaction of CaMKII with one or more of its targets might be an alternative approach. The attenuation of pathological cardiac remodeling after pressure overload in CaMKIIδ-null mice and the specific phosphorylation of HDAC4 by CaMKIIδ suggest that strategies to interrupt specifically the interaction between CaMKII and HDAC4 could be therapeutically beneficial. An inhibitor specific for the HDAC4–CaMKII interaction but not for the interaction of CaMKII with other partners would potentially bypass other biologically essential functions of CaMKII. There is precedent for targeting protein–protein interactions with small molecules in cancer (39).
In the present work we have focused on the specific role of CaMKIIδ in hypertrophy in response to relatively mild pressure overload. In the future, it will be of interest to use CaMKIIδ-KO mice to investigate the importance of this kinase in overt heart failure or after myocardial infarction and to explore the relative importance of CaMKIIδ in different signaling pathways induced by neurohumoral regulators of cardiac growth and function, such as adrenergic agonists, angiotensin II, and endothelin.
Materials and Methods
Generation of CaMKIIδ-KO Mice.
Details of gene targeting and generation of mutant mice are described in supporting information (SI) Materials and Methods.
TAC.
Methods for TAC are described in SI Materials and Methods.
Histology and Quantification of Fibrosis.
Methods for histology and quantification of fibrosis are described in SI Materials and Methods.
RNA Analysis.
Methods for RNA analyis are described in SI Materials and Methods.
Adult Ventricular Myocyte Isolation and Intracellular Ca2+ Measurements.
Methods for Ca2+ measurements on adult myocytes are described in SI Materials and Methods.
HDAC Kinase Activity Assay.
Methods for the HDAC kinase assay are described in SI Materials and Methods.
Plasmids.
Expression constructs for constitutively active CaMKII (Myc-tagged CaMKIIB T287D) and Myc-PKD1 and FLAG-HDAC4 have been described in ref. 18. GFP-AC3-I and GFP-AC3-C expression constructs were a gift from Mark E. Anderson (University of Iowa, Iowa City, IA).
Cell Culture and Transfection Assays.
Methods for cell culture and transfection assays are described in SI Materials and Methods.
Immunostaining and Immunoprecipitation.
Methods for immunostaining and immunoprecipitation are described in SI Materials and Methods.
Transthoracic Echocardiography.
Methods for transthoracic echocardiography are described in SI Materials and Methods.
Statistics.
Differences in HW/BW ratios, morphological parameters, and gene expression between groups were analyzed by one-way ANOVA with Bonferroni's multiple comparison test by using GraphPad Prism; n.s. (nonsignificant), P > 0.05; *, P < 0.05; and **, P < 0.001.
Supplementary Material
Acknowledgments.
We thank Svetlana Bezprozvannaya, Yongli Kong, Claudia Heft, Michaela Oestringer, and Britta Wohlrab for excellent technical assistance, Jens Fielitz and Kunhua Song for helpful discussions, Jennifer Brown for editorial assistance, Jose Cabrera for assistance with graphics, and Muthu Periasamy and Michael Schneider for comments on the manuscript. We thank Mark E. Anderson for the GFP-AC3-I and -C expression constructs and for his comments on the manuscript. J.B. was supported by Emmy Noether Program Grant BA-2258/2-1 of the Deutsche Forschungsgemeinschaft (DFG) and M.M.K. by a Young Investigator Award of the medical faculty at Heidelberg. Work in the laboratory of E.N.O. was supported by the National Institutes of Health, the Donald W. Reynolds Center for Clinical Cardiovascular Research, and the Robert A. Welch Foundation. L.S.M. was supported by the DFG through Clinical Research group KFO155 Grant MA 1982/2-1 and Heisenberg Grant MA 1982/4-1, and by the Deutsche Gesellschaft für Kardiologie Hengstberger grant. S.N. was supported by European Union FP6 Grant LSHM-CT-2005-018833, EUGeneHeart.
Footnotes
Conflict of interest statement: E.N.O. is cofounder of MiRagen Therapeutics. E.N.O. and J.B. are consultants for Gilead Therapeutics and have filed a patent on the modulation of cardiac hypertrophy and CaMKII.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813013106/DCSupplemental.
References
- 1.Saito Y, et al. Augmented expression of atrial natriuretic polypeptide gene in ventricle of human failing heart. J Clin Invest. 1989;83:298–305. doi: 10.1172/JCI113872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oka T, Xu J, Molkentin JD. Reemployment of developmental transcription factors in adult heart disease. Semin Cell Dev Biol. 2007;18:117–131. doi: 10.1016/j.semcdb.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frey N, Olson EN. Cardiac hypertrophy: The good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 4.Frey N, McKinsey TA, Olson EN. Decoding calcium signals involved in cardiac growth and function. Nat Med. 2000;6:1221–1227. doi: 10.1038/81321. [DOI] [PubMed] [Google Scholar]
- 5.McKinsey TA. Derepression of pathological cardiac genes by members of the CaM kinase superfamily. Cardiovasc Res. 2007;73:667–677. doi: 10.1016/j.cardiores.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 6.Fielitz J, et al. Requirement of protein kinase D1 for pathological cardiac remodeling. Proc Natl Acad Sci USA. 2008;105:3059–3063. doi: 10.1073/pnas.0712265105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang R, et al. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 8.Molkentin JD, et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vega RB, et al. Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol Cell Biol. 2004;24:8374–8385. doi: 10.1128/MCB.24.19.8374-8385.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun AP, Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: From form to function. Annu Rev Physiol. 1995;57:417–445. doi: 10.1146/annurev.ph.57.030195.002221. [DOI] [PubMed] [Google Scholar]
- 11.Means AR. Regulatory cascades involving calmodulin-dependent protein kinases. Mol Endocrinol. 2000;14:4–13. doi: 10.1210/mend.14.1.0414. [DOI] [PubMed] [Google Scholar]
- 12.Tombes RM, Faison MO, Turbeville JM. Organization and evolution of multifunctional Ca2+/CaM-dependent protein kinase genes. Gene. 2003;322:17–31. doi: 10.1016/j.gene.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 13.Hudmon A, Schulman H. Structure–function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem J. 2002;364:593–611. doi: 10.1042/BJ20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knott JG, et al. Calmodulin-dependent protein kinase II triggers mouse egg activation and embryo development in the absence of Ca2+ oscillations. Dev Biol. 2006;296:388–395. doi: 10.1016/j.ydbio.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Lorca T, et al. Calmodulin-dependent protein kinase II mediates inactivation of MPF and CSF upon fertilization of Xenopus eggs. Nature. 1993;366:270–273. doi: 10.1038/366270a0. [DOI] [PubMed] [Google Scholar]
- 16.Silva AJ, Paylor R, Wehner JM, Tonegawa S. Impaired spatial learning in α-calcium-cal-modulin kinase II mutant mice. Science. 1992;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- 17.Grueter CE, Colbran RJ, Anderson ME. CaMKII, an emerging molecular driver for calcium homeostasis, arrhythmias, and cardiac dysfunction. J Mol Med. 2007;85:5–14. doi: 10.1007/s00109-006-0125-6. [DOI] [PubMed] [Google Scholar]
- 18.Backs J, Song K, Bezprozvannaya S, Chang S, Olson EN. CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J Clin Invest. 2006;116:1853–1864. doi: 10.1172/JCI27438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu X, et al. Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation–transcription coupling. J Clin Invest. 2006;116:675–682. doi: 10.1172/JCI27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Backs J, Backs T, Bezprozvannaya S, McKinsey TA, Olson EN. Histone deacetylase 5 acquires calcium/calmodulin-dependent kinase II responsiveness by oligomerization with histone deacetylase 4. Mol Cell Biol. 2008;28:3437–3445. doi: 10.1128/MCB.01611-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bers DM. Cardiac excitation–contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 22.Grueter CE, et al. L-type Ca2+ channel facilitation mediated by phosphorylation of the β subunit by CaMKII. Mol Cell. 2006;23:641–650. doi: 10.1016/j.molcel.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Backs J, Olson EN. Control of cardiac growth by histone acetylation/deacetylation. Circ Res. 2006;98:15–24. doi: 10.1161/01.RES.0000197782.21444.8f. [DOI] [PubMed] [Google Scholar]
- 24.Kim Y, et al. The MEF2D transcription factor mediates stress-dependent cardiac remodeling in mice. J Clin Invest. 2008;118:124–132. doi: 10.1172/JCI33255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoch B, Meyer R, Hetzer R, Krause EG, Karczewski P. Identification and expression of δ isoforms of the multifunctional Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human myocardium. Circ Res. 1999;84:713–721. doi: 10.1161/01.res.84.6.713. [DOI] [PubMed] [Google Scholar]
- 26.Colomer JM, Mao L, Rockman HA, Means AR. Pressure overload selectively up-regulates Ca2+/calmodulin-dependent protein kinase II in vivo. Mol Endocrinol. 2003;17:183–192. doi: 10.1210/me.2002-0350. [DOI] [PubMed] [Google Scholar]
- 27.Zhang T, et al. The cardiac-specific nuclear δ(B) isoform of Ca2+/calmodulin-dependent protein kinase II induces hypertrophy and dilated cardiomyopathy associated with increased protein phosphatase 2A activity. J Biol Chem. 2002;277:1261–1267. doi: 10.1074/jbc.M108525200. [DOI] [PubMed] [Google Scholar]
- 28.Zhang T, et al. The δC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res. 2003;92:912–919. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- 29.Elgersma Y, Sweatt JD, Giese KP. Mouse genetic approaches to investigating calcium/calmodulin-dependent protein kinase II function in plasticity and cognition. J Neurosci. 2004;24:8410–8415. doi: 10.1523/JNEUROSCI.3622-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakai K, Miyazaki J. A transgenic mouse line that retains Cre recombinase activity in mature oocytes irrespective of the cre transgene transmission. Biochem Biophys Res Commun. 1997;237:318–324. doi: 10.1006/bbrc.1997.7111. [DOI] [PubMed] [Google Scholar]
- 31.Maier LS, Bers DM. Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation–contraction coupling in the heart. Cardiovasc Res. 2007;73:631–640. doi: 10.1016/j.cardiores.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 32.McKinsey TA, Zhang CL, Olson EN. Activation of the myocyte enhancer factor-2 transcription factor by calcium/calmodulin-dependent protein kinase-stimulated binding of 14-3-3 to histone deacetylase 5. Proc Natl Acad Sci USA. 2000;97:14400–14405. doi: 10.1073/pnas.260501497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maier LS, et al. Transgenic CaMKIIδC overexpression uniquely alters cardiac myocyte Ca2+ handling: Reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res. 2003;92:904–911. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- 34.Erickson JR, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bayer KU, De Koninck P, Schulman H. Alternative splicing modulates the frequency-dependent response of CaMKII to Ca2+ oscillations. EMBO J. 2002;21:3590–3597. doi: 10.1093/emboj/cdf360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill JA, et al. Cardiac hypertrophy is not a required compensatory response to short-term pressure overload. Circulation. 2000;101:2863–2869. doi: 10.1161/01.cir.101.24.2863. [DOI] [PubMed] [Google Scholar]
- 37.Chawla S, Vanhoutte P, Arnold FJ, Huang CL, Bading H. Neuronal activity-dependent nucleocytoplasmic shuttling of HDAC4 and HDAC5. J Neurochem. 2003;85:151–159. doi: 10.1046/j.1471-4159.2003.01648.x. [DOI] [PubMed] [Google Scholar]
- 38.Linseman DA, et al. Inactivation of the myocyte enhancer factor-2 repressor histone deacetylase 5 by endogenous Ca2+/calmodulin-dependent kinase II promotes depolarization-mediated cerebellar granule neuron survival. J Biol Chem. 2003;278:41472–41481. doi: 10.1074/jbc.M307245200. [DOI] [PubMed] [Google Scholar]
- 39.Arkin M. Protein–protein interactions and cancer: Small molecules going in for the kill. Curr Opin Chem Biol. 2005;9:317–324. doi: 10.1016/j.cbpa.2005.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.