Abstract
Transduction of the primate cortex with adeno-associated virus (AAV)-based gene therapy vectors has been challenging, because of the large size of the cortex. We report that a single infusion of AAV2 vector into thalamus results in widespread expression of transgene in the cortex through transduction of widely dispersed thalamocortical projections. This finding has important implications for the treatment of certain genetic and neurodegenerative diseases.
Keywords: adeno-associated viral vector, cortex, gene delivery, thalamocortical
The use of gene therapy vectors to deliver biologics to cortical regions has remained a challenge, due in large part to the physical constraints of effectively delivering a vector specifically to the cerebral cortex. Although multiple direct cortical infusions can be effective in small animal brains, as the architecture and volume of brain tissue increases in primates, it becomes almost impossible to achieve widespread cortical delivery through direct cortical infusions (1, 2).
Over the last decade, adeno-associated virus (AAV)-based vectors have emerged as the preferred vector system for neurologic gene therapy, with an excellent safety record in multiple clinical trials (3–5). To realize clinical efficacy, our group has focused on developing methods designed to distribute AAV vectors efficiently throughout target brain structures (6). Simple injections do not distribute AAV vectors effectively, relying as they do on diffusion, which is effective only within a 1- to 3-mm radius. Our approach, known as convection-enhanced delivery (CED), was pioneered by Oldfield et al. (7) and has been used clinically in gene therapy (AAV2-hAADC) for Parkinson's disease (5). CED also has been used to distribute nonviral particles, such as drug-loaded liposomes (8–13), to treat brain cancer. The underlying principle of CED is that infusate is pumped into brain parenchyma under sufficient pressure to overcome the hydrostatic pressure of interstitial fluid, thereby forcing the infused particles into close contact with the dense perivasculature of the brain. Pulsation of these vessels acts as a pump, distributing the particles over large distances throughout the parenchyma (14). To increase the safety and efficacy of CED, we have developed a reflux-resistant cannula (15) and introduced monitored delivery with real-time MRI (16, 17). Monitored delivery has allowed us to quantify and control aberrant events, such as cannula reflux and leakage of infusate into ventricles (18). Anterograde (19) and retrograde (20) transport along axonal tracts is a consistently observed phenomenon in CED of AAV vectors. This remarkably efficacious process suggests that axonal transport might be able to mediate effective distribution to the primate cortex from the relatively compact thalamus, because axonal projections from the thalamus distribute widely to lamina III and IV of the cerebral cortex.
The prospect of being able to target widespread regions of the human cortex with AAV vectors that drive expression of secreted transgenes has obvious applications in Alzheimer's disease (21, 22), lysosomal storage disorders (1, 23), and perhaps other serious disorders with a strong cortical manifestation. Accordingly, we investigated the axonal transportation of AAV2 vectors along known thalamocortical projections in the rhesus monkey [nonhuman primate (NHP)]. Direct infusion of AAV2 vectors into the thalamus of NHPs resulted in the expression of transgenic reporter proteins by neurons located within the targeted thalamic nuclei and in multiple regions of the frontal cortex well beyond the tissue distribution achieved solely by direct infusion.
Results
Widespread Transgenic Protein Expression After Intrathalamic AAV2 Vector Delivery.
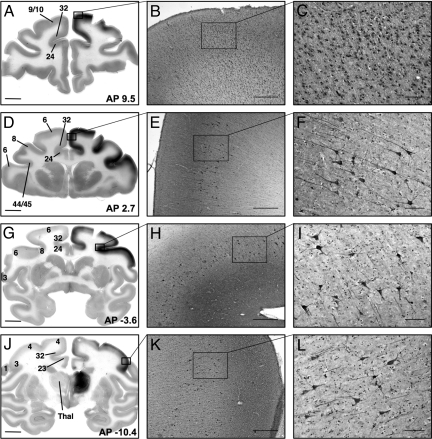
AAV2–glial-derived neurotrophic factor (GDNF) drives abundant secretion of GDNF from transduced neurons, which can be visualized by immunohistochemistry and quantified by ELISA. After infusion of AAV2-GDNF into the thalamus by CED, extensive GDNF immunostaining was detected in the frontal cortex ipsilateral to the infusion site (Fig. 1). The rhesus monkey thalamus is ≈1.0 cm3 in size, as measured by MRI (D. Yin, personal communication), and the human thalamus is estimated to be ≈5.8 cm3 by MRI (24). Given these sizes, a non-CED injection would be very unlikely to distribute effectively within the thalamus. As shown in Fig. 1J, the expression of GDNF within the NHP thalamus was comprehensive but contained within the gray matter bounds of the targeted thalamic nuclei, with no evidence of infusion related leakage or reflux of the AAV2-GDNF vector into adjacent nontargeted areas. GDNF expression also was evident within the cerebral cortex, extending from anterior areas of the frontal lobe (Fig. 1A) to regions of the parietal lobe located above the midbrain (Fig. 1J). This represents an anterior–posterior axis of 25 mm covering prefrontal association cortical areas (cortical areas 9 and 10; Fig. 1 A–C), the premotor cortex (area 6; Fig. 1 D–F), frontal eye fields (area 8; Fig. 1 G–I), primary somatosensory cortical areas (areas 1, 2, and 3; Fig. 1 J–L), and the primary motor cortex (area 4; Fig. 1J), and includes expression in the cingulate cortex (areas 23, 24, and 32; Fig. 1 A, D, G, and J) and Broca's area (areas 44 and 45; Fig. 1D). The cortical expression of GDNF was localized to the neuronal gray matter layers, with a distinct lack of GDNF-positive staining seen in the underlying white matter tracts. No GDNF staining was found in the contralateral hemisphere in any of the sections analyzed, nor was any staining noted in brain regions other than the thalamus and cerebral cortex. In addition to the intense staining of individual neuronal cell bodies and cellular processes, GDNF staining was observed across multiple layers of the frontal cortex with an intensity gradient that was highest in cortical laminae III and IV (Fig. 2).
Fig. 1.
Distribution of GDNF protein after AAV2-GDNF infusion into the thalamus. (A) GDNF expression detected by immunohistochemistry staining in the prefrontal cortex ipsilateral to thalamic infusion. (B and C) Large numbers of nonpyramidal GDNF-positive neurons can be seen across multiple layers in the prefrontal cortex (areas 9 and 10). (D–G) GDNF expression in the premotor cortex (area 6), frontal eye fields (area 8), Broca's area (areas 44 and 45), and cingulate cortex (areas 23, 24, and 32). Pyramidal neurons in laminae V and VI of the premotor cortex (E and F) and frontal eye fields (H and I) expressing GDNF. Strong GDNF-immunopositive staining is evident in the cortical layers above the pyramidal neurons. (J) Intensive GDNF-positive staining in the infused thalamus and cortical GDNF expression in the somatosensory cortex (areas 1, 2, and 3) and primary motor cortex (area 4). (K and L) GDNF-positive neurons in laminae V and VI of the somatosensory cortex. AP, anterior–posterior distance (in mm) from the bregma. Thal, thalamus. Numbers indicate different cortical areas referenced in the text. (Scale bars: 5 mm for A, D, G, and J; 500 μm for B, E, H, and K; 100 μm for C, F, I, and L.)
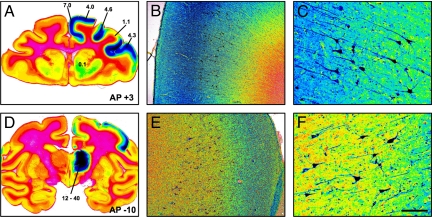
Fig. 2.
Level of GDNF protein expression after infusion of AAV2-GDNF into the right thalamus. Pseudocolor images of GDNF immunohistochemistry-stained sections showing the gradients of GDNF distribution in both the thalamus and cortex. Blue represents the highest intensity of DAB staining; red, the lowest intensity. The numbers in panels A and D represent the level of GDNF protein (μg of GDNF per mg of total protein) in different areas of the brain measured by ELISA from an adjacent tissue block. In panels B, C, E, and F, higher magnification of the cortex shows the high intensity of GDNF staining in lamina III/IV and the high cytoplasmic presence of GDNF in lamina V/VI pyramidal neurons. AP, anterior–posterior distance (in mm) from the bregma. (Scale bars: 10 mm in A and D; 500 μm in B and E; 100 μm in C and F.)
The macroscopically evident GDNF staining of large cortical regions correlated with the presence of GDNF-positive neuronal fibers and cell bodies; however, the overall intensity of immunostaining did not reflect the actual number of GDNF-positive neurons in a specific area when examined microscopically, with extensive noncellular staining indicative of secreted GDNF. Most GDNF-positive neurons within the cortex were identified morphologically as pyramidal neurons located in cortical lamina V/VI, with axonal projections into the overlying layers (Fig. 1E and F). The density of GDNF-positive neurons was particularly high in the prefrontal cortex, where large numbers of nonpyramidal neurons were observed in laminae II–IV (Fig. 1 A–C).
The levels of GDNF protein present in the thalamus, striatum, and various cortical areas were quantified 6 months after AAV2-GDNF delivery. Levels in the vector-infused thalamus ranged from 12 to 40 ng per mg of protein (< 0.6 ng in the contralateral hemisphere), and those in the ipsilateral frontal cortex ranged from 1 to 7 ng per mg of protein (with no GDNF detected in the contralateral cortex). The values shown in Fig. 2 indicate the approximate correlation of GDNF quantification with GDNF immunostaining from an adjacent coronal tissue block.
Thalamocortical Trafficking of AAV2 Vector and Transduction of Cortical Neurons.
Cytoplasmic expression and accumulation of GFP in transduced cells after AAV2-GFP delivery was used to assess the localization of transduced neurons after thalamic infusion in NHPs. GFP expression was analyzed in both the thalamus and frontal cortex to investigate correlations between the distribution of AAV2 vector in the thalamus and the transduction of neurons in specific regions of the cortex indicative of thalamocortical axonal trafficking of AAV2 vectors.
Distribution of AAV2-GFP infusion within the thalamus was assessed in 4 NHPs (ID numbers V422, V632, V655, and V991) with respect to specific thalamic nuclei containing GFP-immunopositive neurons. Because of slight differences in cannula positioning, each animal showed some discrepancy in the thalamic distribution of GFP staining [supporting information (SI) Fig. S1]. In brief, AAV2-GFP transduction within the thalamus was most extensive in monkey V422, with GFP expression seen throughout the ventral lateral, ventral anterior, and mediodorsal nuclei and extending rostrally into the anterior nucleus. Monkey V632 showed a more posterior infusion, with GFP-positive neurons extending from the ventral anterior to ventral posterior nuclei. Monkey V655 exhibited restricted distribution of GFP-positive neurons, contained mainly within the mediodorsal and ventral lateral nuclei. Monkey V991 received a slightly more lateral infusion of AAV2-GFP, resulting in transduction of the ventral lateral and ventral anterior nuclei, with GFP expression also observed in the internal capsule. Evidence of vector leakage/reflex back up the cannula tract into the lateral ventricle was seen in monkeys V655 and V991, but not in the other 2 animals. This leakage resulted in a smaller area of transduction in the thalamus.
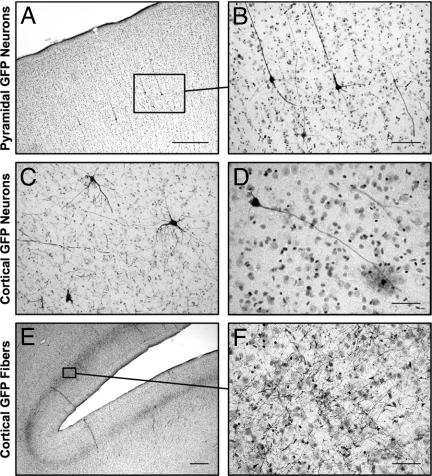
Within the cerebral cortex, GFP-positive neuronal cell bodies and processes were clearly distinguishable by immunocytochemical staining. Most GFP-positive neurons were identified as pyramidal neurons located in lamina V/VI. (Fig. 3A and B); however, other GFP-positive cells with the morphology of nonprojection basket neurons and glia were found in smaller numbers (Fig. 1 C and D). In addition, we also observed areas in which GFP staining was localized to fibers in lamina IV (Fig. 1 E and F). In contrast to the dispersed GDNF staining, all GFP staining was clearly localized to neuronal structures, indicating intracellular accumulation of GFP specifically within neurons, compared with the secretion and extracellular diffusion of GDNF.
Fig. 3.
Cortical expression of GFP after infusion of AAV2-GFP to the left thalamus. Individual GFP-immunopositive neurons are seen within different areas of the cortex. (A and B) Cortical pyramidal neurons are the predominant type of GFP-positive neuron. (C and D) Neurons without pyramidal morphology also are seen in the cortex, including basket-like neurons (C) and glia-like cells (D). (E and F) Extensive GFP-positive fiber networks also are seen in the frontal cortex. (Scale bars: 500 μm in A and E; 100 μm in B and C; 50 μm in D and F.)
Systematic scanning of GFP-immunostained coronal sections from the 4 monkeys identified specific regions of the frontal cortex that contained GFP-positive neurons. As for AAV-GFP transduction of thalamic neurons, the distribution of GFP-positive cells in the cortex was slightly different in each monkey (Table 1). The secondary motor cortex (area 6) and frontal eye fields (area 8) were the main regions in which the majority of GFP-positive neurons were consistently found. In each monkey, the anterior cingulate cortex (areas 24 and 32) also contained GFP-positive neurons. GFP-positive neurons also were found in other cortical areas, including the primary motor cortex (area 4), somatosensory cortex (areas 3 and 2), posterior cingulate cortex (areas 23 and 31), and Broca's area (areas 44 and 45). As summarized in Table 1, monkey V422 had a considerable distribution of GFP-positive neurons in the frontal eye fields and Broca's area. Monkey V632 was the only animal with GFP expression in the primary somatosensory cortex but lacked any expression in Broca's area. Monkey V655 displayed diffuse distribution of GFP-positive cortical neurons across most of the cortical areas analyzed. Monkey V991 had restricted expression, with GFP-positive neurons found only in the anterior cingulate cortex, secondary motor cortex, and frontal eye fields. No GFP-positive cells were found in the hemisphere contralateral to the infusion site or subcortical nuclei other than the AAV2-GFP infused thalamus.
Table 1.
Relative distribution of GFP-positive neurons in the cerebral cortex
| Monkey ID | Thalamic nuclei | Prefrontal cortex 9/10/46 | Broca's area 44/45 | Frontal eye fields 8 | Secondary motor cortex 6 | Anterior cingulate cortex24/32 | Somatosensory cortex 3/1/2 | Primary motor cortex 4 | Posterior cingulate cortex 23/31 |
|---|---|---|---|---|---|---|---|---|---|
| V422 | AN, VA, MD | ** | *** | *** | *** | ** | * | ||
| V632 | VA, VL, VP | ** | *** | ** | ** | ** | ** | ||
| V655 | VL, MD | *** | * | *** | *** | ** | * | * | |
| V991 | VA, VL | * | * | ** | ** | * |
Representation of GFP-positive neuronal distribution in the cortex ipsilateral to the thalamic infusion. Relative distribution of GFP-positive cortical neurons:
*** majority of neurons;
** smaller numbers of neurons;
* few isolated neurons. AN, anterior nucleus; MD, mediodorsal nucleus; VA, ventral anterior nucleus; VL, ventral lateral nucleus; VP, ventral posterior nucleus.
Discussion
Efficient delivery of therapeutic proteins to the brain remains a serious, and frequently unappreciated, obstacle to achieving clinical efficacy while minimizing adverse effects. Developments in gene delivery have provided an opportunity to establish production of biologics within the brain parenchyma. These advances have led to the initiation of multiple clinical trials in which AAV2 vectors have become a preferred vector system for treating neurologic disorders (4). Although focal targeting of a specific nucleus can be reliably accomplished by stereotactic neurosurgical infusion, the extensive convoluted arrangement of the human cortex is not easily targeted by direct infusion of viral vectors. The difficulties in safely achieving widespread gene expression in the brain have hindered the development of potential treatments for lysosomal storage diseases (25, 26), Canavan's disease, and dementia, including Alzheimer's disease. With AAV2 emerging as the preeminent vector for neurologic gene delivery, we investigated an alternative route of AAV2 delivery to the cortex via axonal trafficking along thalamocortical projections. The cerebral cortex is the major recipient of thalamic efferent projections.
Initially, we used GDNF as a secreted reporter protein with no known function in the cerebral cortex. We infused AAV2-GDNF into the thalamus and found high concentrations of GDNF in the frontal cortex. GDNF in the cortex appeared to be largely localized to laminae III and IV, where the majority of thalamocortical axons are known to innervate (27, 28), indicating secretion from the thalamic terminals. In addition to the extracellular GDNF staining, many lamina V/VI pyramidal neurons within the same cortical areas also contained GDNF, suggesting transduction of cortical neurons by AAV2-GDNF. Many of the GDNF-positive neurons in the frontal cortex were located 20 mm from the AAV2-GDNF infusion site, a distance significantly greater than can be explained solely by vector infusion. With no significant GDNF expression detected outside the cortex and thalamus, this specific transportation between the thalamus and cortex suggests axonal transportation of both GDNF protein and AAV2 vector.
We further investigated the axonal trafficking of AAV2 with AAV2-GFP, because, unlike GDNF, GFP remains cytoplasmic and thus is indicative of direct cellular transduction. Theoretically, cytoplasmic staining of cortical neurons for GDNF could result from the uptake of secreted GDNF. By mapping the localization of GFP-positive neurons in the frontal cortex of each monkey and analyzing this transduction of cortical cells in conjunction with the observed thalamic distribution, we were able to infer some of the known topographical organization of the thalamocortical projections (29, 30), suggesting active transportation of AAV2 vector along single axonal projections. In the most restricted thalamic infusion, GFP was contained largely within neurons of the mediodorsal and ventral lateral thalamic nuclei. Therefore, with this restricted infusion as a starting reference, we assumed that the finding of GFP-positive neurons in the secondary motor cortex and prefrontal cortex of each monkey was due, at least in part, to AAV2 vector transport along axonal projections connecting the medial thalamic nucleus and secondary motor cortex. Congruent with our observations, thalamic neurons of the medial nuclear group have been shown to send efferent projections to the areas of the frontal cortex (21, 31). A slightly more anterior infusion that transduced the ventral anterior and ventral lateral thalamic nuclei resulted in a very similar pattern of cortical GFP expression as that from medial thalamic infusion, with GFP-positive cells observed in the anterior secondary motor cortex, cingulate cortex, and frontal eye fields. Although the thalamocortical projections are very topographically organized, there is considerable overlap in cortical connections, especially from adjacent thalamic structures. Spread of AAV2-GFP transduction into the anterior thalamic nucleus generated GFP-positive neurons in Broca's area, whereas a more caudal spread to the ventral posterior thalamic nucleus led to GFP-positive neurons in more posterior cortical areas, such as the primary somatosensory cortex and primary motor cortex.
Reciprocal projections between specific thalamic nuclei and cortical areas hinder an absolute determination, based on our findings, of whether AAV2 is being trafficked anterogradely or retrogradely. Transduction of neurons that project to the thalamus potentially could occur through incorporation of the AAV2 vector particle at the axon terminal and intracellular transportation back to the cell body. Retrograde transport of AAV has been shown to occur via receptor-mediated internalization at the axon terminal, followed by microtubule-mediated transport to the nucleus (32, 33), and has been used in the targeting of motor neurons by intramuscular AAV vector injection (21). Although retrograde-mediated transduction may explain the AAV2 vector transduction of lamina V/VI pyramidal neurons that send their efferent projections to the thalamus (34), we found GFP-positive nonpyramidal neurons as well. To transduce cortical neurons without thalamic projections, intact AAV2 vector itself must be transported to the cortex before cortical cells are transduced. The correlation between the topological organization of thalamocortical projections and the observed areas of cortical transduction suggests that transfer of AAV2 vector to the cortex is mediated by anterograde axonal transportation. The possible anterograde transport of other AAV serotypes 1 and 9 in the mouse brain was reported recently (35); however, reciprocal projections and the small size of the mouse brain precluded a conclusive determination of transport mechanisms. Identifying the mechanisms of axonal transportation of AAV would be valuable; possibly, the relatively poor transduction efficiency of AAV vectors, combined with the need for very high titer infusions, allows extensive transportation of these vectors before host cells are transduced.
The discrepancies in the distribution of cortical neuronal transduction found in the present study demonstrate the potential ability of targeted infusions of specific thalamic nuclei to obtain transgene expression in restricted areas of the cortex. They also highlight the need for intraoperative imaging of gene delivery vector infusions, to ensure correct infusion of the vector into the target structure (36–38). Leakage during vector infusion into nonthalamic areas, such as the internal capsule and lateral ventricle, was observed in 2 of the 4 monkeys. This leakage resulted in reduced transduction of the targeted thalamus and, consequently, decreased transportation to cortical regions.
Overall, our understanding of AAV vector transport is relatively poor, with parenchymal CNS gene delivery viewed largely as a procedure for localized delivery. Nonetheless, our findings underscore the unrealized potential for AAV2 vectors to transduce neurons located a considerable distance from the infusion site. Although the CED approach is potentially useful for gene delivery to dispersed regions of the CNS, it also carries clinical safety concerns associated with the use of AAV vectors intended to exclusively target a localized region of the CNS.
Methods
Surgical Delivery.
Recombinant AAV2 vectors containing either human GDNF cDNA (AAV2-GDNF) or GFP cDNA (AAV2-GFP) under the control of cytomegalovirus promoter were infused into the right thalamus of 6 adult rhesus monkeys using a CED protocol described previously (6). All experiments were performed in accordance with National Institutes of Health guidelines and with protocols approved by the University of California San Francisco's Institutional Animal Care and Use Committee.
Production of AAV.
Recombinant AAV2-GDNF was generated through a triple-transfection technique (39, 40). AAV2-GFP was produced in insect cells with a recombinant baculovirus (41). Both vectors underwent CsCl gradient centrifugation to remove empty capsids. AAV2-GFP and AAV2-GDNF were obtained at stock concentrations of 1.0 × 1013 and 1.1 × 1013 vector genomes per mL in PBS (pH 7.4) and Pluronic F-68 (0.001% vol/vol), respectively.
Immunohistochemistry.
Immunostaining with antibodies against GDNF (1:500, AF-212-NA; R&D Systems) and GFP (1:500, AB3080; Chemicon) was performed on Zamboni fixed 40-μm coronal sections covering the entire frontal cortex and extending in a posterior direction to the level of the thalamus. The localization of GDNF and GFP immunopositive neurons was analyzed with reference to The Rhesus Monkey Brain in Stereotactic Coordinates (42) to identify specific areas of immunostaining in the cortex and thalamus.
GDNF Protein ELISA.
Tissue punches from 3-mm coronal blocks of fresh frozen tissue were obtained from cortical, thalamic, and striatal regions of an AAV2-GDNF–infused monkey, as indicated on the GDNF immunostained sections from adjacent tissue blocks shown in Fig. 1. The level of GDNF protein expression was quantified using a commercial GDNF ELISA kit (Emax GDNF ELISA, Promega) specific for human GDNF.
Supplementary Material
Acknowledgments.
This work was supported by National Institute of Neurological Disorders and Stroke Grant R01 NS056107–01 (to K.S.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810682106/DCSupplemental.
References
- 1.Vite CH, et al. Effective gene therapy for an inherited CNS disease in a large animal model. Ann Neurol. 2005;57:355–364. doi: 10.1002/ana.20392. [DOI] [PubMed] [Google Scholar]
- 2.Vite CH, Passini MA, Haskins ME, Wolfe JH. Adeno-associated virus vector-mediated transduction in the cat brain. Gene Ther. 2003;10:1874–1881. doi: 10.1038/sj.gt.3302087. [DOI] [PubMed] [Google Scholar]
- 3.Kaplitt MG, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: An open-label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 4.Fiandaca M, Forsayeth J, Bankiewicz K. Current status of gene therapy trials for Parkinson's disease. Exp Neurol. 2008;209:51–57. doi: 10.1016/j.expneurol.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Eberling JL, et al. Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology. 2008;70:1980–1983. doi: 10.1212/01.wnl.0000312381.29287.ff. [DOI] [PubMed] [Google Scholar]
- 6.Bankiewicz KS, et al. Convection-enhanced delivery of AAV vector in parkinsonian monkeys: In vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp Neurol. 2000;164:2–14. doi: 10.1006/exnr.2000.7408. [DOI] [PubMed] [Google Scholar]
- 7.Bobo RH, et al. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krauze MT, Forsayeth J, Park JW, Bankiewicz KS. Real-time imaging and quantification of brain delivery of liposomes. Pharm Res. 2006;23:2493–2504. doi: 10.1007/s11095-006-9103-5. [DOI] [PubMed] [Google Scholar]
- 9.Krauze MT, et al. Real-time visualization and characterization of liposomal delivery into the monkey brain by magnetic resonance imaging. Brain Res Brain Res Protoc. 2005;16:20–26. doi: 10.1016/j.brainresprot.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Krauze MT, et al. Convection-enhanced delivery of nanoliposomal CPT-11 (irinotecan) and PEGylated liposomal doxorubicin (Doxil) in rodent intracranial brain tumor xenografts. Neurol Oncol. 2007;9:393–403. doi: 10.1215/15228517-2007-019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krauze MT, et al. Effects of the perivascular space on convection-enhanced delivery of liposomes in primate putamen. Exp Neurol. 2005;196:104–111. doi: 10.1016/j.expneurol.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Sampson JH, et al. Comparison of intratumoral bolus injection and convection-enhanced delivery of radiolabeled antitenascin monoclonal antibodies. Neurosurg Focus. 2006;20:E14. doi: 10.3171/foc.2006.20.4.9. [DOI] [PubMed] [Google Scholar]
- 13.Sampson JH, et al. Intracerebral infusate distribution by convection-enhanced delivery in humans with malignant gliomas: Descriptive effects of target anatomy and catheter positioning. Neurosurgery. 2007;60(2) Suppl 1:ONS89–ONS98. doi: 10.1227/01.NEU.0000249256.09289.5F. [DOI] [PubMed] [Google Scholar]
- 14.Hadaczek P, et al. Convection-enhanced delivery of adeno-associated virus type 2 (AAV2) into the striatum and transport of AAV2 within monkey brain. Hum Gene Ther. 2006;17:291–302. doi: 10.1089/hum.2006.17.291. [DOI] [PubMed] [Google Scholar]
- 15.Krauze MT, et al. Reflux-free cannula for convection-enhanced high-speed delivery of therapeutic agents. J Neurosurg. 2005;103:923–929. doi: 10.3171/jns.2005.103.5.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saito R, et al. Distribution of liposomes into brain and rat brain tumor models by convection-enhanced delivery monitored with magnetic resonance imaging. Cancer Res. 2004;64:2572–2579. doi: 10.1158/0008-5472.can-03-3631. [DOI] [PubMed] [Google Scholar]
- 17.Fiandaca MS, Forsayeth JR, Dickinson PJ, Bankiewicz KS. Image-guided convection-enhanced delivery platform in the treatment of neurological diseases. Neurotherapeutics. 2008;5:123–127. doi: 10.1016/j.nurt.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varenika V, et al. Real-time imaging of CED in the brain permits detection of infusate leakage. J Neurosurg. 2008;109:874–880. doi: 10.3171/JNS/2008/109/11/0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hadaczek P, Mirek H, Bringas J, Cunningham J, Bankiewicz K. Basic fibroblast growth factor enhances transduction, distribution, and axonal transport of adeno-associated virus type 2 vector in rat brain. Hum Gene Ther. 2004;15:469–479. doi: 10.1089/10430340460745793. [DOI] [PubMed] [Google Scholar]
- 20.Kaspar BK, Llado J, Sherkat N, Rothstein JD, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- 21.Tuszynski MH. Intraparenchymal NGF infusions rescue degenerating cholinergic neurons. Cell Transplant. 2000;9:629–636. doi: 10.1177/096368970000900508. [DOI] [PubMed] [Google Scholar]
- 22.Tuszynski MH, et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med. 2005;11:551–555. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- 23.Passini MA, Lee EB, Heuer GG, Wolfe JH. Distribution of a lysosomal enzyme in the adult brain by axonal transport and by cells of the rostral migratory stream. J Neurosci. 2002;22:6437–6446. doi: 10.1523/JNEUROSCI.22-15-06437.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arciniegas D, et al. The thalamus and the schizophrenia phenotype: Failure to replicate reduced volume. Biol Psychiatry. 1999;45:1329–1335. doi: 10.1016/s0006-3223(97)00459-9. [DOI] [PubMed] [Google Scholar]
- 25.Lonser RR, et al. Image-guided, direct convective delivery of glucocerebrosidase for neuronopathic Gaucher disease. Neurology. 2007;68:254–261. doi: 10.1212/01.wnl.0000247744.10990.e6. [DOI] [PubMed] [Google Scholar]
- 26.Schiffmann R, Brady RO. New prospects for the treatment of lysosomal storage diseases. Drugs. 2002;62:733–742. doi: 10.2165/00003495-200262050-00002. [DOI] [PubMed] [Google Scholar]
- 27.Giguere M, Goldman-Rakic PS. Mediodorsal nucleus: Areal, laminar, and tangential distribution of afferents and efferents in the frontal lobe of rhesus monkeys. J Comp Neurol. 1988;277:195–213. doi: 10.1002/cne.902770204. [DOI] [PubMed] [Google Scholar]
- 28.Jacobson S, Trojanowski JQ. Corticothalamic neurons and thalamocortical terminal fields: An investigation in rat using horseradish peroxidase and autoradiography. Brain Res. 1975;85:385–401. doi: 10.1016/0006-8993(75)90815-x. [DOI] [PubMed] [Google Scholar]
- 29.Kievit J, Kuypers HG. Organization of the thalamo-cortical connections to the frontal lobe in the rhesus monkey. Exp Brain Res. 1977;29:299–322. doi: 10.1007/BF00236173. [DOI] [PubMed] [Google Scholar]
- 30.Brysch W, Brysch I, Creutzfeldt OD, Schlingensiepen R, Schlingensiepen KH. The topology of the thalamo-cortical projections in the marmoset monkey (Callithrix jacchus) Exp Brain Res. 1990;81:1–17. doi: 10.1007/BF00230095. [DOI] [PubMed] [Google Scholar]
- 31.Goldman-Rakic PS, Porrino LJ. The primate mediodorsal (MD) nucleus and its projection to the frontal lobe. J Comp Neurol. 1985;242:535–560. doi: 10.1002/cne.902420406. [DOI] [PubMed] [Google Scholar]
- 32.Kaspar BK, et al. Targeted retrograde gene delivery for neuronal protection. Mol Ther. 2002;5:50–56. doi: 10.1006/mthe.2001.0520. [DOI] [PubMed] [Google Scholar]
- 33.Boulis NM, et al. Adeno-associated viral vector gene expression in the adult rat spinal cord following remote vector delivery. Neurobiol Dis. 2003;14:535–541. doi: 10.1016/j.nbd.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 34.Killackey HP, Sherman SM. Corticothalamic projections from the rat primary somatosensory cortex. J Neurosci. 2003;23:7381–7384. doi: 10.1523/JNEUROSCI.23-19-07381.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cearley CN, Wolfe JH. A single injection of an adeno-associated virus vector into nuclei with divergent connections results in widespread vector distribution in the brain and global correction of a neurogenetic disease. J Neurosci. 2007;27:9928–9940. doi: 10.1523/JNEUROSCI.2185-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fiandaca M, et al. Real-time MR imaging of adeno-associated viral vector delivery to the primate brain. Neuroimage. 2008 doi: 10.1016/j.neuroimage.2008.11.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lonser RR, et al. Real-time image-guided direct convective perfusion of intrinsic brainstem lesions: Technical note. J Neurosurg. 2007;107:190–197. doi: 10.3171/JNS-07/07/0190. [DOI] [PubMed] [Google Scholar]
- 38.Szerlip NJ, et al. Real-time imaging of convection-enhanced delivery of viruses and virus-sized particles. J Neurosurg. 2007;107:560–567. doi: 10.3171/JNS-07/09/0560. [DOI] [PubMed] [Google Scholar]
- 39.Matsushita T, et al. Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Ther. 1998;5:938–945. doi: 10.1038/sj.gt.3300680. [DOI] [PubMed] [Google Scholar]
- 40.Wright JF, Qu G, Tang C, Sommer JM. Recombinant adeno-associated virus: formulation challenges and strategies for a gene therapy vector. Curr Opin Drug Discov Devel. 2003;6:174–178. [PubMed] [Google Scholar]
- 41.Urabe M, Ding C, Kotin RM. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum Gene Ther. 2002;13:1935–1943. doi: 10.1089/10430340260355347. [DOI] [PubMed] [Google Scholar]
- 42.Paxinos G, Huang XF, Toga AW. The Rhesus Monkey Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.