Abstract
Chronic regular use of β2-adrenoceptor (β2-AR) agonists in asthma is associated with a loss of disease control and increased risk of death. Conversely, we have found that administration of β2-AR inverse agonists results in attenuation of the asthma phenotype in an allergen-driven murine model. Besides antagonizing agonist-induced signaling and reducing signaling by empty receptors, β-AR inverse agonists can also activate signaling by novel pathways. To determine the mechanism of the β-AR inverse agonists, we compared the asthma phenotype in β2-AR-null and wild-type mice. Antigen challenge of β2-AR-null mice produced results similar to what was observed with chronic β2-AR inverse agonist treatment, namely, reductions in mucous metaplasia, airway hyperresponsiveness (AHR), and inflammatory cells in the lungs. These results indicate that the effects of β2-AR inverse agonists are caused by inhibition of β2-AR signaling rather than by the induction of novel signaling pathways. Chronic administration of alprenolol, a β-blocker without inverse agonist properties, did not attenuate the asthma phenotype, suggesting that it is signaling by empty receptors, rather than agonist-induced β2-AR signaling, that supports the asthma phenotype. In conclusion, our results demonstrate that, in a murine model of asthma, β2-AR signaling is required for the full development of three cardinal features of asthma: mucous metaplasia, AHR, and the presence of inflammatory cells in the lungs.
Keywords: airway hyperresponsiveness, β-blocker, inverse agonist, mucous metaplasia, inflammation
Asthma is a disease characterized by airway inflammation, airway hyperresponsiveness (AHR), and airway remodeling. Current pharmacological management of asthma aims to attenuate AHR, reverse bronchoconstriction, and reduce chronic inflammation. Because of their potent bronchodilating effects, short-acting β-adrenoreceptor (β2-AR) agonists are the standard treatment for the acute relief of asthma. Long-acting β2-AR agonists are traditionally prescribed as add-on therapies to inhaled corticosteroids for maintenance therapy of moderate and severe asthma. However, chronic repetitive administration of long-acting and/or short-acting β-AR agonists has been associated with tolerance (1–4), an increase in AHR to allergen (5), poor asthma control (6), and death (7).
Analogous to asthma, administration of β-AR agonists in congestive heart failure (CHF) also produced acutely beneficial but chronically detrimental effects. In CHF, drugs classified as β-blockers (β-AR antagonists and inverse agonists) were once contraindicated because acute administration decreased cardiac output and produced other negative inotropic effects (8). However, large clinical trials have shown that chronic administration of certain β-blockers improves cardiac output and decreases mortality (9, 10). Presently, β-blockers are contraindicated in asthma because their acute administration may cause airway narrowing (11, 12). Based on the clinical effects of β-blockers in CHF, we hypothesized that the chronic effects of β-blockers in asthma may be different from those observed during their acute administration (13). In previous studies, we chronically administered β-blockers with inverse agonist properties in a well-established antigen-driven murine asthma model and observed a significant decrease in epithelial cell mucous metaplasia, AHR, and airway inflammation (14, 15). β-AR inverse agonists are a subset of β-blockers that in addition to inhibiting β-AR agonist-induced signaling also inhibit signaling produced by spontaneously or constitutively active receptors (16). Constitutive activity is the signaling of a G protein-coupled receptors (GPCRs) in the absence of an agonist. Thus, inverse agonists inhibit all signaling through the receptor's classical signaling pathway. However, it has recently been shown that many inverse agonists are also capable of activating receptor signaling through alternative pathways (17–19). For example, many β-AR inverse agonists are capable of stimulating a G protein-independent, β-arrestin-dependent activation of ERK1/2 (17).
In this work we asked whether targeted disruption of the β2-AR gene can replicate what was observed pharmacologically with the chronic use of inverse agonists. Specifically, we compared the effect of antigen challenge on β2-AR−/− and wild-type mice to determine airway function, the degree of mucous metaplasia by airway epithelial cells, and the number of inflammatory cells in bronchoalveolar lavage fluid (BALF). These experiments were designed to determine whether the beneficial effects of chronic inverse agonist treatment in the murine asthma model are caused by diminished β2-AR signaling or by certain inverse agonists activating alternative pathways to the classical β2-AR-Gs cascade (i.e., producing “biased agonism”) (17–19). The results of these experiments indicate that it is inhibition of all β2-AR signaling, and not biased agonism, that is responsible for the beneficial effects of chronic inverse agonist treatment.
We also chronically administered alprenolol, a β-blocker without inverse agonist properties (15, 20), to probe further the role of β2-AR signaling in the development of the asthma phenotype by determining whether the signal was a result of constitutive receptor activity or activation of the β2-AR by endogenous agonists. The results of these experiments suggest that it is constitutive β2-AR signaling that allows the full development of AHR, mucous metaplasia, and airway inflammation in the murine model of asthma.
Results
Airway Epithelial Cell Mucin Production.
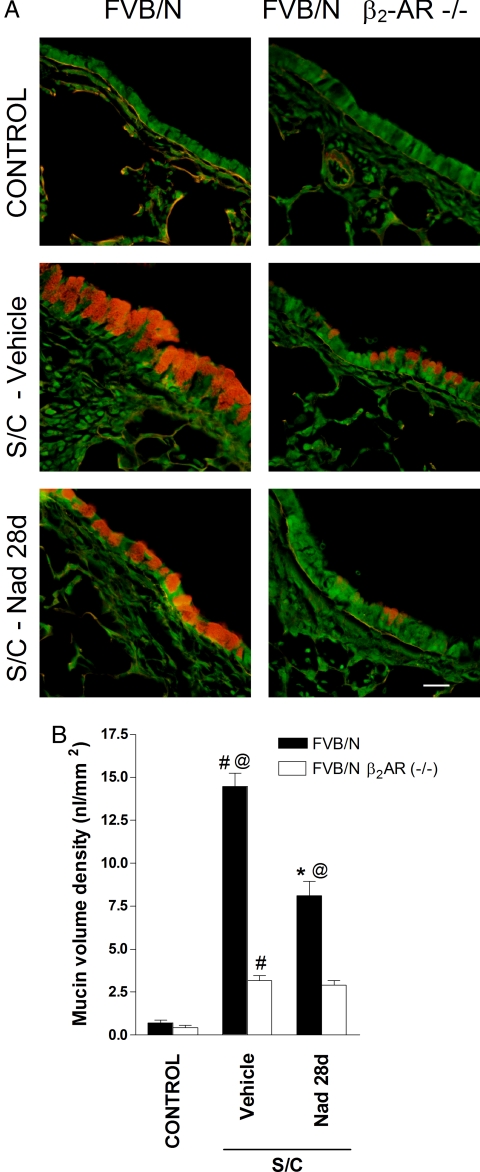
Our previous studies were performed by using BALB/c and C57BL/6J mice (14), whereas the β2-AR−/− mouse was constructed in the wild-type FVB/N background (21). Minimal intracellular mucin was observed in saline-challenged FVB/N mice assessed by using periodic acid fluorescent Schiff (PAFS) staining, whereas in antigen-challenged FVB/N mice there was abundant intracellular mucin (Fig. 1). These results established that the murine model used in the present work exhibits mucous metaplasia, a characteristic of asthma that contributes to airflow obstruction and AHR. Within the antigen-challenged mouse group, administration of the β-AR inverse agonist nadolol (16) for 28 days produced a decrease in mucous metaplasia and partially reversed the changes in airway epithelial cell morphology (Fig. 1A). These results are similar to what we observed with the BALB/c and C57B/6J mice (14). In the FVB/N β2-AR−/− mice group, antigen challenge produced significantly lower mucin volume density (P < 0.05) compared with antigen-challenged FVB/N mice and lower than in FVB/N mice treated with nadolol (P < 0.05) (Fig. 1). Treatment of β2-AR−/− mice with nadolol did not result in any further reduction in mucin volume density (Fig. 1).
Fig. 1.
Effect of β2-AR gene disruption and chronic administration of the inverse agonist nadolol on mucin content in the airway epithelium. (A) Mucin content in the airway epithelia of FVB/N β2-AR−/− and FVB/N mice was measured with PAFS from saline-challenged mice (control), antigen-challenged mice (S/C) administered with either vehicle, or antigen-challenged mice administered nadolol for 28 days (Nad 28 d). (Scale bar, 20 μm.) (B) Morphometric quantification of the mucin volume density was assessed from the various treatment groups. Values represent the mean ± SEM of data from 6–8 mice in each group. #, P < 0.05 vs. control FVB/N β2-AR−/− and FVB/N mice; @, P < 0.05 vs. SC vehicle-treated FVB/N β2-AR−/− mice; *, P < 0.05 vs. SC vehicle-treated FVB/N mice.
Airway Hyperresponsiveness to Methacholine.
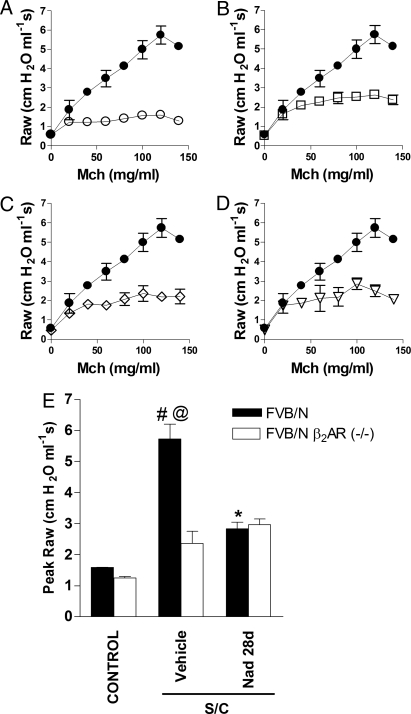
For antigen-challenged vehicle-treated FVB/N mice, the values for methacholine-induced increases in airway resistance (Raw) were significantly higher compared with those in saline-challenged vehicle-treated control FVB/N mice (Fig. 2 A and E). This indicates that this strain of mouse exhibits AHR, a cardinal feature of airway dysfunction in human asthma. Chronic administration of the inverse agonist nadolol in antigen-challenged FVB/N mice caused a significant attenuation of AHR as shown by the values for Raw and peak Raw at methacholine doses ≥80 mg/mL (Fig. 2 B and E). Antigen-challenged vehicle-treated FVB/N β2-AR−/− mice exhibited decreased AHR (P < 0.05) and replicated qualitatively what was observed pharmacologically with the use of nadolol in FVB/N mice (compare Fig. 2 B and C). In antigen-challenged FVB/N β2-AR−/− mice, chronic administration of nadolol yielded no additional benefits as shown by the values for Raw and peak Raw (compare Fig. 2 C, D, and E).
Fig. 2.
Effect of β2-AR gene disruption and chronic administration of the inverse agonist nadolol on AHR. FVB/N β2-AR−/− and FVB/N mice were saline-challenged (control) or antigen-challenged (S/C) and administered either vehicle or nadolol for 28 days before receiving methacholine. (A–D) Values for Raw were recorded by using a computer-controlled ventilator apparatus comparing antigen-challenged FVB/N mice (filled circles) with saline-challenged FVB/N mice (A, open circles), antigen-challenged FVB/N mice treated with nadolol (B, open squares), antigen-challenged FVB/N β2-AR−/− mice (C, open diamonds), or antigen-challenged FVB/N β2-AR−/− mice treated with nadolol (D, open triangles). (E) Values for peak Raw were determined for each mouse by choosing the highest Raw value produced by any of the methacholine doses (most often the next to last dose, 120 mg/mL) from the individual methacholine dose–response curves. Values represent the mean ± SEM of data from 8 mice in each group. #, P < 0.05 vs. control FVB/N β2-AR−/− and FVB/N mice; @, P < 0.05 vs. SC vehicle-treated FVB/N β2-AR−/− mice; *, P < 0.05 vs. SC vehicle-treated FVB/N mice.
Bronchoalveolar Lavage Cellularity.
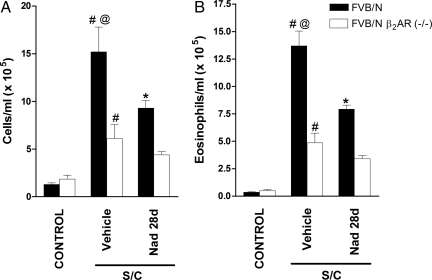
The total cell count in BALF was greatly increased in antigen-challenged FVB/N mice compared with saline-challenged FVB/N mice (Fig. 3A). Chronic administration of nadolol (28 days) significantly reduced total cell counts (Fig. 3A). BALF eosinophil numbers were also significantly increased in antigen-challenged FVB/N mice compared with saline-challenged mice (Fig. 3B), and again, chronic administration of nadolol significantly reduced BALF eosinophil numbers (Fig. 3B). Similar to the changes we observed with chronic administration of nadolol in wild-type FVB/N mice, targeted disruption of the β2-AR gene caused a reduction in total cell counts and eosinophils by ≈60% after antigen challenge (Fig. 3). These findings indicate that disruption of the β2-AR gene or chronic administration of inverse agonists similarly modifies eosinophilic airway inflammation in a murine model of asthma.
Fig. 3.
Effect of β2-AR gene disruption and chronic administration of the inverse agonist nadolol on cell count and eosinophils. Total cell count (A) and eosinophils in BALF (B) from saline-challenged (control) mice, and antigen-challenged (S/C) mice administered with either vehicle or nadolol for 28 days (Nad 28 d). BALF was collected 24 h after the last challenge. Values represent the mean ± SEM of data from 8–12 mice in each group. #, P < 0.05 vs. control FVB/N β2-AR−/− and FVB/N mice; @, P < 0.05 vs. SC vehicle-treated FVB/N β2-AR−/− mice; *, P < 0.05 vs. SC vehicle-treated FVB/N mice.
Effects of Alprenolol With or Without Nadolol Coadministration.
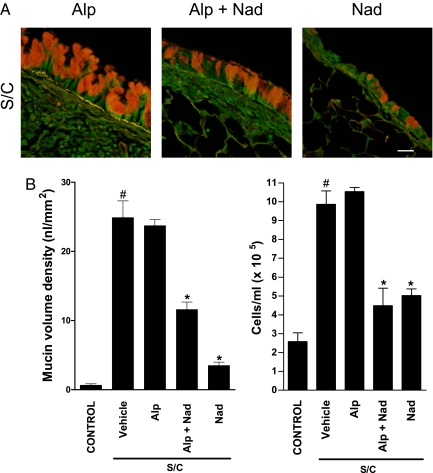
Chronic administration of alprenolol, a β-blocker with no inverse agonist properties, did not reduce mucous metaplasia or BALF inflammatory cell counts in antigen-challenged BALB/cJ mice (Fig. 4). However, chronic coadministration of alprenolol and nadolol produced only a partial reversal of the mucous metaplasia compared with chronic nadolol administration alone but full inhibition of the BALF inflammatory cell counts (Fig. 4). Together, these results suggest that the permissive role of the β2-AR in the asthma phenotype does not require activation by endogenous ligands
Fig. 4.
Effect of chronic administration of nadolol and alprenolol on mucin content in the airway epithelium and BALF cell counts. (A) Mucin content in the airway epithelium of BALB/c mice was measured by using PAFS from antigen-challenged (S/C) mice administered the inverse agonist nadolol (Nad), alprenolol (a β-blocker with weak agonist properties) (Alp), or nadolol and alprenolol for 28 days. (Scale bar, 20 μm.) (B) Morphometric quantification of the mucin volume density (Right) and cell counts (Left) were assessed from the various treatment groups. Values represent the mean ± SEM of data from 5–7 mice in each group. #, P < 0.05 vs. control mice; *, P < 0.05 vs. SC vehicle-treated mice.
Discussion
We have reported that chronic treatment with β2-AR inverse agonists results in reductions of AHR, mucous metaplasia, and inflammatory cells in BALF (14, 15). Here, we report that targeted disruption of the β2-AR gene or chronic treatment with a β-blocker with inverse agonist properties at the β2-AR both produce comparable attenuation of the asthma-like phenotype in a murine antigen-driven model of asthma. Our results surprisingly suggest that constitutive β2-AR signaling is required for the full development of mucous metaplasia, AHR, and inflammatory cells in BALF in a murine model of asthma (Figs. 1–3), although there are residual non-β2-AR inflammatory responses.
It is now known that many if not all GPCRs, including the β2-AR, can signal in the absence of agonist, a phenomenon known as constitutive activity (16, 22). Simultaneous with this discovery of constitutive signaling was the identification of compounds termed inverse agonists. Inverse agonists are a subset of drugs classified as blockers or antagonists, with the difference being that whereas both antagonists and inverse agonists can block agonist-induced activation of the receptor, only inverse agonists are able to inhibit the constitutive signaling of the receptor. Thus, inverse agonists are capable of inhibiting all receptor signaling, whereas antagonists only prevent agonist-induced signaling.
A more recent discovery has been the finding that many inverse agonists are capable of producing what is known as biased agonism (17), a phenomenon originally described by Kenakin as “agonist-directed trafficking of receptor signals” (16), and also described as “functional selectivity” (23). Biased agonism refers to the fact that some ligands may signal through only one of several pathways modulated by a receptor. An example relevant to this work is that some ligands that function as inverse agonists for the classical β2-AR–Gs signaling pathway can nonetheless activate the receptor to signal through a G protein-independent, β-arrestin-dependent, pathway (17). For instance carvedilol acts as a biased ligand at the β2-AR signaling via β-arrestin-dependent ERK1/2 activation in the absence of G protein activation (17). This biased agonism of β-AR ligands such as carvedilol has been hypothesized to be the mechanism by which carvedilol appears to be more effective than other β-AR inverse agonists in the treatment of CHF (17, 24).
Based on the paradigm shift that occurred in chronic CHF therapy, we investigated whether chronic treatment with β2-AR inverse agonists, a class of drugs currently contraindicated in asthma because of their potential to produce acute bronchoconstriction, may be beneficial with long-term administration (14, 15, 25, 26). Indeed, we found that chronic treatment with β2-AR inverse agonists produced a time-dependent decrease in AHR and mucous metaplasia in a murine model of asthma (14, 15), whereas treatment with alprenolol, a β-blocker without inverse agonist properties, did not decrease AHR (15). However, these studies did not determine whether the beneficial effect of certain β-blockers was caused by their inverse agonist properties or by biased agonism of a non-Gs-dependent pathway. Our current results with the β2-AR−/− mice rule out biased agonism as the explanation because these mice lack a functional β2-AR gene and are incapable of producing any β2-AR signaling through any pathway. Further support for this is that we have shown that the biased agonist carvedilol, a drug capable of activating the ERK1/2 pathway and EGFR transactivation, but an inverse agonist at the classical Gs pathway, was not as effective at reducing AHR as nadolol, an inverse agonist that did not activate ERK1/2 or produce EGFR transactivation (15, 17, 18).
We had also reported an up-regulation of β2-ARs after chronic β-AR inverse agonist treatment and speculated this may be partially responsible for the attenuation of the asthma phenotype (25). However, the results with the β2-AR−/− mice also rule out receptor up-regulation as necessary for attenuation of the asthma phenotype.
We next performed experiments to determine whether the required β2-AR signaling was caused by activation by endogenous β2-AR agonists (adrenalin and noradrenalin) or a result of constitutive β2-AR activity. For these experiments, we chronically treated antigen-challenged BALB/cJ mice with alprenolol, a β-blocker with very weak agonist properties at the β2-AR (20). Because alprenolol is not a β2-AR inverse agonist, it would only reduce agonist-produced signaling and not reduce constitutive β2-AR signaling. Alprenolol had no effect on mucous metaplasia or BALF inflammatory cells (Fig. 4), but the inverse agonist nadolol did inhibit the responses, suggesting that it was constitutive β2-AR signaling that was allowing the development of the observed changes in the airways. Because in some circumstances alpenolol can behave as a partial agonist (15, 17, 20), another possible interpretation of these data could be that alprenolol was reducing the β2-AR signaling produced by endogenous agonist but that its weak agonist activity was sufficient to allow mucous metaplasia and airway inflammation to develop. The only accurate method to separate agonist-induced signaling from constitutive signaling is to use a true “neutral” antagonist (not one with weak agonist, or even weak inverse agonist activities). However, such compounds are exceedingly rare (16), and there is no agreement that any neutral antagonists exist for the β2-AR (17).
The fact that the effects of nadolol were attenuated by coadministration of alprenolol is consistent with the hypothesis that the effects of nadalol were caused by binding with the β2-AR (Fig. 4). This hypothesis is further supported by the fact that nadolol was unable to produce any additional effect in the β2-AR-null mice (Figs. 1–3).
The specific findings of this work on the airway epithelium, AHR, and inflammatory cells in BALF are consistent with our previous findings after chronic β2-AR inverse agonist treatment in other strains of mice, and they suggest a possible role for β2-AR inverse agonists in the chronic management of asthma. The airway epithelium in asthma contains increased numbers of mucin-filled goblet cells that contribute significantly to airflow obstruction and play a central role in asthma-related deaths (6, 27–29). Therefore, attenuation of airway mucin content would be expected to play a significant role in managing this disease. Attenuation of mucin content was observed in the FVB/N β2-AR−/− mice as well as after chronic nadolol treatment of wild-type FVB/N (Figs. 1 and 4) or other strains (14). It remains unknown whether similar effects can be replicated in human asthma.
Targeted disruption of the β2-AR gene also produced a significant decrease in AHR (Fig. 2). Although previous studies of the β1- and β2-AR−/− nonallergic mice showed a decreased response to bronchoconstrictors (30), the effect of β2-AR gene deletion has not been studied in an asthma model. Here, we show that the reduction in AHR in FVB β2-AR−/− mice was comparable with what was observed with chronic nadolol treatment in antigen-challenged wild-type FVB/N mice (Fig. 2) and similar to what we have observed with chronic administration of inverse agonists in antigen-driven models of asthma by using other mouse strains (15). Although future investigations are still needed to investigate the relevance of these findings in human asthma, a recent study demonstrated a dose-dependent attenuation of AHR with 9-week administration of oral nadolol in patients with mild asthma (26).
A characteristic of airway inflammation in asthma is the infiltration of cells such as eosinophils and lymphocytes (31, 32), producing a wide range of inflammatory mediators that are responsible for the perpetuation of airway inflammation (31, 32). Our present results show that chronic treatment with nadolol again, as in other strains (Fig. 4) (14), reduced the inflammatory cells in BALF of antigen-challenged FVB/N mice (Fig. 3). These results were qualitatively similar to what was observed by using the FVB/N β2-AR−/− mice (Fig. 3). It remains unknown whether similar effects can be replicated in human asthma.
In conclusion, in a murine model of asthma, both pharmacological and genetic evidence confirm that β2-AR signaling is required for the full development of three cardinal features of asthma: mucous metaplasia, AHR, and inflammatory cell infiltration into the lungs. These findings appear paradoxical in view of the fact that chronic activation of the β2-AR has long been thought to be beneficial in asthma and has led to the development of several long-acting and ultralong-acting β2-AR agonists. Our findings, if confirmed in human studies, may cause a paradigm shift in the future pharmacological management of chronic asthma.
Materials and Methods
Animals.
Six- to 12-week-old BALB/c and FVB/N (male) mice (Jackson Animal Laboratory) and β2-AR-null FVB/N (21) (male) mice (a generous gift from Brian Kobilka, Stanford University) were housed under specific pathogen-free conditions in accordance with the Institutional Animal Care and Use Committee of the University of Houston.
Animal Sensitization and Challenge.
Antigen-challenged BALB/c, FVB/N, and β2-AR-null FVB/N mice were sensitized (weekly i.p. injections, on days 0, 7, and 14) and challenged (once daily intranasally for 5 days on days 41–45) with ovalbumin as described in ref. 14. Saline-challenged mice were sensitized with ovalbumin but challenged with saline.
Drug Administration.
A group of antigen-challenged BALB/c, FVB/N, and FVB/N β2-AR-null mice were fed (ad libitum) mouse chow containing nadolol or alprenolol (a β1/β2-AR antagonist with partial β2-AR agonist activity) (Sigma) (16, 20), between days 18 and 46 (28-day treatment) at concentrations of 250 ppm and 7,200 ppm, respectively (14). These concentrations were chosen because they had been shown to produce effects in mice (33). The β-blocker nadolol, a nonselective β-AR antagonist with equal affinity for both β1- and β2-AR, was chosen because a previous study using transgenic mice with cardiac overexpression of the human β2-AR revealed this drug to be a full inverse agonist at this receptor (16). The half-life and pharmacokinetic profile of nadolol made the drug suitable for dosing orally in the chow. Untreated saline-challenged or antigen-challenged mice received vehicle and were fed with normal mouse chow. For experiments examining constitutive receptor activity, BALB/c mice were used because this strain is the most prevalent antigen-driven murine model of asthma. Experimental mice were killed on day 46 (14).
Bronchoalveolar Lavage.
Cold PBS (1 mL) was infused and drawn back through the tracheal cannula from killed (0.1 mL of 65 mg/mL pentobarbital) mice and repeated once. The determination of total and differential leukocyte counts in the BALF was performed as described in ref. 14.
Histochemistry.
For PAFS reagent staining to examine intracellular mucin, lungs were fixed with 4% paraformaldehyde in PBS (pH 7.0) infused through a tracheal cannula at room temperature, then removed from the thoracic cavity and further fixed overnight at 4 °C before being embedded in paraffin (34). Lungs were then sectioned, stained for fluorescence microscopy, and examined under a 40× objective as described in ref. 34. Images were acquired before any measurements using MagnaFire 2.1 (Optronics) and analyzed using ImagePro Plus by blinded investigators (34).
Lung Physiology.
On day 46, mice were anesthetized, tracheotomized, and connected to computer-controlled ventilator apparatus (Flexivent; Scientific Respiratory Equipment) (35). To induce airway constriction, a solution containing acetyl-β-methylcholine chloride (methacholine) (Sigma) was infused by using the Flexivent nebulizer. The methacholine dose was started at 10 μg/mL and increased stepwise up to a maximum of 140 μg/mL. After each methacholine dose was administered, the central Raw was sampled at 1-min intervals for 4 min and then averaged. The values for Raw were measured by using the force oscillation technique, and the complex input impedance of the respiratory system was computed as described in ref. 36. The values for Raw were plotted as a function of methacholine doses, with the largest value for Raw obtained in response to methacholine airway constriction referred to as peak Raw (15).
Statistical Analysis.
Quantitative data are presented as mean ± SEM (expressed as the percentage SEM). Statistical analysis for multiple groups was performed by using one-way ANOVA followed by Dunnett's multicomparison test (Prism; GraphPad). P < 0.05 was considered statistically significant.
Acknowledgments.
This work was supported by the Strategic Program for Asthma Research of the American Asthma Foundation (R.A.B.) and National Institutes of Health Grant R01HL72984 (to B.F.D.).
Footnotes
Conflict of interest statement: R.A.B. is a scientific founder and shareholder of Inverseon, Inc. S.P. is a shareholder of Inverseon, Inc.
This article is a PNAS Direct Submission.
See Commentary on page 2095.
References
- 1.Lipworth BJ. Airway subsensitivity with long-acting β2-agonists: Is there cause for concern? Drug Saf. 1997;16:295–308. doi: 10.2165/00002018-199716050-00002. [DOI] [PubMed] [Google Scholar]
- 2.Shore SA, Drazen JM. β-Agonists and asthma: Too much of a good thing? J Clin Invest. 2003;112:495–497. doi: 10.1172/JCI19642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE. Meta-analysis: Effect of long-acting β-agonists on severe asthma exacerbations and asthma-related deaths. Ann Intern Med. 2006;144:904–912. doi: 10.7326/0003-4819-144-12-200606200-00126. [DOI] [PubMed] [Google Scholar]
- 4.Abramson MJ, Walters J, Walters EH. Adverse effects of β-agonists: Are they clinically relevant? Am J Respir Med. 2003;2:287–297. doi: 10.1007/BF03256657. [DOI] [PubMed] [Google Scholar]
- 5.Cockcroft DW, McParland CP, Britto SA, Swystun VA, Rutherford BC. Regular inhaled salbutamol and airway responsiveness to allergen. Lancet. 1993;342:833–837. doi: 10.1016/0140-6736(93)92695-p. [DOI] [PubMed] [Google Scholar]
- 6.Drazen JM, et al. Comparison of regularly scheduled with as-needed use of albuterol in mild asthma: Asthma Clinical Research Network. N Engl J Med. 1996;335:841–847. doi: 10.1056/NEJM199609193351202. [DOI] [PubMed] [Google Scholar]
- 7.Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM. The Salmeterol Multicenter Asthma Research Trial: A comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15–26. doi: 10.1378/chest.129.1.15. [DOI] [PubMed] [Google Scholar]
- 8.Bond RA, Spina D, Parra S, Page CP. Getting to the heart of asthma: Can “β-blockers” be useful to treat asthma? Pharmacol Ther. 2007;115:360–374. doi: 10.1016/j.pharmthera.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Lechat P, et al. Clinical effects of β-adrenergic blockade in chronic heart failure: A meta-analysis of double-blind, placebo-controlled, randomized trials. Circulation. 1998;98:1184–1191. doi: 10.1161/01.cir.98.12.1184. [DOI] [PubMed] [Google Scholar]
- 10.Hall SA, et al. Time course of improvement in left ventricular function, mass, and geometry in patients with congestive heart failure treated with β-adrenergic blockade. J Am Coll Cardiol. 1995;25:1154–1161. doi: 10.1016/0735-1097(94)00543-y. [DOI] [PubMed] [Google Scholar]
- 11.Boskabady MH, Snashall PD. Bronchial responsiveness to β-adrenergic stimulation and enhanced β-blockade in asthma. Respirology. 2000;5:111–118. doi: 10.1046/j.1440-1843.2000.00236.x. [DOI] [PubMed] [Google Scholar]
- 12.Singh BN, Whitlock RM, Comber RH, Williams FH, Harris EA. Effects of cardioselective β-adrenoceptor blockade on specific airways resistance in normal subjects and in patients with bronchial asthma. Clin Pharmacol Ther. 1976;19:493–501. doi: 10.1002/cpt1976195part1493. [DOI] [PubMed] [Google Scholar]
- 13.Bond RA. Is paradoxical pharmacology a strategy worth pursuing? Trends Pharmacol Sci. 2001;22:273–276. doi: 10.1016/s0165-6147(00)01711-9. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen LP, et al. Chronic exposure to β-blockers attenuates inflammation and mucin content in a murine asthma model. Am J Respir Cell Mol Biol. 2008;38:256–262. doi: 10.1165/rcmb.2007-0279RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Callaerts-Vegh Z, et al. Effects of acute and chronic administration of β-adrenoceptor ligands on airway function in a murine model of asthma. Proc Natl Acad Sci USA. 2004;101:4948–4953. doi: 10.1073/pnas.0400452101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bond RA, et al. Physiological effects of inverse agonists in transgenic mice with myocardial overexpression of the β2-adrenoceptor. Nature. 1995;374:272–276. doi: 10.1038/374272a0. [DOI] [PubMed] [Google Scholar]
- 17.Wisler J, et al. A unique mechanism of β-blocker action: Carvedilol stimulates β-arrestin signaling. Proc Natl Acad Sci USA. 2007;104:16657–16662. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim IM, et al. β-Blockers alprenolol and carvedilol stimulate β-arrestin-mediated EGFR transactivation. Proc Natl Acad Sci USA. 2008;105:14555–14560. doi: 10.1073/pnas.0804745105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker JG, Hill SJ. Multiple GPCR conformations and signalling pathways: Implications for antagonist affinity estimates. Trends Pharmacol Sci. 2007;28:374–381. doi: 10.1016/j.tips.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jasper JR, Michel MC, Insel PA. Amplification of cyclic AMP generation reveals agonistic effects of certain β-adrenergic antagonists. Mol Pharmacol. 1990;37:44–49. [PubMed] [Google Scholar]
- 21.Chruscinski AJ, et al. Targeted disruption of the β2 adrenergic receptor gene. J Biol Chem. 1999;274:16694–16700. doi: 10.1074/jbc.274.24.16694. [DOI] [PubMed] [Google Scholar]
- 22.Parra S, Bond RA. Inverse agonism: From curiosity to accepted dogma, but is it clinically relevant? Curr Opin Pharmacol. 2007;7:146–150. doi: 10.1016/j.coph.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Penn RB. Embracing emerging paradigms of G protein-coupled receptor agonism and signaling to address airway smooth muscle pathobiology in asthma. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:149–169. doi: 10.1007/s00210-008-0263-1. [DOI] [PubMed] [Google Scholar]
- 24.Poole-Wilson PA, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET) randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 25.Lin R, et al. Changes in β2-adrenoceptor and other signaling proteins produced by chronic administration of “β-blockers” in a murine asthma model. Pulm Pharmacol Ther. 2008;21:115–124. doi: 10.1016/j.pupt.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanania NA, et al. The safety and effects of the β-blocker, nadolol, in mild asthma: An open-label pilot study. Pulm Pharmacol Ther. 2008;21:134–141. doi: 10.1016/j.pupt.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holgate ST, et al. Epithelial–mesenchymal communication in the pathogenesis of chronic asthma. Proc Am Thorac Soc. 2004;1:93–98. doi: 10.1513/pats.2306034. [DOI] [PubMed] [Google Scholar]
- 28.Kuperman DA, et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med. 2002;8:885–889. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]
- 29.Zhen G, et al. IL-13 and epidermal growth factor receptor have critical but distinct roles in epithelial cell mucin production. Am J Respir cell Mol Biol. 2007;36:244–253. doi: 10.1165/rcmb.2006-0180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGraw DW, Almoosa KF, Paul RJ, Kobilka BK, Liggett SB. Antithetic regulation by β-adrenergic receptors of Gq receptor signaling via phospholipase C underlies the airway β-agonist paradox. J Clin Invest. 2003;112:619–626. doi: 10.1172/JCI18193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wills-Karp M, Karp CL. Biomedicine. Eosinophils in asthma: Remodeling a tangled tale. Science. 2004;305:1726–1729. doi: 10.1126/science.1104134. [DOI] [PubMed] [Google Scholar]
- 32.Barnes PJ, Chung KF, Page CP. Inflammatory mediators and asthma. Pharmacol Rev. 1988;40:49–84. [PubMed] [Google Scholar]
- 33.Callaerts-Vegh Z, et al. Effects of different β-adrenoceptor ligands in mice with permanent occlusion of the left anterior descending coronary artery. Br J Pharmacol. 2003;138:1505–1516. doi: 10.1038/sj.bjp.0705205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evans CM, et al. Mucin is produced by Clara cells in the proximal airways of antigen-challenged mice. Am J Respir Cell Mol Biol. 2004;31:382–394. doi: 10.1165/rcmb.2004-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evans KL, Bond RA, Corry DB, Shardonofsky FR. Frequency dependence of respiratory system mechanics during induced constriction in a murine model of asthma. J Appl Physiol. 2003;94:245–252. doi: 10.1152/japplphysiol.00593.2002. [DOI] [PubMed] [Google Scholar]
- 36.Schuessler TF, Bates JH. A computer-controlled research ventilator for small animals: Design and evaluation. IEEE Trans Biomed Eng. 1995;42:860–866. doi: 10.1109/10.412653. [DOI] [PubMed] [Google Scholar]