Abstract
The nonsense-mediated mRNA decay (NMD) pathway is a well-known eukaryotic surveillance mechanism that eliminates aberrant mRNAs that contain a premature termination codon (PTC). The UP-Frameshift (UPF) proteins, UPF1, UPF2, and UPF3, are essential for normal NMD function. Several NMD substrates have been identified, but detailed information on NMD substrates is lacking. Here, we noticed that, in Arabidopsis, most of the mRNA-like nonprotein-coding RNAs (ncRNAs) have the features of an NMD substrate. We examined the expression profiles of 2 Arabidopsis mutants, upf1-1 and upf3-1, using a whole-genome tiling array. The results showed that expression of not only protein-coding transcripts but also many mRNA-like ncRNAs (mlncRNAs), including natural antisense transcript RNAs (nat-RNAs) transcribed from the opposite strands of the coding strands, were up-regulated in both mutants. The percentage of the up-regulated mlncRNAs to all expressed mlncRNAs was much higher than that of the up-regulated protein-coding transcripts to all expressed protein- coding transcripts. This finding demonstrates that one of the most important roles of NMD is the genome-wide suppression of the aberrant mlncRNAs including nat-RNAs.
Keywords: ncRNA, tiling array, UPF1, UPF3
In Arabidopsis thaliana, hundreds of nonprotein-coding RNA (ncRNA) transcripts have been discovered based on the cloning of full-length cDNAs, whole-genome tiling array, and deep-sequencing analysis (1–8). They include microRNA (miRNA) precursors, transacting siRNA (ta-siRNA) precursors, and mRNA-like ncRNAs (mlncRNA). The mlncRNAs were divided into 2 types—natural antisense transcript RNAs (nat-RNAs) that arise from the strands opposite the coding strands and other mlncRNAs.
Nonsense-mediated mRNA decay (NMD) is a eukaryotic mRNA quality-control mechanism that eliminates aberrant mRNAs containing a premature termination codon (PTC) from cells to avoid the production of truncated proteins (9–13). Such transcripts can arise by genomic frameshifts, nonsense mutations, inefficiently spliced premRNAs, and so on. Several proteins involved in NMD have already been discovered (10). Among them, the UP-Frameshift (UPF) proteins, UPF1, UPF2, and UPF3, are core components of mRNA surveillance complexes and are essential for normal NMD function. In yeasts and mammals, aberrant transcripts with nonsense mutations are recognized by the UPF complex and then degraded from the 5′ and 3′ ends by recruiting-decapping and 5′→3′ exonuclease activities and deadenylating and 3′→5′ exonuclease activities (14–16).
Plants also have a sophisticated NMD system (10). The Arabidopsis genome encodes homologues of 3 UPF genes (UPF1, UPF2, and UPF3). Some UPF1 and UPF3 mutants were obtained in Arabidopsis and analyzed to investigate the NMD system in plants and to identify several mRNA substrates for NMD by using microarrays (17–20). For example, the aberrant mRNAs containing PTC were overaccumulated in the upf1 and upf3 mutants (17–19).
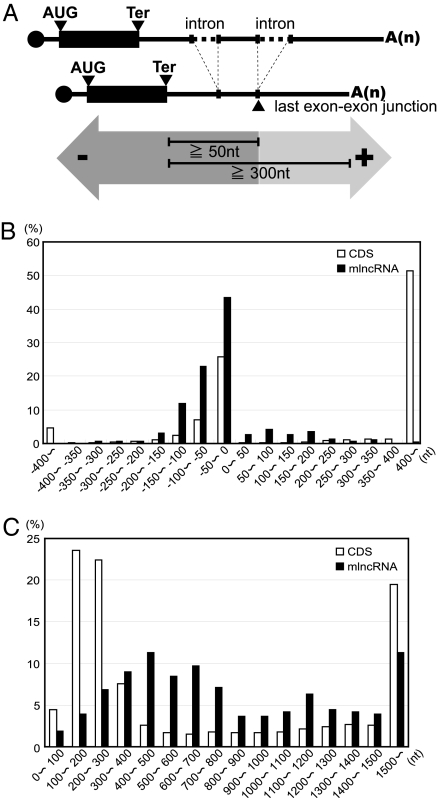
In plants, aberrant mRNAs with termination codons located distant (≧300 nt) from the 3′ termini of mRNAs or ≧50 nt upstream of the last exon–exon junction tend to be recognized as substrates for NMD (21–23) (Fig. 1A). Furthermore, NMD in plants also targets mRNAs that do not possess any introns as in yeast, which is different from NMD in animals (24).
Fig. 1.
The features of mRNA-like ncRNAs. (A) Illustration of the proposed consensus for the NMD target. The mRNAs with termination codons located distant (≧300 nt) from the 3′ termini of mRNAs or ≧50 nt upstream of the last exon–exon junction tend to be recognized as substrates for NMD. Arrows (−) and (+) indicate upstream and downstream from the last exon–exon junction, respectively. (B) Distribution of the distances from the last exon–exon junctions to the 5′-end-closest termination codons of protein-coding mRNAs or mRNA-like ncRNAs (mlncRNAs). (C) Distribution of the distances from the 3′termini of RNAs to the 5′-end-closest termination codons of protein-coding mRNAs or mlncRNAs. Ter, termination codon. CDS, coding sequence.
We hypothesized that many mlncRNAs are degraded by the NMD system, because most of the mlncRNAs have the features of NMD substrates and the potential to be recognized as aberrant transcripts by the UPF complex in Arabidopsis. Here, we performed whole-genome tiling arrays for upf1-1 and upf3-1 mutant plants. The results showed that NMD suppresses not only protein-coding transcripts but also many mlncRNAs. Identification of NMD substrates including many mlncRNAs will aid in understanding the regulatory mechanisms of eukaryotic transcriptomes and elucidating the roles of mlncRNAs.
Results
Features of mlncRNAs as NMD Substrates.
Of the mlncRNAs with Arabidopsis Genome Initiative (AGI) codes in Arabidopsis The Arabidopsis Information Resource 8 (TAIR8) genome version, 74.6% (293/393) have one or more introns. Of the mlncRNAs with introns, 39.9% (117/293) have termination codons that are located ≧50 nt upstream of the last exon–exon junction when the first AUGs near the 5′ termini are recognized as start codons (Fig. 1B). Notice that the majority of the mlncRNAs with intron(s) have a termination codon located upstream of the last exon–exon junction (Fig. 1B, black bars). However, only 17.0% (3,463/20,374) of the protein-coding mRNAs with intron(s) have the 5′-end-closest termination codons that are located ≧50 nt upstream of the last exon–exon junction (Fig. 1B, white bars). They include either the termination codons of the protein-coding ORFs or the termination codons of the upstream ORFs (uORFs) that sometimes exist in front of the major ORFs. Furthermore, of the mlncRNAs with ORFs, 87.3% (331/379) have termination codons that are located >300 nt from the 3′ termini of the mRNA, although, only 49.5% (11,655/23,438) of protein-coding mRNAs have such termination codons (Fig. 1C). Thus, many mlncRNAs have the features of a plant NMD target as described above and may be suppressed by NMD.
Overaccumulation of Many mlncRNAs in upf1-1 and upf3-1.
To examine whether these mlncRNAs are suppressed by NMD, we performed whole-genome-tiling array analysis by using 2 upf mutants: upf1-1 and upf3-1. We focused on the up-regulated transcripts, because the target RNAs should be released and up-regulated when the NMD mechanism is inhibited in the upf mutants. In this study, we used the gene annotations of TAIR8 for gene classification. However, we excluded the AGI codes for conserved uORFs from the analyses described throughout this article, because they represent uORFs in the 5′ untranslated regions (UTRs) of the protein-coding mRNAs but not independent transcripts themselves.
Compared with the wild-type accumulation of 237 and 167, AGI-annotated transcripts were increased ≧1.8-fold in upf1-1 and upf3-1, respectively (P initial ≦10−8, FDR α = 0.05, Fig. 2A and supporting information (SI) Table S1 and Table S2). Of the increased AGI-annotated transcripts, 198 (83.5%) and 138 (82.6%) were protein-coding genes in upf1-1 and upf3-1, respectively (Fig. 2A and Table S1 and Table S2). We estimated that 55.6% and 60.1% of those in upf1-1 and upf3-1, respectively, were genes whose transcripts have uORFs in their 5′UTRs, whereas only 31.8% and 32.0% of the protein-coding genes that were not up-regulated in upf1-1 and upf3-1, respectively, had uORFs. This result is consistent with previous reports that many transcripts with uORFs were regulated by the NMD system in mammals and yeasts (16, 25, 26).
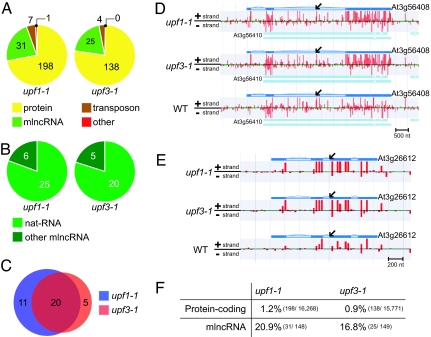
Fig. 2.
AGI-annotated transcripts up-regulated in upf1-1 and upf3-1. (A) Classification of AGI-annotated transcripts up-regulated ≧1.8-fold in upf1-1 and upf3-1, respectively (P initial ≦10−8, FDR α = 0.05). (B) Classification of AGI-annotated mlncRNAs up-regulated >1.8-fold (P initial ≦10−8, FDR α = 0.05) into 2 types, nat-RNA and other mlncRNA. (C) Overlap of AGI-annotated mlncRNAs up-regulated between upf1-1 and upf3-1 (P initial ≦10−8, FDR α = 0.05). (D and E) Detection of a nat-RNA (At3g56408) (D) and another mlncRNA (At3g26612) (E) up-regulated ≧1.8-fold in both upf1-1 and upf3-1 (P initial ≦10−8, FDR α = 0.05). Black arrows indicate the gene structures of nat-RNA or other mlncRNA in TAIR8. The deep-blue regions are exons and the light-blue regions are introns. The red and green bars indicate the relative signal intensity of probes (red ≧400, green <400). The sense gene, At3g56410, is also shown. The tiling-array expression data are available at http://omicspace.riken.jp/gps/group/psca3. (F) Percentages of the protein-coding transcripts and mlncRNAs up-regulated ≧1.8-fold to the expressed protein-coding transcripts and the expressed mlncRNAs (P initial ≦10−8, FDR α = 0.05).
Of the increased AGI-annotated transcripts, 31 (13.1%) and 25 (15.0%) transcripts encoded mlncRNAs in upf1-1 and upf3-1, respectively. Furthermore, of the mlncRNAs that were up-regulated, 25 (80.6%) and 20 (80.0%) were classified as nat-RNAs, and 6 (19.4%) and 5 (20.0%) were classified as other mlncRNAs in upf1-1 and upf3-1 (Fig. 2B). Of the mlncRNAs that were up-regulated in each mutant, 20 were found in both mutants (Fig. 2C). Eighteen (90%) of them were nat-RNAs, and two (10%) were other mlncRNAs. For example, accumulation of a nat-RNA from the gene At3g56408 (Fig. 2D) and an mlncRNA transcribed from the gene At3g26612 (Fig. 2E) are up-regulated in both mutants, but accumulation of the transcripts from the sense gene At3g56410, encoding an unknown protein, is not up-regulated (Fig. 2D).
Importantly, all termination codons of 36 AGI-annotated mlncRNAs up-regulated in upf1-1 and upf3-1 were located >400 nt upstream (average 1,250 nt upstream) of the 3′ termini. Furthermore, 30 of the up-regulated mlncRNAs have exon–exon junction(s). Their termination codons are located upstream (average 59 nt upstream) of the last exon–exon junction. These features of the up-regulated mlncRNAs in the upf mutants almost meet the previously proposed consensus of aberrant RNA molecules targeted by the NMD system (21–23). Therefore, our tiling-array analysis indicated that the mlncRNAs are recognized as the aberrant transcripts and suppressed by the NMD system.
Of all expressed protein-coding AGI-annotated transcripts (16,268 in upf1 and 15,771 in upf3, P initial ≦10−8), the transcripts with ≧1.8-fold increase (FDR α = 0.05) were 1.2% and 0.9%, respectively; however, of all the expressed mlncRNAs with AGI codes (148 in upf1 and 149 in upf3, P initial ≦10−8), the mlncRNAs with ≧1.8-fold increase (FDR α = 0.05) were 20.9% and 16.8% (Fig. 2F). These percentages were much higher than those of the protein-coding transcripts.
We also classified the expressed mlncRNAs (153 in the wild type or upf1-1, and 158 in the wild type or upf3-1) or the protein-coding transcripts (17,004 in the wild type or upf1-1, and 16,800 in the wild type or upf3-1) with AGI codes into some groups based on the fold changes (Fig. S1 A and B, Table S3 and Table S4). The mlncRNAs showed an apparent tendency toward increased accumulation in both upf1-1 and upf3-1 mutants (Fig. S1A), whereas the protein-coding transcripts relatively did not (Fig. S1B). These findings suggested that one of the most important roles of NMD is the genome-wide suppression of aberrant mlncRNAs including nat-RNAs.
Prediction and Characterization of Non-AGI Transcriptional Units (TUs).
The ARTADE program (4, 27) to detect expressed genes and unannotated (non-AGI) TUs from tiling-array data predicted 1,752, 1,707, and 1,894 non-AGI TUs in the wild type, upf1-1 and upf3-1, respectively (P initial ≦10−8). We classified these TUs into 2,980 nonredundant groups. The non-AGI TUs that are located on the same strand of the genome and overlapped each other by more than one base were classified into the same group (Fig. S2). Among the TUs in the same group, 1 TU with the highest intensity was identified as the group-representative TU. We used the predicted gene structure of each group-representative TU for the analyses described below.
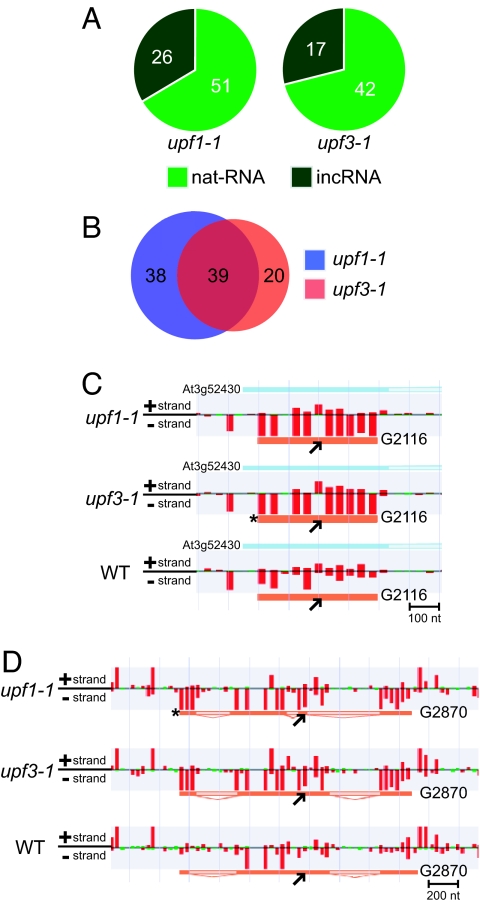
Of all the predicted non-AGI TUs, 77 and 59 TUs were up-regulated ≧1.8-fold in upf1-1 and upf3-1, respectively (P initial ≦10−8, FDR α = 0.05) (Table S5 and Table S6). Of them, at least 46.8% and 47.5% were supported by cDNA clones. Of the increased non-AGI TUs, 51 and 42 in upf1-1 and upf3-1, respectively, were the TUs for putative nat-RNAs, and 26 and 17, respectively, were the TUs for putative intergenic ncRNAs (incRNA) (Fig. 3A). Here, we identified TUs overlapping >1 nt with antisense AGI code genes as nat-RNAs and the rest as incRNAs. Of the non-AGI TUs that were increased, 39 were found in both upf1-1 and upf3-1 (Fig. 3B). For example, G2116, a putative nat-RNA that was derived from the antisense strand of the gene for PHYTOALEXIN-DEFICIENT 4 (PAD4; At3g52430), was up-regulated in both mutants (Fig. 3C), and Group 2870 (G2870), a putative incRNA, was also up-regulated in both mutants (Fig. 3D). These results suggest that more mlncRNAs were regulated by the NMD system than estimated from the analysis of AGI-annotated genes shown in Fig. 2.
Fig. 3.
Predicted non-AGI TUs up-regulated in upf1-1 and upf3-1. (A) Classification of predicted mlncRNAs up-regulated ≧1.8-fold in upf1-1 and upf3-1 into 2 types, nat-RNA and incRNA (P initial ≦10−8, FDR α = 0.05). (B) Overlap of non-AGI TUs up-regulated ≧1.8-fold (P initial ≦10−8, FDR α = 0.05) in upf1-1 and upf3-1. (C and D) Detection of a putative nat-RNA (G2116) (C) and a putative incRNA (G2870) (D) up-regulated >1.8-fold in both upf1-1 and upf3-1 (P initial ≦10−8, FDR α = 0.05). Black arrows indicate the predicted gene structures. The deep-orange regions are putative exons and the light-orange regions are putative introns. The red and green bars indicate the relative signal intensity of probes (Red ≧400, Green <400). Asterisks show the group representatives in G2116 and G2870, respectively. The sense gene, At3g52430 (PAD4), is also shown as a blue bar.
Validation of the Tiling-Array Results.
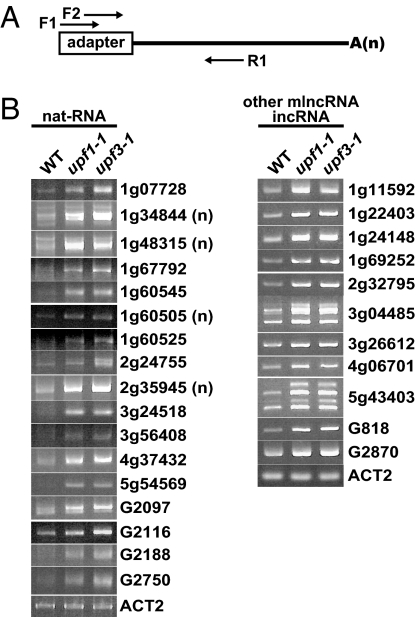
To validate the tiling-array experiments, quantitative reverse transcription-PCR (RT-PCR) of the mlncRNAs with ≧1.8-fold increase in upf1-1 and upf3-1 (P initial ≦10−8) (Table S3 and Table S4) was performed. 5′ rapid amplification of cDNA ends (RACE)-based RT-PCR was used to detect strand-specific bands of nat-RNAs (Fig. 4A), because sense and antisense transcripts should arise from both strands at the same locus. The sample RNAs were dephosphorylated, decapped, ligated with 5′ adapter, and reverse-transcribed followed by quantitative PCR by using a 5′ forward primer annealed to the adapter and gene-specific reverse primer (Fig. 4A). However, the common quantitative RT-PCR was used to analyze the other mlncRNAs and incRNAs. Among 30 mlncRNAs up-regulated in the array analysis, accumulation of at least 28 mlncRNAs including 17 nat-RNAs was increased in upf1-1 and upf3-1 compared with those of the wild type (Fig. 4B). Similar results were obtained by using plants containing another mutant allele of UPF3, upf3-2 (Fig. S3).
Fig. 4.
Quantitative RT-PCR analysis of AGI and non-AGI mlncRNAs. (A) Illustration of 5′RACE-based RT-PCR used for nat-RNA detection. The sample RNA was subjected to dephosphorylation and then decapping reaction. The RNA adapter was ligated to the 5′ end of the RNA. After the RT reaction by using a specific oligo(dT) primer, first PCR was performed by using F1 and R1 primer set. When specific signals were not detectable in the first PCR, additional second (nested) PCR was performed by using the first PCR product as a template and F2 and R1 primer set. (B) Detection of selected nat-RNAs, incRNAs, and other mlncRNAs with ≧1.8-fold increase (P initial ≦10−8). ACT2 mRNA was used as an internal control. (n) indicates the result of the second (nested) PCR.
The 5′RACE-based RT-PCR described above can detect only the capped transcripts but not uncapped transcripts. To examine whether uncapped mlncRNAs were also targeted by NMD, we directly ligated the 5′ adapter to the sample RNAs followed by quantitative RT-PCR. If uncapped mlncRNAs were targeted, this experiment could detect higher signals in the upf mutants than in the wild type. However, no mlncRNA-specific amplified bands were detected in either the wild type, upf1-1 or upf3-1 (Fig. S4). This result indicated that the capped mlncRNAs, but not uncapped mlncRNAs, were the major substrates of NMD.
To compare the sequences of the up-regulated mlncRNAs among the wild type, upf1-1 and upf3-1, we cloned and sequenced the PCR-amplified products of 5 AGI-annotated mlncRNAs. The sequences were highly homologous (≧99%), and the 5′-end-closest termination codons, which are putative triggers of NMD, were conserved among the wild type, upf1-1 and upf3-1. The results described above suggested that the native mlncRNAs were recognized as aberrant transcripts and were suppressed by the NMD system.
Regulation of the mlncRNAs by NMD Depends on Their Translation.
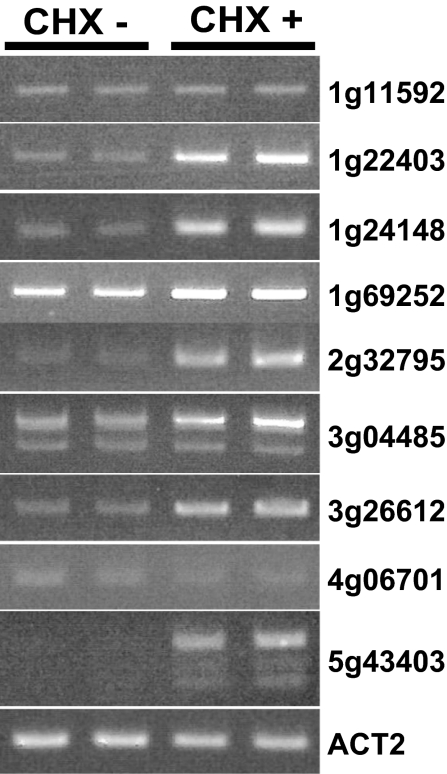
The NMD system is a translation-dependent pathway (9). To obtain further experimental support, we treated wild-type seedlings with cycloheximide (CHX), which inhibits translation and thus temporarily suppresses the NMD system (17, 21, 23), and examined the expression of some mlncRNAs. Of the 9 detected mlncRNAs, accumulation of 7 mlncRNAs in the CHX-treated (CHX+) plants was up-regulated compared with those in the mock-treated (CHX-) plants (Fig. 5). These results, and the data in Fig. 4, indicate that the NMD system suppresses aberrant mlncRNAs.
Fig. 5.
Quantitative RT-PCR analysis of some mlncRNAs in the cycloheximide (CHX)-treated plants. Two independent samples from CHX-untreated (CHX-) and CHX-treated (CHX+) plants, respectively, were loaded. ACT2 mRNA was used as an internal control.
Discussion
In this study, we showed that many mlncRNAs could serve as NMD substrates (Fig. 1) and that not only protein-coding transcripts but also many mlncRNAs, including nat-RNAs, were up-regulated in upf1-1 and upf3-1 (Figs. 2 and 3). The location of the 5′-end-closest termination codons of the up-regulated AGI-annotated mlncRNAs is in agreement with the previously proposed consensus of the aberrant RNA molecules targeted by the NMD system (21–23). This finding suggests that the NMD system genome-widely suppresses aberrant mlncRNAs including nat-RNAs. Identification of the NMD-targeted mlncRNAs may help identify the roles of these mlncRNAs.
Some short ORFs that could encode short peptides have been reported to function in biologically important processes (28, 29). In fact, the short ORFs of many mlncRNAs, which could be recognized by NMD, could produce short peptides. It is unknown whether these putative peptides could be translated and whether they have an important function in biological processes. However, their overaccumulation might have an unfavorable effect on plant cells. Thus, NMD may coordinate the amount of these short peptides by suppressing the short peptide-encoding mlncRNAs before the translation.
The NMD system is considered to be a mechanism involved in the degradation of aberrant mRNAs that contain a premature termination codon (PTC) resulting from unexpected errors such as genomic mutations, transcriptional errors, and missplicing (9). However, the percentage of up-regulated mlncRNAs to all expressed mlncRNAs was much higher than the percentage of up-regulated mRNAs to all expressed mRNAs in upf1-1 and upf3-1 (Fig. 2F and Fig. S3). This result suggests that one of the most important roles of NMD is the suppression of the mlncRNAs that are recognized as aberrant transcripts.
Many transcripts including hundreds of intergenic ncRNAs are overaccumulated in RNAi knockdown lines of core subunits of the exosome in Arabidopsis (3). The transcripts up-regulated in the upf mutants may overlap with exosome substrates identified previously, because, in the NMD pathway, the RNAs recognized as the aberrant transcripts by the UPF complex should be degraded from the 3′ end by deadenylation and subsequent 3′→5′ exonuclease activity, which is probably included in the exosome (14–16). However, only subtle overlaps (6 AGI-annotated transcripts) were found between the transcripts up-regulated in the upf mutants and exosome substrates (Table S1 and Table S2). In addition, the previously identified population of exosome substrates does not include any nat-RNAs. These differences are probably because of the difference of growth condition, age of plants used, and statistical analysis method.
Of the AGI-annotated transcripts up-regulated in upf1-1 and upf3-1, 8 transcripts were derived from transposon genes (Fig. 2A, Table S1 and Table S2). In addition, 4 up-regulated nat-RNAs were derived from the antisense strands of nonexpressed sense genes annotated as transposons or pseudogenes (Table S1 and Table S2). Taken together, of the 97 non-AGI TUs up-regulated (Fig. 3A), 21 (22%) TUs include short segment(s) of the repeat sequence(s) originating from transposable elements (E value <0.01) (Table S5 and Table S6). The transposon-associated ncRNAs have been reported to be suppressed by DNA methylation at the transcriptional level (2, 6). The relationship between DNA methylation and the suppression of mlncRNA by the NMD system is as yet unknown.
Nuclear cap binding protein 80 (CBP80) promotes the interaction of UPF1 with UPF2 in mammals (30). The Arabidopsis CBP80 homolog, ABA HYPERSENSITIVE1 (ABH1), is involved in premRNA splicing and processing of miRNA precursor (7, 8). Inactivation of ABH1 that results in decreased levels of mature miRNAs is accompanied by apparent stabilization of the precursors. However, our tiling-array analysis of upf mutants did not reveal any remarkable accumulation of miRNA precursors. Therefore, the NMD system is probably involved in the suppression of the aberrant mlncRNAs, not in the processing of miRNA.
Materials and Methods
Plant Materials and RNA Extraction.
The Col-0 ecotype of Arabidopsis was used in this study. The mutants upf1-1 (point mutation), upf3-1 (SALK_025175), and upf3-2 (SALK_097931) were as described previously (14–16). Plants were grown in plastic dishes (30 plants per plastic dish) containing GM agar (0.85%) medium supplemented with 1% sucrose <16-h-light/8-h-dark (40–80 μmol of photons m−2 sec−1) essentially as described in ref. 4. Total RNA was extracted from 15-day-old seedlings by using Isogen reagent (NIPPON GENE), precipitated with 1/3 volume of 8M LiCl, and resolved in RNase-free DEPC water.
Whole-Genome-Tiling Array and Analysis.
The GeneChip Arabidopsis tiling-array set (1.0F Array and 1.0R Array, Affymetrix) was used (2, 4). Eight micrograms per array of the total RNA extracted from 15-day-old seedlings was used for probe synthesis. Probe synthesis, array hybridization, and computational analyses of RNA expression were performed as described in ref. 4. Three independent biological replicates were performed for each strand array. The ARTADE-based method (P initial ≦10−8) was used to detect the expressed AGI-annotated genes and predict the non-AGI TUs from the expression data (4, 27). We identified the AGI transcripts and the non-AGI TUs predominantly up-regulated in both upf mutants by Mann–Whitney U test (FDR α = 0.05) as described in ref. 4.
The Arabidopsis genome annotation used in this analysis was based on the TAIR8 genome version (ftp://ftp.arabidopsis.org/home/tair/Genes/TAIR8_genome_release/TAIR8_functional_descriptions) as of May 5, 2008. Of the AGI-annotated genes annotated as “other RNA,” the genes annotated as “Potential natural antisense gene” were identified as nat-RNAs, and the rest except transacting siRNA precursors were as other mlncRNAs in this study. When 1 AGI-annotated gene had some structural variations, 1 variant with the youngest variant number was selected for use in the analyses of array data.
CHX Treatment.
Fifteen-day-old Arabidopsis seedlings were vacuum-infiltrated with 10 μg/ml CHX (Nacalai Tesque) in the buffer (0.046 g/L Murashige and Skoog Plant Salt Mixture, 0.3 g/L sucrose, pH 5.8). The seedlings were incubated on the bench at room temperature for 3 h, followed by total RNA extraction.
Quantitative RT-PCR Analysis.
The total RNA was subjected to DNase I (Takara) treatment before the RT reaction. cDNA was synthesized from 4 μg of the total RNA in 40 μL of mixture by using a Primescript 1st strand cDNA synthesis kit (Takara) and oligo(dT) primer. PCR was performed in 20 μL of mixture with 0.5 μL of the RT product, Ex Taq polymerase (Takara) and the respective gene-specific primer sets. The cycling parameters were 94 °C for 2 min and 30 sec, 25 or 30 cycles of 20 sec at 94 °C, 30 sec at 55 °C, 30 sec at 72 °C, and a final elongation step at 72 °C for 2 min and 30 sec. Primer sets used are listed in Table S7. PCR products were subjected to electrophoresis on 2% agarose gel followed by ethidium bromide staining for visualization.
Quantitative 5′RACE-Based RT-PCR analysis.
The total RNA was subjected to DNase I (Takara) treatment before the series of treatments. GeneRacer Kit (Invitrogen) was used according to the modified protocol described below. The total RNA (5 μg) was treated with calf intestinal phosphatase (CIP) followed by treatment of tobacco acid pyrophosphatase (TAP) to remove the 5′ cap structure. Three micrograms of the resulting RNA were subjected to ligation with GeneRacer RNA oligo adapter to 5′ end of the RNA by using T4 RNA ligase. The ligated RNA was reverse-transcribed to synthesize cDNA by using SuperScript III RT and the GeneRacer Oligo dT primer in 20 μL of mixture. First PCR was performed in 20 μL of mixture with 0.25 μL of RT product, Ex Taq polymerase, GeneRacer 5′ forward primer, and the gene-specific reverse primer. The cycling parameters of first PCR were 94 °C for 2 min and 30 sec, 40 cycles of 20 sec at 94 °C, 30 sec at 60 °C, 30 sec at 72 °C, and a final elongation step at 72 °C for 2 min and 30 sec. Second (nested) PCR was performed in a 20 μL of mixture with 1 μL of the first PCR product, Ex Taq polymerase, GeneRacer 5′ nested forward primer, and the gene-specific reverse primer. The cycling parameters of nested PCR were 94 °C for 2 min and 30 sec, 20 cycles of 20 sec at 94 °C, 30 sec at 60 °C, 30 sec at 72 °C, and a final elongation step at 72 °C for 2 min and 30 sec. Visualization was as described above. The forward and reverse primers used are listed in Table S8.
Supplementary Material
Acknowledgments.
We thank Y. Watanabe (University of Tokyo) and K. Hori (Rikkyo University) for providing the seeds of upf1-1 and upf3-1 (SALK_025175) and helpful discussion and the Arabidopsis Biological Resource Center for the seeds of upf3-2 (SALK_097931). This work was supported by the Japanese Ministry of Education, Culture, Science, Sports, and Technology Grant-in-Aid for Scientific Research on Priority Areas “Systems Genomics” (to M.S.), RIKEN President Discretionary Fund Grant (to M.S.), and RIKEN Genome Research Grant (to K.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE12101). The tiling-array data can also be viewed at http://omicspace.riken.jp/gps/group/psca3. The detailed dataset of supplementary tables can be downloaded at http://pfgweb.psc.riken.jp/download.html.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808902106/DCSupplemental.
References
- 1.Yamada K, et al. Empirical analysis of transcriptional activity in the Arabidopsis genome. Science. 2003;302:842–846. doi: 10.1126/science.1088305. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, et al. Genome-wide high resolution mapping and functional analysis of DNA methylation in Arabidopsis. Cell. 2006;126:1189–1201. doi: 10.1016/j.cell.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Chekanova JA, et al. Genome-wide high resolution mapping of exosome substrates reveals hidden features in the Arabidopsis transcriptome. Cell. 2007;131:1340–1353. doi: 10.1016/j.cell.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 4.Matsui A, et al. Arabidopsis transcriptome analysis under drought, cold, high-salinity and ABA treatment conditions using a tiling array. Plant Cell Physiol. 2008;49:1135–1149. doi: 10.1093/pcp/pcn101. [DOI] [PubMed] [Google Scholar]
- 5.Seki M, et al. Functional annotation of a full-length Arabidopsis cDNA collection. Science. 2002;296:141–145. doi: 10.1126/science.1071006. [DOI] [PubMed] [Google Scholar]
- 6.Lister R, et al. Highly integrated single-base resolution maps of the epigenome in Arabidopsis. Cell. 2008;133:523–536. doi: 10.1016/j.cell.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregory BD, et al. A link between RNA Metabolism and silencing affecting Arabidopsis development. Dev Cell. 2008;14:854–866. doi: 10.1016/j.devcel.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Laubinger S, et al. Dual roles of the nuclear cap-binding complex and SERRATE in pre-mRNA splicing and microRNA processing in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2008;105:8795–8800. doi: 10.1073/pnas.0802493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maquat LE. Nonsense-mediated mRNA decay: Splicing, translation and mRNP dynamics. Nat Rev Mol Cell Biol. 2004;5:89–99. doi: 10.1038/nrm1310. [DOI] [PubMed] [Google Scholar]
- 10.Behm-Ansmant I, et al. mRNA quality control: An ancient machinery recognizes and degrades mRNAs with nonsense codons. FEBS Lett. 2007;581:2845–2853. doi: 10.1016/j.febslet.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 11.Conti E, Izaurralde E. Nonsense-mediated mRNA decay: Molecular insights and mechanistic variations across species. Curr Opin Cell Biol. 2005;17:316–325. doi: 10.1016/j.ceb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Culbertson MR, Leeds PF. Looking at mRNA decay pathways through the window of molecular evolution. Curr Opin Genet Dev. 2003;13:207–214. doi: 10.1016/s0959-437x(03)00014-5. [DOI] [PubMed] [Google Scholar]
- 13.Rehwinkel J, Raes J, Izaurralde E. Nonsense-mediated mRNA decay: Target genes and functional diversification of effectors. Trends Biochem Sci. 2006;31:639–646. doi: 10.1016/j.tibs.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell P, Tollervey D. An NMD pathway in yeast involving accelerated deadenylation and exosome-mediated 3′→5′ degradation. Mol Cell. 2003;11:1405–1413. doi: 10.1016/s1097-2765(03)00190-4. [DOI] [PubMed] [Google Scholar]
- 15.Lejeune F, Li X, Maquat LE. Nonsense-mediated mRNA decay in mammalian cells involves decapping, deadenylating, and exonucleolytic activities. Mol Cell. 2003;12:675–687. doi: 10.1016/s1097-2765(03)00349-6. [DOI] [PubMed] [Google Scholar]
- 16.He F, et al. Genome-wide analysis of mRNAs regulated by the nonsense-mediated and 5′ to 3′ mRNA decay pathways in yeast. Mol Cell. 2003;12:1439–1452. doi: 10.1016/s1097-2765(03)00446-5. [DOI] [PubMed] [Google Scholar]
- 17.Hori K, Watanabe Y. UPF3 suppresses aberrant spliced mRNA in Arabidopsis. Plant J. 2005;43:530–540. doi: 10.1111/j.1365-313X.2005.02473.x. [DOI] [PubMed] [Google Scholar]
- 18.Yoine M, et al. The lba1 mutation of UPF1 RNA helicase involved in nonsense-mediated mRNA decay cause pleiotropic phenotypic changes and altered sugar signaling in Arabidopsis. Plant J. 2006;47:49–62. doi: 10.1111/j.1365-313X.2006.02771.x. [DOI] [PubMed] [Google Scholar]
- 19.Arciga-Reyes L, Wootton L, Kieffer M, Davies B. UPF1 is required for nonsense-mediated mRNA decay (NMD) and RNAi in Arabidopsis. Plant J. 2006;47:480–489. doi: 10.1111/j.1365-313X.2006.02802.x. [DOI] [PubMed] [Google Scholar]
- 20.Yoine M, Nishii T, Nakamura K. Arabidopsis UPF1 RNA helicase for nonsense-mediated mRNA decay is involved in seed size control and is essential for growth. Plant Cell Physiol. 2006;47:572–580. doi: 10.1093/pcp/pcj035. [DOI] [PubMed] [Google Scholar]
- 21.Kertesz S, et al. Both introns and long 3′-UTRs operate as cis-acting elements to trigger nonsense-mediated decay in plants. Nucleic Acids Res. 2006;34:6147–6157. doi: 10.1093/nar/gkl737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz AM, et al. Stability of plant mRNAs depends on the length of the 3′-untranslated region. Biochemistry (Moscow) 2006;71:1377–1384. doi: 10.1134/s0006297906120145. [DOI] [PubMed] [Google Scholar]
- 23.Hori K, Watanabe Y. Context analysis of termination codons in mRNA that are recognized by plant NMD. Plant Cell Physiol. 2007;48:1072–1078. doi: 10.1093/pcp/pcm075. [DOI] [PubMed] [Google Scholar]
- 24.Kerenyi Z, et al. Inter-kingdom conservation of mechanism of nonsense-mediated mRNA decay. EMBO J. 2008;27:1585–1595. doi: 10.1038/emboj.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendell JT, Dietz HC. When the message goes awry: Disease-producing mutations that influence mRNA content and performance. Cell. 2001;107:411–414. doi: 10.1016/s0092-8674(01)00583-9. [DOI] [PubMed] [Google Scholar]
- 26.Mendell JT, et al. Nonsense surveillance regulates expression of diverse classes of mammalian transcripts and mutes genomic noise. Nat Genet. 2004;36:1073–1078. doi: 10.1038/ng1429. [DOI] [PubMed] [Google Scholar]
- 27.Toyoda T, Shinozaki K. Tiling array-driven elucidation of transcriptional structures based on maximum-likelihood and Markov models. Plant J. 2005;43:611–621. doi: 10.1111/j.1365-313X.2005.02470.x. [DOI] [PubMed] [Google Scholar]
- 28.Galindo MI, Pueyo JI, Fouix S, Bishop SA, Couso JP. Peptides encoded by short ORFs control development and define a new eukaryotic gene family. PLoS Biol. 2007;5:e106. doi: 10.1371/journal.pbio.0050106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kondo T, et al. Small peptide regulators of actin-based cell morphogenesis encoded by a polycistronic mRNA. Nat Cell Biol. 2007;9:660–665. doi: 10.1038/ncb1595. [DOI] [PubMed] [Google Scholar]
- 30.Hosoda K, Kim YK, Lejeune F, Maquat LE. CBP80 promotes interaction of Upf1 with Upf2 during nonsense-mediated mRNA decay in mammalian cells. Nat Struct Mol Biol. 2005;12:893–901. doi: 10.1038/nsmb995. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.