Abstract
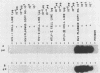
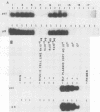
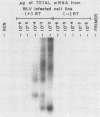
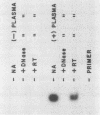
Bovine leukemia virus (BLV) is the etiologic agent of leukemia in cattle and is believed to cause decreases in milk productivity, fertility, and life span in infected cows. BLV is a type C retrovirus in the Oncovirinae subfamily. It is most closely related to human T-cell lymphoma/leukemia virus type I (HTLV-I) and type II (HTLV-II). Since the polymerase chain reaction (PCR) provides rapid and efficient amplification of DNA sequences, primers were designed to amplify regions of the polymerase (pol) and pX genes specific for BLV targets. These sets of primers consistently amplified as few as 10 copies of BLV DNA contained in a plasmid in the background of 1 microgram of either human or bovine chromosomal DNA. In addition, no amplification products were detected from cell lines infected with HTLV-I, HTLV-II, or human immunodeficiency virus type 1 or 2 by the BLV PCR systems. Samples of peripheral blood mononuclear cells from 18 cows, previously determined to be serologically positive or negative, were correctly identified in a blind study as containing proviral DNA by use of the BLV primers and probes. Cloning and sequencing of amplified products revealed finite sequence variations among a previously cloned BLV isolate, the wild-type virus, and the published genome. Reverse transcriptase-directed PCR with the primers for both BLV pol and BLV pX was performed on plasma from a BLV-infected cow and detected in vivo BLV RNA expression. In summary, we have developed a specific and sensitive assay using PCR for the detection and identification of BLV infections; this assay can now be applied to clinical and basic research questions in veterinary medicine.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott M. A., Poiesz B. J., Byrne B. C., Kwok S., Sninsky J. J., Ehrlich G. D. Enzymatic gene amplification: qualitative and quantitative methods for detecting proviral DNA amplified in vitro. J Infect Dis. 1988 Dec;158(6):1158–1169. doi: 10.1093/infdis/158.6.1158. [DOI] [PubMed] [Google Scholar]
- Brenner J., Van-Haam M., Savir D., Trainin Z. The implication of BLV infection in the productivity, reproductive capacity and survival rate of a dairy cow. Vet Immunol Immunopathol. 1989 Oct;22(3):299–305. doi: 10.1016/0165-2427(89)90017-2. [DOI] [PubMed] [Google Scholar]
- Byrne B. C., Li J. J., Sninsky J., Poiesz B. J. Detection of HIV-1 RNA sequences by in vitro DNA amplification. Nucleic Acids Res. 1988 May 11;16(9):4165–4165. doi: 10.1093/nar/16.9.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer G. F., Boerrigter H. M., Akkermans J. P., Brenner J. Use of milk samples and monoclonal antibodies directed against BLV-p24 to identify cattle infected with bovine leukemia virus (BLV). Vet Immunol Immunopathol. 1989 Oct;22(3):283–292. doi: 10.1016/0165-2427(89)90015-9. [DOI] [PubMed] [Google Scholar]
- Deschamps J., Kettmann R., Burny A. Experiments with cloned complete tumor-derived bovine leukemia virus information prove that the virus is totally exogenous to its target animal species. J Virol. 1981 Nov;40(2):605–609. doi: 10.1128/jvi.40.2.605-609.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer J. F., Abt D. A., Bhatt D. M., Marshak R. R. Studies on the relationship between infection with bovine C-type virus, leukemia, and persistent lymphocytosis in cattle. Cancer Res. 1974 Apr;34(4):893–900. [PubMed] [Google Scholar]
- Ferrer J. F. Bovine lymphosarcoma. Adv Vet Sci Comp Med. 1980;24:1–68. [PubMed] [Google Scholar]
- Ferrer J. F., Stock N. D., Lin P. Detection of replicating C-type viruses in continuous cell cultures established from cows with leukemia: effect of the culture medium. J Natl Cancer Inst. 1971 Sep;47(3):613–621. [PubMed] [Google Scholar]
- Gupta P., Ferrer J. F. Comparison of various serological and direct methods for the diagnosis of BLV infection in cattle. Int J Cancer. 1981 Aug 15;28(2):179–184. doi: 10.1002/ijc.2910280211. [DOI] [PubMed] [Google Scholar]
- Hoff-Jørgensen R. An international comparison of different laboratory tests for the diagnosis of bovine leukosis: suggestions for international standardization. Vet Immunol Immunopathol. 1989 Oct;22(3):293–297. doi: 10.1016/0165-2427(89)90016-0. [DOI] [PubMed] [Google Scholar]
- Kettmann R., Cleuter Y., Gregoire D., Burny A. Role of the 3' long open reading frame region of bovine leukemia virus in the maintenance of cell transformation. J Virol. 1985 Jun;54(3):899–901. doi: 10.1128/jvi.54.3.899-901.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettmann R., Deschamps J., Cleuter Y., Couez D., Burny A., Marbaix G. Leukemogenesis by bovine leukemia virus: proviral DNA integration and lack of RNA expression of viral long terminal repeat and 3' proximate cellular sequences. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2465–2469. doi: 10.1073/pnas.79.8.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettmann R., Marbaix G., Cleuter Y., Portetelle D., Mammerickx M., Burny A. Genomic integration of bovine leukemia provirus and lack of viral RNA expression in the target cells of cattle with different responses to BLV infection. Leuk Res. 1980;4(6):509–519. doi: 10.1016/0145-2126(80)90062-4. [DOI] [PubMed] [Google Scholar]
- McDonald H. C., Ferrer J. F. Detection, quantitation, and characterization of the major internal virion antigen of the bovine leukemia virus by radioimmunoassay. J Natl Cancer Inst. 1976 Oct;57(4):875–882. doi: 10.1093/jnci/57.4.875. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Montagna R. A., Papsidero L., Poiesz B. J. Evaluation of a solid-phase immunoassay for the simultaneous detection of antibodies to human immunodeficiency virus type 1 and human T-cell lymphotropic virus type I. J Clin Microbiol. 1991 May;29(5):897–900. doi: 10.1128/jcm.29.5.897-900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper C. E., Abt D. A., Ferrer J. F., Marshak R. R. Seroepidemiological evidence for horizontal transmission of bovine C-type virus. Cancer Res. 1975 Oct;35(10):2714–2716. [PubMed] [Google Scholar]
- Piper C. E., Ferrer J. F., Abt D. A., Marshak R. R. Postnatal and prenatal transmission of the bovine leukemia virus under natural conditions. J Natl Cancer Inst. 1979 Jan;62(1):165–168. [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Willems L., Kettmann R., Campbell K., Zaya R., Burny A., Haseltine W. A. The 3' region of bovine leukemia virus genome encodes a trans-activator protein. EMBO J. 1986 Oct;5(10):2585–2589. doi: 10.1002/j.1460-2075.1986.tb04538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagata N., Yasunaga T., Tsuzuku-Kawamura J., Ohishi K., Ogawa Y., Ikawa Y. Complete nucleotide sequence of the genome of bovine leukemia virus: its evolutionary relationship to other retroviruses. Proc Natl Acad Sci U S A. 1985 Feb;82(3):677–681. doi: 10.1073/pnas.82.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock N. D., Ferrer J. F. Replicating C-type virus in phytohemagglutinin-treated buffy-coat cultures of bovine origin. J Natl Cancer Inst. 1972 Apr;48(4):985–996. [PubMed] [Google Scholar]
- Van Der Maaten M. J., Miller J. M. Replication of bovine leukemia virus in monolayer cell cultures. Bibl Haematol. 1975 Oct;(43):360–362. doi: 10.1159/000399166. [DOI] [PubMed] [Google Scholar]
- Williams A. E., Fang C. T., Slamon D. J., Poiesz B. J., Sandler S. G., Darr W. F., 2nd, Shulman G., McGowan E. I., Douglas D. K., Bowman R. J. Seroprevalence and epidemiological correlates of HTLV-I infection in U.S. blood donors. Science. 1988 Apr 29;240(4852):643–646. doi: 10.1126/science.2896386. [DOI] [PubMed] [Google Scholar]
- Wu M. C., Shanks R. D., Lewin H. A. Milk and fat production in dairy cattle influenced by advanced subclinical bovine leukemia virus infection. Proc Natl Acad Sci U S A. 1989 Feb;86(3):993–996. doi: 10.1073/pnas.86.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broeke A., Cleuter Y., Chen G., Portetelle D., Mammerickx M., Zagury D., Fouchard M., Coulombel L., Kettmann R., Burny A. Even transcriptionally competent proviruses are silent in bovine leukemia virus induced tumor cells. Haematol Blood Transfus. 1989;32:428–432. doi: 10.1007/978-3-642-74621-5_74. [DOI] [PubMed] [Google Scholar]