Abstract
Cell-based therapeutics are currently being developed for a wide array of unmet medical needs. As obstructive vascular disease is the major cause of mortality in the world, cell-based strategies aimed at developing novel therapies or improving current therapies are currently under study. These studies are based on the evolving understanding of the biology of vascular progenitor cells, which has in turn led to the availability of well-defined sources of vascular cells for delivery. Crucial to the development of these approaches is the preclinical testing of cell delivery in animal models. This review highlights the crucial steps involved in the selection of cell sources and generation, delivery approaches, animal models to be used, and endpoints to be studied, in the context of cell delivery for vascular disease. Furthermore, the development of cell delivery to induce angiogenesis in ischemic limbs and to improve the response to large vessel injury will be discussed.
Introduction
As the burden of cardiovascular disease grows worldwide, the search for new therapies includes the consideration of strategies based on delivery of cellular products. The development of these new therapeutics will require careful preclinical investigation involving the use of animal models of human disease. In many cell-based strategies as the patient is often the source of the cells, each therapeutic interaction is complicated by the qualities of the agent and the recipient. This factor and the fact that many reagents are not suitable for nonhuman species make preclinical studies potentially more difficult for cellular approaches. This perspective will focus on the importance of preclinical animal studies in the development of cell-based therapies for vascular disease.
Defining the burden and unmet needs of vascular disease
Vascular disease is the proximate cause of the major sources of morbidity and mortality in the western world and accounts for up to 30% of all deaths worldwide (International Cardiovascular Disease Statistics, 2008a). One third of Americans have some form of cardiovascular disease, most with a vascular basis (International Cardiovascular Disease Statistics, 2008b). The cost to care for these disorders in the USA is over $450,000,000,000 yearly. Atherosclerosis, atherothrombosis and hypertension may lead to myocardial infarction, stroke, renal failure and limb ischemia. Venous insufficiency, deep venous thrombosis and pulmonary embolism also account for significant morbidity and mortality as well. In spite of technologically advanced approaches to treat advanced vascular disease and some decline in recent mortality, the clinical burden remains enormous. Primary prevention strategies albeit effective are often limited by patient awareness, compliance and resources. As such, there are many opportunities in multiple vascular beds to improve current technologies or to develop novel therapies for patient populations for which no effective treatments exist.
In the area of coronary arterial circulation, some patients with symptomatic occlusive atherosclerosis are not be amenable to standard percutaneous or surgical approaches, and new ways to treat theses patients are required. We have recently identified a group of patients in a population-based study determined to be nonrevascularizable for symptomatic ischemic heart disease (Kiernan et al., 2007). Estimates suggest that 50,000-100,000 such individuals would be identified yearly in the USA. Reasons for individuals not being suitable for revascularization include co-morbidities, lack of suitable surgical targets and small vessel disease. Patients who have ischemic myocardial or peripheral territories subtended by vessels that cannot be stented or bypassed might benefit from the development of therapies that stimulate local angiogenesis or growth of new blood vessels into the ischemic territory (Takeshita et al., 1994). Alternatively, improving the results of angioplasty or stenting in vascular segments prone to restenose might extend therapies to new populations of patients.
In addition to obstructive vascular disease, ischemia resulting from microvascular or endothelial dysfunction is associated with morbidity and mortality (Suwaidi et al., 2000). In spite of multiple pharmacological studies including ACE (angiotensin-converting enzyme) inhibitors (Mancini et al., 1996), statins (Egashira et al., 1994), and L-arginine (Lerman et al., 1998) as well as recent and ongoing studies of spinal cord stimulation (Eddicks et al., 2007), no effective form of therapy for this disorder has been established.
The syndrome of pulmonary hypertension is a complex of disorders that includes primary disorders, such as primary pulmonary hypertension, and possibly secondary disorders as a result of chronic thromboembolism or hypoxia. Long-term therapies with prostacyclin or vasodilators may require chronic intravenous treatment. New approaches are needed to treat pulmonary hypertension broadly (Michelakis et al., 2008).
Obstructive peripheral vascular disease may lead to claudication, gangrene and amputation. Diabetes leads to microvascular dysfunction and ischemia. Bypass and stenting or angioplasty of peripheral vessels has the additional limitations of the external forces and compression. Therapeutic angiogenesis of the lower extremity has also been proposed and tested to improve blood supply to an ischemic limb (Powell et al., 2008). Taken together and in spite of major advances in the treatment of obstructive vascular disease, there is a high likelihood that considerable unmet needs will exist in this area for the foreseeable future. As such, the search for novel therapeutics is critically important.
Cells as therapeutics
The history of cellular therapies is as old as blood transfusion. As such, it is an essential and commonplace part of modern medical practice. Of course the concept of cellular replacement therapy (and tissue transplantation) has been expanded to include transfer of numerous cell and tissue types for a vast array of indications. The key feature of these original cellular replacement therapies is that the cell of interest is generally reintroduced into the patient to serve its original function (i.e. red blood cell transfusion). Cellular therapeutics that utilize cells for a heterologous function are regulated as drugs in the USA and as such require a greater degree of preclinical data and approval prior to clinical use. Thus, whether autologous or allogeneic, isolated, cultured or modified, cellular products intended for functions different from their native function are met by high production and preclinical standards before human use.
Cells are attractive as therapeutics for many reasons. First, cells are multifunctional and their functions are highly regulated. Cells may have distinct autocrine, paracrine and endocrine effects or combinations thereof. The secretome of cells is complex and may be modified in a regulated and reproducible manner. Second, cells may have a natural tropism for target tissues. Third, multiple sources of donor cells exist. A single donor may provide a cell source that could be used for thousands of allogeneic doses. Alternatively, autologous sources of cells may provide a single dose for one time use. Fourth, cells may be modified in culture or genetically to provide unique potencies for therapy. These features have led to great enthusiasm for cellular therapies for meeting clinical needs.
Potential for cell therapy of vascular disease
How might cellular therapy be used in the vasculature? In consideration of unmet needs, cellular therapy might be used to broaden the spectrum of potential therapies or improve the efficacy of standard therapies. In the former category would be the delivery of endothelial cells or progenitors to induce angiogenesis in ischemic limbs. In the latter case, using cells to improve the vascular remodeling response to acute or chronic vascular injury (stenting, angioplasty, etc.) might allow for improved treatment of vascular beds with poor response to injury. Thus, arguably the two major vascular clinical needs – deficient small vessel angiogenesis in ischemic tissue and detrimental larger vessel arterial response to injury – might be addressable by cell therapy approaches. These approaches will be the focus of this perspective.
The primary concern of regulatory agencies (for Phase 1 studies) is the demonstration of safety, not efficacy, in preclinical studies. That being said, it is generally and broadly preferred to provide demonstration of efficacy of cell delivery in a clinically relevant preclinical model of disease prior to latter phase studies. As such, many questions must be answered before initiating such a study (or series of studies) and the following discussion is meant to highlight and clarify these questions. The crucial issues to address include defining the cell type and source (including species), the animal model and endpoints to be studied and the method of delivery (Box 1). These issues will be discussed in the context of angiogenesis for chronic limb ischemia and large vessel repair following vascular injury.
Box 1. Points to consider for preclinical animal models of cell delivery.
Selection of cell type
Do the tissues need to be autologous or should they be nonautologous/allogeneic?
What reagents are available in the selected species?
Selection of cell source
Which species is the most appropiate source?
Which tissue will be used?
How will cells be isolated, selected and cultured?
Delivery methods
Is the delivery of cells compatible with relevant clinical applications?
Will a clinical device be used?
Animal model – mechanism of vascular injury
Which is best: genetic model, environmental manipulation or a combination?
How well does it resemble the clinical disease?
Will it require endothelial injury (e.g. wire, balloon or stent)?
What is the size of the vessel and its clinical relevance?
Animal model – cell generation and delivery
Does it need to be immunocompetent or immunodeficient?
Does it have an adequate volume of blood or amount of tissue substrate?
What is the proliferative capacity of the cells that will be generated?
How accessible is the vessel for device placement and/or regional cell delivery?
Study endpoints
How will efficacy be determined?
How safe are the methods?
What biodistribution of the stem cells is expected and how will it be measured?
Cell types and sources
The vascular wall is composed of finite types of cells. Vascular smooth muscle cells in the tunica media provide structural and functional support. Endothelial cells lining the vessel and in the adventitia regulate thromboresistance and vascular tone. Adventitial fibroblasts are found in the loose connective tissue that surrounds the vessel. As such, these cell types may be candidates for cellular repair therapies. In addition, since all tissues contain vasculature, every tissue is a potential source (Box 2). The focus of this discussion is on the use of endothelial cells, or cells with the potential to develop an endothelial phenotype, for large vessel repair and therapeutic angiogenesis.
Box 2. Potential sources of therapeutic endothelium.
Mature endothelium
Large arteries: phenotypically suitable for arterial to arterial delivery. If autologous, cells are likely to be dysfunctional given systemic nature of atherosclerosis. Whether delivery of large numbers of dysfunctional cells offers therapeutic benefit remains to be determined. Limited proliferative capacity compared with progenitor and microvascular endothelial cells. Require surgical excision with/without re-anastomosis, a major clinical limitation.
Large veins: phenotypically different from arterial endothelium, less adherent in the face of arterial pressures. Limited proliferative capacity. Require excision, but relatively less invasive than arteries. Considerable venous redundancy is an asset.
Vascularized tissues (including adipose): microvascular endothelial cells from capillaries predominantly. Higher proliferative potential and high tube-forming capacity, suitable for enhancement of angiogenesis in ischemic tissue. Lower levels of endothelial nitric oxide synthase expression compared with arterial cells; of undetermined relevance. Some tissues can be obtained with minimally invasive harvest procedure, e.g. needle biopsy of subcutaneous adipose.
Endothelial precursors
Blood: at least three types of endothelial-like cell. (1) Mature endothelial (sloughed) cells, low in number, low proliferative capacity. (2) Culture-modified mononuclear cells, monocyte-endothelial phenotypic characteristics, limited proliferative capacity. (3) Outgrowth endothelial cells, probably progenitor derived. Similar surface antigen phenotype to microvascular endothelial cells. High proliferative potential and tube-forming capacity. Can be generated from relatively small volumes of peripheral blood, minimally invasive. Require prolonged culture times.
Bone marrow: probably contains precursors at different levels of multipotency and maturity. Higher precursor yield compared with any other tissue. Outgrowth endothelial cells generated from bone-marrow precursor similar in phenotype to blood-derived cells. Require invasive bone marrow harvest.
Adipose tissue: probably contains precursors in addition to mature microvascular endothelial cells (see vascularized tissue section above). Generates large numbers of microvascular-like endothelial cells. Minimally invasive harvest procedure.
A logical starting point for selection or isolation of endothelial cells would be to harvest mature endothelial cells for transplantation. Harvesting of vascular tissue or vascularized tissues may provide a source of endothelial cells that can be transplanted for therapeutic purposes. Autologous sources may provide immune compatibility but might not provide the ‘off-the-shelf’ dosing that allogeneic sources would provide. Two major issues must be considered with regard to mature endothelial cells. First, will they have the plasticity to affect a therapeutic endpoint, and second have they been altered as a result of the underlying disease process. Nonetheless, multiple sources of mature endothelial cells exist. Venous endothelium has been harvested and used to populate biomaterial or synthetic vascular grafts (Herring et al., 1978; Graham et al., 1979; Belden et al., 1982; Noishiki et al., 1990a; Noishiki et al., 1990b; Noishiki et al., 1992), although endothelial cells may be quite limited in these settings (Zilla et al., 1987).
Adipose tissue contains two potent sources of endothelial cells, namely resident microvascular endothelial cells and adipose-derived stromal cells (Miranville et al., 2004; Planat-Benard et al., 2004; Fraser et al., 2006; Wosnitza et al., 2007; Traktuev et al., 2008). In humans, the former may be defined as CD34+/CD45− cells that express CD31 and CD144. The adipose-derived stem cells (CD34+/CD45−/CD31−) share many properties with marrow stromal cells, are capable of endothelial differentiation, and are found in close proximity to endothelial cells (ECs) within adipose tissue.
Given differences in endothelial cell phenotype and function according to tissue, matching the cell source to the intended cell phenotype may be important. For example, microvascular endothelial cells derived from tissues such as adipose may be relevant for therapeutic angiogenesis, given their highly proliferative nature and higher tube-forming capacity compared with large artery endothelial cells. By contrast, using artery-derived endothelial cells for relocation to another arterial system might be advantageous given the relatively poor ability to form new capillaries. Highly angiogenic cells might in theory promote adventitial and neointimal proliferation, promoting plaque expansion. In addition, large arterial endothelial cells express much higher amounts of the anti-atherosclerotic protein endothelial-specific nitric oxide synthase (eNOS) compared with microvascular endothelium (Gulati et al., 2003a). However, clinical applications of this approach may be limited by access to large arterial tissue.
The ability to generate cells with an endothelial phenotype from peripheral blood would greatly simplify the harvesting process. In 1963 Stump and colleagues (Stump et al., 1963) demonstrated that endothelial colonies could be generated on small patches of Dacron within the lumen of an aortic interposition graft, which led the authors to postulate circulating blood as the endothelial source. Modern studies of animal and human chimeras using genetic markers and tagging have revealed that bone marrow-derived circulating progenitors may contribute to both endothelial and intimal smooth muscle cell formation in multiple models of vascular injury (Shi et al., 1998; Sata and Walsh, 2003).
Asahara’s landmark paper suggested that a CD34+ circulating cell subset contained endothelial precursor cells (EPCs) (Asahara et al., 1997), and changed the field forever. They demonstrated that cells that were enriched for CD34 expression (at 15% purity) could be used to generate cells with an endothelial phenotype. Harraz and colleagues have suggested that the majority of cells exhibiting this phenotype were CD34 negative (Harraz et al., 2001). We have demonstrated that the majority of these EPCs come from CD14+ monocytic cells in vitro (Gulati et al., 2003a). This lack of clear and specific lineage definitions for EPCs has hindered the field significantly.
Data from these studies provide indirect evidence for the existence of circulating endothelial progenitor cells. The precise identification of immature circulating endothelial precursors, however, remains an ongoing area of investigation. Using blood from volunteers who had previously undergone sex-mismatched bone marrow transplantation, Lin and colleagues cultured peripheral blood mononuclear cells obtained (Lin et al., 2000). Early in the culture period, they identified endothelial cells of the recipient’s genotype and that were of low proliferative capacity, suggestive of mature endothelium. Following prolonged culture, endothelial colonies were generated that demonstrated a donor genotype. These so-called outgrowth endothelial cells (OEC) exhibited a high proliferative capacity beyond that of mature cells, which is consistent with the OECs originating from immature precursor cells derived from donor bone marrow. In addition, within circulating blood there are mature endothelial cells that are probably senescent and sloughed from resident vasculature (Solovey et al., 1997).
With regard to cells from peripheral blood, when peripheral blood mononuclear cells (PBMCs) are cultured in endothelial conditions for 4–7 days, the population of cells is mixed, consisting predominantly of CD14+ monocytes, some of which express endothelial markers such as CD31 and von Willebrand factor (vWF) and take up acetylated low-density lipoprotein (LDL) and bind Bandeiraea simplicifolia (BS)-lectin. These cells, which are phenotypically identical to those originally defined by Asahara et al. (Asahara et al., 1997), have been referred to as EPCs. At later times under defined culture conditions, rare colonies of OECs arise from precursors within the original mixed population. OECs are both more homogeneous and more distinctly endothelial than EPCs morphologically, phenotypically and functionally (Gulati et al., 2003a; Hur et al., 2004).
Morphologically, OECs produce colonies that resemble proliferating cobblestones in a monolayer which exhibit contact inhibition, and which are indistinguishable from mature human aortic endothelial cells in culture. By contrast, EPCs vary in morphology, show little or no proliferative capacity and do not form monolayers.
Phenotypically, unlike EPCs, OECs express endothelial-specific nitric oxide and caveolin-1, markers of a mature endothelial phenotype. In addition, OECs show more pronounced expression of VEGFR-2, VE-cadherin and CD-31 as examples and are uniformly negative for expression of CD14, a marker of the monocyte-macrophage lineage. EPCs, however, strongly express CD14 (Harraz et al., 2001; Gulati et al., 2003a; Hur et al., 2004).
Functionally, both cell types have been used in animal models of vascular disease with success in promoting angiogenesis in ischemic tissue and in limiting the proliferative response to injury in large arteries, but probably through different mechanisms in each cell type.
When considering the cell source, the issue of species must be carefully considered for several reasons. If one is testing a strategy intended to be autologous in humans, it must be considered whether it should also be tested in an autologous fashion in animal studies. If so, one might be limited by species-specific reagents if cell isolation techniques are necessary. Alternatively, one could consider using a human cell product in an immunodeficient animal model. Yet, the immunodeficiency may affect the outcomes of the model selected and the response to the cell product. Finally for acute studies such as biodistribution, one might use an immunocompetent animal to provide this information. Obviously if one is using an inbred strain of animal, the autologous nature of cells could be replaced by using a syngeneic source, which might include harvesting from multiple animals.
Type of model
The ideal animal model for vascular cell therapy research should develop human-like atherosclerotic or ischemic disease in an accelerated fashion, have human-like cardiovascular physiology, have enough tissue reserve to allow generation of adequate numbers of autologous cells (e.g. blood volume, adipose mass) without physiological compromise, and have anatomy suitable for device access to the area under study. Clearly no all-encompassing experimental model exists. With regards to atherosclerosis, in humans this is a multifactorial disease involving a complex interplay between genetic and environmental components., In the case of large vessel injury, the goal in humans is the prevention of thrombosis and restenosis following angioplasty or stenting of an artery. Although combined genetic-environmental rodent models may more closely resemble human atherosclerotic disease, their size has a number of limitations.
Sites and mechanisms of injury
Murine injury models such as wire trauma to an iliac artery or tie-off of a carotid artery may be clinically relevant as sites of injury, but the mechanisms of injury differ significantly from dilation barotrauma produced during human angioplasty. In this regard, the murine aorta is of sufficient caliber to allow for balloon injury and stent placement, and is a widely studied model as a result, but the aortic site differs in response to injury compared with peripheral arteries and is therefore less clinically relevant. By virtue of size, the rat carotid artery appears appropriate in terms of site (carotid relevance) and diameter (accessible to balloon catheters), but is less appealing mechanistically in terms of resemblance to human atherosclerotic disease. Again, by virtue of size, when considering adjunctive device therapy, alternatives to clinical devices are used which may lack fidelity in replicating the process in humans. Finally, the rodents used are often relatively young compared with the age of human adults with clinically important disease. These differences may account for the lack of efficacy of most therapeutics tested in these models.
Generation of adequate cell numbers
With currently available in vitro cell harvest and culture techniques, post-natal murine tissues have in general been disappointing in terms of ability to generate adequate endothelial cell or progenitor numbers for study. Useable blood volume or tissue mass substrate is clearly limited given the animal size. Moreover, although we and others have had considerable success in generating highly proliferative endothelial colonies from rabbit, canine, porcine and human blood (Gulati et al., 2003a; Gulati et al., 2003b; Gulati et al., 2004), our experience has been that murine peripheral blood, even when pooled to volumes of 10 milliliters or more, generate few colonies, which proliferate for one to two passages only (R.G. and R.D.S., unpublished).
In contrast to rodent models, larger animal models such as the pig lend themselves well to appropriate site and type-specific mechanical injury, device therapy and cell generation. However, such models generally lack underlying atheroma. Exceptions include cholesterol-fed and genetically mutant pigs, which produce atheroma that is not always consistent between animals (Lowe et al., 1988; Herrmann et al., 2003), including hypercholesterolemia and hypertension. In studying chronic limb ischemia, similar issues apply. Induction of ischemia through ligation, embolization or removal of arteries is effective but acute and lacks the chronic disease background in humans. Given the limitations outlined above, in general, preclinical testing in multiple animal models is preferred during development of clinical applications.
Methods of cell delivery
In preclinical models of cell therapy, the methods of cell delivery should be as similar as possible to the proposed clinical application. However, even then species differences may exist, particularly if cells are given intravascularly. In the case of intended catheter delivery in humans, the size of the devices precludes rodent testing. In that case alternatives need to be selected for rodents and the catheter might be used in a larger animal such as a pig to determine catheter/cell compatibility, safety and biodistribution (Naimark et al., 2003).
Cell-enhanced repair following vascular injury
In 1978, Herring and colleagues were the first to evaluate the therapeutic use of autologous endothelial cells in a preclinical vascular model (Herring et al., 1978). Harvested canine external jugular veins were digested to yield an endothelial cell suspension, and these cells were used to pre-coat (seed) Dacron vascular grafts ex vivo. Four weeks after implantation of grafts in the abdominal aortic position, 76% of the grafts that had been seeded with autologous venous endothelial cells were patent, compared with only 22% of the unseeded control grafts. This landmark study allowed the field to advance to clinical trials. Although venous cell-seeded prosthetic infrainguinal graft studies have yielded promising initial results, the delay incurred by the burden of necessary culture expansion of endothelial cells may prove limiting (Meinhart et al., 2001).
Wilson and colleagues used the approach of autologous endothelial cell delivery to demonstrate the feasibility of gene delivery with cells as vectors (Wilson et al., 1989). Prosthetic vascular grafts seeded with genetically modified canine endothelial cells, derived from autologous vein, were found to express the transgene 5 weeks after implantation. In the same year, Nabel and colleagues locally infused genetically modified endothelial cells following ilio-femoral arterial injury in a porcine model with transgene expression being evident at 4 weeks (Nabel et al., 1989).
Following proof of principle that autologous endothelial cells could be delivered to native vasculature after injury (Nabel et al., 1989), a series of studies were undertaken to evaluate the effect of venous cell delivery upon arterial structure and function (Berinyi et al., 1992; Thompson et al., 1993; Conte et al., 1994; Thompson et al., 1994; Conte et al., 1995). Reimplantation of cells after arterial injury was associated with enhanced endothelialization. However, despite beneficial effects upon early vascular remodeling, one study showed no effect of venous endothelial cell delivery upon subsequent neointimal formation (Conte et al., 1995).
More recently, Edelman and colleagues have extended the use of endothelial cell implants to include nonautologous and extraluminal delivery of endothelial cells (Nugent et al., 1999; Nugent and Edelman, 2001). In a porcine model, porcine or bovine aortic endothelial cells were implanted in Gelfoam matrices in the perivascular space following injury. Cell delivery from both sources was shown to inhibit both restenosis and vascular thrombosis. However, bovine cell implants provoked an immune response at the site of implantation. Given the perivascular location of delivered cells, the studies suggest that the effects demonstrated are probably paracrine in nature.
With the re-ignited interest in the circulation as a source of endothelial cells, many studies have been performed highlighting the potential use of circulating cells to modify healing at sites of arterial injury. The first of these studies was performed in a splenectomized murine model. Werner and colleagues cultured spleen-derived cells toward an endothelial phenotype and demonstrated that intravenously infused cells homed to injured arterial segments. Systemic cell infusion was also associated with enhanced arterial re-endothelialization and inhibited neointimal formation following denuding injury (Werner et al., 2003). Of note, without splenectomy, cell homing was predominantly to the spleen. Moreover, despite re-delivery to the spleen, vascular effects were not seen, suggesting that the mechanism of benefit in the splenectomy model was not endocrine in nature, i.e. cell localization at the artery wall is necessary for therapeutic efficacy. In a xenogeneic model, Fujiyama and colleagues transplanted defined populations of human mononuclear cells into balloon-injured arteries of athymic nude rats (Fujiyama et al., 2003). This model system included systemic gene transfer of monocyte chemoattractant protein-1 (MCP-1) prior to cell transplantation. It was shown that local delivery of monocytes (CD34−CD14+) was associated with enhanced re-endothelialization and reduced neointimal formation compared with delivery of CD34+ cells and of unselected mononuclear cells. The coexpression of endothelial (but not monocytic) markers on pre-labeled cells detected at the site of injury was thought to indicate transdifferentiation of delivered monocytes into mature endothelium. However, fusion of delivered cells with resident endothelium was not excluded. Moreover, the role of MCP-1 is unclear. The complex model systems in both studies may limit both interpretation and relevance to clinical translation.
We and others have demonstrated the potential use of circulation-derived endothelial cells to improve vascular healing in a variety of pre-clinical models (Griese et al., 2003; Gulati et al., 2003b; He et al., 2004; Kong et al., 2004). In our initial experiments, we used a New Zealand White rabbit model to generate cells with partial endothelial phenotype from peripheral blood by culturing isolated mononuclear cells for 7 days under defined conditions. These cells, similar to EPCs defined by Asahara and colleagues (Asahara et al., 1997), were referred to as culture-modified mononuclear cells (CMMC). This population contains both monocyte-derived cells with endothelial features and a minority of precursors to outgrowth endothelial cells (OECs) (Gulati et al., 2003a). A segment of carotid artery was clamp-isolated and injured with three passes of an inflated balloon catheter passed under direct vision through an arteriotomy. Vessel injury was followed by infusion of autologous, fluorescently labeled CMMCs through the arteriotomy with subsequent dwelling of the cell suspension in the absence of flow for 20 minutes. Following closure of the arteriotomy and removal of clamps to restore normal blood flow, animals were allowed to recover. At 4 weeks, evaluation of carotid cross sections revealed labeled cells lining the lumen and expressing markers of endothelial but not monocytic lineage. However, labeled cells were also detected in deeper layers expressing monocytic but not endothelial markers. Local infusion of CMMCs, therefore, appeared to result in cells in the arterial walls of both endothelial and macrophage phenotypes.
We then evaluated the effect of CMMC delivery on the response to vascular injury by comparing local delivery of a saline cell suspension immediately after balloon injury with saline alone as a control (Figs 1 and 2). Carotid rings were examined in an organ chamber 4 weeks after balloon injury and cell delivery. CMMC delivery was associated with a marked improvement in endothelial-dependent arterial relaxation compared with saline alone, although these responses did not achieve those of uninjured vessels. CMMC delivery was also associated with significantly enhanced re-endothelialization and an ~50% reduction in neointimal formation as indexed by cross-sectional intima to media ratios (Gulati et al., 2003b). However, the finding that delivered (labeled) cells were only a small minority of the resident cells 4 weeks after introduction might suggest a paracrine effect of the cells upon subsequent arterial behavior.
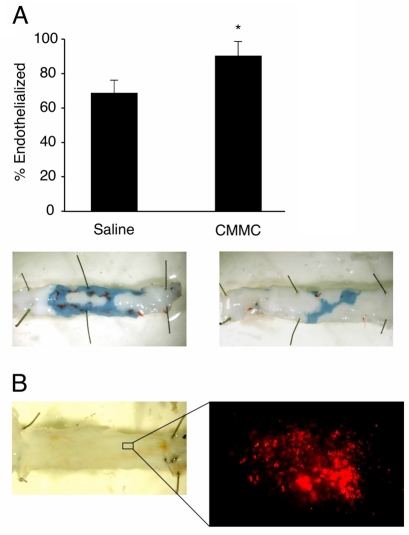
Fig. 1.
Local CMMC delivery accelerates re-endothelialization after balloon injury. Rabbits underwent balloon carotid injury and delivery of autologous CMMCs or saline. (A) Evans Blue dye was systemically administered just before euthanization at 4 weeks, to identify non-endothelialized areas. The re-endothelialized area is significantly greater in carotids from CMMC-treated animals than those treated with just saline. *P<0.05. Representative photographs are shown of exposed carotid lumens; re-endothelialized areas do not stain blue. (B) En face lumen microscopy 4 weeks after injury and delivery of fluorescently labeled CMMCs. The absence of Evans Blue staining indicates complete re-endothelialization of the luminal surface. Many fluorescently labeled colonies were seen on the luminal surface suggesting direct participation of CMMCs in re-endothelialization (a representative example of one colony is shown on the right). Figure reprinted with permission from the American Heart Association journals (Gulati et al., 2003b).
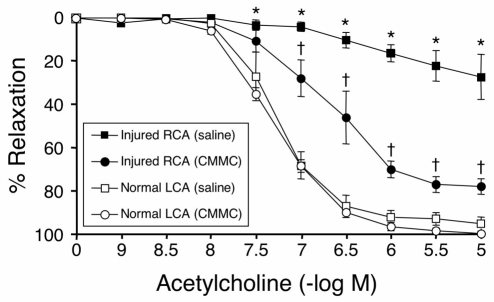
Fig. 2.
CMMC delivery enhances endothelial-dependent vasorelaxation 4 weeks after balloon injury. To investigate the effect of CMMC delivery on vascular function, endothelial-dependent vasorelaxation of excised carotid rings was examined in an organ chamber at 4 weeks after injury. Following pre-contraction with phenylephrine, ring relaxation in response to incremental doses of acetylcholine was assessed. Vessels from CMMC-treated rabbits showed markedly enhanced vasoreactivity to acetylcholine. Data are expressed as mean % relaxation ±s.e.m. (*P<0.05 for CMMC vs saline-treated injured arteries). Uninjured left carotid arteries retained the largest responses (†P<0.05 for maximal relaxation and EC50 compared with CMMC rings). Figure reprinted with permission from the American Heart Association journals (Gulati et al., 2003b).
In a study by He and colleagues, also using the New Zealand White rabbit balloon injury model, near identical effects were reported (He et al., 2004). Moreover, this group also demonstrated that the generated cells expressed a range of angiogenic factors including VEGF and FGF, raising the possibility that these may in part mediate the effects noted in both studies.
In addition to the delivery of a heterogeneous population of CMMCs (similar to EPCs reported by Asahara and colleagues), the delivery of outgrowth endothelial cells (OECs), a homogeneous population of distinct endothelial cells generated from peripheral blood, has also been studied. Kaushal and colleagues demonstrated that seeding of decellularized vascular grafts with OECs generated from sheep blood dramatically improved graft patency following implantation in an autologous fashion (Kaushal et al., 2001). In fact, the carotid interposition grafts were shown to remain patent up to 130 days after implantation. We and others have used the New Zealand White rabbit balloon injury model to evaluate the effect of local autologous OEC delivery upon subsequent arterial healing (Griese et al., 2003; Gulati et al., 2004). Given the distinctly endothelial nature of these cells, we hypothesized that delivery may modify the vascular response in an endothelial-dependent manner. We also evaluated the effect of (non-cultured) peripheral blood mononuclear cell (PBMC) delivery as an important control for cultured cell therapy. Our results, together with those of Griese and colleagues, indicated that OEC delivery reduced neointimal formation compared with delivery of both saline (Griese et al., 2003; Gulati et al., 2004) and PBMCs (Gulati et al., 2004). Once again, despite potent effects, all studies indicated relatively poor long-term residence in comparison to input numbers, suggesting again that paracrine mechanisms probably underlie the vascular effects seen.
Although studies have compared the effects of CMMCs and OECs upon capillary neogenesis in a murine model, a comparison has not been undertaken in a larger arterial injury model. Retrospectively evaluating the functional and structural effects of OEC delivery and CMMC delivery (acknowledging that these were separate studies), the effects of each cell type appear almost identical (Gulati et al., 2003b; Gulati et al., 2004). Given the more distinct and potent endothelial properties of OECs, as well as their delivery in ~10-fold greater numbers, it is perhaps surprising that OECs were not clearly superior in terms of modulating vascular healing. Whether there is a threshold for maximum benefit or whether CMMCs exhibit a more potent effect per cell, perhaps through paracrine factor secretion, remains to be determined.
Cell-mediated therapeutic angiogenesis
Within the original description of ‘endothelial progenitor cells’ by Asahara in 1997, there was demonstration of delivery of those cells in areas of hind limb ischemia resulting in angiogenesis in the affected limb (Asahara et al., 1997). These studies used tail vein injection of labeled human cells into athymic mice and used histological assessment of the ischemic limb. Histology confirmed that the labeled cells assumed a perivascular position. That landmark paper concluded with a statement suggesting that these cells might provide ‘treatment of regional ischemia.’ In a follow-up paper Kalka (Kalka et al., 2000) used laser doppler scanning to compare the effect of labeled human EPC delivery in a hind limb ischemia model in athymic mice. In this study cells were delivered via intramuscular injections and were found to localize in perivascular areas associated with enhanced angiogenesis. These papers set the stage for further studies utilizing different animal models, cell types and cell sources.
Endothelial cells from tissue sources have also been used. Adipose-derived stem cells have been shown to have distinct angiogenic properties, which include the ability to improve blood flow in a murine hind limb ischemia model (Miranville et al., 2004). Additionally, adipose tissue contains mature endothelium which may also be used.
Although direct cellular effects have been implicated in the angiogenic effects of cell delivery, paracrine effects may also play a role. Iba and colleagues demonstrated that delivery of peripheral blood mononuclear cells and platelets had a significant angiogenic effect without any incorporation of cells into vascular structures (Iba et al., 2002). Although often overlooked, the paracrine effect may be significant or even primary in other studies of cell delivery. Similarly Wragg and colleagues recently demonstrated that delivery of so-called multipotent adult progenitor cell-derived progenitor cells (MDPCs) induced angiogenesis by stimulating inflammation through an SDF-1-dependent mechanism (Wragg et al., 2008).
Cell-mediated gene transfer has also been used to enhance angiogenesis (Iwaguro et al., 2002). EPCs have been transduced to deliver VEGF to sites of ischemia. Transduction with VEGF allowed for lower doses of EPCs to be effective in the hind limb ischemia model in athymic mice. These studies lend great hope for developing cell-based therapeutics to induce angiogenesis.
Conclusion
Cell-based therapies for vascular disease are being developed to meet the needs of patients with severe vascular disease. Development of these therapies requires careful selection of cells that might possess the functional qualities required and testing of these cells in the most relevant animal models. Careful consideration should be given to the cells, cell source, species used, and delivery systems when designing preclinical animal studies. Additionally, the purpose of the study, determination of efficacy, safety, or biodistribution may dictate which animal models to utilize. When carefully considered, these studies should provide evidence to support the timely translation of these cell-based therapeutics to humans.
Footnotes
COMPETING INTERESTS
The authors declare no competing financial interests.
This Perspective is part of an ongoing series on stem cells. Please see the related articles: 'Cell therapy for the diseased liver:from stem cell biology to novel models for hepatotropic human pathogens' in DMM Volume 1, Issue 2/3, pages 113-130 and 'Progenitor cell therapies for traumatic brain injury: barriers and opportunities in translation' in DMM Volume 2, Issue 1/2, pages 23-38.
REFERENCES
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner J. (1997). Isolation of putative progenitor endothelial cells for angiogensis. Science 275, 964–967 [DOI] [PubMed] [Google Scholar]
- Belden TA, Schmidt SP, Falkow LJ, Sharp WV. (1982). Endothelial cell seeding of small-diameter vascular grafts. Trans. Am. Soc. Artif. Intern. Organs 28, 173–177 [PubMed] [Google Scholar]
- Berinyi L, Conte M, Mulligan R. (1992). Repopulation of injured arteries with genetically modified endothelial cells. J. Vasc. Surg. 15, 932–934 [DOI] [PubMed] [Google Scholar]
- Conte M, Birinyi L, Miyata T, Fallon J, Gold H, Whittemore A, Mulligan R. (1994). Efficient repopulation of denuded rabbit arteries with autologous gentically modified endothelial cells. Circulation 23, 2161–2169 [DOI] [PubMed] [Google Scholar]
- Conte M, Belkin M, Donaldson M, Baum P, Mannick J, Whittemore A. (1995). Femorotibial bypass for claudication: do results justify an aggressive approach? J. Vasc. Surg. 21, 873–880 [DOI] [PubMed] [Google Scholar]
- Eddicks S, Maier-Hauff K, Schenk M, Muller A, Baumann G, Theres H. (2007). Thoracic spinal cord stimulation improves functional status and relieves symptoms in patients with refractory angina pectoris: the first placebo-controlled randomised study. Heart 93, 585–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egashira K, Hirooka Y, Kai H, Sugimachi M, Suzuki S, Inou T, Takeshita A. (1994). Reduction in serum cholesterol with pravastatin improves endothelium-dependent coronary vasomotion in patients with hypercholesterolemia. Circulation 89, 2519–2524 [DOI] [PubMed] [Google Scholar]
- Fraser JK, Schreiber R, Strem B, Zhu M, Alfonso Z, Wulur I, Hedrick MH. (2006). Plasticity of human adipose stem cells toward endothelial cells and cardiomyocytes. Nat. Clin. Pract. Cardiovasc. Med. 3 Suppl. 1, S33–S37 [DOI] [PubMed] [Google Scholar]
- Fujiyama S, Amano K, Uehira K, Yoshida M, Nishiwaki Y, Nozawa Y, Jin D, Takai S, Miyazaki M, Egashira K, et al. (2003). Bone marrow monocyte lineage cells adhere on injured endothelium in a monocyte chemoattractant protein-1-dependent manner and accelerate reendothelialization as endothelial progenitor cells. Circ. Res. 93, 980–989 [DOI] [PubMed] [Google Scholar]
- Graham LM, Vinter DW, Ford JW, Kahn RH, Burkel WE, Stanley JC. (1979). Cultured autogenous endothelial cell seeding of prosthetic vascular grafts. Surg. Forum 30, 204–206 [PubMed] [Google Scholar]
- Griese DP, Ehsan A, Melo LG, Kong D, Zhang L, Mann MJ, Pratt RE, Mulligan RC, Dzau VJ. (2003). Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: implications for cell-based vascular therapy. Circulation 108, 2710–2715 [DOI] [PubMed] [Google Scholar]
- Gulati R, Jevremovic D, Peterson TE, Chatterjee S, Shah V, Vile RG, Simari RD. (2003a). Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ. Res. 93, 1023–1025 [DOI] [PubMed] [Google Scholar]
- Gulati R, Jevremovic D, Peterson TE, Witt TA, Kleppe LS, Mueske CS, Lerman A, Vile RG, Simari RD. (2003b). Autologous culture-modified mononuclear cells confer vascular protection after arterial injury. Circulation 108, 1520–1526 [DOI] [PubMed] [Google Scholar]
- Gulati R, Jevremovic D, Witt TA, Kleppe LS, Vile RG, Lerman A, Simari RD. (2004). Modulation of the vascular response to injury by autologous blood-derived outgrowth endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 287, H512–H517 [DOI] [PubMed] [Google Scholar]
- Harraz M, Jiao C, Hanlon HD, Hartley RS, Schatteman GC. (2001). CD34−blood-derived human endothelial progenitors. Stem Cells 19, 304–312 [DOI] [PubMed] [Google Scholar]
- He T, Smith LA, Harrington S, Nath KA, Caplice NM, Katusic ZS. (2004). Transplantation of circulating endothelial progenitor cells restores endothelial function of denuded rabbit carotid arteries. Stroke 35, 2378–2384 [DOI] [PubMed] [Google Scholar]
- Herring M, Gardner A, Glover J. (1978). A single-staged technique for seeding vascular grafts with autogenous endothelium. Surgery 84, 498–504 [PubMed] [Google Scholar]
- Herrmann J, Gulati R, Napoli C, Woodrum JE, Lerman LO, Rodriguez-Porcel M, Sica V, Simari RD, Ciechanover A, Lerman A. (2003). Oxidative stress-related increase in ubiquitination in early coronary atherogenesis. FASEB J. 17, 1730–1732 [DOI] [PubMed] [Google Scholar]
- Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. (2004). Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler. Thromb. Vasc. Biol. 24, 288–293 [DOI] [PubMed] [Google Scholar]
- Iba O, Matsubara H, Nozawa Y, Fujiyama S, Amano K, Mori Y, Kojima H, Iwasaka T. (2002). Angiogenesis by implantation of peripheral blood mononuclear cells and platelets into ischemic limbs. Circulation 106, 2019–2025 [DOI] [PubMed] [Google Scholar]
- International Cardiovascular Disease Statistics (2008a). Statistical fact sheet: populations (2008 update). American Heart Association. www.americanheart.org/downloadable/heart/1201543457735FS06INT08.pdf
- International Cardiovascular Disease Statistics (2008b). Heart disease and stroke statistics (2008 update at-a-glance). American Heart Association. www.americanheart.org/downloadable/heart/1200078608862HS_Stats%202008.final.pdf
- Iwaguro H, Yamaguchi J, Kalka C, Murasawa S, Masuda H, Hayashi S, Silver M, Li T, Isner JM, Asahara T. (2002). Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation 105, 732–738 [DOI] [PubMed] [Google Scholar]
- Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. (2000). Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc. Natl. Acad. Sci. USA 97, 3422–3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal S, Amiel GE, Guleserian KJ, Shapira OM, Perry T, Sutherland FW, Rabkin E, Moran AM, Schoen FJ, Atala A, et al. (2001). Functional small-diameter neovesels created using endothelial progenitor cells expanded ex vivo. Nat. Med. 7, 1035–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan T, Sandhu G, Boilson B, Rihal C, Simari R. (2007). Cellular interventional therapy for non-revascularizable coronary artery disease: how many patients are eligible? Am. J. Cardiol. 100, 8A Suppl. S, 2L [Google Scholar]
- Kong D, Melo LG, Mangi AA, Zhang L, Lopez-Ilasaca M, Perrella MA, Liew CC, Pratt RE, Dzau VJ. (2004). Enhanced inhibition of neointimal hyperplasia by genetically engineered endothelial progenitor cells. Circulation 109, 1769–1775 [DOI] [PubMed] [Google Scholar]
- Lerman A, Burnett JC, Jr, Higano ST, McKinley LJ, Holmes DR., Jr (1998). Long-term L-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation 97, 2123–2128 [DOI] [PubMed] [Google Scholar]
- Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. (2000). Origins of circulating endothelial cells and endothelial outgrowth from blood. J. Clin. Invest. 105, 71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe SW, Checovich WJ, Rapacz J, Attie AD. (1988). Defective receptor binding of low density lipoprotein from pigs possessing mutant apolipoprotein B alleles. J. Biol. Chem. 263, 15467–15473 [PubMed] [Google Scholar]
- Mancini D, Katz S, Donchez L, Aaronson K. (1996). Coupling of hemodynamic measurements with oxygen consumption during exercise does not improve risk stratification in patients with heart failure. Circulation 94, 2492–2496 [DOI] [PubMed] [Google Scholar]
- Meinhart JG, Deutsch M, Fischlein T, Howanietz N, Froschl A, Zilla P. (2001). Clinical autologous in vitro endothelialization of 153 infrainguinal ePTFE grafts. Ann. Thorac. Surg. 71, S327–S331 [DOI] [PubMed] [Google Scholar]
- Michelakis ED, Wilkins MR, Rabinovitch M. (2008). Emerging concepts and translational priorities in pulmonary arterial hypertension. Circulation 118, 1486–1495 [DOI] [PubMed] [Google Scholar]
- Miranville A, Heeschen C, Sengenes C, Curat CA, Busse R, Bouloumie A. (2004). Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation 110, 349–355 [DOI] [PubMed] [Google Scholar]
- Nabel E, Plautz G, Boyce F, Stanley J, Nabel G. (1989). Recombinant gene expression in vivo within endothelial cells of the arterial wall. Science 244, 1342–1344 [DOI] [PubMed] [Google Scholar]
- Naimark WA, Lepore JJ, Klugherz BD, Wang Z, Guy TS, Osman H, Moainie SL, Gorman RC, Reed G, Gorman JH, 3rd, et al. (2003). Adenovirus-catheter compatibility increases gene expression after delivery to porcine myocardium. Hum. Gene Ther. 14, 161–166 [DOI] [PubMed] [Google Scholar]
- Noishiki Y, Yamane Y, Miyata T, Okoshi T, Tomizawa Y. (1990a). Development of a soft, pliable, slow heparin release venous graft. ASAIO Trans. 36, M343–M346 [PubMed] [Google Scholar]
- Noishiki Y, Yamane Y, Tomizawa Y, Okoshi T, Satoh S, Wildevuur CR. (1990b). Endothelialization of vascular prostheses by transplantation of venous tissue fragments. ASAIO Trans. 36, M346–M348 [PubMed] [Google Scholar]
- Noishiki Y, Yamane Y, Tomizawa Y, Okoshi T, Satoh S, Wildevuur CR, Suzuki K. (1992). Rapid endothelialization of vascular prostheses by seeding autologous venous tissue fragments. J. Thorac. Cardiovasc. Surg. 104, 770–778 [PubMed] [Google Scholar]
- Nugent HM, Edelman ER. (2001). Endothelial implants provide long-term control of vascular repair in a porcine model of arterial injury. J. Surg. Res. 99, 228–234 [DOI] [PubMed] [Google Scholar]
- Nugent HM, Rogers C, Edelman ER. (1999). Endothelial implants inhibit intimal hyperplasia after porcine angioplasty. Circ. Res. 84, 384–391 [DOI] [PubMed] [Google Scholar]
- Planat-Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, et al. (2004). Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation 109, 656–663 [DOI] [PubMed] [Google Scholar]
- Powell RJ, Simons M, Mendelsohn FO, Daniel G, Henry TD, Koga M, Morishita R, Annex BH. (2008). Results of a double-blind, placebo-controlled study to assess the safety of intramuscular injection of hepatocyte growth factor plasmid to improve limb perfusion in patients with critical limb ischemia. Circulation 118, 58–65 [DOI] [PubMed] [Google Scholar]
- Sata M, Walsh K. (2003). Circulating vascular progenitor cells contribute to vascular repair, remodeling, and lesion formation. Trends Cardiovasc. Med. 13, 249–253 [DOI] [PubMed] [Google Scholar]
- Shi Q, Rafii S, Wu M-D, Wijelath E, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage L, et al. (1998). Evidence for circulating bone marrow-derived endothelial cells. Blood 92, 362–367 [PubMed] [Google Scholar]
- Solovey A, Lin Y, Browne P, Choong S, Wayner E, Hebbel RP. (1997). Circulating activated endothelial cells in sickle cell anemia. N. Engl. J. Med. 337, 1584–1590 [DOI] [PubMed] [Google Scholar]
- Stump MM, Jordan GL, DeBakey ME, Halpert B. (1963). Endothelium grown from circulating blood on isolated intravascular dacron hub. Am. J. Pathol. 43, 361–363 [PMC free article] [PubMed] [Google Scholar]
- Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. (2000). Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 101, 948–954 [DOI] [PubMed] [Google Scholar]
- Takeshita S, Zheng L, Brogi E, Kearney M, Pu L, Bunting S, Ferrara N, Symes J, Isner J. (1994). Therapeutic angiogenesis: a single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J. Clin. Invest. 93, 662–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M, Bud J, Eady S, James R, Bell P. (1993). A method to transluminally seed angioplasty sites with endothelial cells using a double balloon catheter. Eur. J. Vasc. Surg. 7, 113–121 [DOI] [PubMed] [Google Scholar]
- Thompson M, Budd J, Eady S, Hartley G, Early M, James R, Bell P. (1994). Platelet deposition after angioplasty is abolished by restoration of the endothelial cell monolayer. J. Vasc. Surg. 19, 478–486 [DOI] [PubMed] [Google Scholar]
- Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH, March KL. (2008). A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ. Res. 102, 77–85 [DOI] [PubMed] [Google Scholar]
- Werner N, Junk S, Laufs U, Link A, Walenta K, Bohm M, Nickenig G. (2003). Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ. Res. 93, e17–e24 [DOI] [PubMed] [Google Scholar]
- Wilson J, Birinyi L, Salomon R, Libby P, Callow A, Mulligan R. (1989). Implantation of vascular grafts lined with genetically modified endothelial cells. Science 244, 1344–1346 [DOI] [PubMed] [Google Scholar]
- Wosnitza M, Hemmrich K, Groger A, Graber S, Pallua N. (2007). Plasticity of human adipose stem cells to perform adipogenic and endothelial differentiation. Differentiation 75, 12–23 [DOI] [PubMed] [Google Scholar]
- Wragg A, Mellad JA, Beltran LE, Konoplyannikov M, San H, Boozer S, Deans RJ, Mathur A, Lederman RJ, Kovacic JC, et al. (2008). VEGFR1/CXCR4-positive progenitor cells modulate local inflammation and augment tissue perfusion by a SDF-1-dependent mechanism. J. Mol. Med. 86, 1221–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilla P, Fasol R, Deutsch M, Fischlein T, Minar E, Hammerle A, Krupicka O, Kadletz M. (1987). Endothelial cell seeding of polytetrafluoroethylene vascular grafts in humans: a preliminary report. J. Vasc. Surg. 6, 535–541 [DOI] [PubMed] [Google Scholar]