SUMMARY
Human embryonic stem cell (hESC)-derived neurons have the potential to model neurodegenerative disorders. Here, we demonstrate the expression of a mutant gene, superoxide dismutase 1(SOD1), linked to familial amyotrophic lateral sclerosis (ALS) in hESC-derived motor neurons. Green fluorescent protein (GFP) expression under the control of the HB9 enhancer was used to identify SOD1-transfected motor neurons that express human wild-type SOD1 or one of three different mutants (G93A, A4V and I113T) of SOD1. Neurons transfected with mutant SOD1 exhibited reduced cell survival and shortened axonal processes as compared with control-transfected cells, which could survive for 3 weeks or more. The results indicate that hESC-derived cell populations can be directed to express disease-relevant genes and to display characteristics of the disease-specific cell type. These genetically manipulated hESC-derived motor neurons can facilitate and advance the study of disease-specific cellular pathways, and serve as a model system to test new therapeutic approaches.
INTRODUCTION
Stem cell-derived neurons are becoming increasingly important in biological research and in devising therapeutic strategies for neurodegenerative disorders, i.e. as replacements for degenerating cells in the central nervous system (CNS) (Gao et al., 2005; Suzuki et al., 2007) and for high-throughput drug screens (Broom et al., 2006; Rubin, 2008). Several groups have generated motor neurons from human embryonic stem cell (hESC) lines (Li et al., 2005; Roy et al., 2005). These motor neurons might be useful for research of amyotrophic lateral sclerosis (ALS), a progressive and fatal neurodegenerative disorder characterized by motor neuron loss, which occurs in sporadic (~90% of cases) and familial forms (Simpson and Al-Chalabi, 2006; Brown, 1995). About 10-20% of familial cases carry a mutation in the superoxide dismutase 1 (SOD1) gene, which encodes a ubiquitous antioxidant protein. More than 100 SOD1 mutations have been linked to familial ALS, however, a single disease mechanism associated with SOD1 has not been established. Some SOD1 mutations are known to cause specific symptoms, such as rapid disease progression (A4V mutation) or late disease onset (I113T mutation) (Cudkowicz et al., 1997; Simpson and Al-Chalabi, 2006). Most research on the pathological mechanisms in ALS has been performed on rodent models that express mutant genes linked to familial ALS, and on animal and human non-neuronal or immortalized neuronal cell lines. Increasingly, it is becoming recognized that the disease biology and progression of ALS is very different in animal models and non-neuronal cells when compared with the human condition, which is exemplified by the difficulty in successfully translating animal drug studies to human patients (Schnabel, 2008). The expression of disease-relevant genes in hESC-derived neurons will provide new disease models that are more relevant to human biology.
Here, we generated hESC-derived, electrophysiologically active motor neurons. These motor neurons were manipulated to model familial ALS by combining stem cell differentiation techniques with timed cell dissociation and directed mutant SOD1 gene expression under an HB9 regulator and a green fluorescent protein (GFP) reporter. This easily identifiable population of mutant SOD1-expressing motor neurons was confirmed by immunocytochemical and RT-PCR detection of motor neuron-specific markers, as well as by electrophysiology. We report that mutant SOD1 overexpression causes alterations in neuronal morphology and reduces cell viability. These findings demonstrate the validity and utility of developing human disease-specific cell culture models.
RESULTS
Directed differentiation of motor neurons
Retinoic acid (RA)-caudalizing and Sonic hedgehog (Shh)-ventralizing signals have previously been demonstrated to direct the specification of motor neurons from hESCs (Li et al., 2005). Motor neurons have been produced from the hESC lines H1, H9, BG1, BG2 and HES 3.1, 3.2 and 3.3. (Li et al., 2005; Lim et al., 2006; Shin et al., 2005; Roy et al., 2005). Here, we generated motor neurons from the HSF-1 and HSF-6 hESC lines (supplementary material Fig. S1) using a modification of previously published methods (Goldman and Sim, 2005; Li et al., 2005; Roy et al., 2005). A subset of TUJ1-positive neurons was immunoreactive for the transcription factors HB9 and ISLET1 and for choline acetyltransferase (ChAT) (supplementary material Fig. S1D-F). To determine the stage-specific expression of motor neuron markers in the differentiating population and to confirm the immunocytochemistry results, we performed RT-PCR analysis at several time points. HB9 and ISLET1 expression was detected after the addition of N2 and RA to the medium. However, ChAT expression was detected only after the addition of Shh and motor neuron maturation factors (BDNF, GDNF and CNTF) (supplementary material Fig. S1G). Both immunostaining and RT-PCR analysis are consistent with the differentiation of motor neurons from hESC.
We then counted the numbers of neuralized TUJ1-positive cells and motor neurons in the total population of hESC-derived cells. We detected an average of 31.34% TUJ1- and 10.07% ChAT-positive cells (n=3) in the entire hESC-derived cell population and calculated that 39.13% of the TUJ1-positive cell population were also ChAT positive.
Mutant SOD1-transfected, hESC-derived motor neurons are identified by HB9-driven GFP fluorescence
In order to identify mutant SOD1-expressing motor neurons in a mixed population of differentiating cells, we used a selection cassette in which the expression of GFP and either wild-type SOD1 or one of three different SOD1 mutations (G93A, A4V and I113T) are driven by a 3.6 kb motor neuron-specific enhancer within the gene encoding the motor neuron transcription factor HB9 (Roy et al., 2005; Roy et al., 2004) (Fig. 1A). The control vector expressed GFP only. We dissociated and replated the differentiated cells before transfection, which increased the transfection efficiency 100-fold compared with transfection in the undissociated culture. Immunocytochemical staining of HB9-driven, GFP-expressing mutant SOD1 cells showed co-expression of the motor neuron-specific markers HB9, ISLET1 and ChAT (Fig. 1B–K). Only a G93A-specific antibody is currently available for identifying cells that express the three mutant SOD1 constructs (Urushitani et al., 2007). This antibody specifically recognized the cells that were transfected with the G93A mutant (Fig. 2A–C), but not with I113T, A4V or wild-type SOD1 (data not shown). These data indicate that GFP expression reliably reports the expression of mutant SOD1. In order to analyze whether mutant or wild-type SOD1 transfection results in different expression rates of the protein, we analyzed the numbers of green fluorescent cells by fluorescence-activated cell sorting (FACS). The difference in fluorescent cell numbers was evaluated using the chi-square test and was not statistically significant (supplementary material Fig. S2).
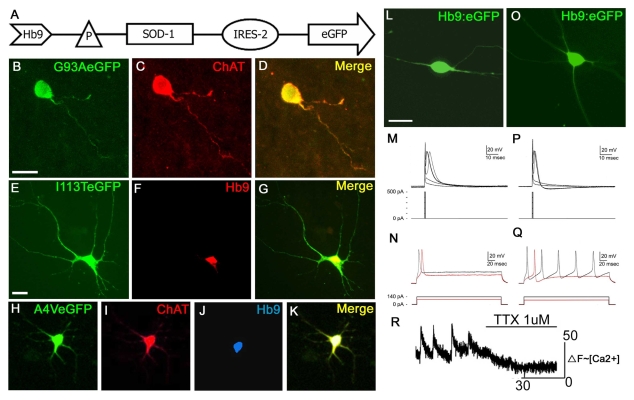
Fig. 1.
Generation of SOD1-transgenic motor neurons from hESCs. (A) Wild-type SOD1 and mutant G93A, I113T and A4V SOD1 cassettes were cloned into the BamHI site of the E/Hb9-3.6kb:EGFP vector containing the HB9 enhancer linked to the h-globin gene basal promoter. (B-D) G93A–GFP-transfected motor neurons co-stained for ChAT. (E-G) I113T–GFP-transfected motor neurons co-stained for HB9. (H-K) A4V–GFP-transfected motor neurons co-stained for ChAT and HB9. (L-Q) Action potential properties of hESC-derived motor neurons fall into two distinct classes that are typical of developing motor neurons. Panels (L-N) represent less differentiated cells with neurites extending =100 μm. Panels (O-Q) represent more highly differentiated cells with extensive axonal arborizations. Hb9:EGFP-positive cells were whole-cell patched in the current-clamp mode. Short duration (0.5 milliseconds) current pulses of increasing amplitude evoke single action potentials with a distinct threshold (M,P). Longer duration (250 milliseconds) current pulses elicit single action potentials (red) when the amplitude of the injected current is near to the threshold [(N) 100 nA; (Q) 80 nA]. When the amplitude of the stimulus pulse is increased, the more mature hESC-derived motor neurons respond with repetitive firing (Q). (R) Representative trace of fluo-3 ΔF (~[Ca2+]i) versus time for a single cell. Bath application of tetrodotoxin (TTX, 1 μM) abolishes spontaneous Ca2+ oscillations. Abbreviations: P, promoter; IRES-2, internal ribosomal entry site 2. Bars, 10 μm (B-D); 20 μm (E-O).
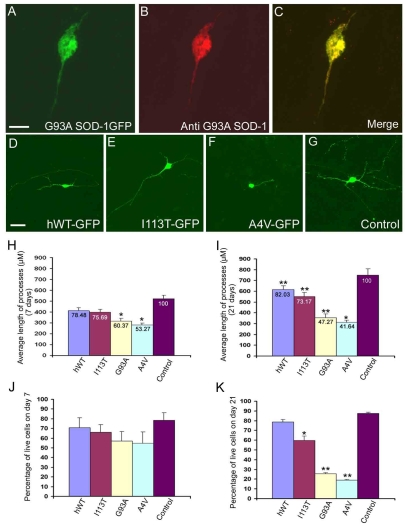
Fig. 2.
Mutant SOD1 alters cell morphology and viability.(A-C) Staining of G93A–GFP-transfected cells with the G93A-specific antibody shows an accumulation of G93A SOD1 cells in the soma and processes at 21 days after transfection.(D-G) Representative fluorescent micrograph of cells transfected with wild-type SOD1, the SOD1 mutants or the control vector after 21 days. (H,I) Average length of neurites on days 7 and 21 after transfection. The values at the top of the bars represent the relative percentage difference between motor neurons transfected with the respective mutants or wild-type SOD1 compared with those expressing the control GFP vector. The length of neurites at 7 days after transfection was reduced by 21.52% (F=3.49, P>0.05) in cells expressing wild-type SOD1, 24.31% (F=6.69, P>0.05) in cells expressing I113T SOD1, 39.63% (F=14.26, P<0.05) in cells expressing G93A SOD1 and by 46.73% (F=11.49, P<0.05) in cells expressing A4V SOD1. (J) Percentage cell survival from day 4 to day 7 after transfection. (K) Percentage cell survival from day 4 to day 21 after transfection; *P<0.05 and **P<0.01 compared with the control. All experiments were executed in triplicate by a blinded observer. Statistical analysis of the percentage of GFP-positive cells compared with wild-type cells shows significant differences with the G93A (F=13.10, P<0.05) and A4V (F=19.18, P<0.01) mutations. Abbreviation: hWT, human wild type. Bars, 10 μm (A-C); 20 μm (D-G).
hESC-derived motor neurons are electrophysiologically active
The excitability of hESC-derived motor neurons was assayed electrophysiologically after reducing the cell density by papain dissociation and replating. hESC-derived cells that were cultured for 48-62 days with RA and Shh were whole-cell patch clamped in the current-clamp mode. Of the 16 cells patched in 10 different cultures, 14 cells exhibited action potential responses to injected pulses of current. [The two electrically passive cells were enhanced GFP (eGFP)-positive cells from 48- and 49-day-old cultures.] The responses of the electrically active cells fell into two distinct categories. Typical of electrically excitable cells, the action potentials in both of these categories (Fig. 1M,P) had a discrete threshold for activation that was dependent on the amplitude of the injected current. In morphologically less-developed hESC-derived motor neurons (Fig. 1L), the action potentials had a broader waveform and, following an increase in stimulus duration and strength, the cells did not fire repetitive action potentials (Fig. 1M,N). In contrast, hESC-derived motor neurons with more extensive axonal arborizations (Fig. 1O) had a distinctively different action potential waveform that was accompanied by repetitive firing (Fig. 1P,Q). Both types of responses could be found in different cells from the same culture, indicating a mixed progression of neuronal differentiation. However, repetitive firing was more commonly observed in older cultures (with a mean age of 56 days in culture versus a mean of 51 days in culture for cells that fire single action potentials).
hESC-derived, HB9–GFP-expressing motor neurons display spontaneous calcium transients
A small proportion (17 of 300 cells in 10 recordings) of HB9–GFP-transfected motor neurons displayed spontaneous, transient changes in intracellular calcium [Ca2+]i under resting conditions, whose characteristics were consistent with the firing of action potentials. These increases in [Ca2+]i involved the entire cell simultaneously, showed a rapid upstroke and slow decay, and were reversibly abolished by removal of extracellular calcium or by tetrodotoxin (TTX, 1 μM) (Fig. 1L). In three out of 10 recordings,spontaneous [Ca2+]i transients were synchronized in three or four adjacent cells. These observations suggest that hESC-derived motor neurons are capable of spontaneous activity similar to in vivo observations in the developing spinal cord (Hanson and Landmesser, 2003; Rosato-Siri et al., 2004).
Mutant SOD1 alters the morphology of hESC-derived motor neurons
Reduced axonal branching has been reported in transgenic mutant SOD1 mouse strains (Pun et al., 2006), therefore we assessed the effect of mutant SOD1 on neurite morphology. HSF-1-derived motor neurons expressing mutant G93A, A4V or I113T SOD1-GFP and controls expressing wild-type SOD1-GFP or GFP alone were digitally captured at 7 and 21 days after transfection. When measured 7 days after transfection, neurite length was significantly diminished in cells expressing G93A SOD1 and A4V SOD1 when compared with motor neurons expressing the control GFP vector (Fig. 2H).
On day 21 following transfection, neurite length was diminished in motor neurons expressing each SOD1 mutant and wild-type SOD1 when compared with the vector control. However, according to Scheffe’s multiple comparison F-test, there was a difference in the magnitude of reductions among the different groups, with reductions of 18% (F=14.2, P<0.05) in wild-type SOD1-expressing cells, 27% (F=31.41, P<0.01) in I113T-expressing cells, 53% (F=110.83, P<0.01) in G93A-expressing cells and 57% (F=134.83, P<0.01) in A4V-expressing cells (Fig. 2I). Furthermore, the length of the processes was significantly reduced to 58% (F=46.00, P<0.01) in G93A-expressing cells and 51% (F=61.88, P<0.01) in A4V-expressing cells when compared to wild type. These findings demonstrate a reduction in neurite length in mutant SOD1-expressing neurons relative to the vector control.
Mutant SOD1 reduces cell survival in hESC-derived motor neurons
In order to investigate the effect of mutant SOD1 on motor neuron survival, we determined the numbers of GFP-positive cells in transfected cultures over time. The highest number of fluorescent cells was found on day 4 post-transfection in all strains. Analysis of GFP-positive cells at day 7 after transfection did not yield any statistically significant difference between the mutants and the control (Fig. 2J), whereas analysis on day 21 showed survival rates of 92% (F=3.28, P>0.05) in wild-type SOD1-expressing cells, 69% (F=30.36, P<0.05) in I113T-expressing cells, 27.76% (F=70.47, P<0.01) in G93A-expressing cells and 22% (F=80.95, P<0.01) in A4V-expressing cells compared with the mock-transfected control cells (Fig. 2K). These data indicate that all three ALS-linked, mutant SOD1 proteins induced motor neuron death.
DISCUSSION
Here, we demonstrate that the expression of ALS-linked SOD1 mutations in human motor neurons results in altered neurite morphology and survival. These studies should serve as a platform for in vitro studies of motor neuron pathology and potential treatments for motor neuron degeneration.
The motor neurons generated in these studies are functionally competent. Adult motor neurons generate multiple action potentials at a rate that increases in proportion to the injected current (Granit et al., 1963). In vivo recordings from lumbar spinal motor neurons have demonstrated that repetitive firing of action potentials is a characteristic acquired during maturation (Gao and Ziskind-Conhaim, 1998). Embryonic spinal motor neurons at embryonic day (E)16 exhibit action potentials with relatively broad waveform properties and, regardless of increasing stimulus strength, only respond with single action potentials. After birth, the action potentials of spinal motor neurons have faster kinetics, and a prolonged injection of current generates repetitive firing that is typical of mature motor neurons (Gao and Ziskind-Conhaim, 1998). Thus, the progression of electrical excitability that we measured in hESC-derived motor neurons reflects what occurs during normal development. Similar patterns have been observed in vitro in neurons derived from either human H9 ES cells (Johnson et al., 2007) or mouse ES cells (Miles et al., 2004).
An independent method of assaying cell excitability is through calcium imaging. Motor neurons in the developing spinal cord exhibit spontaneous bursts of action potentials that are mediated by excitatory input via interneurons (Hanson and Landmesser, 2003; Rosato-Siri et al., 2004). Unlike the patch-clamp experiments, the calcium imaging was performed on dense cultures permitting extensive contact among cells. The calcium imaging results suggest that functional excitatory synaptic connections can influence the activity of HB9–GFP-positive motor neurons. Taken together, the methods used to verify motor neuron lineage, including immunohistochemical and PCR detection of motor neuron markers, patch clamping and calcium imaging indicate that we generated functional motor neurons.
The ALS-associated G93A, A4V and I113T SOD1 mutations were expressed in hESC-derived motor neurons resulting in motor neurons with characteristics of ALS-related degeneration, i.e. reduced neurite extension and enhanced cell death. Interestingly, when assessing neurite length and cell survival, the severity of the specific SOD1 mutants in cell culture corresponds with reports in patients with familial ALS. Of the three mutants, the A4V mutation causes the most severe disease phenotype with a very rapid disease course, whereas the G93A and I113T mutations cause a moderate phenotype and a milder phenotype, respectively (Juneja et al., 1997; Cudkowicz et al., 1997). These findings support the notion that the phenotype of familial ALS is based, at least in part, on the direct toxic effect of mutant SOD1 on motor neurons. Recent studies have suggested that cell populations other than motor neurons, such as glial cells and interneurons, might contribute to motor neuron degeneration (Clement et al., 2003; Boilee et al., 2006; Beers et al., 2006; Yamanaka et al., 2008). Before the direct role of mutant SOD1 in motor neuron degeneration can be fully understood, additional studies will be required to better define the respective roles of glia that express mutant SOD1 and ancillary neuronal phenotypes in motor neuron loss. Ultimately, this in vitro system may be used to develop the means to inhibit SOD1-induced motor neuron degeneration.
In addition to promoting motor neuron death, expression of mutant SOD1 resulted in reduced neurite length. Although this observation is consistent with in vivo observations that axonal degeneration appears to be an early defect that precedes the onset of ALS symptoms and motor neuron death (Fischer and Glass, 2007; Bradley et al., 1983; Fischer et al., 2004), the stem-cell-based culture system used in this study does not allow us to distinguish between developmental and degenerative changes. This issue will be addressed in future work. Nevertheless, the present cell strain may facilitate the study of factors that mitigate axonal degeneration.
Current studies focus on a known genetic cause of ALS, but most ALS patients do not have SOD1 mutations so one might question the utility of SOD1-centered studies. However, the present study does potentially have broader implications for other forms of ALS. First, the final pathways of neurodegeneration, such as apoptosis, are similar among different types of ALS, as are the clinical and neuropathological presentations (Cleveland and Rothstein, 2001; Hirano, 1996; Shibata et al., 2007). Second, studying interventions that inhibit SOD1-induced cell death may be applied more broadly to other mechanisms of motor neuron cell death.
The reported strategy of expressing disease-causing mutations in hESC-derived neurons might also be useful for studying genetic forms of other neurodegenerative diseases with patient subpopulations that carry known gene defects such as spinal muscular atrophy, inherited dementias and Parkinson’s disease. Importantly, such human-cell-specific disease models will facilitate the investigation of disease mechanisms, including known cellular factors that contribute to ALS such as mitochondrial vacuolization, ubiquitin inclusions and neurofilament accumulations. Understanding disease mechanisms is one prerequisite for identifying new therapeutic targets. These neurons, along with neurons derived from induced pluripotent stem cells (iPS) (Dimos et al., 2008), provide the opportunity to study early disease pathways in human material, whereas traditionally human CNS tissue had to be obtained from post-mortem material. Moreover, this cell model is especially useful for studying diseases that lack rodent models that accurately reflect the human pathology. hESC-derived neuronal disease models might prove to be a viable alternative or addition to non-neuronal cell culture and animal studies.
METHODS
Differentiation of hESCs into motor neurons
HSF-1 (XY, 46, NIH No. UC01) and HSF-6 (XX, 46, NIH No. UC06) (www.wicell.org) (Wu et al., 2007) cells were cultured on mouse embryonic feeder cells in growth medium with DMEM/F12, 20% knockout serum replacement (KSR) and 8 ng/ml of basic fibroblast growth factor (bFGF). Differentiation of neural precursors was induced by holding the hESC aggregates as suspension cultures in the absence of bFGF for 6 days, followed by cell plating on laminin-coated dishes in N2 medium. After 6 days, 1 μM RA was added and cells were differentiated for 3 days until columnar cells formed. The rosettes were mechanically isolated and cultured on 6-well laminin-coated plates with 200 nM Shh (R&D Biosystems) for 6 days. Further differentiation occurred over the next 2-3 weeks with 50 nM Shh and 20 ng/ml BDNF, GDNF and CNTF (Peprotech) (Roy et al., 2005). All culture supplements were purchase from Invitrogen unless specified otherwise.
Immunocytochemistry
Cells fixed in 4% paraformaldehyde for 20 minutes at room temperature (RT) were permeabilized with 0.2% triton in PBS and blocked with the appropriate serum. The primary antibody was incubated overnight at 4°C, the secondary antibody was then incubated for 1-2 hours at RT. Anti-human nestin (1:200, Chemicon), βIII-tubulin (1:500, Covance), ChAT (1:200, Chemicon), anti-GFP (1:500, Aves Labs), MNR2/Hb9 (1:50, DSHBH) and Islet1 (1:50, DSHB) were used. Anti-G93ASOD-1 was a gift from Jean-Pierre Julien. All fluorescent secondary antibodies were purchased from Invitrogen. Cells were viewed under a confocal microscope (Zeiss, LSM 510 META). For quantitative studies, GFP-expressing cells were counted by a blinded observer, in at least three wells per strain, after capture using an Olympus IX50 fluorescent microscope.
Semi-quantitative RT-PCR
The cells were collected in Trizol. RT-PCR was carried out at as described previously (Saravanan et al., 2007). The PCR program consisted of an initial denaturation step at 95°C for 2 minutes, then 30 cycles of denaturation at 95°C for 30 seconds, annealing at 55°C for 30 seconds and extension at 72°C for 30 seconds. The specificity of all PCR reactions was tested by parallel reactions omitting cDNA (not shown). The PCR products were separated by 2% agarose gel electrophoresis and visualized by ethidium bromide staining.
Fluorescence-activated cell sorting (FACS)
Differentiated and hESC cultures that were transfected to express G93A SOD1, wild-type SOD1 or empty vector were collected from the dishes by TripLE (Invitrogen, Carlsbad, CA) treatment and were washed twice with DMEM/F12 containing B27. The cells were collected in 5 milliliter tubes fitted with cell strainer caps (BD falcon) and were then subjected to GFP sorting.
Cloning
Wild-type and SOD1 mutants (G93A, A4V and I113T) were PCR amplified from the YEP 351 vector (Roe et al., 2002). BamHI restriction sites were introduced at either end of the PCR product and subcloned into the BamHI site of the Hb9:eGFP vector. The orientation of the insert was confirmed by sequencing.
Generation and analysis of mutant SOD1-expressing motor neurons from hESCs
Motor neurons were differentiated as described above, dissociated by incubation with papain (Wu et al., 2007) and replated onto laminin-coated 24-well plates at a density of 0.2 million cells/cm2. The cells were grown for 3-5 days and then transfected with the GFP control vector, or with wild-type or mutant SOD1 plasmids using lipofectamine, as per the manufacturer’s protocol. Neuron dissociation and replating at low density promoted neurite extension within 24-48 hours. The transfection efficiency of the replated cells increased 100-fold compared with the transfection in culture before dissociation. GFP-expressing cells were counted by a blinded observer on days 2, 4, 7 and 21 and captured under an Olympus IX50 fluorescent microscope on days 7 and 21. A blinded observer measured the length of the processes of 996 cells and 878 cells on days 7 and 21, respectively, with the aid of SimplePCI software. Statistical analysis was performed by one-way analysis of variance (ANOVA) with post hoc Scheffe’s multiple comparison F-test. A difference below, or equal to, the probability level of 0.05 was considered statistically significant.
Electrophysiology
The ability of differentiated neurons to generate action potentials was determined using standard whole-cell, current-clamp techniques. hESC-derived motor neurons were viewed with an Olympus IX70 inverted microscope using phase contrast and fluorescence optics to identify hESC-derived motor neurons before recordings were obtained. Motor neurons were also identified morphologically and by immunostaining with an antibody to ChAT. All experiments were performed at RT (20–23°C). The bath solution contained 120 mM NaCl, 1.2 mM KH2PO4, 1.9 mM KCl, 26 mM NaHCO3, 2.2 mM CaCl2, 1.4 mM MgSO4, 10 mM D-glucose and 7.5 mM Na HEPES, was adjusted to pH 7.2 (with NaOH), and was equilibrated with 95% O2 and 5% CO2. Patch pipettes (with a resistance of 3–5 MΩ) were filled with internal recording solution containing 140 mM potassium gluconate, 10 mM Na HEPES, 1 mM EGTA, 4 mM ATP-Mg and 0.3 mM GTP; pH 7.2 (adjusted with KOH). To optimize excitability, the resting potential was maintained at −70 mV. The membrane voltage was monitored in response to current injections with a duration of either 0.5 or 250 milliseconds. The 0.5-millisecond pulses were injected in steps of 100 pA, from 0 to 500 pA. The 250-milliseconds pulses were applied in steps of 20 pA, from 0 to 180 pA. The current-clamp recordings were amplified by using an Axopatch 2B patch-clamp amplifier with 4-pole Bessel filtering at 5 kHz. The signals were sampled at 10 kHz by using a Digidata 1322A analog to digital converter, then acquired and stored on a computer hard drive and analyzed offline using pClamp 6 (Clampfit) software.
Calcium recording
Cells on glass cover slips were loaded with the fluorescent calcium indicator, fluo-4, by bath application [2.5 μM fluo-4 AM in Hanks’ balanced salt solution (HBSS) for 30 minutes]. Experiments were performed at RT in HBSS, which was exchanged by superfusion in an open chamber.
Fluorescence imaging was performed using a custom-built video-rate confocal microscope, as described previously (Beltran-Parrazal et al., 2006). In brief, a 475 nm diode laser was scanned across the specimen with oscillating mirrors into an inverted microscope. The ensuing fluorescence was detected by a photomultiplier tube (PMT Hammatsu) and digitized by a video frame capture board (Raven, Bit Flow) using the Video Savant software. Within the images, changes in fluorescence intensity were analyzed by selecting regions of interest on individual cells and plotting fluorescence versus time using ImageJ software.
Supplementary Material
Acknowledgments
We thank Dr Joan S. Valentine and Dr Edie Gralla at the UCLA Dept. of Chemistry for kindly providing the SOD1 DNA and thank Dr Jean-Pierre Julien at Université Laval, Quebec, CA for providing the G93A SOD1 antibody. Funding was provided by Miriam and Sheldon Adelson Program in Neural Repair Research (S.K., J.A.U., S.A.G., H.I.K. and M.W.-P.), David Vickter Foundation (M.W.-P.), NIH/NINDS K08 NS002240 (M.W.-P.) and UCLA Department of Molecular and Medical Pharmacology (J.A.U.).
Footnotes
COMPETING INTERESTS
The authors declare no competing financial interests.
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/content/2/3-4/189/suppl/DC1
REFERENCES
- Beers DR, Henkel JS, Xiao Q, Zhao W, Wang J, Yen AA, Siklos L, McKercher SR, Appel SH. (2006). Wild-type microglia extend survival in PU.1 knockout mice with familial ALS. Proc. Natl. Acad. Sci. USA 103, 16021–16026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran-Parrazal L, Lopez-Valdes HE, Brennan KC, Diaz-Munoz M, de Vellis J, Charles AC. (2006). Mitochondrial transport in processes of cortical neurons is independent of intracellular calcium. Am. J. Physiol. Cell Physiol. 291, C1193–C1197 [DOI] [PubMed] [Google Scholar]
- Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. (2006). Onset and progression in inherited ALS determined by motor neurons and microglia. Science 312, 1389–1392 [DOI] [PubMed] [Google Scholar]
- Bradley WG, Good P, Rasool CG, Adelman LS. (1983). Morphometric and biochemical studies of peripheral nerves in ALS. Ann. Neurol. 14, 267–277 [DOI] [PubMed] [Google Scholar]
- Broom WJ, Auwarter KE, Ni J, Russel DE, Yeh LA, Maxwell MM, Glicksman M, Kazantsev AG, Brown RH., Jr (2006). Two approaches to drug discovery in SOD1-mediated ALS. J. Biomol. Screen 11, 729–735 [DOI] [PubMed] [Google Scholar]
- Brown RH., Jr (1995). ALS: recent insights from genetics and transgenic mice. Cell 80, 687–692 [DOI] [PubMed] [Google Scholar]
- Clement AM, Nguyen MD, Roberts EA, Garcia ML, Boillee S, Rule M, McMahon AP, Doucette W, Siwek D, Ferrante RJ, et al. (2003). Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice.Science 302, 113–117 [DOI] [PubMed] [Google Scholar]
- Cleveland DW, Rothstein JD. (2001). From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat. Rev. Neurosci. 2, 806–819 [DOI] [PubMed] [Google Scholar]
- Cudkowicz ME, McKenna-Yasek D, Sapp PE, Chin W, Geller B, Hayden DL, Schoenfeld DA, Hosler BA, Horvitz HR, Brown RH. (1997). Epidemiology of mutations in superoxide dismutase in ALS. Ann. Neurol. 41, 210–221 [DOI] [PubMed] [Google Scholar]
- Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, et al. (2008). Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science 321, 1218–1221 [DOI] [PubMed] [Google Scholar]
- Fischer LR, Glass JD. (2007). Axonal degeneration in motor neuron disease. Neurodegener. Dis. 4, 431–442 [DOI] [PubMed] [Google Scholar]
- Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, Polak MA, Glass JD. (2004). ALS is a distal axonopathy: evidence in mice and man. Exp. Neurol. 185, 232–240 [DOI] [PubMed] [Google Scholar]
- Gao BX, Ziskind-Conhaim L. (1998). Development of ionic currents underlying changes in action potential waveforms in rat spinal motoneurons. J. Neurophysiol. 80, 3047–3061 [DOI] [PubMed] [Google Scholar]
- Gao J, Coggeshall RE, Tarasenko YI, Wu P. (2005). Human neural stem cell-derived cholinergic neurons innervate muscle in motoneuron deficient adult rats. Neuroscience 131, 257–262 [DOI] [PubMed] [Google Scholar]
- Goldman SA, Sim F. (2005). Neural progenitor cells of the adult brain. Novartis Found. Symp. 265, 66–80 [PubMed] [Google Scholar]
- Granit R, Kernell D, Shortess GK. (1963). Quantitative aspects of repetitive firing of mammalian motoneurones, caused by injected currents. J. Physiol. 168, 911–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MG, Landmesser LT. (2003). Characterization of the circuits that generate spontaneous episodes of activity in the early embryonic mouse spinal cord. J. Neurosci. 23, 587–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano A. (1996). Neuropathology of ALS: an overview. Neurology 47, S63–S66 [DOI] [PubMed] [Google Scholar]
- Johnson MA, Weick JP, Pearce RA, Zhang SC. (2007). Functional neural development from human embryonic stem cells: accelerated synaptic activity via astrocyte coculture. J. Neurosci. 27, 3069–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juneja T, Pericak-Vance MA, Laing NG, Dave S, Siddique T. (1997). Prognosis in familial ALS: progression and survival in patients with glu100gly and ala4val mutations in Cu,Zn superoxide dismutase. Neurology 48, 55–57 [DOI] [PubMed] [Google Scholar]
- Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, Zhang SC. (2005). Specification of motoneurons from human embryonic stem cells. Nat. Biotechnol. 23, 215–221 [DOI] [PubMed] [Google Scholar]
- Lim UM, Sidhu KS, Tuch BE. (2006). Derivation of motor neurons from three clonal human embryonic stem cell lines. Curr. Neurovasc. Res. 3, 281–288 [DOI] [PubMed] [Google Scholar]
- Miles GB, Yohn DC, Wichterle H, Jessell TM, Rafuse VF, Brownstone RM. (2004). Functional properties of motoneurons derived from mouse embryonic stem cells. J. Neurosci. 24, 7848–7858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pun S, Santos AF, Saxena S, Xu L, Caroni P. (2006). Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat. Neurosci. 9, 408–419 [DOI] [PubMed] [Google Scholar]
- Roe JA, Wiedau-Pazos M, Moy VN, Goto JJ, Gralla EB, Valentine JS. (2002). In vivo peroxidative activity of FALS-mutant human CuZnSODs expressed in yeast. Free Radic. Biol. Med. 32, 169–174 [DOI] [PubMed] [Google Scholar]
- Rosato-Siri MD, Zoccolan D, Furlan F, Ballerini L. (2004). Interneurone bursts are spontaneously associated with muscle contractions only during early phases of mouse spinal network development: a study in organotypic cultures. Eur. J. Neurosci. 20, 2697–2710 [DOI] [PubMed] [Google Scholar]
- Roy NS, Nakano T, Keyoung H, Rashbaum W, Carpenter M, Kang J, Nedergaard M, Goldman SA. (2004). Telomerase-immortalization of the human fetal spinal cord ventricular zone generates stable lines of lineage-restricted spinal progenitor cells. Nat. Biotechnol. 22, 297–305 [DOI] [PubMed] [Google Scholar]
- Roy NS, Nakano T, Xuing L, Kang J, Nedergaard M, Goldman SA. (2005). Enhancer-specified GFP-based FACS purification of human spinal motor neurons from embryonic stem cells. Exp. Neurol. 196, 224–234 [DOI] [PubMed] [Google Scholar]
- Rubin LL. (2008). Stem cells and drug discovery: the beginning of a new era? Cell 132, 549–552 [DOI] [PubMed] [Google Scholar]
- Saravanan K, Bussow H, Weiler N, Gieselmann V, Franken S. (2007). A spontaneously immortalized Schwann cell line to study the molecular aspects of metachromatic leukodystrophy. J. Neurosci. Methods 161, 223–233 [DOI] [PubMed] [Google Scholar]
- Schnabel J. (2008). Standard model. Nature 454, 682–685 [DOI] [PubMed] [Google Scholar]
- Shibata N, Kawaguchi M, Uchida K, Kakita A, Takahashi H, Nakano R, Fujimura H, Sakoda S, Ihara Y, Nobukuni K, et al. (2007). Protein-bound crotonaldehyde accumulates in the spinal cord of superoxide dismutase-1 mutation-associated familial ALS and its transgenic mouse model. Neuropathology 27, 49–61 [DOI] [PubMed] [Google Scholar]
- Shin S, Dalton S, Stice SL. (2005). Human motor neuron differentiation from human embryonic stem cells. Stem Cells Dev. 14, 266–269 [DOI] [PubMed] [Google Scholar]
- Simpson CL, Al-Chalabi A. (2006). ALS as a complex genetic disease. Biochim. Biophys. Acta 1762, 973–985 [DOI] [PubMed] [Google Scholar]
- Suzuki M, McHugh J, Tork C, Shelley B, Klein SM, Aebischer P, Svendsen CN. (2007). GDNF secreting human neural progenitor cells protect dying motor neurons, but not their projection to muscle, in a rat model of familial ALS. PLoS ONE 2, e689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushitani M, Ezzi SA, Julien JP. (2007). Therapeutic effects of immunization with mutant superoxide dismutase in mice models of ALS. Proc. Natl. Acad. Sci. USA 104, 2495–2500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Xu J, Pang ZP, Ge W, Kim KJ, Blanchi B, Chen C, Sudhof TC, Sun YE. (2007). Integrative genomic and functional analyses reveal neuronal subtype differentiation bias in human embryonic stem cell lines. Proc. Natl. Acad. Sci. USA 104, 13821–13826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW. (2008). Astrocytes as determinants of disease progression in inherited ALS. Nat. Neurosci. 11, 251–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.