Abstract
Lesch-Nyhan disease (LND) is a rare inherited disorder caused by mutations in the gene encoding hypoxanthine-guanine phosphoribosyltransferase (HPRT). LND is characterized by overproduction of uric acid, leading to gouty arthritis and nephrolithiasis. Affected patients also have characteristic neurological and behavioral anomalies. Multiple cell models have been developed to study the molecular and metabolic aspects of LND, and several animal models have been developed to elucidate the basis for the neurobehavioral syndrome. The models have different strengths and weaknesses rendering them suitable for studying different aspects of the disease. The extensive modeling efforts in LND have questioned the concept that an ‘ideal’ disease model is one that replicates all of its features because the pathogenesis of different elements of the disease involves different mechanisms. Instead, the modeling efforts have suggested a more fruitful approach that involves developing specific models, each tailored for addressing specific experimental questions.
Clinical features of Lesch-Nyhan disease (LND)
LND is an inherited disorder wherein affected patients exhibit marked overproduction of uric acid, a normal by-product of purine metabolism (Jinnah and Friedmann, 2001). Normally, uric acid is close to its limit of solubility in biological fluids; therefore, the increased production of uric acid leads to precipitation of uric acid crystals in certain areas of the body such as the joints, where they cause gouty arthritis, and the urogenital system, where they lead to a sandy sludge or stones in the urinary collecting system. These materials can also obstruct the flow of urine, leading to renal failure. In addition, uric acid crystals precipitate in the subcutaneous tissues where they form visible masses known as tophi.
In addition to overproduction of uric acid, LND patients exhibit a characteristic neurobehavioral syndrome. All of the patients have a severe motor handicap associated with generalized dystonia; this is sometimes accompanied by choreoathetosis or spasticity (Jinnah et al., 2006). Most also have cognitive disability, although limitations are often not severe (Schretlen et al., 2001). Patients also show an unusual tendency towards difficult behaviors that include recurrent self-injury, with self-biting being particularly prominent (Schretlen et al., 2005; Preston, 2007). They may also exhibit other difficult behaviors such as impulsive acts of aggression, spitting, or use of foul or sexually charged language.
Some patients affected by LND exhibit other problems. The stature of LND patients is typically short and puberty is often delayed or absent. Further, an asymptomatic macrocytic anemia is common. Gastroesophageal reflux and recurrent emesis can be severe enough to lead to malnutrition. Finally, exceptional patients are affected by a milder phenotype in which some aspects of the clinical phenotype are attenuated or even absent – these patients are known as the Lesch-Nyhan variants.
Treatments for LND
Excellent treatment is available for controlling the overproduction of uric acid in LND patients (Jinnah and Friedmann, 2001). Virtually all patients take allopurinol, which inhibits the conversion of hypoxanthine and guanine to uric acid by the enzyme xanthine oxidase. Since allopurinol simultaneously increases hypoxanthine and related oxypurine metabolites, generous hydration is required at all times to maintain an active urine flow that continuously washes out all purine metabolites. With allopurinol and adequate hydration, there is a markedly reduced risk of gout, kidney stones and subcutaneous tophi.
Unfortunately, controlling the overproduction of uric acid does not address the other clinical problems, which have an independent pathogenesis. Motor impairments are addressed with a wheelchair and other appropriate supportive devices, and the discomfort associated with increased muscle tone can be alleviated with muscle relaxants such as benzodiazepines or baclofen. The behavioral problems are managed with behavior therapy, which can be supplemented with medications if required. Self-injurious behavior is among the most vexing problems. Self-hitting or biting of the fingers and hands often requires restraints that prevent the hands from reaching the face; however, self-biting of the tongue or lips cannot be so readily restrained. When behavior modifications and medications fail, dental extraction is required.
Etiology and pathogenesis of LND
LND is inherited as an X-linked recessive disorder that results from mutations in the HPRT gene (Jinnah et al., 2000). Virtually all cases, therefore, are in males. The gene encodes a housekeeping enzyme, hypoxanthine-guanine phosphoribosyltransferase (HPRT), which plays a role in purine metabolism in virtually all cells of the body. HPRT recycles the purine bases, hypoxanthine and guanine, into the usable purine nucleotide pools. In the absence of HPRT, hypoxanthine and guanine cannot be recycled and, instead, they are degraded to uric acid. Secondary biochemical changes result in enhanced endogenous purine synthesis. The failure of purine recycling together with enhanced purine synthesis is responsible for the overproduction of uric acid in LND.
Case study.
A healthy boy was born uneventfully to apparently normal parents. Delayed acquisition of motor skills was suspected by 3 months of age. Over the next year, his parents noted yellow sandy material in the diapers, but its significance was not appreciated. At 12 months of age, involuntary twisting and stiffening movements emerged, leading to a diagnosis of cerebral palsy. Benzodiazepines and baclofen were instituted to attenuate the involuntary movements, but he required full assistance for all voluntary activities. Shortly after the eruption of teeth at 3 years of age, he began to chew on the inside of his lip and occasionally on his index finger. The development of this unusual but telltale feature led to a suspicion of LND. At screening, the level of serum uric acid was found to be elevated, adding further to the suspicion of this disease. The orange sand in the diapers was also identified as being composed of uric acid crystals, and allopurinol was started to reduce uric acid production. Genetic testing revealed a point mutation in the HPRT gene, which had not previously been associated with disease. The functional significance of the mutation was verified by demonstrating a lack of HPRT enzyme activity in fibroblast cultures from a skin biopsy. Attempts to control the lip biting with multiple medications failed, leading to extreme family stress. After dental extraction, the boy and his family were much relieved, although he required limb restraints to prevent other forms of self-injury. He lived until 35 years of age when he succumbed to aspiration pneumonia.
The pathogenesis of the neurobehavioral syndrome is less well understood. Aside from being slightly smaller than age-matched controls, the brains of LND patients appear structurally normal (Harris et al., 1998). However, the neurological and behavioral problems point to dysfunction of a particular region known as the basal ganglia (Visser et al., 2000). The dopamine neurons of this brain region in particular seem to be adversely affected by the deficiency in HPRT. Neurochemical studies in autopsied LND brains reveal a 60–80% loss of dopamine in the basal ganglia (Lloyd et al., 1981; Saito et al., 1999). Neuroimaging studies of affected patients reveal similar reductions in other markers of dopamine neurons in the same region (Ernst et al., 1996; Wong et al., 1996). The reasons for the selective dysfunction of dopamine pathways of the basal ganglia are unknown, although there is probably a developmental defect (Egami et al., 2007; Lewers et al., 2008).
The pathogenesis for the other clinical features in LND are also not well understood. However, the clinical expression of the disease indicates that the failure of purine recycling owing to HPRT deficiency has adverse effects on somatic growth, the maturation of bone marrow stem cells into erythrocytes, and on gastrointestinal motility.
The need for surrogate models
Surrogate experimental models are essential for LND because the disorder is extraordinarily rare, with a prevalence of approximately three people in every million (Jinnah and Friedmann, 2001). This rarity makes patient studies very challenging because even if sufficient patients could be found, the types of studies needed to elucidate the biological basis for neurobehavioral dysfunction cannot be conducted with human subjects.
Fortunately, multiple experimental models have been developed. There are several tissue culture models available for exploring the metabolic and cellular consequences of HPRT deficiency in vitro. There are also multiple animal models for exploring the influence of HPRT deficiency in vivo. These various models have different strengths and limitations, as summarized below.
Tissue culture models: neural and non-neural
Some of the earliest cell models developed for LND were based on non-neural cells such as erythrocytes, lymphocytes and fibroblasts (Jinnah and Friedmann, 2000). These materials can be obtained from affected patients, so an obvious strength is their direct relevance to affected humans. These cell models have proven to be extremely valuable for understanding the consequences of HPRT deficiency on purine metabolism and cell function. The HPRT-deficient cells fail to recycle hypoxanthine and guanine, resulting in an accumulation of purine waste products. An unexpected finding has been the lack of an obvious purine deficiency, which most investigators expected would result from the failure of purine recycling. Some studies reveal small decreases or increases in one or another purine, but results are inconsistent and many studies reveal normal levels of purines (Shirley et al., 2007).
The non-neural cell models have also made it possible to explore the mechanisms responsible for several secondary changes in purine metabolism, such as accelerated synthesis of purines. They have also been used to explore the mechanisms responsible for other secondary and tertiary metabolic abnormalities, including the accumulation of HPRT co-substrates and the effects of this accumulation on other processes using the same substrates. These studies have raised appreciation for the idea that an unexpectedly complex metabolic cascade can result from a single gene defect.
Several investigators have sought, more specifically, to model the processes responsible for neural dysfunction by studying HPRT-deficient neuronal or glial cells in culture (Shirley et al., 2007). Such cells cannot be obtained readily from affected human patients, so most were developed by creating HPRT-deficient sublines of established neuroblastoma or glioma cell lines. In addition to revealing many of the same changes found in primary source cells, these models have revealed abnormalities that could not be identified with non-neural cells. HPRT-deficient dopamine neuron-like lines show a loss of dopamine content that is analogous to that reported for the LND brain (Bitler and Howard, 1986; Yeh et al., 1998; Lewers et al., 2008). These models also reveal changes in neuronal microstructure in the form of abnormal numbers or morphology of neurites, which are comparable to dendrites or axons in vivo (Stacey et al., 1999; Connolly et al., 2001; Shirley et al., 2007).
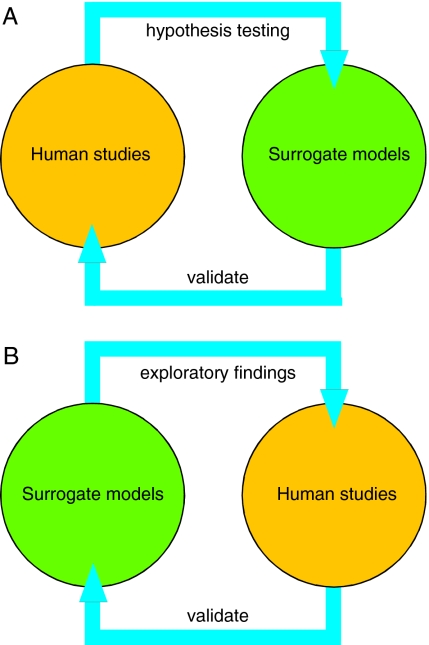
A major strength of the cell models is the ability to explore specific aspects of metabolism and cell biology in a very tightly controlled experimental environment. The loss of dopamine in the neural models provides valuable confirmation of a connection between HPRT and brain dopamine neurons, which was originally suggested by limited human autopsy studies. The microstructural defects in the cell models suggest the possibility of similar defects in the LND brain that could have been missed in the prior human neuropathological studies. Therefore, the cell models can validate limited findings from human studies, and can provide new findings that require validation from human studies (Fig. 1).
Fig. 1.
Validation of hypotheses versus validation of exploratory findings. (A) Experimental findings from human studies often generate hypotheses that cannot be tested in humans. Surrogate experimental models can be very useful to validate these hypotheses. For example, both animal and tissue culture models have been exploited to validate the hypothesis that dopamine loss in the LND brain results from degeneration of dopaminergic axonal projections in LND. (B) Surrogate experimental models can be used in exploratory studies to discover novel directions. Ideally, these findings should be validated by returning to human studies to ensure that they are relevant to the disease being studied. For example, recent findings from both animal and tissue culture models have suggested a defect in the developmental programming of the dopamine neuron neurochemical phenotype. These findings must now be validated by seeking similar defects in selected studies of the LND brain.
The cell models also have their limitations. The most obvious is that results obtained under artificial in vitro conditions may not apply in vivo. The best solution to this problem is to attempt to replicate key findings from cell models using more complex in vivo models or LND patients. Another limitation of cell models is that the results obtained with one type of cell might not apply to other types of cells. Many aspects of purine metabolism are shared by virtually all cells, but some aspects are peculiar to specific cell types or tissues. One example is the use of specific purines, such as adenosine or ATP, by neurons and glia as neural transmitters. Non-neural cell models of LND might, therefore, have limited utility in exploring the mechanisms responsible for these aspects of neural dysfunction. For the same reasons, neural cell models may have limited applicability for understanding the mechanisms responsible for other aspects of LND, such as megaloblastic anemia. The solution to this problem is to select the cell model that is most appropriate for the question that is being addressed.
A final limitation is that findings might be idiosyncratic to the cell model being evaluated (Shirley et al., 2007; Lewers et al., 2008). Because LND is so rare, models derived from blood samples or fibroblast biopsies often come from a single patient, or very small numbers of patients. The small numbers of experimental samples superimposed on the relatively large variation that is inherent in human material can make it challenging to obtain meaningful results. A related problem affects research involving established cell lines. Preparing HPRT-deficient subclones is labor intensive, so most studies examine only one or a small number of mutant lines. Inherent variation in the parent cell line combined with random phenotypic drift in isolated subcultures can produce sublines that differ from the parent line in many respects that are independent of the HPRT deficiency. Conclusions derived from comparisons between small numbers of independent samples must, therefore, be interpreted with caution. These limitations are best addressed by evaluating as many independent cell samples or cell line derivatives as possible.
Animal models: genes, lesions and drugs
Multiple animal models have been developed for LND, each with complementary strengths and weaknesses. HPRT-deficient mice provided the first demonstration that genetically engineered mice could be produced as models for a specific human disease (Hooper et al., 1987; Kuehn et al., 1987). These mice exhibit metabolic abnormalities that are strikingly similar to abnormalities that have been reported for LND and its cell models, including loss of HPRT enzyme activity, failure of purine recycling, and accelerated synthesis of purines (Jinnah et al., 1992b; Jinnah et al., 1993). Studies of the brains of these mice demonstrate reduced dopamine along with microstructural anatomical abnormalities that are analogous to those demonstrated in the LND cell models (Jinnah et al., 1994; Jinnah et al., 1999b; Mikolaenko et al., 2005). These mice have been useful for demonstrating that HPRT deficiency leads to a loss of basal ganglia dopamine through a metabolic process rather than a degenerative one (Egami et al., 2007). The main limitation of these mice is that they do not exhibit some of the more complex aspects of the LND phenotype, such as the consequences of uric acid overproduction or the neurobehavioral syndrome. As a result, they are of limited value for directly addressing these aspects of the disorder.
Another animal model has been developed to address the functional significance of dopamine loss to the neurobehavioral syndrome (Breese et al., 2004). Rats given the dopaminergic neurotoxin 6-hydroxydopamine as adults display a severe loss of dopamine and a reduction in mobility that resembles Parkinson’s disease. The dopamine precursor L-DOPA and other dopamine agonists can be therapeutic in that they restore normal behavior. By contrast, rats given the same lesions as neonates become hyperactive. Administration of L-DOPA or dopamine agonists in the neonatally-lesioned rats is not therapeutic; instead, it exaggerates their hyperactivity and causes self-injurious biting and aggressiveness which are strikingly similar to the difficult behaviors observed with LND. This model has been very useful for establishing a link between early brain dopamine loss and the major neurobehavioral abnormalities in LND. The main limitation of this model is that it is not possible to explore the mechanisms by which HPRT deficiency might trigger the dopamine defect.
Clinical terms.
Choreoathetosis – involuntary movements involving chorea (irregular, rapid flowing movements) and athetosis (slow, writhing, sinuous movements)
Dystonia – excessive involuntary muscle contractions resulting in twisting movements or abnormal postures
Emesis – vomiting
Megaloblastic/macrocytic anemia – a hemoglobin deficiency owing to the presence of enlarged immature/dysfunctional red blood cells
Nephrolithiasis – aggregation of uric acid crystals into kidney stones
Spasticity – an abnormality of increased muscle tone at rest, with a rate-dependent quality on examination
Tophi – deposits of uric acid crystals that aggregate in tissue, particularly cartilage and bone
Several other pharmacological models have been used to study self-injurious biting behavior in rats and mice, including chronic administration of high doses of amphetamine and related psychostimulants, caffeine or opiates (Jinnah and Breese, 1997). Self-injurious biting might also be provoked by a single high dose of clonidine or the L-type calcium channel agonist BayK-8644 (Jinnah et al., 1999a). These models have been useful for elucidating the neuropharmacological basis for self-injurious behavior. The challenge in these models is to work backwards from the known or suspected actions of the drug, to the more complex operations of the intact brain that are required to generate the abnormal behavior.
The ‘ideal’ model: a holy grail?
It is often implied, and sometimes stated explicitly, that the ‘ideal’ model for a disease is one that most closely replicates all of the clinical features seen in its human counterpart. This ideal model encompasses all levels of disease: genetic, biochemical, cellular, pharmacological, anatomical, physiological and behavioral. Models that only partly recapitulate the disease are criticized as incomplete and sometimes declared as outright failures. Considerable time and effort are often devoted to improving these models so that they more closely resemble the human disease.
A major flaw in this conceptualization of the ‘ideal’ model becomes apparent when we ask a simple question: what is the goal of modeling? We have argued that the primary purpose of developing experimental models is not to make them look like their human counterpart, but to use them as tools to elucidated disease pathogenesis so that better treatments may be developed (Jinnah and Breese, 1997; Jinnah et al., 2005; Jinnah et al., 2008). When viewed as tools rather than replicas, the value of models that do not recapitulate the entire human disease becomes more obvious (Fig. 2). Here, the crucial issue is to select the model that is most appropriate for the experimental question of interest.
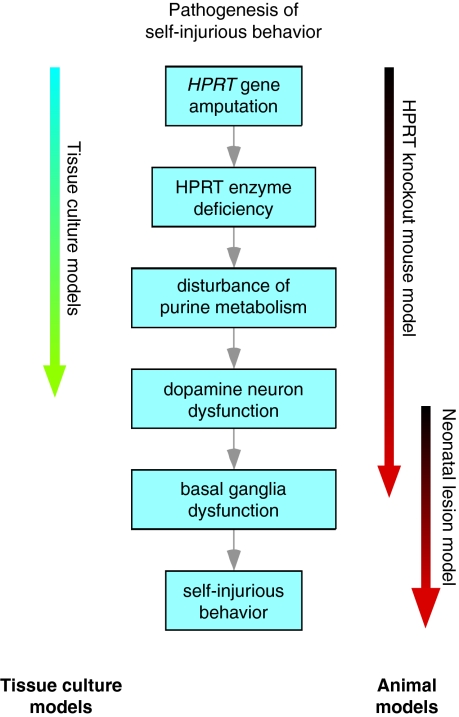
Fig. 2.
The roles of different surrogate experimental models in LND. The blue boxes depict the sequence of events that are thought currently to be involved in the pathogenesis of self-injurious behavior in LND. Tissue culture models have been particularly useful in elucidating proximal steps of the pathogenic pathway involving the effects of HPRT deficiency on purine metabolism. Further, some neuronal models have been useful in addressing the steps relating to dopamine neuron dysfunction, but no tissue culture model is suitable for addressing the more complex physiological interactions that occur among different neural elements of the basal ganglia, which potentially lead to the behavioral defects in the more distal steps of the pathway. Similarly, the HPRT-deficient mouse model has been useful for elucidating the more proximal steps in this pathway in an in vivo setting, but it has not been useful for understanding the behavioral abnormality. However, the neonatal dopamine lesion model has been more useful in distal steps of the pathway, beginning with dopamine lesions and ending with expression of the behavior. Each model has characteristics that might render it particularly well suited for studying specific steps in the pathway. There is no ‘ideal’ model that combines all of these strengths, but proper selection of the most appropriate model can address most experimental questions.
The importance of selecting an appropriate model is illustrated most clearly by the cell models. Models based on neural cells may be required to address the effects of HPRT deficiency on processes that are unique to neurons, such as neural transmission or elaboration of neurites. However, the neural cell models are not the best choices for addressing the mechanisms responsible for megaloblastic anemia. Here, a cell model of hematological origin seems preferable. Although neither neural nor hematological models are likely to replicate all of the cellular consequences of HPRT deficiency, they are nonetheless invaluable for specific experimental questions.
The value of partial models addressing specific experimental needs is often overlooked when considering animals. The HPRT-deficient knockout mouse model was initially discarded as a failure because it did not show any overt symptoms resembling those seen in LND. However, subsequent studies demonstrated multiple metabolic and neurochemical abnormalities that were analogous to the human disorder (Jinnah et al., 1992b; Jinnah et al., 1993; Jinnah et al., 1994; Jinnah et al., 1999b). The mice proved extremely useful for establishing a causal link between HPRT deficiency and a functional disturbance in brain dopamine neurons. They also led to the discovery that behavioral abnormalities could be elicited through appropriate pharmacological challenges (Jinnah et al., 1991; Jinnah et al., 1992a). Thus, the HPRT-deficient mouse has proven to be a viable model for addressing the enigmatic relationship between HPRT deficiency and brain dopamine neurons in vivo, despite the lack of overt symptoms. However, the lack of an overt neurobehavioral phenotype renders this model less than ideal as a vehicle to screen for therapies for these problems. Among the many homologous genetic mouse models now available for human disorders, it is now recognized that few faithfully replicate all aspects of the disease, whereas most replicate only selected portions (Erickson, 1989; Elsea and Lucas, 2002). The partial models can nonetheless be very useful for addressing particular issues.
Moving forward
There are many unanswered questions in LND. They span virtually all of the fields in the biological sciences from molecular genetics through metabolism to complex behavior. They also span many different organs and regions, from gout that occurs in the big toe to the complex neurobehavioral phenotype of the brain. Rather than trying to fantasize over the production of one ‘ideal’ model that appropriately addresses all of these questions, it seems more useful to work with different models, each optimally suited for a specific question. It is not necessary to have a single ideal model that replicates exhaustively all aspects of the disease. Indeed, the best models for gout are likely to be very different from those for the neurobehavioral problems. There is little value in desiring a model for exploring neurobehavioral dysfunction that also exhibits overproduction of uric acid, since these problems are mechanistically unrelated.
Clinical and basic research opportunities.
To understand the unique roles of purine metabolism among different cell and tissue types
Using in vivo models to replicate key findings from cell models
Investigating the influence of HPRT deficiency in models of dopamine neuron development
Determining the mechanisms by which HPRT deficiency triggers dopamine defects
Exploring the mechanisms underlying self-injurious behavior in LND
Use of models to develop more effective treatments for the neurological and behavioral symptoms of LND
Further, modeling should not be viewed as an objective with fixed criteria that are used to define success or failure, but rather as an iterative process wherein information gained is re-invested to modify the starting model or to generate new ones that are even more informative. For example, several currently available cell and animal models have pointed to a defect in developmental specification of the neurochemical phenotype of dopamine neurons in HPRT deficiency (Egami et al., 2007; Lewers et al., 2008). These studies have pointed to the need for models of dopamine neuron development in which the influence of HPRT deficiency can be studied more dynamically. These processes might be studied in early embryos of HPRT-deficient mice, but a more rigorously controlled model might involve the in vitro differentiation of HPRT-deficient embryonic stem cells towards dopaminergic neurons.
Another pressing question is: how can the models help to develop more effective treatments for the neurological and behavioral impairments in LND? The neonatal 6-hydroxydopamine rat model has led to proposals for using D1-dopamine receptor antagonists for self-injurious behavior in LND (Gualtieri and Schroeder, 1991), whereas the BayK 8644 model has led to suggestions regarding the use of L-type calcium channel antagonists (Blake et al., 2006). These and related drugs provide rational choices for clinical trials. A better understanding of the pathogenic mechanisms obtained through exploitation of the various experimental models that are currently available is likely to identify other biological targets that might prove useful for treating the neurobehavioral difficulties in LND.
Acknowledgments
Supported in part by the Lesch-Nyhan Syndrome Children’s Research Foundation and by NIH grants DK082840, HD053312 and NS40470.
Footnotes
COMPETING INTERESTS
The authors declare no competing financial interests.
REFERENCES
- Bitler CM, Howard BD. (1986). Dopamine metabolism in hypoxanthine-guanine phosphoribosyltransferase-deficient variants of PC12 cells. J. Neurochem. 47, 107–112 [DOI] [PubMed] [Google Scholar]
- Blake BL, Muehlmann AM, Egami K, Breese GR, Devine DP, Jinnah HA. (2006). Nifedipine suppresses self-injurious behavior in animals. Dev. Neurosci. 29, 241–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Criswell HE, Moy SS, Papadeas ST, Blake BL. (2004). The neonate 6-hydroxydopamine lesioned rat: a model for clinical neuroscience and neurobiological principles. Brain Res. Rev. 48, 57–73 [DOI] [PubMed] [Google Scholar]
- Connolly GP, Duley JA, Stacey NC. (2001). Abnormal development of hypoxanthine-guanine-phosphoribosyltransferase-deficient CNS neuroblastoma. Brain Res. 918, 20–27 [DOI] [PubMed] [Google Scholar]
- Egami K, Yitta S, Kasim S, Lewers JC, Roberts RC, Lehar M, Jinnah HA. (2007). Basal ganglia dopamine loss due to defect in purine recycling. Neurobiol. Dis. 26, 396–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsea SH, Lucas RE. (2002). The mousetrap: what we can learn when the mouse model does not mimic the human disease. ILAR J. 43, 66–79 [DOI] [PubMed] [Google Scholar]
- Erickson RP. (1989). Why isn’t a mouse more like a man? Trends Genet. 5, 1–3 [DOI] [PubMed] [Google Scholar]
- Ernst M, Zametkin AJ, Matochik JA, Pascualvaca D, Jons PH, Hardy K, Hankerson JG, Doudet DJ, Cohen RM. (1996). Presynaptic dopaminergic deficits in Lesch-Nyhan disease. N. Engl. J. Med. 334, 1568–1572 [DOI] [PubMed] [Google Scholar]
- Gualtieri CT, Schroeder SR. (1991). Pharmacotherapy for self-injurious behavior: preliminary tests of the D1 hypothesis. Prog. Neuropsychopharmacol. Biol. Psychiatry 14, S81–S107 [DOI] [PubMed] [Google Scholar]
- Harris JC, Lee RR, Jinnah HA, Wong DF, Yaster M, Bryan N. (1998). Craniocerebral magnetic resonance imaging measurement and findings in Lesch-Nyhan syndrome. Arch. Neurol. 55, 547–553 [DOI] [PubMed] [Google Scholar]
- Hooper M, Hardy K, Handyside A, Hunter S, Monk M. (1987). HPRT-deficient (Lesch-Nyhan) mouse embryos derived from germline colonization by cultured cells. Nature 326, 292–295 [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Breese GR. (1997). Animal models for Lesch-Nyhan disease. In Biological Aspects of Disease: Contributions From Animal Models (ed. Iannocconne PM, Scarpelli DG. ), pp. 93–143 Amsterdam: Harwood Academic Publishers [Google Scholar]
- Jinnah HA, Friedmann T. (2001). Lesch-Nyhan disease and its variants. In The Metabolic and Molecular Bases of Inherited Disease (ed. Scriver CR, Beaudet AL, Sly WS, Valle D. ), pp. 2537–2570 New York: McGraw-Hill [Google Scholar]
- Jinnah HA, Gage FH, Friedmann T. (1991). Amphetamine-induced behavioral phenotype in a hypoxanthine-guanine phosphoribosyltransferase-deficient mouse model of Lesch-Nyhan syndrome. Behav. Neurosci. 105, 1004–1012 [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Langlais PJ, Friedmann T. (1992a). Functional analysis of brain dopamine systems in a genetic mouse model of Lesch-Nyhan syndrome. J. Pharmacol. Exp. Ther. 263, 596–607 [PubMed] [Google Scholar]
- Jinnah HA, Hess EJ, Wilson MC, Gage FH, Friedmann T. (1992b). Localization of hypoxanthine-guanine phosphoribosyltransferase mRNA in the mouse brain by in situ hybridization. Mol. Cell. Neurosci. 3, 64–78 [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Page T, Friedmann T. (1993). Brain purines in a genetic mouse model of Lesch-Nyhan disease. J. Neurochem. 60, 2036–2045 [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Wojcik BE, Hunt MA, Narang N, Lee KY, Goldstein M, Wamsley JK, Langlais PJ, Friedmann T. (1994). Dopamine deficiency in a genetic mouse model of Lesch-Nyhan disease. J. Neurosci. 14, 1164–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinnah HA, Yitta S, Drew T, Kim BS, Visser JE, Rothstein JD. (1999a). Calcium channel activation and self-biting in mice. Proc. Natl. Acad. Sci. USA 96, 15228–15232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinnah HA, Jones MD, Wojcik BE, Rothstein JD, Hess EJ, Friedmann T, Breese GR. (1999b). Influence of age and strain on striatal dopamine loss in a genetic mouse model of Lesch-Nyhan disease. J. Neurochem. 72, 225–229 [DOI] [PubMed] [Google Scholar]
- Jinnah HA, DeGregorio L, Harris JC, Nyhan WL, O’Neill JP. (2000). The spectrum of inherited mutations causing HPRT deficiency: 75 new cases and a review of 196 previously reported cases. Mutat. Res. 463, 309–326 [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Hess EJ, LeDoux MS, Sharma N, Baxter MG, DeLong MR. (2005). Rodent models for dystonia research: characteristics, evaluation, and utility. Mov. Disord. 20, 283–292 [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Visser JE, Harris JC, Verdu A, Larovere L, Ceballos-Picot I, Neychev V, Torres RJ, Dulac O, Desguerre I, et al. (2006). Delineation of the motor disorder of Lesch-Nyhan disease. Brain 129, 1201–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinnah HA, Richter A, Mink JW, Caldwell GA, Caldwell KA, Gonzalez-Alegre P, Cookson MR, Breakefield XO, Delong MR, Hess EJ. (2008). Animal models for drug discovery in dystonia. Expert Opin. Drug Discovery 3, 83–97 [DOI] [PubMed] [Google Scholar]
- Kuehn MR, Bradley A, Robertson EJ, Evans MJ. (1987). A potential animal model for Lesch-Nyhan syndrome through introduction of HPRT mutations into mice. Nature 326, 295–298 [DOI] [PubMed] [Google Scholar]
- Lewers JC, Ceballos-Picot I, Shirley TL, Mockel L, Egami K, Jinnah HA. (2008). Consequences of impaired purine recycling in dopaminergic neurons. Neuroscience 152, 761–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd KG, Hornykiewicz O, Davidson L, Shannak K, Farley I, Goldstein M, Shibuya M, Kelley WN, Fox IH. (1981). Biochemical evidence of dysfunction of brain neurotransmitters in the Lesch-Nyhan syndrome. N. Engl. J. Med. 305, 1106–1111 [DOI] [PubMed] [Google Scholar]
- Mikolaenko I, Rao LM, Roberts RC, Kolb B, Jinnah HA. (2005). A Golgi study of neuronal architecture in a genetic mouse model for Lesch-Nyhan disease. Neurobiol. Dis. 20, 479–490 [DOI] [PubMed] [Google Scholar]
- Preston R. (2007). An error in the code: what can a rare disorder tell us about human behavior? New Yorker 30–36 [PubMed] [Google Scholar]
- Saito Y, Ito M, Hanaoka S, Ohama E, Akaboshi S, Takashima S. (1999). Dopamine receptor upregulation in Lesch-Nyhan syndrome: a postmortem study. Neuropediatrics 30, 66–71 [DOI] [PubMed] [Google Scholar]
- Schretlen DS, Harris JC, Park KS, Jinnah HA, Ojeda del Pozo N. (2001). Neurocognitive functioning in Lesch-Nyhan disease and partial hypoxanthine-guanine phosphoribosyltransferase deficiency. J. Int. Neuropsychol. Soc. 7, 805–812 [DOI] [PubMed] [Google Scholar]
- Schretlen DS, Ward J, Meyer SM, Yun J, Puig JG, Nyhan WL, Jinnah HA, Harris JC. (2005). Behavioral aspects of Lesch-Nyhan disease and it variants. Dev. Med. Child Neurol. 47, 673–677 [DOI] [PubMed] [Google Scholar]
- Shirley TL, Lewers JC, Egami K, Majumdar A, Kelly M, Ceballos-Picot I, Seidman MM, Jinnah HA. (2007). A human neuronal tissue culture model for Lesch-Nyhan disease. J. Neurochem. 101, 841–853 [DOI] [PubMed] [Google Scholar]
- Stacey N, Ma MHY, Duley JA, Connolly GP. (1999). Morphological abnormalities of HGPRT-deficient mouse N2a neuroblastoma: implications for neuronal development in Lesch-Nyhan syndrome. Cell. Mol. Biol. Lett. 4, 480–481 [Google Scholar]
- Visser JE, Baer PR, Jinnah HA. (2000). Lesch-Nyhan syndrome and the basal ganglia. Brain Res. Rev. 32, 449–475 [DOI] [PubMed] [Google Scholar]
- Wong DF, Harris JC, Naidu S, Yokoi F, Marenco S, Dannals RF, Ravert HT, Yaster M, Evans A, Rousset O, et al. (1996). Dopamine transporters are markedly reduced in Lesch-Nyhan disease in vivo. Proc. Natl. Acad. Sci. USA 93, 5539–5543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J, Zheng S, Howard BD. (1998). Impaired differentiation of HPRT-deficient dopaminergic neurons: a possible mechanism underlying neuronal dysfunction in Lesch-Nyhan syndrome. J. Neurosci. Res. 53, 78–85 [DOI] [PubMed] [Google Scholar]