SUMMARY
The essential role of connexin43 (Cx43) during oogenesis has been demonstrated by the severe germ cell deficiency and arrested folliculogenesis observed in Cx43 knockout mice. Recently, another mutant mouse strain became available (Gja1Jrt/+) that carries the dominant loss-of-function Cx43 mutation, Cx43G60S. Gja1Jrt/+ mice display features of the human disease oculodentodigital dysplasia (ODDD), which is caused by mutations in the GJA1 gene. We used this new mutant strain to study how a disease-linked Cx43 mutant affects oogenesis. We found that female mutant mice are subfertile with significantly reduced mating success and small litters. The phosphorylated species of the Cx43 protein are reduced in the mutant ovaries in association with impaired trafficking and assembly of gap junctions in the membranes of granulosa cells, confirming that the mutant protein acts dominantly on its wild-type counterpart. Correspondingly, although starting with a normal abundance of germ cells, ovaries of the mutant mice contain significantly fewer pre-ovulatory follicles and do not respond to superovulation by gonadotropins, which is at least partially the result of reduced proliferation and increased apoptosis of granulosa cells. We conclude that the Gja1Jrt mutation has a dominant negative effect on Cx43 function in the ovary, rendering the females subfertile. Given these findings, closer examination of reproductive function in ODDD human females is warranted.
INTRODUCTION
Connexins are the protein subunits of gap junction channels, which enable neighboring cells to exchange small signaling molecules (less than ~1 kDa) and to synchronize electrical activities. Six identical or different connexins hexamerize to form a homomeric or heteromeric connexon (hemichannel), respectively. Two connexons (hemichannels) from adjacent cells dock with each other to form an intercellular gap junction channel (reviewed by Laird, 2006). So far, 20 and 21 connexin genes have been found in the mouse and the human genomes, respectively, which have distinct but overlapping patterns of expression (Laird, 2006).
The ovarian follicle provides a good example of a multicellular unit that exhibits expression of multiple connexins and is considered to be reliant upon gap junctional intercellular communication (GJIC) for proper development (reviewed by Kidder, 2005). As the most abundant connexin in the ovary, connexin43 (Cx43) is continuously expressed in the mouse fetal ovary from the onset of ovarian differentiation (Perez-Armendariz et al., 2003). After birth, Cx43 forms numerous large gap junctions among granulosa cells in primordial, primary, secondary and antral follicles with the expression level increasing in parallel with follicle development (reviewed by Granot and Dekel, 2002). The essential role of Cx43 during oogenesis and folliculogenesis has been clearly demonstrated by the Cx43 knockout mice (Gja1–/–). Offspring (both male and female) homozygous for the null mutation have very few primordial germ cells (PGCs) (Juneja et al., 1999). The marked loss of PGCs is at least partially because of increased apoptosis associated with abnormal P53 activation in the germ cells, demonstrating that Cx43 is required for PGC survival in the genital ridges (Francis and Lo, 2006). However, the gonads of Cx43-null fetuses do contain about 10% of the normal number of germ cells (Juneja et al., 1999). Owing to the neonatal death of Cx43-null mice, late fetal ovaries were grafted into the kidney capsule of ovariectomized adult mice to allow postnatal follicular development. In contrast to the full range of follicles, from primordial through to large preovulatory follicles, that are observed in grafted wild-type ovaries, folliculogenesis in mutant ovaries of the C57BL/6 strain did not proceed beyond the primary unilaminar stage (Ackert et al., 2001). Further studies demonstrated that intercellular coupling among granulosa cells was totally abolished in Cx43-null mice, indicating that cell coupling through Cx43 channels is required to sustain granulosa cell proliferation (Gittens et al., 2003; Gittens et al., 2005; Tong et al., 2006). Failure of the Cx43-null follicles to develop multiple layers of granulosa cells was correlated with reduced growth of the oocytes, which were morphologically abnormal, meiotically incompetent and could not be fertilized (Ackert et al., 2001).
Oculodentodigital dysplasia (ODDD) is a rare, human autosomal dominant disorder that is caused by mutations in the GJA1 gene encoding Cx43. Common symptoms include syndactyly of hands and feet, enamel hypoplasia, craniofacial abnormalities, ophthalmic defects and occasionally heart and neurological dysfunction (Loddenkemper et al., 2002; Paznekas et al., 2003). More than 39 different ODDD-causing mutations have been identified in the GJA1 gene so far (reviewed by Laird, 2006). In vitro studies of the ODDD-linked Cx43 mutants have shown that, to varying degrees, most mutants will assemble into gap junction plaque-like structures at the cell surface, however, all of the mutants have severely reduced GJIC compared with wild-type Cx43. Furthermore, when co-expressed with wild-type Cx43, the mutants often act in a dominant negative fashion (Roscoe et al., 2005; Shibayama et al., 2005; McLachlan et al., 2005).
In 2005, a mouse model of ODDD became available when an N-ethyl-N-nitrosourea (ENU) mutagenesis screen resulted in the generation of a mouse that exhibits many classic symptoms of ODDD including syndactyly, enamel hypoplasia and craniofacial bone anomalies (Flenniken et al., 2005). This mouse carries a G60S point mutation (the product of the Gja1Jrt allele) in the first extracellular loop of Cx43, which is one residue away from the P59H mutation identified in human ODDD patients (Vasconcellos et al., 2005). Our previous work demonstrated that the granulosa cells isolated from these mice (Gja1Jrt/+) exhibit either very weak coupling (10–20% versus wild type) or a complete lack of coupling, indicating that the G60S mutant dominantly inhibits the function of co-expressed wild-type Cx43 in vivo (Flenniken et al., 2005). Given the defects observed in Cx43-null ovaries, we hypothesized that Cx43G60S affects oogenesis by inhibiting the function of wild-type Cx43 during germ cell migration and folliculogenesis. To test this hypothesis, we compared the fertility, germ cell numbers and follicular development of Gja1Jrt/+ females with their wild-type littermates. We found that the mutant mice are subfertile with reduced mating success and have smaller litter sizes compared with wild-type siblings. Although they have normal germ cell numbers, the ovaries of the mutant mice contain significantly fewer pre-ovulatory follicles and are impaired in their response to gonadotropins. This was associated with impaired phosphorylation of the Cx43 protein and reduced GJIC among granulosa cells.
RESULTS
Female Gja1Jrt/+ mice have reduced fertility
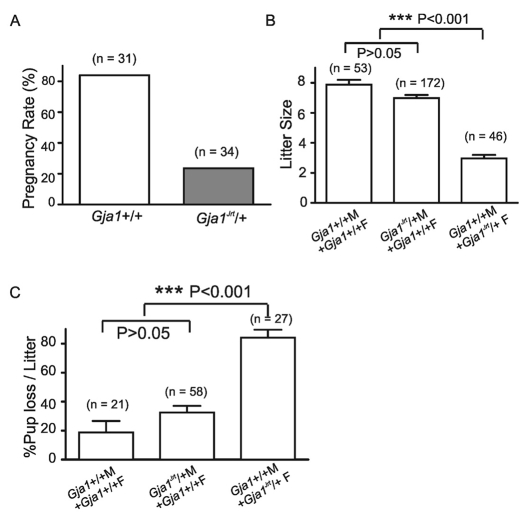
After mating with wild-type males, the pregnancy rate of female Gja1Jrt/+ mice (23.5%, n=34 matings) was much lower than their wild-type littermates (83.9%, n=31) (Fig. 1A). Furthermore, when Gja1Jrt/+ females were mated with wild-type males, their mean litter size (3.0±0.2, n=46 litters) was significantly smaller (P<0.001) compared with those resulting from matings between wild-type females and wild-type males (7.9±0.3, n=53). Surprisingly, litter sizes were normal (7.0±0.2, n=172; P>0.05 compared with wild type) if only the males were carrying the mutation (Fig. 1B), indicating that the mutation selectively affects female fertility. In addition, if the mother carried the mutation, litter sizes were further reduced given the fact that only around 16% of the pups survived beyond postnatal day 1 (Fig. 1C), which was at least partially because of a co-existent lactation problem that has been described elsewhere (Plante and Laird, 2008).
Fig. 1.
Female Gja1Jrt/+ mice have reduced fertility. (A) After mating with wild-type males, the pregnancy rate of female Gja1Jrt/+ mice was much lower than that of their wild-type littermates. (B) Litters resulting from mating between Gja1Jrt/+ females and wild-type males were significantly smaller (***P<0.001) compared with those from mating between wild-type females and wild-type males, and between wild-type females and Gja1Jrt/+ males. (C) The loss of pups was significantly higher (***P<0.001) when Gja1Jrt/+ females were mated with wild-type males compared with mating between wild-type females and wild-type males, and between wild-type females and Gja1Jrt/+ males. There was no significant difference between the latter two groups (P>0.05).
Gja1Jrt/+ ovaries contain normal numbers of germ cells
To determine whether the abundance of germ cells might be affected by the Gja1Jrt mutation, we counted germ cell numbers in the postnatal day 1 ovaries using germ cell nuclear antigen (GCNA) as a specific germ cell marker (Juneja et al., 1999). In contrast to the severe germ cell deficiency observed in Gja1–/– ovaries, there was no significant difference (P=0.71) in germ cell numbers between Gja1Jrt/+ females (n=3639±988) and their wild-type littermates (n=4123±227) (Fig. 2B). Three ovaries from three different mice were counted for each genotype. As shown in Fig. 2A, germ cells in the ovaries of both genotypes showed similar peripheral distribution in the ovarian cortex, however, the numerous Cx43 gap junction ‘plaques’ present in the somatic cells were barely observed in the mutant ovaries.
Fig. 2.
Gja1Jrt/+ ovaries contain normal numbers of germ cells. (A) Ovaries from postnatal day 1 wild-type (top row) and Gja1Jrt/+ (bottom row) females were co-immunolabeled for the germ cell marker GCNA (red) and Cx43 (green). Nuclei were stained with Hoechst (blue). The higher magnification picture (far right column) shows that the gap junction ‘plaques’ (white arrows), which formed in wild-type ovaries, are rarely present in Gja1Jrt/+ ovaries. Bars, 20 μm. (B) Quantification of germ cell numbers per ovary from wild-type and Gja1Jrt/+ females (P=0.71).
Gja1Jrt/+ ovaries contain fewer pre-ovulatory follicles and have reduced response to gonadotropins
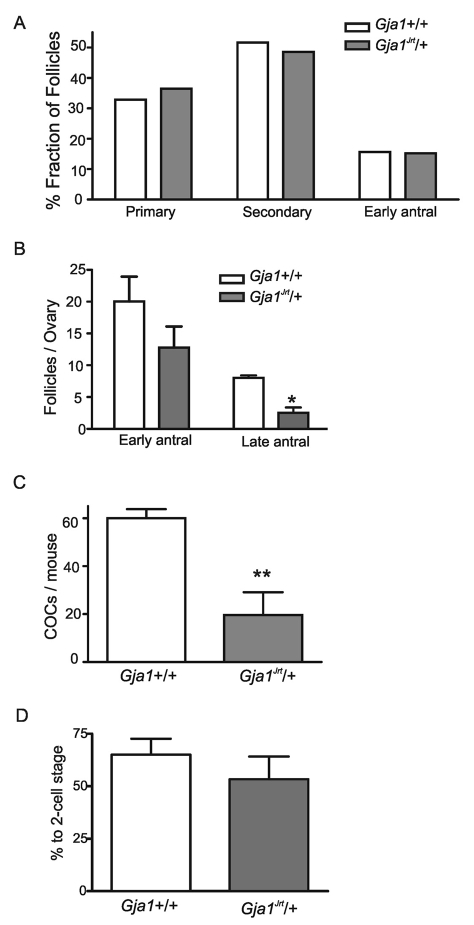
Although starting with similar numbers of germ cells, ovaries of sexually mature Gja1Jrt/+ females contain significantly fewer pre-ovulatory follicles at proestrus (Fig. 3B) (P<0.05 versus wild-type littermates, two-way ANOVA and Bonferroni post hoc test, n=4 ovaries from four mice). However, there was no significant difference in the distribution of the earlier follicle stages (Fig. 3A) (P>0.05, chi-square test, n=3 ovaries from three mice for each group), indicating that the mutation mainly affected the final maturation of the follicles. Morphologically atretic follicles were rarely seen in ovaries of both groups. To test their response to gonadotropins, mice from both genotypes were primed with equine chorionic gonadotropin (eCG), a peptide hormone with follicle-stimulating hormone (FSH) activity in the mouse, and induced to ovulate with human chorionic gonadotropin (hCG) as a source of luteinizing hormone (LH) activity. Significantly fewer oocytes were collected from the oviducts of the Gja1Jrt/+ females (19.6±9.5, n=5 mice versus 60.0±3.8, n=4 wild-type mice, unpaired t-test, P<0.01), indicating that the response of the mutant mice to gonadotropins is reduced (Fig. 3C).
Fig. 3.
Gja1Jrt/+ ovaries contain fewer pre-ovulatory follicles and have reduced response to gonadotropins. (A) Distribution of follicles at different developmental stages in the wild-type and Gja1Jrt/+ ovaries (P >0.05). (B) Quantification of early and late antral follicles in the wild-type and Gja1Jrt/+ ovaries during proestrus (wild-type vs Gja1Jrt/+, P>0.05 for early antral, *P<0.05 for late antral). (C) Quantification of cumulus-oocyte complexes (COCs) collected from the oviducts of wild-type and Gja1Jrt/+ mice after priming with eCG and hCG (**P<0.01). (D) Percentage of oocytes that cleaved to the 2-cell stage after in vitro fertilization (P>0.05).
Oocytes from Gja1Jrt/+ females are developmentally competent
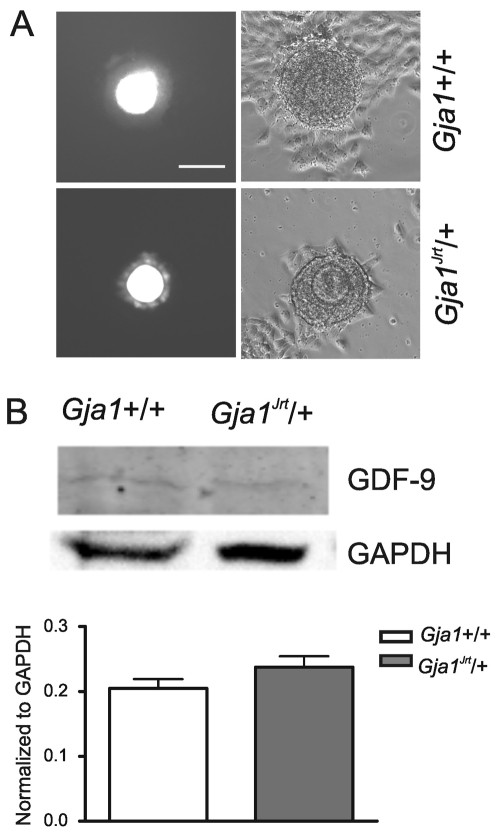
To test the meiotic competence of the oocytes, cumulus-oocyte complexes (COCs) were obtained from punctured antral follicles of females primed with eCG. After Hoechst nuclear staining, it was apparent that oocytes from the Gja1Jrt/+ females are equally as competent as wild-type oocytes in undergoing the first meiotic division to produce the first polar body (Table 1). Although fewer oocytes can be collected from the oviducts of Gja1Jrt/+ females that have been primed with eCG and hCG, after fertilization these oocytes were able to develop to the two-cell stage at a frequency similar to that observed with wild-type oocytes (Fig. 3D) (n=50 wild-type and n=20 mutant oocytes from three mice of each genotype). This indicates that female Gja1Jrt/+ mice produce competent oocytes.
Table 1.
In vitro maturation of oocytes isolated from wild-type and Gja1Jrt/+ females
| Gja1+/+ | Gja1Jrt/+ | |
|---|---|---|
| GV | 1 | 2 |
| MI | 3 | 5 |
| MII | 40 | 28 |
GV, germinal vesicle intact; MI, metaphase I; MII, metaphase II (3rst polar body).
Phosphorylation and trafficking of Cx43 are aberrant in Gja1Jrt/+ granulosa cells
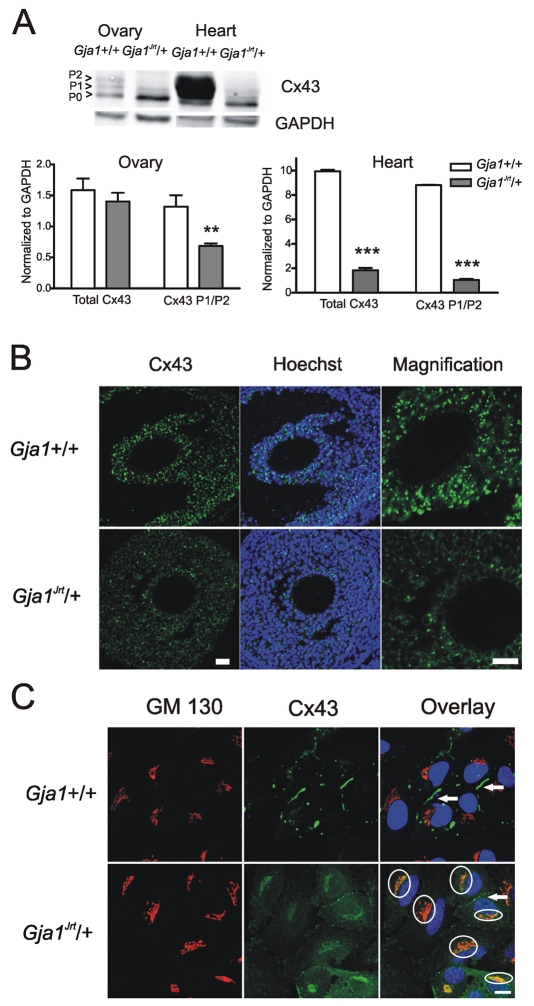
Western blot analysis demonstrated that the abundance of total Cx43 protein in the ovaries from Gja1Jrt/+ mice is not significantly different from that of wild-type mice (1.5±0.2 versus 1.1±0.1, P>0.05) (Fig. 4A). However, the phosphorylated P1 and P2 forms of Cx43 were significantly reduced in the mutant ovaries (1.3±0.2 for wild type versus 0.7±0.1 for Gja1Jrt/+, P<0.01, two-way ANOVA, n=9 blots from six mice for each group), suggesting that the mutation impairs phosphorylation of Cx43 in the ovaries. This result is slightly different from that of our previous study (Flenniken et al., 2005), which showed that the level of both total Cx43 and its phosphorylated forms are significantly downregulated in the mutant ovaries. This is probably because of differences in the strain backgrounds and ages of the mice used in the two studies. To confirm this further, the ovary and heart from the same mice were loaded on gels for western blotting. As shown in Fig. 4A, the abundance of Cx43 in the heart is significantly reduced in terms of both total Cx43 and its phosphorylated forms (P<0.001 for both, two-way ANOVA, n=3 blots from three mice for each group), which is consistent with previous studies (Flenniken et al., 2005; Manias et al., 2008). The consequence of aberrant phosphorylation on gap junction formation in the ovaries was evaluated by immunostaining. As shown in Fig. 4B, there was a marked reduction in the accumulation of Cx43 at the junctional membrane compared with wild-type controls, as visualized by Cx43 gap junction ‘plaques’ in the follicles. To further visualize the trafficking of Cx43 on a cellular level, we isolated granulosa cells from the ovaries of wild-type and Gja1Jrt/+ mice and examined the Cx43 expression profile. Consistent with tissue immunostaining, very few gap junction plaques were evident in the Gja1Jrt/+ granulosa cells, whereas a much larger population of Cx43 was localized to the Golgi apparatus, indicating that the Gja1Jrt mutation affected the normal trafficking of Cx43 to the cell membrane (Fig. 4C).
Fig. 4.
Aberrant phosphorylation and trafficking of Cx43 in Gja1Jrt/+ granulosa cells. (A) Western blot analysis demonstrated that the abundance of total Cx43 protein in the ovaries from Gja1Jrt/+ mice is not significantly different (P>0.05) from wild-type littermates. However, the phosphorylated P1 and P2 forms of Cx43 are significantly reduced in the mutant ovaries (**P<0.01). In contrast, the abundance of Cx43 in the hearts from Gja1Jrt/+ mice is significantly lower than in wild-type hearts in terms of both total Cx43 and its phosphorylated forms (***P<0.001). Cx43 abundance is expressed relative to GAPDH. (B) Representative micrographs showing ovaries from 6–8-week-old wild-type (top row) and Gja1Jrt/+ (bottom row) females immunolabeled for Cx43 (green). Nuclei were stained with Hoechst (blue). The magnified micrograph (far right column) shows that there are numerous gap junction ‘plaques’ in wild-type ovarian follicles, whereas very few similar structures are seen in Gja1Jrt/+ follicles. Bars, 20 μm. (C) Primary granulosa cells isolated from the ovaries of wild-type (top row) or Gja1Jrt/+ (bottom row) mice were co-immunolabeled for Cx43 (green) and the Golgi marker GM130 (red). Nuclei were stained with Hoechst (blue). The merged image shows co-localization (yellow within white circles) in Gja1Jrt/+ cells. White arrows indicate the gap junction plaques that have formed between neighboring cells in both cell populations. Bar, 20 μm.
Proliferation of granulosa cells is reduced in Gja1Jrt/+ ovaries
The absence of Cx43 has been associated with reduced granulosa cell proliferation (Gittens et al., 2005). Therefore, we evaluated the effect of the Gja1Jrt mutation on granulosa cell growth. As demonstrated by using a BrdU uptake assay, only 14.7±3.1% of granulosa cells isolated from Gja1Jrt/+ ovaries took up BrdU after a 4-hour incubation, compared with 26.5±3.3% of the wild-type granulosa cells (P<0.05, t-test, n=15 and n=13 fields from six mice in the wild-type and mutant groups, respectively) (Fig. 5A). To confirm this further, we quantified the expression of a cell proliferation marker, proliferating cell nuclear antigen (PCNA), and found that PCNA expression is significantly reduced in the mutant cells (0.7±0.1 versus 1.0±0.04 for wild-type cells, P<0.001, t-test, n=52 fields from six mice for each group) (Fig. 5B).
Fig. 5.
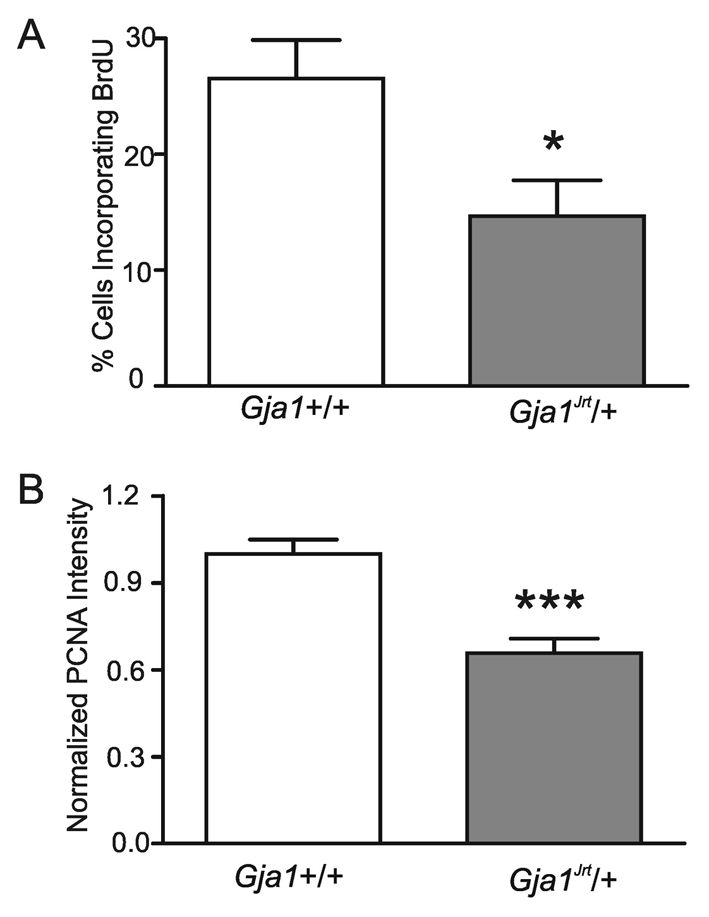
Reduced proliferation of granulosa cells. (A) Data are expressed as the percentage of granulosa cells that incorporated BrdU relative to the total number of cells in a field of view, counted after Hoechst staining (*P<0.05). (B) Primary cultured granulosa cells were immunolabeled for PCNA and the signal intensity was quantified and normalized to one of the wild-type granulosa cell readings from the same experiment (***P<0.001).
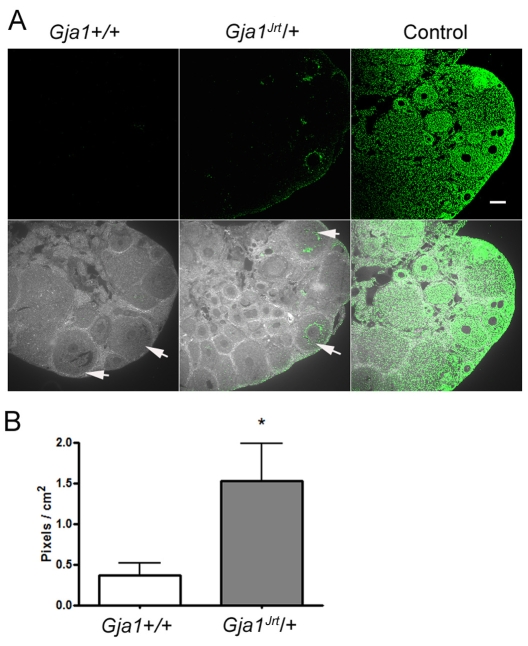
Apoptosis rate is elevated in Gja1Jrt/+ antral follicles
To determine whether the reduced proportion of mature follicles observed in the mutant ovaries was also the result of an elevated apoptosis rate in granulosa cells, we used the TUNEL assay to fluorescently label fragmented DNA from apoptotic cells. Gja1Jrt/+ and wild-type ovaries were examined in parallel. As shown in Fig. 6A, apoptotic granulosa cells were prominent in the early antral follicles of Gja1Jrt/+ ovaries but were infrequent in wild-type follicles. The fluorescent intensities of TUNEL staining in antral follicles are quantified in Fig. 6B (n=14 wild-type follicles from five mice versus n=17 Gja1Jrt/+ follicles from five mice, P<0.05, unpaired t-test). It appears that granulosa cell apoptosis is significantly elevated in the antral follicles of Gja1Jrt/+ ovaries.
Fig. 6.
Elevated apoptosis in Gja1Jrt/+ antral follicles indicated by the TUNEL assay. (A) Representative pictures showing apoptotic cells in the ovaries from 6–8-week-old wild-type (left column) and Gja1Jrt/+ (middle column) females. The right column shows the positive control pretreated with DNase. Fluorescent images are shown in the top row, whereas the corresponding phase contrast pictures are merged in the bottom row to show the tissue structure. White arrows indicate antral follicles. Bar, 100 μm. (B) The fluorescence intensities in the antral follicles were quantified relative to the follicle area and found to differ significantly between genotypes (*P<0.05).
Oocyte-granulosa cell coupling and GDF9 expression are not altered in Gja1Jrt/+ ovaries
Previous work had indicated that the gap junctions coupling the oocyte with the surrounding granulosa cells are composed of Cx37, with little or no contribution from Cx43 (Veitch et al., 2004) (reviewed by Kidder, 2005). To test whether the Gja1Jrt/+ mutation might also affect oocyte-granulosa cell coupling, a gap junction channel-permeant fluorescent dye, Lucifer Yellow, was injected into granulosa cell-enclosed oocytes. As shown in Fig. 7A, in both wild-type and Gja1Jrt/+ follicles, the injected dye passed from oocytes to the surrounding granulosa cells (n=10 for wild type versus n=13 for Gja1Jrt/+), indicating that oocyte-granulosa cell coupling is still intact in Gja1Jrt/+ ovaries. There was no obvious difference between genotypes in the rate of dye passage. However, less extensive dye spreading among the granulosa cells was observed in the Gja1Jrt/+ follicles, confirming that the Gja1Jrt/+ mutation preferentially impairs coupling among the granulosa cells.
Fig. 7.
Unchanged oocyte-granulosa cell coupling and GDF9 expression in Gja1Jrt/+ ovaries. (A) Microinjection of Lucifer Yellow into cumulus-enclosed oocytes demonstrated that the injected dye can pass readily from the oocyte to the surrounding cumulus cells in both wild-type and Gja1Jrt/+ follicles. Bar, 100 μm. (B) Western blot analysis demonstrated that the abundance of GDF9 in ovaries from Gja1Jrt/+ mice is not significantly different from wild-type littermates (P>0.05). GDF9 abundance is expressed relative to GAPDH.
Previous work with the Cx43-null mutant line (Gja1–/–) had indicated an interaction between Cx43-mediated gap junctional communication and intra-ovarian paracrine signaling, with the mutant granulosa cells having reduced responsiveness to the oocyte-derived mitogen, GDF9 (Gittens et al., 2005). GDF9 expression, however, was not reduced. Likewise, the expression of GDF9 in the Gja1Jrt/+ ovaries did not differ from that in wild-type littermates (0.20±0.01 for wild type, n=6 versus 0.23±0.02 for Gja1Jrt/+, n=6, P>0.05, unpaired t-test) (Fig. 7B). Thus, it is unlikely that the impaired oogenesis observed in Gja1Jrt/+ ovaries is the result of altered GDF9 expression.
DISCUSSION
The essential role of Cx43 during oogenesis has been demonstrated in previous studies using Cx43 knockout mice in which the absence of Cx43 caused severe germ cell deficiency and arrested folliculogenesis (Juneja et al., 1999; Ackert et al., 2001). Germ cells are known to express Cx43 and are well-coupled to surrounding cells during their development (Francis and Lo, 2006). Cx43 is also present at all stages of ovary development, where it contributes to coupling between the somatic cells. Therefore, it is believed that the intercellular communication mediated by Cx43 gap junctions is crucial for germ cell survival and ovarian folliculogenesis. However, the knockout mouse model does not mimic any human condition since complete loss of Cx43 is lethal (Reaume et al., 1995). The Gja1Jrt/+ mice, which have a phenotype resembling the human disease ODDD, provided us with a good model to study how a disease-linked Cx43 mutation can affect oogenesis.
Several in vitro studies have demonstrated that ODDD-linked Cx43 mutations exert dominant negative effects on wild-type Cx43, severely limiting Cx43-mediated intercellular communication (Laird, 2006). Our data indicate that the mutation causes aberrant Cx43 phosphorylation, as demonstrated by marked reduction in the phosphorylated forms P1 and P2 and an increased amount of the P0 form in the ovaries. This is consistent with the immunostaining results showing that most of the Cx43 is retained in the intracellular compartment, with very few gap junction plaques being visible in the junctional membranes. This finding indicates that the Gja1Jrt mutation impairs the normal trafficking and assembly of Cx43 gap junction channels in the granulosa cells, presumably through the oligomerization of mutant and wild-type connexins and the impaired assembly of the resulting complex. However, the detailed mechanism underlying this anomaly awaits further investigation. Hypophosphorylation and reduced junctional plaque formation are consistent with our previous work showing that granulosa cells isolated from Gja1Jrt/+ mice are very weakly coupled (10-20% versus wild type), or exhibit a complete lack of coupling (Flenniken et al., 2005), indicating a dominant negative effect of the Gja1Jrt/+ mutation. A similarly severe reduction of intercellular coupling has been observed in other cell types from the same Gja1Jrt/+ mice including osteoblasts, mammary epithelium and cardiomyocytes (McLachlan et al., 2008; Plante and Laird, 2008; Manias et al., 2008) (see Table 2).
Table 2.
Features of the three current ODDD mouse models
| G60S1 | G138R2 | I130T3 | ||
|---|---|---|---|---|
| ODDD syndrome | Craniofacial abnormalities | Yes | Yes | NR |
| Microophthalmia | Yes | Yes | NR | |
| Enamel hypoplasia | Yes | Yes | NR | |
| Syndactyly | Yes | Yes | Yes | |
| Cx43 expression(western blot) | Total | ↓ in heart, epidermis, mammary gland; NC in ovary*, calvaria | ↓ in heart | ↓ in heart |
| P0 band | NC in heart, mammary gland; ↑ in ovary*, calvaria | IC in heart | NC in heart | |
| P1/2 bands | ↓ in heart, mammary gland, ovary*, calvaria | ↓ in heart | ↓ in heart | |
| Cx43 subcellular localization | ↓ in membrane plaques, ↑ intracellularly (granulosa cells, cardiomyocytes, osteoblasts, keratinocytes, mammary myoepithelium) | ↓ in intercalated disks; partial redistribution to lateral cardiomyocyte membranes | ↓ in intercalated disks of cardiomyocytes | |
| GJ conductance | Transfected cell line Cultured primary cells or intact hearts | No electrical coupling (N2A) ↓ in granulosa cells, cardiomyocytes, osteoblasts | No electrical coupling (HeLa) ↓ in cardiomyocytes | Weak electrical coupling (N2A) ↓ in cardiomyocytes |
| Heart pathology | Gross morphology | Mostly normal; patent foramen ovale in some individuals | Normal | Normal |
| Function | Prolonged electrical wave in some individuals, bradycardia, AV block | Prolonged electrical wave, reduced R-wave amplitude, spontaneous ventricular arrhythmia | Reduced conduction velocity, reduced QRS amplitude, spontaneous or induced ventricular arrhythmia | |
| Reproductive capacity | Pregnancy rate (mutant female) | ↓* | NR | NR |
| Litter size (mutant female) | ↓* | ↓ | ↓ | |
| Ovulation rate | ↓* | NR | NR | |
| Mortality | Prenatal death | Yes | Yes | IC |
| Premature postnatal death | Yes | Yes | NR | |
Flenniken et al., 2005; Langlois et al., 2007; McLachlan et al., 2008; Plante and Laird, 2008; Manias et al., 2008;
this study.
NC, no change; IC, inconclusive from the available data; NR, not reported.
Given these findings, it is surprising that germ cell abundance was not affected in the Gja1Jrt/+ mice. It is known that Cx43 is expressed in germ cells and plays an essential role in maintaining their survival, since increased germ cell apoptosis was observed in Cx43-null genital ridges starting from embryonic day (E)11.5 (Francis and Lo, 2006). It is possible that the residual low level of coupling found in the mutant granulosa cells from Gja1Jrt/+ mice is also present in migrating primordial germ cells, and that it is sufficient for maintaining the survival of those cells. Another possibility is that the role of Cx43 in migrating germ cells does not involve GJIC, but is based on other functions of the connexin molecule (reviewed by Jiang and Gu, 2005). For example, it was demonstrated that Cx43 can interact with the growth factor NOV/CCN3 (nephroblastoma overexpressed gene/connective tissue growth factor) through its C-terminal (CT) domain and thereby regulate cell proliferation (Fu et al., 2004; Gellhaus et al., 2004). Since the mutated Cx43 still has an intact CT domain, the functions of the molecule that depend on binding to other cellular proteins via this segment may well remain intact. Indeed, it was recently reported that some ODDD-linked mutations, including G21R, G138R and G60S, still interact with caveolin, whereas fs260, a frameshift mutation in which the CT region is altered and truncated, did not co-immunoprecipitate with caveolin (Langlois et al., 2008). Therefore, it will be interesting to examine oogenesis in a mouse model carrying the ODDD fs260 mutation. The same explanation might also apply to the presence of follicles of all developmental stages in Gja1Jrt/+ ovaries, in contrast to the arrested folliculogenesis observed in Cx43 knockout females (Ackert et al., 2001).
In any case, Gja1Jrt/+ females are subfertile with significantly reduced mating success and smaller litter sizes compared with their wild-type siblings. Although the ovaries of the mutant mice start with normal numbers of germ cells, they contain significantly fewer pre-ovulatory follicles and have a reduced response to gonadotropins, which can at least partially explain the observed reduction of fertility. As with Cx43-null granulosa cells (Gittens et al., 2005), granulosa cells isolated from Gja1Jrt/+ females have a reduced proliferation rate. However, unlike Cx43-null granulosa cells, granulosa cells in Gja1Jrt/+ antral follicles demonstrated a significantly higher apoptosis frequency. This difference is possibly associated with the additional dominant negative effect of the mutant Cx43 protein on wild-type Cx43 function in granulosa cells. Another possibility is that the role of Cx43 in preventing granulosa cells from undergoing apoptosis is follicle-stage-dependent since few, if any, follicles develop to antral stages in Cx43-null ovaries (Ackert et al., 2001; Tong et al., 2006). This too must await further investigation. These factors might, collectively, contribute to the reduced number of mature follicles observed in the Gja1Jrt/+ ovaries. Previous work demonstrated that Cx43 is required for granulosa cells to fully respond to the oocyte-derived mitogen, GDF9 (Gittens et al., 2005), and another study showed that GDF9 protects granulosa cells from apoptosis and is required for the maintenance of FSH receptor expression (Orisaka et al., 2006). The present study confirmed that the expression of GDF9 is not altered in Gja1Jrt/+ ovaries and is the same as that observed in Gja1–/–ovaries (Gittens et al., 2005). Therefore, the Gja1Jrt mutation might affect the normal response of granulosa cells to endocrine and paracrine signals, thus restricting final maturation of the follicles.
Our finding that the Gja1Jrt mutation preferentially affects coupling among the granulosa cells, and leaves oocyte-cumulus cell coupling intact, is consistent with the accumulated evidence that Cx43 is preferentially assembled into the gap junctions that connect granulosa cells, whereas Cx37 forms the gap junctions connecting oocytes with granulosa cells (reviewed by Kidder, 2005). This view was supported recently by Gershon et al. (Gershon et al., 2008), who studied conditional knockout mice in which Cx43 was depleted from oocytes by using the oocyte-specific Zp3 promoter to drive Cre recombinase expression in growing oocytes that carried floxed Gja1 alleles (there was also some reduction of Cx43 expression in the granulosa cells). Gershon et al. reported that the Cx43-deficient oocytes retained gap junctional coupling with the granulosa cells, underwent meiotic maturation in response to LH and, after fertilization, developed normally up to the blastocyst stage. However, the females producing Cx43-deficient oocytes showed a decrease in litter size, an effect that was traced to a reduction in the ability of their blastocysts to implant in the uterus. Thus, it remains possible that the Cx43 deficiency in developing oocytes has an impact on oocyte/embryo developmental competence, which does not manifest itself until implantation. Further experiments will be needed to explore the possibility that implantation failure contributes to the reduced litter sizes of Gja1Jrt/+ females.
To date, two mutant mouse models have been generated that carry human GJA1 mutations: I130T and G138R (Kalcheva et al., 2007; Dobrowolski et al., 2008); it is of interest to compare the phenotype of these two mutants with that of the Gja1Jrt/+ phenotype. Overall, the G138R and I130T mutants demonstrated ODDD-like phenotypes that are similar to the Gja1Jrt/+ mice (summarized in Table 2). Studies of G138R and I130T mice have so far focused on the heart, where the same negative effects that were found in G60S mice have been reported (Flenniken et al., 2005; Manias et al., 2008), including effects on Cx43 expression level, phosphorylation state, subcellular localization and function in cardiomyocytes. However, the cardiac electrical waves described in the three mutant strains were not identical; the differences will need to be examined more carefully within a single laboratory to confirm that they represent different effects of the three mutant alleles. A reduction in litter size was reported for I130T and G138R females, but in both cases it was attributed to prenatal death; ovulation rate was not investigated (Kalcheva et al., 2007; Dobrowolski et al., 2008). It will be important to investigate whether defects in oogenesis, similar to those observed in G60S females, are also present in the I130T and G138R mutants.
Finally, we note that phenotypic descriptions of ODDD patients to date have not included the reproductive system, which has presumably not been investigated since neither men nor women with the ODDD syndrome have been reported to be infertile. The reduced fertility observed in Gja1Jrt/+ female mice raises a concern that some human ODDD females may have unrecognized reproductive deficiencies that are related to GJA1 mutations. Given this uncertainty and the prominent expression of Cx43 in human ovarian follicles (Wang et al., 2008), we believe that further clinical investigations of ODDD females are warranted.
METHODS
Mouse breeding and genotyping
All animal experiments were approved by the Animal Use Subcommittee of the University Council on Animal Care at the University of Western Ontario. The Gja1Jrt/+ mice were generated at the Centre for Modeling Human Disease, University of Toronto, and were kindly provided by Dr Janet Rossant. The original mice were on a mixed C57BL/6J and C3H/HeJ background (Flenniken et al., 2005) and were backcrossed to C57BL/6J for up to four generations. The genotypes were determined by polymerase chain reaction (PCR), as described previously (Flenniken et al., 2005).
Fertility testing
Mice were housed under controlled lighting (12 hours of light, 12 hours of dark) and temperature (21-24°C) conditions. To evaluate the fertility of mice with different genotypes, three mating groups were set up separately: wild-type male with wild-type female mice; Gja1Jrt/+ male with wild-type female mice; and wild-type male with Gja1Jrt/+ female mice. All of the mice were sexually mature by at least 6 weeks of age. One male and one female mouse were housed together for up to 7 days for mating. Offspring were counted on postnatal day 1, including both living and identifiable dead pups. The survival rate was calculated by comparing pup numbers on postnatal days 1 and 7.
Superovulation
To test the ability to ovulate in response to gonadotropins, 3–4-week-old female mice were injected intraperitoneally with 5 IU of eCG (Sigma-Aldrich, Canada) at around 18.00 h, followed 48 hours later with 5 IU of hCG (Sigma-Aldrich). The females were killed 14 to 15 hours later by cervical dislocation following CO2 anesthesia. The COCs were collected from the oviductal ampullae and counted.
In vitro oocyte maturation
Female mice (3-4 weeks old) were killed 24 hours after eCG (5 IU) injection. Ovaries were removed and placed in Waymouth’s MB 752/1 medium plus 5% fetal bovine serum (FBS) and 0.23 mM pyruvic acid (sodium salt; Sigma-Aldrich). Follicles were pierced with 25-gauge needles to liberate COCs. Oocytes enclosed by a complete layer of cumulus cells were washed through culture medium and transferred to a 35 mm Petri dish containing 3 ng/ml FSH (Puregon 100 i.u. follitropin β; Organon Canada, Scarborough, ON) in 3 mls of Waymouth’s medium/5% FBS. Oocytes were matured for 18 hours in a 5% CO2/5% O2/90% N2(5/5/90) atmosphere at 37°C and stained with Hoechst 33342 (Molecular Probes, Eugene, OR), diluted 1:1000 in Waymouth’s medium/5% FBS, to evaluate oocyte maturation.
In vitro fertilization
Female mice (3-4 weeks old) were superovulated as described above. The COCs were collected from the oviductal ampullae in pre-gassed human tubal fluid (HTF) medium (Jackson Laboratory) with 4 mg/ml bovine serum albumin (BSA), and transferred to 500 μl droplets of HTF/BSA under mineral oil (Sigma-Aldrich) containing approximately 2×105 sperm/ml. Sperm were collected from the cauda epididymi of mice at 3-5 months of age. Inseminated oocytes were incubated for 4 hours at 37°C in a 5/5/90 atmosphere. They were then washed through three dishes of HTF/BSA and cultured for 24 hours in 1 ml of HTF/BSA in four-well plates (Nunc) in a 5/5/90 atmosphere at 37°C to yield two-cell stage embryos.
Ovarian histology and follicle counts
Ovaries were collected from 6–8-week-old females to determine follicle distribution. Ovaries were fixed in Bouin’s solution, embedded in paraffin and sectioned serially at a thickness of 5 μm. For histological examination, sections were deparaffinized in xylene and rehydrated in graduated ethanol solutions followed by staining with hematoxylin and eosin. Follicles were classified as primary (a single layer of cuboidal granulosa cells), secondary (multiple layers of granulosa cells but lacking any sign of an antral cavity), early antral (with one or more small fluid-filled cavities) and late antral (having a single large cavity with the oocyte against one side of the follicle). Care was taken to ensure that only follicles sectioned through the nucleus of the oocyte were counted. For early stage (primary and secondary) follicle counts, 3-4 representative sections from each ovary were examined. For antral (early and late) follicle counts, ovaries were collected from mice selected at proestrus based on vaginal smears (Fox and Laird, 1970), and all sections from each ovary were examined. One ovary from each mouse was counted, with 3-5 ovaries from each genotype being examined.
Granulosa cell culture
Follicles were isolated and cultured as described previously (Tong et al., 2006). Briefly, the ovaries of 6–8-week-old female mice were placed in culture in Waymouth MB 752/1 medium containing 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen, Canada). The surrounding fat and connective tissue was removed by using fine 30-gauge needles. The ovaries were then digested in medium containing 2 mg/ml type I collagenase (Sigma-Aldrich) to facilitate follicle release. Follicles were liberated by repeated aspiration and expulsion with a 1 ml pipettor. Follicles were then washed with culture medium and transferred to another dish where oocytes and granulosa cells were separated by treatment with trypsin-EDTA (0.05% trypsin, 0.53 mM EDTA tetrasodium salt; Invitrogen) for 5 minutes followed by centrifugation at 780 g for 5 minutes. The supernatant (containing oocytes) was removed and the granulosa cells resuspended in culture medium. They were then transferred to 12 mm glass coverslips and cultured at 37°C, in 5% CO2/95% air, for less than 48 hours.
Immunofluorescent staining
Granulosa cells grown on glass coverslips were fixed with ice-cold 80% methanol/20% acetone for 20 minutes before blocking with 2% BSA (Sigma-Aldrich) in PBS for 1 hour. Cx43 was detected using a rabbit polyclonal antibody (1:500; Sigma-Aldrich). The Golgi apparatus was labeled with a mouse antibody directed against a resident protein, GM130 (1:100; BD Transduction Laboratories, Mississauga, ON). Appropriate Alexa 594- or Alexa 488-conjugated anti-mouse and anti-rabbit secondary antibodies (Molecular Probes) were used at a 1:200 dilution. Nuclei were labeled with Hoechst 33342 (1:1000; Molecular Probes). Slides were imaged on a Zeiss (Thornwood, NY) LSM 510 META confocal microscope.
For germ cell counting, ovaries were collected from postnatal day 1 female littermates and fixed, embedded and sectioned as described above. Ovary sections were deparaffinized and washed three times with PBS before immunolabeling. GCNA, a specific marker for germ cells, was detected with undiluted rat anti-GCNA antibody (a gift from Dr George Enders, University of Kansas Medical Center) and Texas Red-conjugated anti-rat secondary antibody (1:200; Molecular Probes). To determine germ cell numbers, GCNA-positive cells were counted on consecutive sections for each ovary. Care was taken to ensure that only cells sectioned through the largest diameter of the nucleus were counted. Ovary sections from 6–8-week-old females were also immunolabeled with Cx43 antibody as described above.
Proliferation assays
Granulosa cells were grown on glass coverslips at a confluency of 50–70%. For the bromodeoxyuridine (BrdU) uptake assay, BrdU (Sigma-Aldrich) was added into the culture medium to reach a final concentration of 10 μM. The cells were then incubated for 4 hours at 37°C in 5% CO2/95% air. They were washed three times with PBS before being fixed with ice-cold 80% methanol/20% acetone for 20 minutes. Following incubation with 2 M HCl for 20 minutes to denature DNA, granulosa cells were immunolabeled as described above. BrdU detection was carried out using a mouse anti-BrdU antibody (1:100; Sigma-Aldrich) and Texas Red-conjugated anti-mouse secondary antibody (1:200). Nuclei were labeled with Hoechst 33342 (1:1000). Cell counts were performed after Hoechst staining and expressed as the percentage of cells that were positive for BrdU staining relative to the total number of cells in view. The proliferation rate of granulosa cells was also evaluated by immunostaining with mouse anti-PCNA antibody (1:200; Sigma-Aldrich) and Texas Red-conjugated anti-mouse secondary antibody (1:200). The densities of the PCNA signals were quantified using Zeiss LSM software (Thornwood, NY). To minimize variation between experiments, each measurement was normalized to one of the wild-type granulosa cell readings from the same experiment.
Apoptosis assay
Ovary sections were stained for apoptotic cells by incorporating fluorescein-12-dUTP at the 3′-OH ends of fragmented DNA using the TUNEL (TdT-mediated dUTP nick-end labeling) assay kit (Promega, Madison, WI), according to the manufacturer’s instructions. Sections treated with DNase (1 mg/ml, 10 minutes) (Sigma-Aldrich) were used as positive controls. To quantify the TUNEL staining in antral follicles, the average pixel intensity for the area of antral follicles was determined using ImageJ 1.38x software (National Institutes of Health, USA).
Western blot analysis
Ovaries or hearts were homogenized in single-detergent lysis buffer supplemented with 1 mM NaF, 1 mM Na3VO4 and a protease inhibitor cocktail (1 tablet per 10 ml of buffer) (Sigma-Aldrich). Protein concentrations were determined by using the bicinchonic acid (BCA) assay (Pierce Biotechnology Inc., Rockford IL). 50 μg of protein was separated by electrophoresis on 12% SDS-PAGE gels and transferred to a nitrocellulose membrane. The membrane was incubated with either a rabbit anti-Cx43 antibody (1:1000; Sigma-Aldrich), a goat anti-GDF9 antibody (c-20) (1:400; Santa Cruz, CA) or a mouse anti-GAPDH antibody (1:10,000; Chemicon, Temecula, CA) overnight at 4°C. Following three washes with Tris-buffered saline containing Tween 20 (5 minutes each at room temperature), infra-red fluorescent-labeled secondary antibodies (IRDye 800 anti-rabbit, Rockland Immunochemicals, Gilbertsville, PA; Alexa 680-conjugated anti-mouse, Molecular Probes) were incubated at room temperature for 1 hour and immunoblots were processed and quantified using the Odyssey infrared-imaging system (Li-Cor).
Oocyte microinjection
Ovarian follicles were isolated as described above and transferred to 12 mm glass coverslips then cultured at 37°C in 5% CO2/95% air for 24 hours. The oocytes were impaled for 10 minutes with a 1 mm thin-wall glass capillary (World Precision Instruments, Sarasota, FL), tip diameter 1 μm, which was backfilled with 5% Lucifer Yellow (Molecular Probes) in ddH2O through capillary action. The dye was injected using an Eppendorf InjectMan NI 2 microinjection system and a Leica DMIRE2 inverted microscope with a 20×, 0.5 numerical aperture objective. Images were captured using a Hamamatsu C4742-95ER camera with Openlab software. Only those injections in which the dye filled the oocyte within 1 minute, as evidenced by bright fluorescence, were recorded.
Data analysis and statistics
The data are expressed as mean±s.e.m., with ‘n’ denoting the number of independent experiments. Comparisons of two groups were carried out using a two-tailed unpaired t-test, with a P value below 0.05 indicating significance. Comparisons of more than two groups were carried out with one-way ANOVA followed by a Tukey test. Two-way ANOVA and the Bonferroni test were used to compare two groups under different experimental conditions. Statistical significance is indicated in the graphs with a single asterisk (*) for P<0.05, double asterisk (**) for P<0.01 and triple asterisk (***) for P<0.001.
Acknowledgments
We thank Dr Janet Rossant and the Centre for Modeling Human Disease for generously providing the Gja1Jrt/+ mice. We are grateful to Kevin Barr for his expert advice, technical assistance and management of the mouse colony. We thank Crystal Lounsbury for her assistance with management of the mouse colony and Dr Hong-Xing Wang, Dr Elizabeth McLachlan and Janet Manias for their technical assistance. This work was funded by grants from the Canadian Institutes of Health Research (CIHR) to D.B., G.M.K. and D.W.L. D.T. was supported by a CIHR Canada Graduate Scholarship Doctoral Award.
Footnotes
COMPETING INTERESTS
The authors declare no competing financial interests.
REFERENCES
- Ackert CL, Gittens JE, O’Brien MJ, Eppig JJ, Kidder GM. (2001). Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev. Biol. 233, 258–270 [DOI] [PubMed] [Google Scholar]
- Dobrowolski R, Sasse P, Schrickel JW, Watkins M, Kim JS, Rackauskas M, Troatz C, Ghanem A, Tiemann K, Degen J, et al. (2008). The conditional connexin43G138R mouse mutant represents a new model of hereditary oculodentodigital dysplasia in humans. Hum. Mol. Genet. 17, 539–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flenniken AM, Osborne LR, Anderson N, Ciliberti N, Fleming C, Gittens JE, Gong XQ, Kelsey LB, Lounsbury C, Moreno L, et al. (2005). A Gja1 missense mutation in a mouse model of oculodentodigital dysplasia. Development 132, 4375–4386 [DOI] [PubMed] [Google Scholar]
- Fox R, Laird C. (1970). Sexual cycles. In Reproduction and Breeding Techniques For Laboratory Animals (ed. Hafez ESE.), pp. 107–122 Philadelphia, PA: Lea and Febiger [Google Scholar]
- Francis RJ, Lo CW. (2006). Primordial germ cell deficiency in the connexin 43 knockout mouse arises from apoptosis associated with abnormal p53 activation. Development 133, 3451–3460 [DOI] [PubMed] [Google Scholar]
- Fu CT, Bechberger JF, Ozog MA, Perbal B, Naus CC. (2004). CCN3 (NOV) interacts with connexin43 in C6 glioma cells: possible mechanism of connexin-mediated growth suppression. J. Biol. Chem. 279, 36943–36950 [DOI] [PubMed] [Google Scholar]
- Gellhaus A, Dong X, Propson S, Maass K, Klein-Hitpass L, Kibschull M, Traub O, Willecke K, Perbal B, Lye SJ, et al. (2004). Connexin43 interacts with NOV: a possible mechanism for negative regulation of cell growth in choriocarcinoma cells. J. Biol. Chem. 279, 36931–36942 [DOI] [PubMed] [Google Scholar]
- Gershon E, Plaks V, Aharon I, Galiani D, Reizel Y, Sela-Abramovich S, Granot I, Winterhager E, Dekel N. (2008). Oocyte-directed depletion of connexin43 using the Cre-LoxP system leads to subfertility in female mice. Dev. Biol. 313, 1–12 [DOI] [PubMed] [Google Scholar]
- Gittens JE, Mhawi AA, Lidington D, Ouellette Y, Kidder GM. (2003). Functional analysis of gap junctions in ovarian granulosa cells: distinct role for connexin43 in early stages of folliculogenesis. Am. J. Physiol. Cell. Physiol. 284, C880–C887 [DOI] [PubMed] [Google Scholar]
- Gittens JE, Barr KJ, Vanderhyden BC, Kidder GM. (2005). Interplay between paracrine signaling and gap junctional communication in ovarian follicles. J. Cell. Sci. 118, 113–122 [DOI] [PubMed] [Google Scholar]
- Granot I, Dekel N. (2002). The ovarian gap junction protein connexin43: regulation by gonadotropins. Trends Endocrinol. Metab. 13, 310–313 [DOI] [PubMed] [Google Scholar]
- Jiang JX, Gu S. (2005). Gap junction- and hemichannel-independent actions of connexins. Biochim. Biophys. Acta 1711, 208–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juneja SC, Barr KJ, Enders GC, Kidder GM. (1999). Defects in the germ line and gonads of mice lacking connexin43. Biol. Reprod. 60, 1263–1270 [DOI] [PubMed] [Google Scholar]
- Kalcheva N, Qu J, Sandeep N, Garcia L, Zhang J, Wang Z, Lampe PD, Suadicani SO, Spray DC, Fishman GI. (2007). Gap junction remodeling and cardiac arrhythmogenesis in a murine model of oculodentodigital dysplasia. Proc. Natl. Acad. Sci. USA 104, 20512–20516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidder GM. (2005). Roles of gap junctions in ovarian folliculogenesis: implications for female infertility. In Gap Junctions in Development and Disease (ed. Winterhager E.), pp. 223–237 Berlin: Springer-Verlag [Google Scholar]
- Laird DW. (2006). Life cycle of connexins in health and disease. Biochem. J. 394, 527–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois S, Maher AC, Manias JL, Shao Q, Kidder GM, Laird DW. (2007). Connexin levels regulate keratinocyte differentiation in the epidermis. J. Biol. Chem. 282, 30171–30180 [DOI] [PubMed] [Google Scholar]
- Langlois S, Cowan KN, Shao Q, Cowan BJ, Laird DW. (2008). Caveolin-1 and -2 interact with connexin43 and regulate gap junctional intercellular communication in keratinocytes. Mol. Biol. Cell 19, 912–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loddenkemper T, Grote K, Evers S, Oelerich M, Stogbauer F. (2002). Neurological manifestations of the oculodentodigital dysplasia syndrome. J. Neurol. 249, 584–595 [DOI] [PubMed] [Google Scholar]
- Manias JL, Plante I, Gong XQ, Shao Q, Churko J, Bai D, Laird DW. (2008). Fate of connexin43 in cardiac tissue harbouring a disease-linked connexin43 mutant. Cardiovasc. Res. 80, 385–395 [DOI] [PubMed] [Google Scholar]
- McLachlan E, Manias JL, Gong XQ, Lounsbury CS, Shao Q, Bernier SM, Bai D, Laird DW. (2005). Functional characterization of oculodentodigital dysplasia-associated Cx43 mutants. Cell. Commun. Adhes. 12, 279–292 [DOI] [PubMed] [Google Scholar]
- McLachlan E, Plante I, Shao Q, Tong D, Kidder GM, Bernier SM, Laird DW. (2008). ODDD-linked Cx43 mutants reduce endogenous Cx43 expression and function in osteoblasts and inhibit late stage differentiation. J. Bone Miner. Res. 23, 928–938 [DOI] [PubMed] [Google Scholar]
- Orisaka M, Orisaka S, Jiang JY, Craig J, Wang Y, Kotsuji F, Tsang BK. (2006). Growth differentiation factor 9 is antiapoptotic during follicular development from preantral to early antral stage. Mol. Endocrinol. 20, 2456–2468 [DOI] [PubMed] [Google Scholar]
- Paznekas WA, Boyadjiev SA, Shapiro RE, Daniels O, Wollnik B, Keegan CE, Innis JW, Dinulos MB, Christian C, Hannibal MC, et al. (2003). Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am. J. Hum. Genet. 72, 408–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Armendariz EM, Saez JC, Bravo-Moreno JF, Lopez-Olmos V, Enders GC, Villalpando I. (2003). Connexin43 is expressed in mouse fetal ovary. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 271, 360–367 [DOI] [PubMed] [Google Scholar]
- Plante I, Laird DW. (2008). Decreased levels of connexin43 result in impaired development of the mammary gland in a mouse model of oculodentodigital dysplasia. Dev. Biol. 318, 312–322 [DOI] [PubMed] [Google Scholar]
- Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. (1995). Cardiac malformation in neonatal mice lacking connexin43. Science 267, 1831–1834 [DOI] [PubMed] [Google Scholar]
- Roscoe W, Veitch GI, Gong XQ, Pellegrino E, Bai D, McLachlan E, Shao Q, Kidder GM, Laird DW. (2005). Oculodentodigital dysplasia-causing connexin43 mutants are non-functional and exhibit dominant effects on wild-type connexin43. J. Biol. Chem. 280, 11458–11466 [DOI] [PubMed] [Google Scholar]
- Shibayama J, Paznekas W, Seki A, Taffet S, Jabs EW, Delmar M, Musa H. (2005). Functional characterization of connexin43 mutations found in patients with oculodentodigital dysplasia. Circ. Res. 96, e83–e91 [DOI] [PubMed] [Google Scholar]
- Tong D, Gittens JE, Kidder GM, Bai D. (2006). Patch-clamp study reveals that the importance of connexin43-mediated gap junctional communication for ovarian folliculogenesis is strain specific in the mouse. Am. J. Physiol. Cell. Physiol. 290, C290–C297 [DOI] [PubMed] [Google Scholar]
- Vasconcellos JP, Melo MB, Schimiti RB, Bressanim NC, Costa FF, Costa VP. (2005). A novel mutation in the GJA1 gene in a family with oculodentodigital dysplasia. Arch. Ophthalmol. 123, 1422–1426 [DOI] [PubMed] [Google Scholar]
- Veitch GI, Gittens JE, Shao Q, Laird DW, Kidder GM. (2004). Selective assembly of connexin37 into heterocellular gap junctions at the oocyte/granulosa cell interface. J. Cell. Sci. 117, 2699–2707 [DOI] [PubMed] [Google Scholar]
- Wang HX, Tong D, El-Gehani F, Tekpetey FR, Kidder GM. (2008). Connexin expression and gap junctional coupling in human cumulus cells: Contribution to embryo quality. J. Cell. Mol. Med. May 24 [Epub ahead of print] [doi:10.1111/j.1582–4934.2008.00373.x]. [DOI] [PMC free article] [PubMed] [Google Scholar]