Abstract
Duchenne muscular dystrophy (DMD) is a lethal disease characterized by rapid, progressive atrophy of muscle tissues. Timely screening of therapeutic interventions is necessary for the development of effective treatment approaches for DMD. We have developed an in vitro model using a combination of micropatterning of C2C12 skeletal muscle cells and cell traction force microscopy (CTFM). In this model, C2C12 cells were micropatterned on a highly elongated adhesive island such that the cells assumed a shape typical of a myotube. During differentiation, these cells gradually fused together and began expressing dystrophin, a structural protein of myotubes; meanwhile, their contractile forces, represented by cell traction forces, continually increased until the myotubes reached maturation. In addition, the high-degree alignment of cells favored myotube differentiation and dystrophin expression. Since the fundamental structural unit of muscle tissue is myofiber, which is responsible for muscle contraction, such a technology that can directly quantify the contractile forces of the myotube, a precursor of myofiber, may constitute a fast and efficient screening approach for DMD therapies.
Keywords: Micropatterning, C2C12 cells, Myotube, Dystrophin, Cell traction force
1. Introduction
Muscular dystrophy refers to a group of genetic, hereditary muscle diseases that cause progressive skeletal muscle weakness and affect a large portion of population. The most commonly lethal form of muscular dystrophy, Duchenne muscular dystrophy (DMD), affects about 1 in 3500 male births in the United States every year (Sussman 2002). Patients are generally wheelchair-bound by 6-10 years of age and die in their early twenties. It is well known that DMD is caused by mutations in the Xp21 gene, which encodes dystrophin, a critical component of a membrane-spanning protein complex which structurally connects cortical actin to extracellular matrix (ECM) and transmits the forces generated in muscle sarcomeres to extracellular connective tissues (Monaco 1989; Sweeney and Barton 2000). Absence of dystrophin causes breakdown of muscle fibers in DMD patients, leading to the loss of muscle contractility (Horowits et al. 1990). Therefore, repairing the dystrophin gene and recovering dystrophin protein expression in muscle cells appear to be the keys to treatment for DMD (Wells 2006). Current therapeutic approaches to DMD treatment include cell implantation (Sukhikh et al. 2001), cytokine treatments (Kapsa et al. 2003), gene therapies (van Deutekom and van Ommen 2003; Wang et al. 2000), and pharmacological or nutritional interventions (Radley et al. 2007). However, development of effective DMD therapies has been hindered by lack of a suitable in vitro model that permits direct functional evaluation of the effect of treatments in a timely fashion.
At present, the approaches for evaluating the efficacy of treatment are largely limited to animal models (Bogdanovich et al. 2004; Cooper 1989) or routine cell cultures. The problems with these approaches are that they are highly experimentally demanding, expensive, and not very quantitative. Since contractility is the ultimate function of muscles, and the fundamental structural unit of muscle tissue is the myofiber, a technique that can directly quantify the contractile forces of myofibers might afford an efficient yet concise method for fast screening of gene therapies for DMD. While a variety of techniques have been developed to effectively determine the contractility of individual myocyte or myoblast cells (Balaban et al. 2001; Palmer et al. 1996; Yin et al. 2005) and skinned fibers from muscle tissues (Bottinelli et al. 1996; Horowits et al. 1990; Ochala et al. 2007), there are few methods specifically targeted for quantifying contractility of individual myofibers or myotubes, which are precursors of myofibers. Existing techniques include direct contractile force measurement using microtools (McMahon et al. 1994; Wakayama and Yamada 2000) and prestress measurement through a cell peeling method (Griffin et al. 2004).
In this study, we aimed to develop an in vitro model which could control myotube differentiation of C2C12 mouse muscle cells, a skeletal muscle cell line, and meanwhile allow functional assessment of contractile forces of cells through cell traction force microscopy (CTFM), a fluorescence microscopy-based technology that can quantify traction forces of individual cells (Chen et al. 2007). We used the C2C12 cell line because it is well studied and widely considered to be an effective model for in vitro muscle studies. In differentiation medium, these cells fuse together and form myotubes. Because myotubes will mature into myofibrils, the smallest contractile systems directly responsible for muscle contraction, determination of myotube contractile forces may represent an efficient method for in vitro evaluation of DMD treatments.
2. Materials and methods
2.1. Fabrication of poly(dimethylsiloxane) (PDMS) stamps
PDMS stamps were fabricated by replica molding technique using a silicon wafer as a mold to cast PDMS. The wafer was fabricated using a standard photolithography process and contained an array of 10 μm × 200 μm micro-islands spaced 50 μm apart. A 10:1 (w/w) mixture of Sylgard 184 PDMS prepolymer (Dow Corning) was spin-coated onto the wafer at 3000 rpm for 45 sec, cured at 65 °C for 24 hrs, and then peeled off from the wafer.
2.2. Fabrication of polyacrylamide gel (PAG)
PAG embedded with 0.5 μm red fluorescent micro-beads (Molecular Probes) was fabricated as previously described (Wang et al. 2002). The gel disk was 120 μm in thickness, 10 mm in diameter, and attached to a glass-bottomed 35 mm Petri dish (MatTek). The PAGs contained 5% acrylamide (BioRad) and 0.6% N, N′-methylenebisacrylamide (bis-acrylamide, BioRad) and had a Young's modulus of 15 kPa and a Poisson's ratio of 0.48 (Wang and Pelham 1998).
2.3. Micropatterning collagen on PAG
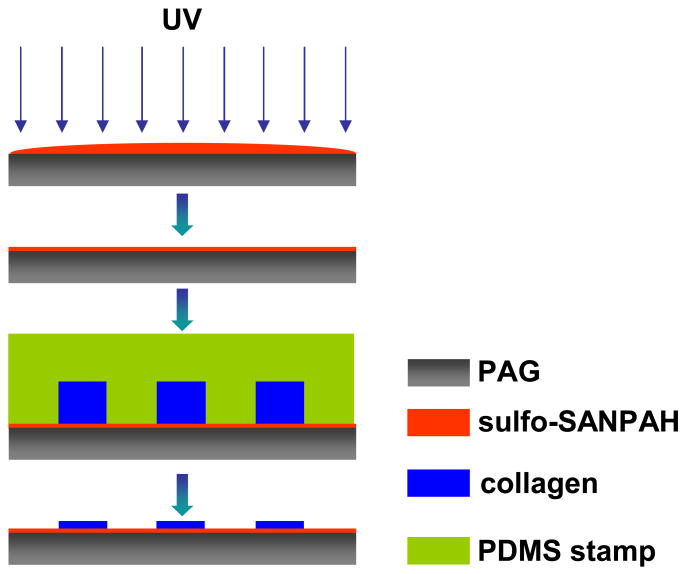
Micropatterns of collagen were fabricated on PAG surface through the following process (Fig. 1). A solution of sulfosuccinimidyl-6-(4′-azido-2′-nitrophenylamino)hexanoate (sulfo-SANPAH, Pierce Biotechnology) was prepared as follows: sulfo-SANPAH was first dissolved in dimethyl sulfoxide (DMSO) at 0.025 mg/μl and then diluted to 1 mg/ml with 1M HEPES buffer. The freshly prepared sulfo-SANPAH solution was placed onto PAG, followed by exposure to UV light for 10 min. Then the PAG was briefly washed with PBS twice. Meanwhile, type I collagen solution (100 μg/ml, Angiotech BioMaterials) was pipetted onto an oxidized PDMS stamp and allowed to fully spread and wet the stamp. The collagen solution was then scraped off the stamp using a cell scraper, and the stamp was placed in conformal contact with PAG and incubated at 4 °C overnight. After that, the stamp was removed, and the PAG was washed three times with PBS before cell culture.
Fig. 1.
Schematic of the process for micropatterning collagen on polyacrylamide gel (PAG). Sulfo-SANPAH solution was put on PAG and upon UV activation, a layer of sulfo-SANPAH was immobilized on the PAG surface. An oxidized PDMS stamp inked with collagen solution was then placed on PAG. After incubation overnight, collagen micro-islands were formed on the PAG surface through conjugation with sulfo-SANPAH.
2.4. Cell culture
C2C12 cells (#CRL-1772, ATCC) were maintained in growth medium consisting of Dulbecco's modified Eagle's medium (DMEM), 10% fetal bovine serum (FBS), and 1% penicillin-streptomycin (P/S) at 37 °C in a 5% CO2 atmosphere. For cell culture on PAG, 200 μl cell suspension (3 × 105 cells/ml) was pipetted onto the PAG region in each dish. After 1 hr, non-attached cells were aspirated, and fresh growth medium was supplied to the culture dish. One day later, growth medium was replaced with differentiation medium, which contained DMEM, 2% horse serum, and 1% P/S. After 1 to 5 days, cells were analyzed using cell traction force microscopy (CTFM) or fixed for immunofluorescence assays.
2.5. Immunofluorescence for dystrophin expression
Cells were fixed for 10 min using 4% paraformaldehyde and washed 2 × 5 min with PBS. They were then blocked with 10% horse serum in PBS solution for 1 hr and subsequently incubated with rabbit anti-dystrophin antibody (#ab15277, Abcam, 1:200 in 5% horse serum solution) for 1 hr. After that, the cells were washed with PBS 3 × 5 min before incubating with FITC-conjugated rabbit anti-mouse antibody (Jackson ImmunoResearch Laboratories, 1:300 in PBS) for 40 min. Once cells were washed with PBS another 3 × 5 min, they were incubated with DAPI (Chemicon, 1:1000 in PBS) for 10 min and then washed with PBS 2 × 2 min. Finally, cell images were acquired on an Eclipse TE2000-U fluorescence microscope (Nikon).
2.6. Cell traction force microscopy
Phase contrast images of individual cells/myotubes as well as fluorescence images of the underlying embedded fluorescent beads in PAG were taken with a fluorescence microscope. After cells on the PAG were trypsinized and detached from the substrate, fluorescence images of the fluorescent beads in the same view were taken again. Cell traction forces were then computed based on these pairs of “force-loaded” and “null-force” fluorescence images using a MATLAB program based on a published method (Butler et al. 2002).
3. Results
3.1. Micropatterned C2C12 cells underwent enhanced myotube differentiation
Using micropatterning technique, an array of highly elongated, rectangular micro-islands (10 μm × 200 μm) of collagen was fabricated on PAG dishes. Upon seeding, C2C12 cells adhered exclusively to the cell-adhesive micro-islands within 1 hr of seeding. These cells then adapted to a rectangular shape during growth and differentiation thereafter (Fig. 2A). Although the number of cells varied on each island at the time of seeding, cells normally spread and grew until they completely occupied the micropatterned islands within 1 day (Fig. 2B), at which time the culture medium was replaced with differentiation medium to induce myotube differentiation of C2C12 cells. After 4 days of culture (3 days in differentiation medium), the majority of cells fused together and started forming distinct myotubes (Fig. 2C).
Fig. 2.
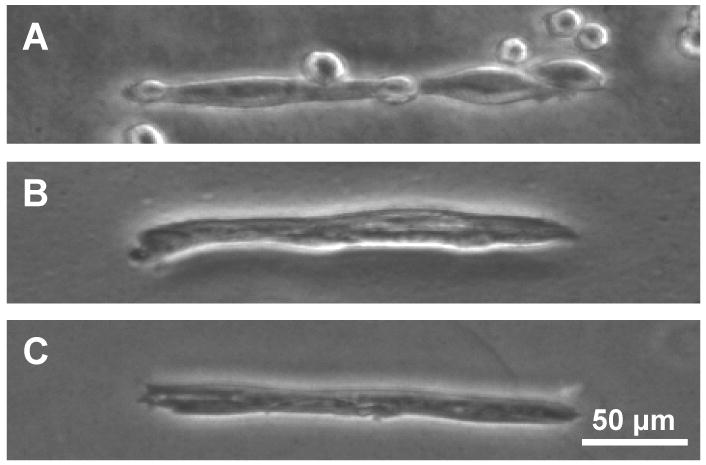
Micropatterned C2C12 cells at various culture times on PAG. (A) 1 hr. (B) 1 day. (C) 4 days.
Formation of myotubes was further confirmed by immunofluorescence for expression of dystrophin protein, a structural protein of myotubes. While individual cells and cells in intermediate myotube fusion stages presented little or no dystrophin, more mature myotubes on highly elongated rectangular islands did express dystrophin (Fig. 3). To examine if the micropatterned cell morphology had an effect on dystrophin expression, we also fabricated cells on circular islands which had identical area as rectangular ones. Not surprisingly, cells on these circular islands, while of similar spreading areas as on elongated rectangular islands, had much lower level of dystrophin expression (data not shown). Similarly, non-patterned cells did not show apparent dystrophin expression when their density was low, and no myotubes were formed. However, at high cell density (close to confluence), some adjacent non-patterned cells fused into myotubes and exhibited strong dystrophin expression.
Fig. 3.
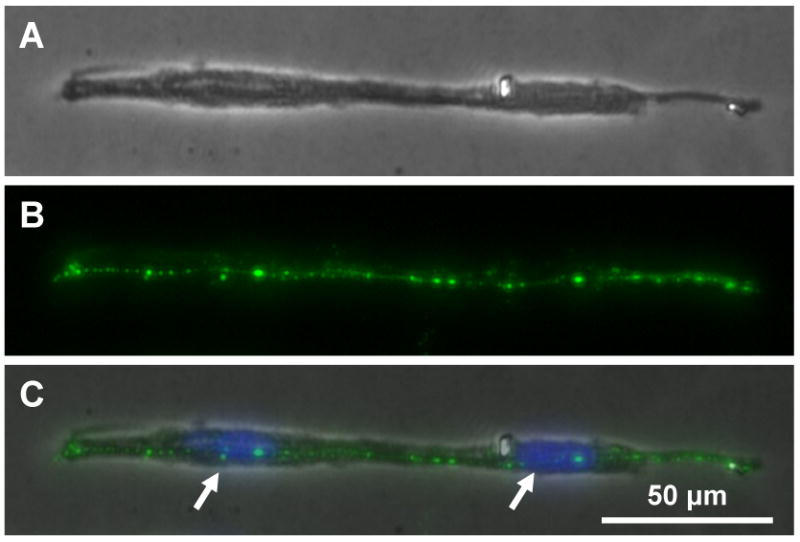
Immunofluorescence microscopy of dystrophin protein expression in a myotube at 5 days of culture. (A) Image of a myotube. (B) Immunofluorescence for dystrophin expression. (C) Merged image of (A) and (B). Arrows in (C) point to nuclei of fused cells.
3.2. Traction force measurement of micropatterned C2C12 cells
As anchorage-dependent cells, myoblasts/myotubes must balance their contraction against adequate adhesion to the substrate. Measuring the forces between these cells and the cell-adhering substrate indirectly measures the contractility of myoblasts/myotubes. Cell traction force microscopy (CTFM) was used in this study to determine the traction forces of myoblasts/myotubes, which indicated the contractile forces of these cells. In brief, CTFM measured cell contraction-caused displacement of fluorescent micro-beads embedded in PAG and then determined cell traction forces by computation. Fig. 4 shows an example of a myotube and the displacement field and traction forces generated by the myotube.
Fig. 4.
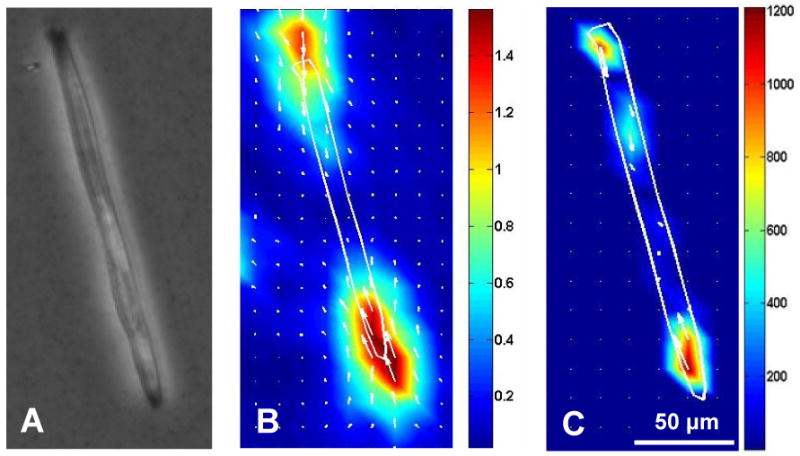
Traction forces of a myotube determined using cell traction force microscopy (CTFM). (A-C) Phase contrast image, displacement field, and traction forces of a myotube on PAG, respectively, at 5 days of culture. The displacement field and traction force map are color coded, with red representing the highest displacement/force and blue representing the lowest.
3.3. Traction forces of micropatterned C2C12 cells increased during differentiation
The C2C12 cells generated different levels of traction forces at progressive stages of differentiation. Using time lapse microscopy (TLM) along with CTFM, the traction forces of micropatterned C2C12 cells at different time points were determined. As an example, we have shown here the traction force development of C2C12 cells on a single island during cell division, spreading, and fusion (Fig. 5). As can be seen from the phase contrast microscopy images and traction force fields of micropatterned cells (Fig. 5A), the overall traction forces of cells continually increased during the course of cell division (from 2 cells to 3 cells) and spreading from 0 to 8 hrs (Fig. 5B). Moreover, after the cells fully spread, the traction forces of these cells still continued to increase. This may indicate ongoing cell fusion and myotube differentiation, processes during which cell contractile force increases. In addition, at later stages of myotube differentiation, cell traction forces propagated more evenly throughout the entire myotube instead of being mainly concentrated at the two ends.
Fig. 5.
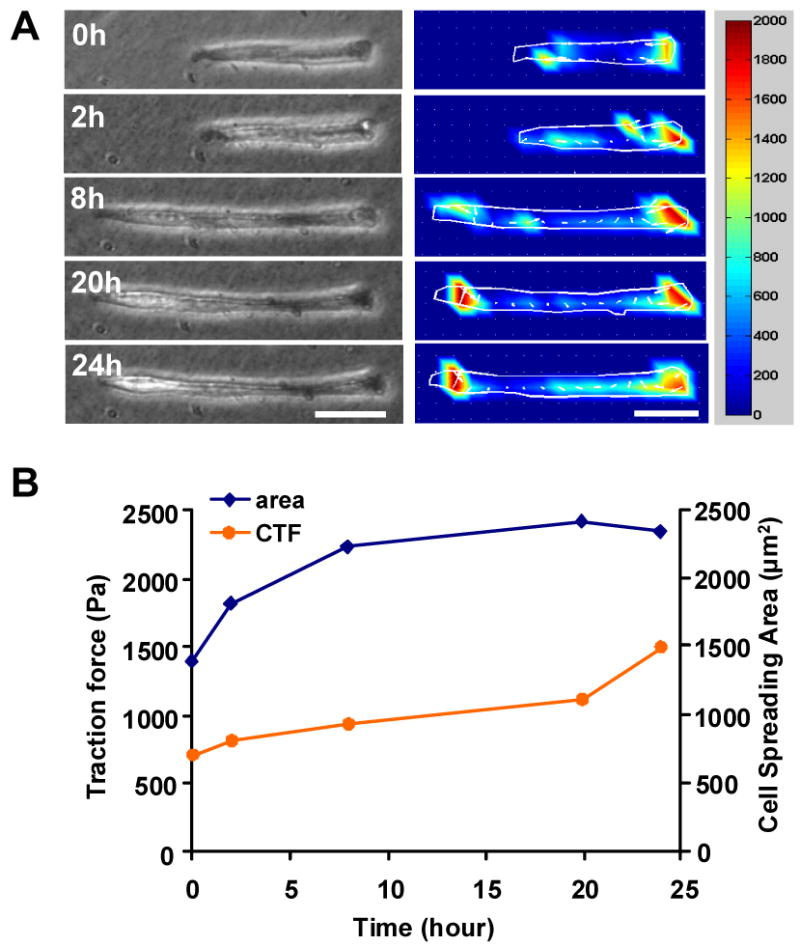
Traction force development of micropatterned C2C12 cells during differentiation. (A) Phase contrast images (left panel) and corresponding traction force maps (middle panel), respectively. The color bar represents traction force level (right panel). Prior to plating on PAG, cells were pre-differentiated for 2 days in differentiation medium containing 2% horse serum. For real time monitoring of cell morphology changes and traction force development, the culture dishes were placed on a fluorescence microscope equipped with an on-stage incubator. Image acquisition started after 1 day of culture on PAG in differentiation medium and lasted for 24 hrs. Note the phase contrast images were slightly out of focus due to the requirement of in situ fluorescence image acquisition. Scale bars, 50 μm. (B) Changes in cell spreading area and cell traction force during C2C12 differentiation between 1 and 2 days of culture.
4. Discussion
In this study, we used a combination of micropatterning and cell traction force microscopy (CTFM) technologies to establish an in vitro model which can potentially be used as a rapid and cost-effective approach for screening gene therapies for DMD. The C2C12 cell line is capable of stable transfection of cDNAs that encode mutant skeletal muscle proteins (McMahon et al. 1994), which provides a valuable model for investigating DMD-targeted gene therapies. Specifically, we looked at the expression of dystrophin protein because it structurally connects the cytoplasmic F-actin of muscle cells to their ECM through a membrane glycoprotein complex and therefore is responsible for preserving muscle cell membrane integrity and muscle cell contractility (Fabbrizio et al. 1995; Horowits et al. 1990). The capability of differentiated C2C12 cells or myotubes to contract and generate measurable forces permits functional assessment of the effects of gene therapies and pharmacological treatments, as well as the effects of native and mutated contractile proteins on the contractile function of the myotube.
We first demonstrated that C2C12 cells can be micropatterned into specific geometry with consistent alignment on collagen-coated PAGs, which facilitate differentiation of these cells into myotubes (Engler et al. 2004). The micropatterning technique may provide a novel way to control the size and morphology of C2C12 cells/myotubes, which not only is beneficial in terms of providing a consistent platform for studies, but more importantly, favors differentiation of C2C12 cells into myotubes by forcing the cells to align in a highly ordered manner (Coletti et al. 2007; Engler et al. 2004; Huang et al. 2006). For instance, the expression of dystrophin was seen in micropatterned C2C12 cells on rectangular islands as early as 3 days after being subjected to differentiation medium, while non-patterned C2C12 cells of similar cell density did not show dystrophin signal. On the other hand, myotubes that had strong dystrophin expression and were composed of greater numbers of cells were formed by C2C12 cells plated at high density. These results suggest that sufficient cell-cell contact favors cell fusion and therefore myotube formation. However, in the case of cells growing in circular islands of similar area as rectangular islands, there was little dystrophin expression in the cells, even though the cells were tightly contacting each other and occupied the same areas as those of cells on rectangular islands. Therefore, cell-cell contact is necessary for proper cell fusion and myotube differentiation but is not sufficient for myotube formation. The alignment of cells in a manner resembling the tube-like shape of native myotubes, on the other hand, may be crucial for the increased dystrophin expression in cells on rectangular islands.
Using CTFM, we also showed that the contractile forces of individual cells/myotubes can be assessed during the course of C2C12 cell differentiation and myotube formation. We found that there was a marked difference in the traction forces of cells at progressive differentiation stages. In general, traction force monotonically increased during the course of C2C12 differentiation. Compared with other existing methods for determining contractile force of single myofibers (Griffin et al. 2004; McMahon et al. 1994; Wakayama and Yamada 2000), the CTFM approach differs in that the contraction of a single myotube is determined by measuring its traction forces on the underlying substrate. A significant advantage of this method is that it permits real time monitoring the development of contraction at various differentiation stages, including cell fusion and myotube maturation. It is worth noting that, however, CTFM measures traction forces on the underlying substrate, not cell contraction per se, although they are closely coupled. Future studies should determine their quantitative relationship.
While different in their technical approaches, all DMD therapies share the ultimate goal of regaining the contractility of muscles. Since myotubes mature into myofibers, the smallest contractile systems directly responsible for muscle contraction, determination of the contractile forces of myotubes may constitute a fast functional in vitro evaluation of treatments for DMD. Therefore, the present study demonstrated that a micropatterned C2C12 cells-based in vitro model together with CTFM technique makes it possible to perform fast yet effective functional assessment of DMD therapies without the difficulties of using an animal model. This in vitro model can be potentially used, for example, to screen the effects of gene insertion or deletion on the function, or generation of contraction, of single myotubes. In addition, simultaneous determination of expression of dystrophin protein along with force monitoring during C2C12 cell differentiation may possibly correlate dystrophin expression with force development during the course of myotube development. This may lead to a better understanding of the role of dystrophin protein in contractility of muscle cells on a quantitative basis.
Acknowledgments
This work was supported by NIH grant AR 049921 (JHW).
Footnotes
Conflict of interest: All authors declare that no financial and personal relationships with other people or organizations could have inappropriately influenced this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nature Cell Biology. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- Bogdanovich S, Perkins KJ, Krag TO, Khurana TS. Therapeutics for Duchenne muscular dystrophy: current approaches and future directions. Journal of Molecular Medicine. 2004;82:102–115. doi: 10.1007/s00109-003-0484-1. [DOI] [PubMed] [Google Scholar]
- Bottinelli R, Canepari M, Pellegrino MA, Reggiani C. Force-velocity properties of human skeletal muscle fibres: myosin heavy chain isoform and temperature dependence. Journal of Physiology. 1996;495(Pt 2):573–586. doi: 10.1113/jphysiol.1996.sp021617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JP, Tolic-Norrelykke IM, Fabry B, Fredberg JJ. Traction fields, moments, and strain energy that cells exert on their surroundings. American Journal of Physiology - Cell Physiology. 2002;282:C595–605. doi: 10.1152/ajpcell.00270.2001. [DOI] [PubMed] [Google Scholar]
- Chen J, Li H, SundarRaj N, Wang JH. Alpha-smooth muscle actin expression enhances cell traction force. Cell Motility and the Cytoskeleton. 2007;64:248–257. doi: 10.1002/cm.20178. [DOI] [PubMed] [Google Scholar]
- Coletti D, Teodori L, Albertini MC, Rocchi M, Pristera A, Fini M, Molinaro M, Adamo S. Static magnetic fields enhance skeletal muscle differentiation in vitro by improving myoblast alignment. Cytometry A. 2007;71:846–856. doi: 10.1002/cyto.a.20447. [DOI] [PubMed] [Google Scholar]
- Cooper BJ. Animal models of Duchenne and Becker muscular dystrophy. British Medical Bulletin. 1989;45:703–718. doi: 10.1093/oxfordjournals.bmb.a072353. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. Journal of Cell Biology. 2004;166:877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbrizio E, Bonet-Kerrache A, Limas F, Hugon G, Mornet D. Dystrophin, the protein that promotes membrane resistance. Biochemical and Biophysical Research Communications. 1995;213:295–301. doi: 10.1006/bbrc.1995.2129. [DOI] [PubMed] [Google Scholar]
- Griffin MA, Engler AJ, Barber TA, Healy KE, Sweeney HL, Discher DE. Patterning, prestress, and peeling dynamics of myocytes. Biophysics Journal. 2004;86:1209–1222. doi: 10.1016/S0006-3495(04)74195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowits R, Dalakas MC, Podolsky RJ. Single skinned muscle fibers in Duchenne muscular dystrophy generate normal force. Annals of Neurology. 1990;27:636–641. doi: 10.1002/ana.410270609. [DOI] [PubMed] [Google Scholar]
- Huang NF, Patel S, Thakar RG, Wu J, Hsiao BS, Chu B, Lee RJ, Li S. Myotube assembly on nanofibrous and micropatterned polymers. Nano Letters. 2006;6:537–542. doi: 10.1021/nl060060o. [DOI] [PubMed] [Google Scholar]
- Kapsa R, Kornberg AJ, Byrne E. Novel therapies for Duchenne muscular dystrophy. Lancet Neurology. 2003;2:299–310. doi: 10.1016/s1474-4422(03)00382-x. [DOI] [PubMed] [Google Scholar]
- McMahon DK, Anderson PA, Nassar R, Bunting JB, Saba Z, Oakeley AE, Malouf NN. C2C12 cells: biophysical, biochemical, and immunocytochemical properties. American Journal of Physiology. 1994;266:C1795–1802. doi: 10.1152/ajpcell.1994.266.6.C1795. [DOI] [PubMed] [Google Scholar]
- Monaco AP. Dystrophin, the protein product of the Duchenne/Becker muscular dystrophy gene. Trends in Biochemical Sciences. 1989;14:412–415. doi: 10.1016/0968-0004(89)90290-9. [DOI] [PubMed] [Google Scholar]
- Ochala J, Frontera WR, Dorer DJ, Van Hoecke J, Krivickas LS. Single skeletal muscle fiber elastic and contractile characteristics in young and older men. Journals of Gerontology A: Biological Sciences and Medical Sciences. 2007;62:375–381. doi: 10.1093/gerona/62.4.375. [DOI] [PubMed] [Google Scholar]
- Palmer RE, Brady AJ, Roos KP. Mechanical measurements from isolated cardiac myocytes using a pipette attachment system. American Journal of Physiology-Cell Physiology. 1996;39:C697–C704. doi: 10.1152/ajpcell.1996.270.2.C697. [DOI] [PubMed] [Google Scholar]
- Radley HG, De Luca A, Lynch GS, Grounds MD. Duchenne muscular dystrophy: focus on pharmaceutical and nutritional interventions. International Journal of Biochemistry & Cell Biology. 2007;39:469–477. doi: 10.1016/j.biocel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Sukhikh GT, Malaitsev VV, Bogdanova IM, Dubrovina IV, Sitnikov VF. A new approach to the therapy of Duchenne muscular dystrophy with early precursors of myogenesis. Bulletin of Experimental Biology and Medicine. 2001;132:1131–1138. doi: 10.1023/a:1014517525951. [DOI] [PubMed] [Google Scholar]
- Sussman M. Duchenne muscular dystrophy. Journal of the American Academy of Orthopaedic Surgeons. 2002;10:138–151. doi: 10.5435/00124635-200203000-00009. [DOI] [PubMed] [Google Scholar]
- Sweeney HL, Barton ER. The dystrophin-associated glycoprotein complex: what parts can you do without? Proceedings of the National Academy of Sciences USA. 2000;97:13464–13466. doi: 10.1073/pnas.011510597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deutekom JC, van Ommen GJ. Advances in Duchenne muscular dystrophy gene therapy. Nature Reviews Genetics. 2003;4:774–783. doi: 10.1038/nrg1180. [DOI] [PubMed] [Google Scholar]
- Wakayama J, Yamada T. Contractility of single myofibrils of rabbit skeletal muscle studied at various MgATP concentrations. Japanese Journal of Physiology. 2000;50:533–542. doi: 10.2170/jjphysiol.50.533. [DOI] [PubMed] [Google Scholar]
- Wang B, Li J, Xiao X. Adeno-associated virus vector carrying human minidystrophin genes effectively ameliorates muscular dystrophy in mdx mouse model. Proceedings of the National Academy of Sciences USA. 2000;97:13714–13719. doi: 10.1073/pnas.240335297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Ostuni E, Whitesides GM, Ingber DE. Micropatterning tractional forces in living cells. Cell Motility and the Cytoskeleton. 2002;52:97–106. doi: 10.1002/cm.10037. [DOI] [PubMed] [Google Scholar]
- Wang YL, Pelham RJ., Jr Preparation of a flexible, porous polyacrylamide substrate for mechanical studies of cultured cells. Methods in Enzymology. 1998;298:489–496. doi: 10.1016/s0076-6879(98)98041-7. [DOI] [PubMed] [Google Scholar]
- Wells DJ. Therapeutic restoration of dystrophin expression in Duchenne muscular dystrophy. Journal of Muscle Research and Cell Motility. 2006;27:387–398. doi: 10.1007/s10974-006-9081-6. [DOI] [PubMed] [Google Scholar]
- Yin SH, Zhang XQ, Zhan C, Wu JT, Xu JC, Cheung J. Measuring single cardiac myocyte contractile force via moving a magnetic bead. Biophysical Journal. 2005;88:1489–1495. doi: 10.1529/biophysj.104.048157. [DOI] [PMC free article] [PubMed] [Google Scholar]