Abstract
Stereocilia, the mechanosensory organelles of hair cells, are a distinctive class of actin-based cellular protrusions with an unparalleled ability to regulate their lengths over time. Studies on actin turnover in stereocilia as well as the identification of several deafness-related proteins essential for proper stereocilia structure and function provide new insights into the mechanisms and molecules involved in stereocilia length regulation and long-term maintenance. Comparisons of ongoing investigations on stereocilia with studies on other actin protrusions offers new opportunities to further understand common principles for length regulation, the diversity of its mechanisms, and how the specific needs of each cell are met.
Keywords: length regulation, stereocilia, hair cells, myosins, actin protrusions, hearing, actin treadmilling
1. Introduction
Actin-based cellular protrusions such as microvilli, filopodia, and stereocilia serve a broad range of functions in eukaryotic cells. The length and lifespan of these cellular protrusions - often matched to their specific functions - depend on regulatory mechanisms acting on the protrusions’ dominant structure, the core bundle of actin filaments. Although much has been revealed about the signaling mechanisms and assembly processes that initiate the formation of these structures, considerably less is known about their length regulation.
Stereocilia, the mechanosensory actin protrusions on the surface of hair cells, are the key players in the transduction of sound waves or motion into electrical signals that underlie our senses of hearing and balance. Each stereocilium is supported by a rigid paracrystalline array of parallel, uniformly polarized and regularly cross-linked actin filaments. Stereocilia share many construction principles with the actin formations in microvilli and filopodia, yet different stereocilia in a single cell can be from 1 up to 120 µm in length and in the hair cells of the mammalian organ of Corti they persist for a lifetime, as the hair cells are not replaced. In each hair cell, stereocilia are graded in length and organized into a characteristic staircase shape (Figure 1). Also, each stereocilia bundle displays a tightly regulated size and shape that depends on the location of the hair cell within the tissue. For example, the vertebrate auditory epithelium displays a tonotopic gradient of stereocilia bundles with lengths inversely proportional to the frequency of sound the cell is tuned to detect. The overall organization gives the impression of an array of ‘strings’ organized like a piano, with a systematic increase in length from one end of the instrument to the other. Although stereocilia are exquisitely sensitive to mechanical vibration, orderly structured, and easily damaged by over-stimulation, many are maintained in proper working order for a lifetime. The precision and the range of operation of the stereocilia length regulation machinery in hair cells is a challenging but excellent system for asking the general cell biology question: How can the length of an organelle or cytoskeletal ensemble be controlled?
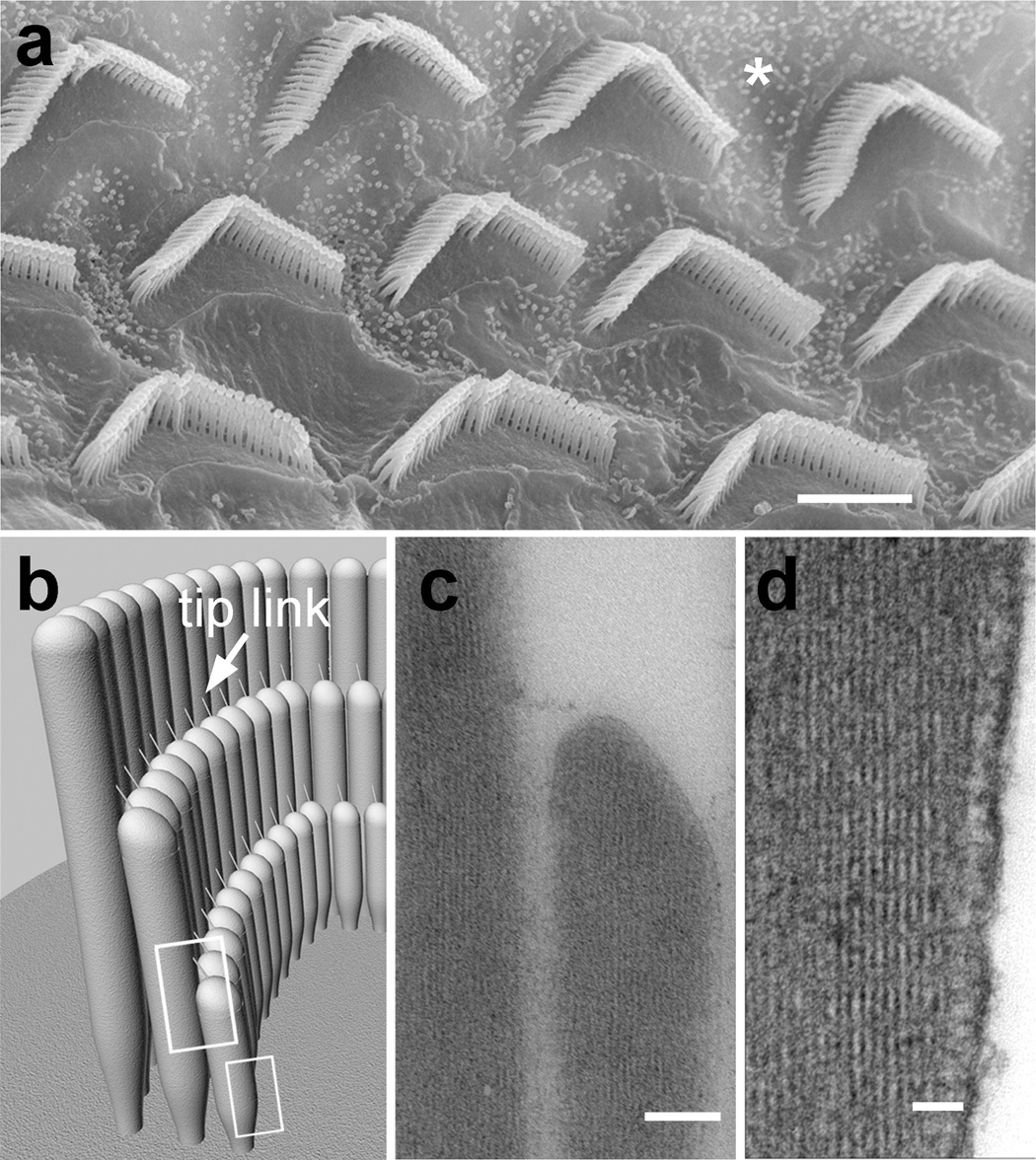
Figure 1.
Hair cell stereocilia appear in a precisely regulated staircase pattern of rows of increasing length. (a) Scanning electron micrograph showing a top view of stereocilia bundles on the apical surface of the inner ear sensory epithelium in the rat cochlea. The asterisk indicates an area on the surface of a non-sensory cell, which displays normal microvilli much shorter than stereocilia. Bar = 5 µm (b) A side view cartoon of a cochlear hair cell. Every bundle in the mammalian cochlea has three rows of stereocilia with rigorously defined lengths. Each stereocilia has a tip-link connecting it to its neighbor in the next shortest row. (c) A thin section electron micrograph showing a cross-section of the upper region of two stereocilia in a rat hair cell, as indicated by the large rectangle in (b). Notice that the tip of the shorter stereocilia is tented due to tip link tension, and that the actin filaments closely match the prolate profile of the membrane. Bar = 150 nm (d) A thin section electron micrograph of the tapered base region of a frog hair cell stereocilium. Notice that the filaments terminate immediately on the plasma membrane. Bar = 50 nm
Like other actin protrusions, stereocilia structure and properties emerge largely from the intrinsic and extrinsic factors modulating the structure and dynamic properties of the actin core. Factors intrinsic to the actin core include: 1) molecules that influence the rate of actin polymerization and depolymerization at the plus and minus ends of the actin filaments, respectively; 2) crosslinking within the actin core; and 3) the action of myosin motors and their binding partners. Factors extrinsic to the actin core, such as plasma membrane tension, interstereociliary links, and overlying extracellular structures, also influence stereocilia lengths and actin dynamics. We do not yet have a completely integrated molecular understanding of the architecture, dynamics, function, and renewal of these specialized cellular structures. However, several pieces of this complex system are being unraveled in mouse model and overexpression studies that are often supported and inspired by the discovery and characterization of novel mouse and human deafness genes. Here we review recent and ongoing investigations on the molecules and processes underlying stereocilia self-renewal and dynamic length regulation.
2. Actin turnover and actin core treadmilling
The formation of stereocilia [3] and functional maturation [4] of the hair bundle occur during the differentiation of the hair cell through a precisely choreographed process of elongation, thickening and reduction of stereocilia number to form an ordered and stable structure with a characteristic staircase pattern. Upon maturation, structural stability is deemed important because the mechanoelectrical transduction apparatus, presumably anchored to the actin core [5], is sensitive to nanometer displacements [6].
Despite the requirement for relatively rigorous structural stability, stereocilia undergo continuous actin turnover [7] and length regulation similar to what has been described for actin protrusions such as microvilli [8] or filopodia [1], albeit at much slower rates. When the incorporation and turnover of actin in the stereocilia was examined using the expression of GFP-tagged proteins in cultured hair cells, it was observed that the actin filament core is renewed and remodeled continuously [7, 9]. In these experiments, hair cells showed incorporation of actin-GFP that appears at the tips of the stereocilia within hours after transfection and progresses towards the base [7]. In developing hair cells in culture (postnatal days 3 to 5) where the stereocilia are still elongating, actin-GFP fluorescence was observed along the entire length of the stereocilia as early as 6 hours after transfection [7]. During this early phase of stereocilia maturation, actin filaments polymerize at rates ~50 times higher than the rate of stereocilia elongation [3]. This shows that stereocilia elongation proceeds while the actin core is undergoing continuous turnover. In the cultures of well-developed hair cells (10–15 days old) with presumably mature bundles, actin-GFP incorporation continues from the tips to the base at estimated flux rates ranging from ~1–10 µm/24 h (~0.004 – 0.04 monomers/second/filament) depending on the length of the stereocilia [9]. These treadmilling rates are within an order of magnitude of the treadmilling rates observed for individual actin filaments in in vitro systems [10, 11]. By comparison, the treadmilling rates measured in parallel actin bundles of microvilli (0.27 µm/min, [2]) and filopodia (0.63 µm/min, [1]) are up to 100 times faster. The slower treadmilling rates in stereocilia compared to microvilli and filopodia are in part a reflection of the differences in number of filaments, density of packing, and nature of the crosslinking proteins. Stereocilia can have over 200 actin filaments tightly packed into a dense paracrystal by the crosslinking activity of fimbrin [12] and espin [13], while microvilli may have only ~20 loosely crosslinked filaments [14, 15]. In Drosophila bristles, which have actin cores similar to stereocilia, the extent and nature of crosslinking can stabilize their actin filaments and regulate their turnover rates [16, 17].
Transfection of cultured hair cells with GFP-espin shows that espin is also incorporated at the tip of the stereocilia dense actin paracrystal and moves rearwards at the same rate as actin[9]. These results indicate that the entire stereocilia actin paracrystalline core is being constantly assembled at the tip, treadmilling downward, and disassembling at the base (Figure 2). The matching rates of actin and espin incorporation suggest that the processes of actin polymerization and actin crosslinking in stereocilia are coupled, similar to what has been described for the in vitro formation of parallel actin filament bundles crosslinked by fimbrin [18].
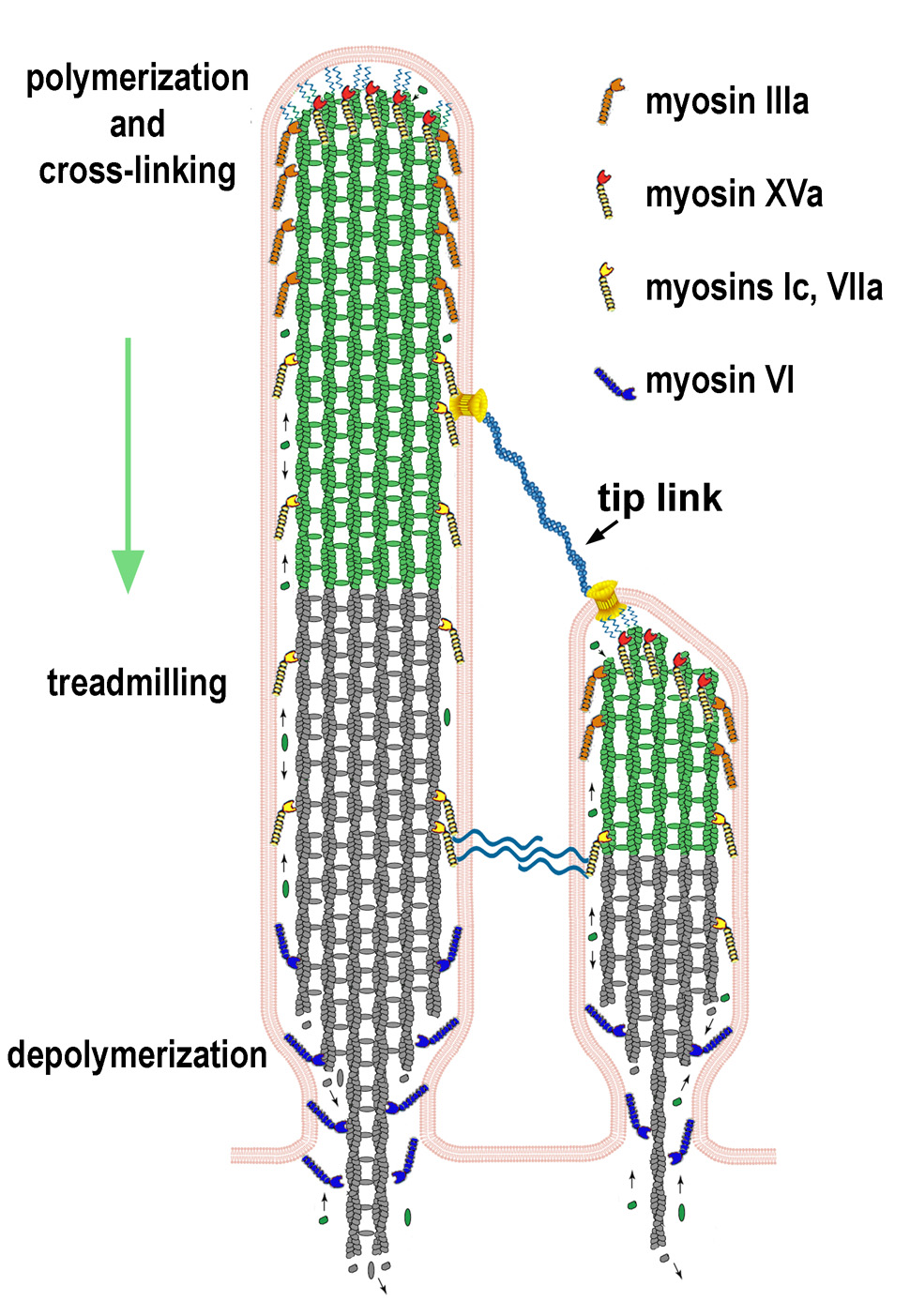
Figure 2.
Cartoon diagram of two neighboring stereocilia. Actin filaments and crosslinkers are continuously assembling at the tips and disassembling at the bases at closely matched rates, resulting in an overall treadmilling core. Note that actin monomers at the tips of stereocilia may be recycled monomers from the depolymerizing filaments at the stereocilia base, from the cytoplasm, or some combination of both. Also note that the shorter stereocilium has graded lengths of F-actin that tightly match the membrane profile, which is tented due to tip-link tension. Adjacent to the tip link is the putative mechanotransduction channel, which is indicated along with hypothetical cytoplasmic links that connect to the actin core. Myosins XVa and IIIa both localize at the stereocilia tip, but myosin XVa localizes to the extreme tip region, while myosin IIIa extends further down the stereocilia shaft. Myosin VI localizes to the base, suggesting a possible role in regulating the minus-ends of actin filaments. Myosins VIIa and Ic localize along the shaft of the stereocilia, implicating a possible role in regulating the retrograde flow of the stereocilia core. Myosins VIIa and Ic also interact with interstereociliary links, which tether neighboring stereocilia actin cores.
While it is clear that actin-GFP monomers newly synthesized in the cytoplasm are quickly incorporated into the stereocilia tip [7], it remains undetermined to what extent the stereocilia actin monomer pool is segregated from the cytoplasmic pool. The entire actin paracrystalline core is enclosed by a membrane, save for a relatively small opening to the cytoplasm at the base. The stereocilia taper and the narrow opening at the base may be an anatomical feature that facilitates retention and recycling of monomers within stereocilia. This might allow for self-renewal of the actin core by the recycling of actin monomers within each stereocilium, as has been shown to occur in filopodial and lamellipodial protrusions in neuronal growth cones [19] and fibroblasts [20].
Recent experiments using multi-isotope imaging mass spectrometry [21] in adult frogs and mice indicate a slow rate (on the order of months) of incorporation of newly synthesized protein in the stereocilia [21, 22]. If these results hold true, then either turnover is slow in stereocilia in vivo, or a large fraction of actin monomers are recycled within each stereocilia with minimal cost in terms of protein synthesis. Future studies elucidating the relative contribution of recycled versus newly synthesized actin monomers to the continual renewal of stereocilia may provide further insight into the hair cell’s mechanisms for stereocilia renewal and length regulation over time.
3. Concerted actin turnover and length regulation
Analyses of the actin incorporation and treadmilling rates in relation to stereocilia length reveal that longer stereocilia have faster actin incorporation and treadmilling rates [9]. In other words, the treadmill rates of stereocilia actin cores are matched to their lengths. One consequence of this is that actin monomers take the same amount of time to treadmill through the length of the shortest and longest stereocilia in a single bundle, such that the entire bundle renews synchronously [9]. The direct relationship between stereocilia length and polymerization rates may reveal new insights into the mechanisms of assembly and maintenance of the stereocilia actin core. Theoretical studies indicate that faster polymerization rates can help push out longer membrane protrusions [23–25], suggesting that the faster assembly rates observed in longer stereocilia are an inherent requirement for attaining their prescribed lengths. However, the precise molecular mechanisms that control the rate of assembly and disassembly of the stereocilia actin cores remain to be determined. One possible mechanism is the accumulation of graded distributions of regulatory proteins at the tips or along the shafts of different length stereocilia, as has been shown for myosin XVa [9]. Alternatively, one theoretical study suggests that the direct relationship between treadmilling rates and length is controlled by depolymerization rates at the base [23].
Experiments using cytochalasin D to inhibit actin polymerization show that disassembly continues at the same treadmilling rates measured in the actin-GFP transfection experiments, independently of whether assembly is occurring at the tip [9, 26]. While these experiments indicate that disassembly at the base proceeds independently of assembly at the tip, these processes likely influence each other over time because mature hair cells maintain stereocilia at steady-state lengths. One mechanism that might help maintain the steady-state balance between polymerization at the tip and depolymerization at the base is one in which the pool of actin monomers is maintained constant and the polymerization at the tips depends on a continuous supply of recycled actin monomers provided by disassembly at the base. In support of this hypothesis, such dependence of polymerization activity on recycled actin monomers has been described in neuronal growth cones [19] and fibroblasts [20].
Close observation of the bundle shows that stereocilia of the same rank in the staircase organization often show slight differences in length [9]. During actin-GFP incorporation experiments, the transient rate of incorporation can vary and consistently appears to be slightly faster at the slightly more elongated stereocilia and slightly shorter at the slightly shorter stereocilia [9]. These observations suggest that the length of each stereocilium fluctuates but is regulated to maintain an average steady-state length matched to its position in the staircase organization. Fluctuations in actin assembly rates are also observed as part of the regulation of actin assembly in filopodia [1].
4. Intrinsic factors regulating stereocilia length
Considering that neighboring stereocilia are regulated to form the staircase bundle and that the entire stereocilia bundle structure is dynamically maintained, it is clear that intrinsic processes of actin regulation and the extrinsic constraints of the bundle organization coexist and mutually influence each other. In the following section we discuss the proteins that are intrinsic to the actin core and are involved in the length regulation process.
4.1 Crosslinkers play multiple roles in stereocilia structural organization
Studies performed in a variety of in vitro and in vivo systems show that crosslinking can increase the stiffness [27, 28], stability [12, 13, 29–32], and length [2, 33] of parallel actin filament bundles. Stereocilia have particularly rigid paracrystalline cores [15]. Recent analysis of espin-crosslinked actin bundles in vitro demonstrates that specific amino acid changes associated with deafness mutations disrupt the paracrystalline organization of the actin filamant bundles, thereby decreasing their bending modulus by three orders of magnitude [34]. Clearly, crosslinkers play a critical role in maintaining stiffness, which is particularly important for generating long protrusions such as the stereocilia.
Crosslinking can also regulate the depolymerization rates of actin bundles. In densely crosslinked actin bundles (e.g., Drosophila bristles), crosslinkers act as minus-end ‘cappers’ due to their binding to one or more of the other actin filaments in a parallel bundle, resulting in a decrease in probability of a crosslinker-bound monomer from completely dissociating from the actin bundle [16, 17]. A recent modeling study of stereocilia actin dynamics and length regulation showed that hypothetical F-actin minus-end ‘cappers’ that down-regulate the effective depolymerization rates could be involved in regulating stereocilia lengths and treadmilling rates proportionally [23].
Mutations in the gene encoding espin causes deafness in jerker mice [13] and humans [35], and examination of espin-null jerker mouse hair cells shows that their stereocilia are abnormally thin and short and degrade after several days [13]. Espin is not only likely to cross-link and stabilize the actin core, but also to play an important role in regulating its length. Crosslinking-mediated upregulation of actin polymerization [2] or downregulation of depolymerization rates [16, 17] are likely to explain the elongation of stereocilia and microvilli when espin is overexpressed [2, 33]. That the jerker mice hair cells are able to form stereocilia at all in the absence of espin is probably due to the presence of fimbrin [12]. Genetic studies on Drosophila bristles indicate that the presence of more than one crosslinker is critical for efficient and stable actin bundling [29]. The specific contributions of espin and fimbrin to the stereocilia actin core remain to be elucidated, but one possibly important difference is that fimbrin is calcium sensitive [36]. Thus, espin may be the more stable of the two actin crosslinkers in stereocilia, especially near the tips, where calcium is thought to enter the stereocilia through the mechanoelectrical transduction (MET) channel (Figure 2) [37].
4.2 Actin regulatory proteins at the plus and minus ends of the actin filament core
Despite the large number of actin-regulatory proteins involved in other actin protrusions (reviewed in [38], [39], [40] and [41]), very few of these proteins have been proven to be involved in stereocilia length regulation. However, evidence exists that some of these proteins, as well as some novel proteins initially linked to inherited deafness, may be involved in actin regulation at either the plus ends (espin and the formin protein mDia1) or minus ends (radixin, PTPRQ, triobp, and tropomyosin) of stereocilia actin cores, and are discussed below.
A recent study showed that the actin crosslinker espin contains a WH2 domain essential for the formation of actin bundles in transfected cells, supposedly via up-regulation of actin polymerization activity [42]. This WH2-dependent activity points to yet another mechanism by which espin crosslinkers can regulate stereocilia length, since espin incorporates into the stereocilia actin core at the polymerizing tip [9, 33], where WH2- domains must be localized in order for their polymerization up-regulation activity to occur [43]. Espin has several similarities to the Ena/VASP proteins, and both of these proteins are implicated in the initiation and elongation of actin protrusions [15, 40]. For example, espin and Ena/VASP contain G-actin binding motifs, actin bundling motifs, profilin-binding and proline-rich domains [15, 40], all of which are implicated in actin regulation [38–41]. Ena/VASP is thought to mediate filopodial protrusion via G-actin-binding [44, 45], actin bundling [45, 46], and is also thought to have anti-capping activity [47]. Thus, it should not be surprising if, like Ena/VASP, espin regulates or influences actin dynamics and stereocilia lengths via multiple mechanisms [9, 23, 24, 48].
The well studied roles of formins in actin polymerizarion and their widespread presence in actin protrusions makes formins attractive candidates for regulation of stereocilia length. While no formins have yet been reported to be localized in stereocilia, one member of the formin family, mDia1, which is implicated in nonsyndromic progressive deafness DFNA1 [49], has been suggested to be involved in stereocilia elongation [50] and [51]. Since DFNA1 is a progressive hearing loss phenotype, it is possible that mDia1 is involved in the long-term maintenance of stereocilia structure.
Stereocilia length can also be affected by the minus-end actin dynamics at the stereocilia base [23]. Radixin localizes to the stereocilia base [52], and is required for normal hearing and is essential for normal stereocilia structure in cochlear hair cells [53]. Radixin is a member of the ERM (ezrin, radixin, moesin) family, which is important for regulation of morphogenesis and the actin cytoskeleton in a variety of cells [54]. The tethering of actin filaments to the plasma membrane is important for regulating actin structures [55], and radixin is known to directly mediate this process in neuronal cells [56, 57]. Thus, it is likely that radixin regulates stereocilia actin by anchoring the minus-end of actin filaments to the plasma membrane in the tapered region of the stereocilia, given its localization at the stereocilia base in between the actin core and the plasma membrane [52]. Interestingly, ezrin can compensate for radixin in vestibular, but not cochlear hair cell stereocilia [53], suggesting an important role for ERM proteins in stereocilia structure.
Protein tyrosine phosphatase receptor Q (PTPRQ) [58] is another protein localized to the stereocilia base [59, 60] that is required for normal stereocilia structure [60, 61], function [59] and hearing [61]. PTPRQ is a multifunctional protein with a long extracellular domain containing 18 fibronectin III repeats, a membrane spanning domain, and an unusual cytoplasmic phosphatase domain with phosphatidylinositol phosphatase (PIPase) and tyrosine phosphatase activity [58]. Phosphatidylinositol is a lipid that binds to, and influences the activity of several actin associated proteins that regulate the architecture of the actin cytoskeleton [62]. Mutations in PTPRQ lead to a loss of the tapered base normally seen in wild-type stereocilia and result in giant stereocilia [60, 61]. PTPRQ is likely a source of PIPase activity [58] at the stereocilia base [59] and therefore may be involved in minus-end actin regulation [62].
Several mutations in the gene coding for Triobp (previously named Tara), cause nonsyndromic deafness DFNB28 [63, 64]. Triobp is an F-actin binding protein that associates with the Trio guanine nucleotide exchange factor, and is involved in regulating actin cytoskeletal organization [65]. Triobp immunoreactivity has been shown along the entire length of stereocilia [64], while an antibody specific to one of the Triobp isoforms (isoform 5) shows reactivity only at the stereocilia rootlets [66], which are electron dense regions in the most central portion of the actin core that extend from the basal region of stereocilia into the cortical actin layer below the apical surface of the hair cell [67]. While little is known about the role of this protein in stereocilia and the cellular basis for DFNB28, in vitro experiments in HeLa cells demonstrate that Triobp can stabilize and bundle actin filaments [65]. Thus, it is likely that Triobp is involved in regulating the stability of the minus-end of the stereocilia actin core.
Tropomyosin is another actin-binding protein that localizes to the stereocilia rootlet [68, 69]. Tropomyosin has been implicated as an actin minus-end capper that can slow down depolymerization of actin filaments in vitro [70]. It has been suggested that tropomyosin activity can affect actin cytoskeleton dynamics in migrating epithelial cells [71], and normal retrograde flow and stability of actin filaments in yeast has been shown to be dependent on tropomyosin activity [72]. All of these data suggest that tropomyosin may be regulating actin dynamics at the stereocilia rootlet. The role the rootlet plays in stereocilia length regulation is unclear, but the electron dense region near the minus-ends of stereocilia actin filaments suggests the presence of large amounts of proteins, any of which may be involved in regulating the overall minus-end dynamics of the stereocilia actin core.
4.3 Regulation of stereocilia length mediated by myosin activity
Myosins are involved in actin protrusion initiation and length regulation via a number of mechanisms, including actin-filament convergence [73], retrograde flow of actin filaments [72], and dynamic localization of cargo that directly or indirectly regulate actin protrusion length [74, 75]. Unconventional myosins XVa, IIIa, VI, and VIIa were first identified as products of genes causing nonsyndromic deafness. These myosins have been localized in stereocilia and shown to cause deafness through disruption of stereocilia structure and dynamic properties. Recent reports have begun to describe the specific roles of these myosins in actin dynamics, length regulation, and the active maintenance of stereocilia bundle structure.
Myosin XVa is essential for the normal staircase formation and elongation of stereocilia of the organ of Corti and vestibular hair cells [76]. Myosin XVa deficient mice (Shaker 2 mice) have very short stereocilia [76] that lack a clearly defined tip density [9]. Myosin XVa localizes at the tips of stereocilia in concentrations proportional to the stereocilia length [9]. The mechanism by which hair cells sort myosin XVa, depending on the length of stereocilia [9], as well as the precise mechanism by which myosin XVa regulates stereocilia lengths, are likely interdependent and remain to be fully elucidated.
Myosin XVa has been shown to transport whirlin, a PDZ domain-containing scaffolding protein required for normal hearing in humans and mice [77], to the tips of stereocilia [74]. Studies suggest that whirlin recruits the membrane-associated guanylate kinase (MAGUK) proteins p55 and Ca2+-calmodulin serine kinase (CASK), and protein 4.1R to the stereocilia tip-complex [78], any of which may be involved in regulating actin assembly[78]. Whirler mice, which have a mutation in the gene coding for whirlin [77], display very short stereocilia, indicating a defect in the stereocilia elongation machinery [79]. A more recent study showed that while both shaker-2 and whirler mice both have short stereocilia, the shaker-2 mice have a more severe phenotype suggesting that myosin XVa has additional roles other than transporting whirlin [80]. Interestingly, the abnormally short stereocilia in shaker-2 mouse inner hair cells are still able to mechanotransduce in culture [81], emphasizing the importance of stereocilia length regulation in vivo - although the shaker-2 hair cells are able to mechanotransduce in isolated tissue preparations, the mice are deaf [82].
Myosin IIIa, one of two human class III myosins [83, 84], was identified as essential for normal hearing when it was shown that loss-of-function mutations in MYO3A cause nonsyndromic, progressive hearing loss DFNB30 [85]. Recent studies show that myosin IIIa is localized at the tips of stereocilia [4, 86]. In contrast to myosin XVa, whose localization forms a cap at the extreme tips of stereocilia [9], myosin IIIa localizes around the tip-density and extends further down the length of the stereocilia [86], suggesting distinct roles for these two proteins. Myosin IIIa has a kinase domain that allows for self-regulation of myosin IIIa’s phosphorylation state [87] and affinity for actin [88]. When hair cells are transfected with myosin IIIa constructs without the kinase domain, the stereocilia display enhanced elongation and bulging tips [86]. This phenotype demonstrates that regulated amounts of myosin IIIa activity in stereocilia are required for proper length maintenance. Myosin IIIa possesses two tail homology domains (designated as 3THDI and 3THDII) that are highly conserved among different species[89]. Current studies (Salles et al., in preparation) suggest that 3THDI is involved in transporting espin isoform 1 to the stereocilia tips, and that this activity profoundly affects the lengths of actin protrusions in both hair cells and transfected COS-7 cells. Interestingly, immunostaining studies show that myosin IIIa expression levels correlate with the onset and maturation of hair cells’ ability for adaptation to mechanotransduction [4]. Not only does this suggest that myosin IIIa may serve multiple roles in stereocilia function, but it also raises the question of whether mechanotransduction channel activity and length regulation affect one another.
Myosin VI is a minus-end directed motor whose loss of function causes deafness in Snell’s waltzer mice [90] and humans [91]. Snell’s waltzer mice hair cells have malformed bundles with giant stereocilia[60, 92]. In Snell’s waltzer mice stereocilia, PTPRQ localizes all along the length of the stereocilia, suggesting that myosin VI activity is required to actively confine PTPRQ to the base of the stereocilia in wild-type mice[60]. A combined role for myosin VI and PTPRQ in regulating stereocilia structure is likely given that these two proteins both localize to the base of the stereocilia, and similar giant stereocilia form when either of these proteins are not functioning in the stereocilia [60]. The active transport of actin regulating proteins to the plus and minus ends of actin filaments is one way myosins could regulate actin dynamics and, ultimately, stereocilia length. Immunolocalization and transfection studies show that myosin VI [60] and PTPRQ [60] display an exponentially decaying gradient in distribution (notably, radixin displays a graded distribution as well [52]). These distributions reveal an active localization process involving a combination of minus-end (myosin VI) directed processive motion, transient unbinding, and diffusion of these molecules[60]. Therefore, it appears that myosins shuttle actin regulatory proteins to increase their local concentrations and catalytic activites at the ends of actin filaments, which in turn could influence stereocilia lengths.
Mutations in myosin VIIa are responsible for deafness in the shaker 1 mouse [93] and USH1B humans[94]. Recently it was observed that stereocilia lacking myosin VIIa displayed longer stereocilia [26]. Blocking actin polymerization with cytochalasin D shows that the longer stereocilia in myosin VIIa mutants are indeed treadmilling faster [26], in accordance with previous stereocilia actin treadmilling studies [7, 9]. The localization of myosin VIIa along the length of stereocilia makes it a reasonable candidate for regulating the actin core’s rearward flow rates. This rearward flow could be in part due to myosin moving upwards on the actin filaments while attached to the plasma membrane, thereby pulling the actin core down [95]. The effects on stereocilia length of myosin VIIa’s tethering of the actin cytoskeleton to extracellular links, were not investigated. However, it was proposed that myosin VIIa regulates length via some other mechanism, e.g., competing with actin polymerization factors for F-actin barbed-ends [26]. In support of this hypothesis, there seemed to be enhanced localization of whirlin at the tips of myosin VIIa deficient hair cells. Thus, myosin VIIa may influence or regulate length and stereocilia function through a variety of mechanisms.
Myosin-1c is also well known for its role in maintaining tension in interstereociliary links, which is required for proper adaptation of the hair cell’s mechanoelectrical transduction responses [96]. The localization of myosin-Ic along the shaft of the stereocilia in mammalian hair cells [4] also makes it a candidate for regulating rearward flow rates [95]. In support of this notion, the closely related myosin-1a, another myosin- 1 isoform expressed in the inner ear [97], was recently shown to be involved in the relative movement of the plasma membrane and the actin cytoskeleton in brush border microvilli [98]. Myosin-1a is essential for proper hearing [99], and is also critical for the proper formation of brush border microvilli [100]. Interestingly, when myosin-1a is absent in brush border microvilli it is partially compensated for by myosin-1c activity [100]. Although the biophysical characterization of a myosin-1a mutation that causes nonsyndromic deafness has begun [101], future studies will be necessary to determine the precise role of myosin-1a in stereocilia structure and function.
5. Extrinsic factors that influence stereocilia length
Hair cell stereocilia are not free from external influences in vivo. For example, the tallest row of stereocilia in mammalian cochlear hair cells is constrained by an overlying tectorial membrane, while the shorter stereocilia are connected to their taller neighbors by the tip link. In addition, all stereocilia are laterally interconnected by various other links. Finally, the stereocilia plasma membrane mechanically interacts with the tips of the actin cores. Any of these factors may exert mechanical or biochemical influences on actin polymerization and stereocilia length. Not only could extrinsic biochemical and biomechanical factors influence actin turnover and length regulation in stereocilia, but they could also alter response properties of MET. What is the interplay between these critical and dynamic hair cell bundle processes? How does mechanical stimulation, either directly to the membrane or indirectly via MET, modulate stereocilia treadmilling?
Membrane tension may mechanically regulate actin polymerization at the tip [23, 48, 102, 103]. Moreover, actin polymerization and elongation at the ends of each filament exert a reciprocal force on the membrane and influence membrane shape [23]. In principle, it is possible to couple actin polymerization to an external load in order to drive movement and generate force [41]. In such a model, actin polymerization and protrusion elongation would stall when a balance between opposing forces produced by the membrane tension and the actin polymerization is attained [23, 24, 48, 102]. Examination of the tip shape and structure of stereocilia provides evidence in support of this concept. Thin sections of directly frozen stereocilia show a smooth profile for the stereocilia-encapsulating membrane as if the membrane is under tension, with actin filaments graded in length and exactly matching the membrane profile (Figure 1). In order for the actin filaments to display such ordered length gradations, the compressive force normal to the membrane tension must influence the actin elongation process. The re-tailoring of actin filaments and progressive rounding of the stereocilia tip that follows the disruption of the tip link with the calcium chelator BAPTA [9] supports the hypothesis that membrane tension can influence actin turnover rates. However, we cannot exclude that other signaling mechanisms such as Ca2+ entry through the mechanotransduction channel are involved in producing this effect, since BAPTA treatment inhibits Ca2+ entry into the stereocilia. All of the myosins known to be present in stereocilia possess calmodulin-binding IQ domains, pointing towards a role for Ca2+ in regulating their activity [104], which could in turn directly influence stereocilia length. Since many actin-regulatory proteins are calcium-dependent [105], tip link tension and the corresponding influx of Ca2+ may regulate the stereocilia actin core via both physical and chemical mechanisms.
Extracellular structures may also influence stereocilia length. For example, the height of the tallest row of stereocilia in the organ of Corti perfectly matches the separation of the overlying tectorial membrane and the reticular lamina[106], suggesting that the load exerted by the tectorial membrane serves as a mechanical feedback that influences stereocilia length. Alternatively, chemical signaling from the tectorial membrane to stereocilia membrane proteins regulates stereocilia lengths. For example, α8β1 integrin[107], tetraspan membrane protein of hair cell stereocilia (Tmhs) [108], and transmembrane inner ear (Tmie) protein [109, 110] are all membrane proteins that localize to stereocilia tips and are required for normal hearing and stereocilia structure. Regardless of the precise mechanism, it is expected that the tectorial membrane regulates stereocilia lengths given that stereocilia lengths are critically maintained at least in part due to a need for close coupling of stereocilia tips to the tectorial membrane [106].
In the vestibular system, the tallest row of stereocilia isn’t always defined by the overlying structures, which are directly coupled to a single true cilium called kinocilium [111, 112]. Instead, the height of the tallest stereocilia appears to be marked by a densely linked region connecting the stereocilia tips to the kinocilium shaft [113]. Interestingly, the kinocilium is immediately adjacent to the tallest row of stereocilia, and is critical for the proper orientation of the staircase shape of the bundle [3]. It has been suggested that links between the kinocilium and the stereocilia mechanically influence the elongation and orientation of the tallest row of stereocilia, which in turn mechanically induce the elongation of the next row of stereocilia via their tip link connections, thereby resulting in the distinctive staircase shaped bundle [3].
Although tip links and kinocilial links are inherently correlated with stereocilia lengths by virtue of their location at stereocilia tips, other interstereociliary links and their binding partners localized along the stereocilia shafts may also be involved in regulating the overall stereocilia actin dynamics. In particular, the scaffolding proteins vezatin, harmonin, SANS and whirlin along with the transmembrane proteins cadherin 23, protocadherin 15, VLGR1, and usherin all form complexes that bind to myosin VIIa and tether the actin cytoskeleton to interstereociliary links (for a recent review, see [114]). Since tethering actin filaments to the plasma membrane can affect filament stability and dynamics [55], the tethering of treadmilling stereocilia actin filaments to the membrane via active myosin motors may be an important process for regulating the dynamic stereocilia actin cores. Also, the fact that these interstereociliary links may be coupled to two different treadmilling cores raises the question as to whether two moving actin cores can mechanically influence and communicate with one another via interstereociliary link tension.
6. Conclusions and future directions
Several processes and molecular players involved in stereocilia length regulation have been uncovered in recent years. However, only about one-third of the genes associated with the 130 loci linked to nonsyndromic deafness are known, and about half of those genes (~23) express proteins localized to and are required for normal stereocilia structures [114]. Thus, new proteins involved in the regulation of stereocilia lengths are likely to be revealed by current and future studies on deafness genes that code for actin cytoskeleton associated proteins.
What can deafness mutations tell us about stereocilia maintenance? Perhaps the most interesting mutations are the ones that lead to progressive hearing loss, as they could indicate subtle, yet significant changes in the actin core dynamics, and some evidence already exists for this notion. For example, mutations in γ-actin are associated with nonsyndromic progressive deafness [115–118], and the localization of γ- versus β-actin in stereocilia may be slightly different [119], suggesting that γ- and β-actin may have non-overlapping roles and dynamics in stereocilia structure. Of course, future studies will also have to be done on ‘canonical’ actin-associated proteins, but candidates fortunately do not have to be completely guessed upon – recent mass spectrometry proteomic studies on hair cell bundles have already demonstrated their power in identifying new stereocilia proteins and have helped expand the inventory of known stereocilia essential proteins [120].
The rigorous organization displayed by the hair cell bundle leads us to believe that what may seem to be parallel processes are likely interconnected by a multitude of processes. For example, different length stereocilia may need the same concentration of certain proteins essential for proper inner ear function. This is evident for the plasma membrane Ca2+ ATP-ase-2 (PMCA2), which is essential for normal hearing [121], and appears in equal concentrations in each stereocilia [122]. This means that the cell must be sorting higher amounts of PMCA2 to longer stereocilia [122]. The differential actin treadmilling rates in stereocilia demonstrate that even though a cytoskeletal structure may be built out of identical components, each of which have intrinsic biochemical properties, the ensemble dynamics can be highly variable, perhaps as a result of differences in the relative amounts of just a few key components. The question of how the hair cell manages length regulation is likely to yield some interesting insights into how the cell regulates different processes in parallel to one another.
Acknowledgements
The authors would like to thank Ronald Petralia, Cole Graydon, and Aurea De Sousa for their critical review of the manuscript and helpful suggestions. This work was funded by NIDCD/DIR, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mallavarapu A, Mitchison T. Regulated actin cytoskeleton assembly at filopodium tips controls their extension and retraction. J Cell Biol. 1999;146(5):1097–1106. doi: 10.1083/jcb.146.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loomis PA, et al. Espin cross-links cause the elongation of microvillus-type parallel actin bundles in vivo. J Cell Biol. 2003;163(5):1045–1055. doi: 10.1083/jcb.200309093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tilney LG, Tilney MS, DeRosier DJ. Actin filaments, stereocilia, and hair cells: how cells count and measure. Annu Rev Cell Biol. 1992;8:257–274. doi: 10.1146/annurev.cb.08.110192.001353. [DOI] [PubMed] [Google Scholar]
- 4.Waguespack J, Salles FT, Kachar B, Ricci AJ. Stepwise morphological and functional maturation of mechanotransduction in rat outer hair cells. J Neurosci. 2007;27(50):13890–13902. doi: 10.1523/JNEUROSCI.2159-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kros CJ, et al. Reduced climbing and increased slipping adaptation in cochlear hair cells of mice with Myo7a mutations. Nat Neurosci. 2002;5(1):41–47. doi: 10.1038/nn784. [DOI] [PubMed] [Google Scholar]
- 6.Robles L, Ruggero MA. Mechanics of the mammalian cochlea. Physiol Rev. 2001;81(3):1305–1352. doi: 10.1152/physrev.2001.81.3.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneider ME, Belyantseva IA, Azevedo RB, Kachar B. Rapid renewal of auditory hair bundles. Nature. 2002;418(6900):837–838. doi: 10.1038/418837a. [DOI] [PubMed] [Google Scholar]
- 8.Tyska MJ, Mooseker MS. MYO1A (brush border myosin I) dynamics in the brush border of LLC-PK1-CL4 cells. Biophys J. 2002;82(4):1869–1883. doi: 10.1016/S0006-3495(02)75537-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rzadzinska AK, Schneider ME, Davies C, Riordan GP, Kachar B. An actin molecular treadmill and myosins maintain stereocilia functional architecture and self-renewal. J Cell Biol. 2004;164(6):887–897. doi: 10.1083/jcb.200310055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wegner A. Head to tail polymerization of actin. J Mol Biol. 1976;108(1):139–150. doi: 10.1016/s0022-2836(76)80100-3. [DOI] [PubMed] [Google Scholar]
- 11.Fujiwara I, Takahashi S, Tadakuma H, Funatsu T, Ishiwata S. Microscopic analysis of polymerization dynamics with individual actin filaments. Nat Cell Biol. 2002;4(9):666–673. doi: 10.1038/ncb841. [DOI] [PubMed] [Google Scholar]
- 12.Tilney MS, et al. Preliminary biochemical characterization of the stereocilia and cuticular plate of hair cells of the chick cochlea. J Cell Biol. 1989;109(4 Pt 1):1711–1723. doi: 10.1083/jcb.109.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng L, et al. The deaf jerker mouse has a mutation in the gene encoding the espin actin-bundling proteins of hair cell stereocilia and lacks espins. Cell. 2000;102(3):377–385. doi: 10.1016/s0092-8674(00)00042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartles JR. Parallel actin bundles and their multiple actin-bundling proteins. Curr Opin Cell Biol. 2000;12(1):72–78. doi: 10.1016/s0955-0674(99)00059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sekerkova G, Zheng L, Loomis PA, Mugnaini E, Bartles JR. Espins and the actin cytoskeleton of hair cell stereocilia and sensory cell microvilli. Cell Mol Life Sci. 2006;63(19–20):2329–2341. doi: 10.1007/s00018-006-6148-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guild GM, Connelly PS, Vranich KA, Shaw MK, Tilney LG. Actin filament turnover removes bundles from Drosophila bristle cells. J Cell Sci. 2002;115(Pt 3):641–653. doi: 10.1242/jcs.115.3.641. [DOI] [PubMed] [Google Scholar]
- 17.Tilney LG, Connelly PS, Ruggiero L, Vranich KA, Guild GM. Actin filament turnover regulated by cross-linking accounts for the size, shape, location, and number of actin bundles in Drosophila bristles. Mol Biol Cell. 2003;14(10):3953–3966. doi: 10.1091/mbc.E03-03-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volkmann N, DeRosier D, Matsudaira P, Hanein D. An atomic model of actin filaments cross-linked by fimbrin and its implications for bundle assembly and function. J Cell Biol. 2001;153(5):947–956. doi: 10.1083/jcb.153.5.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallo G, Yee HF, Jr, Letourneau PC. Actin turnover is required to prevent axon retraction driven by endogenous actomyosin contractility. J Cell Biol. 2002;158(7):1219–1228. doi: 10.1083/jcb.200204140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cramer LP. Role of actin-filament disassembly in lamellipodium protrusion in motile cells revealed using the drug jasplakinolide. Curr Biol. 1999;9(19):1095–1105. doi: 10.1016/s0960-9822(99)80478-3. [DOI] [PubMed] [Google Scholar]
- 21.Lechene C, et al. High-resolution quantitative imaging of mammalian and bacterial cells using stable isotope mass spectrometry. J Biol. 2006;5(6):20. doi: 10.1186/jbiol42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piazza V, et al. Multi-Isotope Imaging Mass Spectrometry (MIMS) Mapping of Protein Turnover in Hair Cells Reveals Highly Stable Stereocilia; Association for Research in Otolaryngology 30th midwinter research meeting; 2007. [Google Scholar]
- 23.Prost J, Barbetta C, Joanny JF. Dynamical control of the shape and size of stereocilia and microvilli. Biophys J. 2007;93(4):1124–1133. doi: 10.1529/biophysj.106.098038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mogilner A, Rubinstein B. The physics of filopodial protrusion. Biophys J. 2005;89(2):782–795. doi: 10.1529/biophysj.104.056515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peskin CS, Odell GM, Oster GF. Cellular motions and thermal fluctuations: the Brownian ratchet. Biophys J. 1993;65(1):316–324. doi: 10.1016/S0006-3495(93)81035-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prosser HM, Rzadzinska AK, Steel KP, Bradley A. Mosaic complementation demonstrates a regulatory role for myosin VIIa in actin dynamics of stereocilia. Mol Cell Biol. 2008;28(5):1702–1712. doi: 10.1128/MCB.01282-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Claessens MM, Bathe M, Frey E, Bausch AR. Actin-binding proteins sensitively mediate F-actin bundle stiffness. Nat Mater. 2006;5(9):748–753. doi: 10.1038/nmat1718. [DOI] [PubMed] [Google Scholar]
- 28.Gardel ML, et al. Elastic behavior of cross-linked and bundled actin networks. Science. 2004;304(5675):1301–1305. doi: 10.1126/science.1095087. [DOI] [PubMed] [Google Scholar]
- 29.Tilney LG, Connelly PS, Vranich KA, Shaw MK, Guild GM. Why are two different cross-linkers necessary for actin bundle formation in vivo and what does each cross-link contribute? J Cell Biol. 1998;143(1):121–133. doi: 10.1083/jcb.143.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tilney LG, Tilney MS, Guild GM. F actin bundles in Drosophila bristles. I. Two filament cross-links are involved in bundling. J Cell Biol. 1995;130(3):629–638. doi: 10.1083/jcb.130.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanders MC, Way M, Sakai J, Matsudaira P. Characterization of the actin cross-linking properties of the scruin-calmodulin complex from the acrosomal process of Limulus sperm. J Biol Chem. 1996;271(5):2651–2657. doi: 10.1074/jbc.271.5.2651. [DOI] [PubMed] [Google Scholar]
- 32.Mahajan-Miklos S, Cooley L. The villin-like protein encoded by the Drosophila quail gene is required for actin bundle assembly during oogenesis. Cell. 1994;78(2):291–301. doi: 10.1016/0092-8674(94)90298-4. [DOI] [PubMed] [Google Scholar]
- 33.Rzadzinska A, Schneider M, Noben-Trauth K, Bartles JR, Kachar B. Balanced levels of Espin are critical for stereociliary growth and length maintenance. Cell Motil Cytoskeleton. 2005;62(3):157–165. doi: 10.1002/cm.20094. [DOI] [PubMed] [Google Scholar]
- 34.Purdy KR, Bartles JR, Wong GC. Structural polymorphism of the actin-espin system: a prototypical system of filaments and linkers in stereocilia. Phys Rev Lett. 2007;98(5):058105. doi: 10.1103/PhysRevLett.98.058105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naz S, et al. Mutations of ESPN cause autosomal recessive deafness and vestibular dysfunction. J Med Genet. 2004;41(8):591–595. doi: 10.1136/jmg.2004.018523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glenney JR, Jr, Kaulfus P, Matsudaira P, Weber K. F-actin binding and bundling properties of fimbrin, a major cytoskeletal protein of microvillus core filaments. J Biol Chem. 1981;256(17):9283–9288. [PubMed] [Google Scholar]
- 37.Ricci AJ, Kachar B, Gale J, Van Netten SM. Mechano-electrical transduction: new insights into old ideas. J Membr Biol. 2006;209(2–3):71–88. doi: 10.1007/s00232-005-0834-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol. 2008;9(6):446–454. doi: 10.1038/nrm2406. [DOI] [PubMed] [Google Scholar]
- 39.Chhabra ES, Higgs HN. The many faces of actin: matching assembly factors with cellular structures. Nat Cell Biol. 2007;9(10):1110–1121. doi: 10.1038/ncb1007-1110. [DOI] [PubMed] [Google Scholar]
- 40.Drees F, Gertler FB. Ena/VASP: proteins at the tip of the nervous system. Curr Opin Neurobiol. 2008;18(1):53–59. doi: 10.1016/j.conb.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112(4):453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 42.Loomis PA, et al. Targeted wild-type and jerker espins reveal a novel, WH2-domain-dependent way to make actin bundles in cells. J Cell Sci. 2006;119(Pt 8):1655–1665. doi: 10.1242/jcs.02869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chereau D, et al. Actin-bound structures of Wiskott-Aldrich syndrome protein (WASP)-homology domain 2 and the implications for filament assembly. Proc Natl Acad Sci U S A. 2005;102(46):16644–16649. doi: 10.1073/pnas.0507021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walders-Harbeck B, Khaitlina SY, Hinssen H, Jockusch BM, Illenberger S. The vasodilator-stimulated phosphoprotein promotes actin polymerisation through direct binding to monomeric actin. FEBS Lett. 2002;529(2–3):275–280. doi: 10.1016/s0014-5793(02)03356-2. [DOI] [PubMed] [Google Scholar]
- 45.Applewhite DA, et al. Ena/VASP proteins have an anti-capping independent function in filopodia formation. Mol Biol Cell. 2007;18(7):2579–2591. doi: 10.1091/mbc.E06-11-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schirenbeck A, et al. The bundling activity of vasodilator-stimulated phosphoprotein is required for filopodium formation. Proc Natl Acad Sci U S A. 2006;103(20):7694–7699. doi: 10.1073/pnas.0511243103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barzik M, et al. Ena/VASP proteins enhance actin polymerization in the presence of barbed end capping proteins. J Biol Chem. 2005;280(31):28653–28662. doi: 10.1074/jbc.M503957200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Atilgan E, Wirtz D, Sun SX. Mechanics and dynamics of actin-driven thin membrane protrusions. Biophys J. 2006;90(1):65–76. doi: 10.1529/biophysj.105.071480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lynch ED, et al. Nonsyndromic deafness DFNA1 associated with mutation of a human homolog of the Drosophila gene diaphanous. Science. 1997;278(5341):1315–1318. [PubMed] [Google Scholar]
- 50.Evangelista M, Zigmond S, Boone C. Formins: signaling effectors for assembly and polarization of actin filaments. J Cell Sci. 2003;116(Pt 13):2603–2611. doi: 10.1242/jcs.00611. [DOI] [PubMed] [Google Scholar]
- 51.Zigmond SH. Formin-induced nucleation of actin filaments. Curr Opin Cell Biol. 2004;16(1):99–105. doi: 10.1016/j.ceb.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 52.Pataky F, Pironkova R, Hudspeth AJ. Radixin is a constituent of stereocilia in hair cells. Proc Natl Acad Sci U S A. 2004;101(8):2601–2606. doi: 10.1073/pnas.0308620100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kitajiri S, et al. Radixin deficiency causes deafness associated with progressive degeneration of cochlear stereocilia. J Cell Biol. 2004;166(4):559–570. doi: 10.1083/jcb.200402007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hughes SC, Fehon RG. Understanding ERM proteins--the awesome power of genetics finally brought to bear. Curr Opin Cell Biol. 2007;19(1):51–56. doi: 10.1016/j.ceb.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 55.Pak CW, Flynn KC, Bamburg JR. Actin-binding proteins take the reins in growth cones. Nat Rev Neurosci. 2008;9(2):136–147. doi: 10.1038/nrn2236. [DOI] [PubMed] [Google Scholar]
- 56.Paglini G, Kunda P, Quiroga S, Kosik K, Caceres A. Suppression of radixin and moesin alters growth cone morphology, motility, and process formation in primary cultured neurons. J Cell Biol. 1998;143(2):443–455. doi: 10.1083/jcb.143.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castelo L, Jay DG. Radixin is involved in lamellipodial stability during nerve growth cone motility. Mol Biol Cell. 1999;10(5):1511–1520. doi: 10.1091/mbc.10.5.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wright MB, Hugo C, Seifert R, Disteche CM, Bowen-Pope DF. Proliferating and migrating mesangial cells responding to injury express a novel receptor protein-tyrosine phosphatase in experimental mesangial proliferative glomerulonephritis. J Biol Chem. 1998;273(37):23929–23937. doi: 10.1074/jbc.273.37.23929. [DOI] [PubMed] [Google Scholar]
- 59.Hirono M, Denis CS, Richardson GP, Gillespie PG. Hair cells require phosphatidylinositol 4,5-bisphosphate for mechanical transduction and adaptation. Neuron. 2004;44(2):309–320. doi: 10.1016/j.neuron.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 60.Sakaguchi H, et al. Dynamic compartmentalization of protein tyrosine phosphatase receptor Q at the proximal end of stereocilia: implication of myosin VI-based transport. Cell Motil Cytoskeleton. 2008;65(7):528–538. doi: 10.1002/cm.20275. [DOI] [PubMed] [Google Scholar]
- 61.Goodyear RJ, et al. A receptor-like inositol lipid phosphatase is required for the maturation of developing cochlear hair bundles. J Neurosci. 2003;23(27):9208–9219. doi: 10.1523/JNEUROSCI.23-27-09208.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takenawa T, Itoh T. Phosphoinositides, key molecules for regulation of actin cytoskeletal organization and membrane traffic from the plasma membrane. Biochim Biophys Acta. 2001;1533(3):190–206. doi: 10.1016/s1388-1981(01)00165-2. [DOI] [PubMed] [Google Scholar]
- 63.Riazuddin S, et al. Mutations in TRIOBP, which encodes a putative cytoskeletal-organizing protein, are associated with nonsyndromic recessive deafness. Am J Hum Genet. 2006;78(1):137–143. doi: 10.1086/499164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shahin H, et al. Mutations in a novel isoform of TRIOBP that encodes a filamentous-actin binding protein are responsible for DFNB28 recessive nonsyndromic hearing loss. Am J Hum Genet. 2006;78(1):144–152. doi: 10.1086/499495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seipel K, O'Brien SP, Iannotti E, Medley QG, Streuli M. Tara, a novel F-actin binding protein, associates with the Trio guanine nucleotide exchange factor and regulates actin cytoskeletal organization. J Cell Sci. 2001;114(Pt 2):389–399. doi: 10.1242/jcs.114.2.389. [DOI] [PubMed] [Google Scholar]
- 66.Kitajiri S-i, et al. Genetic, Cell biological and Physiological Analyses of a Mouse Model for DFNB28 Deafness; Abstracts of the 31st Annual Mid Winter Research Meeting of the Association for Research in Otolaryngology; 2008. [Google Scholar]
- 67.Tilney LG, Derosier DJ, Mulroy MJ. The organization of actin filaments in the stereocilia of cochlear hair cells. J Cell Biol. 1980;86(1):244–259. doi: 10.1083/jcb.86.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Drenckhahn D, et al. Three different actin filament assemblies occur in every hair cell: each contains a specific actin crosslinking protein. J Cell Biol. 1991;112(4):641–651. doi: 10.1083/jcb.112.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Furness DN, Mahendrasingam S, Ohashi M, Fettiplace R, Hackney CM. The dimensions and composition of stereociliary rootlets in mammalian cochlear hair cells: comparison between high- and low-frequency cells and evidence for a connection to the lateral membrane. J Neurosci. 2008;28(25):6342–6353. doi: 10.1523/JNEUROSCI.1154-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Broschat KO. Tropomyosin prevents depolymerization of actin filaments from the pointed end. J Biol Chem. 1990;265(34):21323–21329. [PubMed] [Google Scholar]
- 71.Gupton SL, et al. Cell migration without a lamellipodium: translation of actin dynamics into cell movement mediated by tropomyosin. J Cell Biol. 2005;168(4):619–631. doi: 10.1083/jcb.200406063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huckaba TM, Lipkin T, Pon LA. Roles of type II myosin and a tropomyosin isoform in retrograde actin flow in budding yeast. J Cell Biol. 2006;175(6):957–969. doi: 10.1083/jcb.200609155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tokuo H, Mabuchi K, Ikebe M. The motor activity of myosin-X promotes actin fiber convergence at the cell periphery to initiate filopodia formation. J Cell Biol. 2007;179(2):229–238. doi: 10.1083/jcb.200703178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Belyantseva IA, et al. Myosin-XVa is required for tip localization of whirlin and differential elongation of hair-cell stereocilia. Nat Cell Biol. 2005;7(2):148–156. doi: 10.1038/ncb1219. [DOI] [PubMed] [Google Scholar]
- 75.Tokuo H, Ikebe M. Myosin X transports Mena/VASP to the tip of filopodia. Biochem Biophys Res Commun. 2004;319(1):214–220. doi: 10.1016/j.bbrc.2004.04.167. [DOI] [PubMed] [Google Scholar]
- 76.Anderson DW, et al. The motor and tail regions of myosin XV are critical for normal structure and function of auditory and vestibular hair cells. Hum Mol Genet. 2000;9(12):1729–1738. doi: 10.1093/hmg/9.12.1729. [DOI] [PubMed] [Google Scholar]
- 77.Mburu P, et al. Defects in whirlin, a PDZ domain molecule involved in stereocilia elongation, cause deafness in the whirler mouse and families with DFNB31. Nat Genet. 2003;34(4):421–428. doi: 10.1038/ng1208. [DOI] [PubMed] [Google Scholar]
- 78.Mburu P, et al. Whirlin complexes with p55 at the stereocilia tip during hair cell development. Proc Natl Acad Sci U S A. 2006;103(29):10973–10978. doi: 10.1073/pnas.0600923103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Holme RH, Kiernan BW, Brown SD, Steel KP. Elongation of hair cell stereocilia is defective in the mouse mutant whirler. J Comp Neurol. 2002;450(1):94–102. doi: 10.1002/cne.10301. [DOI] [PubMed] [Google Scholar]
- 80.Mustapha M, et al. Whirler mutant hair cells have less severe pathology than shaker 2 or double mutants. J Assoc Res Otolaryngol. 2007;8(3):329–337. doi: 10.1007/s10162-007-0083-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stepanyan R, Belyantseva IA, Griffith AJ, Friedman TB, Frolenkov GI. Auditory mechanotransduction in the absence of functional myosin-XVa. J Physiol. 2006;576(Pt 3):801–808. doi: 10.1113/jphysiol.2006.118547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Probst FJ, et al. Correction of deafness in shaker-2 mice by an unconventional myosin in a BAC transgene. Science. 1998;280(5368):1444–1447. doi: 10.1126/science.280.5368.1444. [DOI] [PubMed] [Google Scholar]
- 83.Dose AC, Burnside B. A class III myosin expressed in the retina is a potential candidate for Bardet-Biedl syndrome. Genomics. 2002;79(5):621–624. doi: 10.1006/geno.2002.6749. [DOI] [PubMed] [Google Scholar]
- 84.Dose AC, Burnside B. Cloning and chromosomal localization of a human class III myosin. Genomics. 2000;67(3):333–342. doi: 10.1006/geno.2000.6256. [DOI] [PubMed] [Google Scholar]
- 85.Walsh T, et al. From flies' eyes to our ears: mutations in a human class III myosin cause progressive nonsyndromic hearing loss DFNB30. Proc Natl Acad Sci U S A. 2002;99(11):7518–7523. doi: 10.1073/pnas.102091699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schneider ME, et al. A new compartment at stereocilia tips defined by spatial and temporal patterns of myosin IIIa expression. J Neurosci. 2006;26(40):10243–10252. doi: 10.1523/JNEUROSCI.2812-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dose AC, et al. The kinase domain alters the kinetic properties of the myosin IIIA motor. Biochemistry. 2008;47(8):2485–2496. doi: 10.1021/bi7021574. [DOI] [PubMed] [Google Scholar]
- 88.Kambara T, Komaba S, Ikebe M. Human myosin III is a motor having an extremely high affinity for actin. J Biol Chem. 2006;281(49):37291–37301. doi: 10.1074/jbc.M603823200. [DOI] [PubMed] [Google Scholar]
- 89.Dose AC, et al. Myo3A, one of two class III myosin genes expressed in vertebrate retina, is localized to the calycal processes of rod and cone photoreceptors and is expressed in the sacculus. Mol Biol Cell. 2003;14(3):1058–1073. doi: 10.1091/mbc.E02-06-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Avraham KB, et al. The mouse Snell's waltzer deafness gene encodes an unconventional myosin required for structural integrity of inner ear hair cells. Nat Genet. 1995;11(4):369–375. doi: 10.1038/ng1295-369. [DOI] [PubMed] [Google Scholar]
- 91.Melchionda S, et al. MYO6, the human homologue of the gene responsible for deafness in Snell's waltzer mice, is mutated in autosomal dominant nonsyndromic hearing loss. Am J Hum Genet. 2001;69(3):635–640. doi: 10.1086/323156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Self T, et al. Role of myosin VI in the differentiation of cochlear hair cells. Dev Biol. 1999;214(2):331–341. doi: 10.1006/dbio.1999.9424. [DOI] [PubMed] [Google Scholar]
- 93.Gibson F, et al. A type VII myosin encoded by the mouse deafness gene shaker-1. Nature. 1995;374(6517):62–64. doi: 10.1038/374062a0. [DOI] [PubMed] [Google Scholar]
- 94.Weil D, et al. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature. 1995;374(6517):60–61. doi: 10.1038/374060a0. [DOI] [PubMed] [Google Scholar]
- 95.Welch MD, Mallavarapu A, Rosenblatt J, Mitchison TJ. Actin dynamics in vivo. Curr Opin Cell Biol. 1997;9(1):54–61. doi: 10.1016/s0955-0674(97)80152-4. [DOI] [PubMed] [Google Scholar]
- 96.Gillespie PG, Cyr JL. Myosin-1c, the hair cell's adaptation motor. Annu Rev Physiol. 2004;66:521–545. doi: 10.1146/annurev.physiol.66.032102.112842. [DOI] [PubMed] [Google Scholar]
- 97.Dumont RA, Zhao YD, Holt JR, Bahler M, Gillespie PG. Myosin-I isozymes in neonatal rodent auditory and vestibular epithelia. J Assoc Res Otolaryngol. 2002;3(4):375–389. doi: 10.1007/s101620020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McConnell RE, Tyska MJ. Myosin-1a powers the sliding of apical membrane along microvillar actin bundles. J Cell Biol. 2007;177(4):671–681. doi: 10.1083/jcb.200701144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Donaudy F, et al. Multiple mutations of MYO1A, a cochlear-expressed gene, in sensorineural hearing loss. Am J Hum Genet. 2003;72(6):1571–1577. doi: 10.1086/375654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tyska MJ, et al. Myosin-1a is critical for normal brush border structure and composition. Mol Biol Cell. 2005;16(5):2443–2457. doi: 10.1091/mbc.E04-12-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yengo CM, Ananthanarayanan SK, Brosey CA, Mao S, Tyska MJ. Human deafness mutation E385D disrupts the mechanochemical coupling and subcellular targeting of myosin-1a. Biophys J. 2008;94(2):L5–L7. doi: 10.1529/biophysj.107.122689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mogilner A, Oster G. Cell motility driven by actin polymerization. Biophys J. 1996;71(6):3030–3045. doi: 10.1016/S0006-3495(96)79496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mogilner A, Oster G. Force generation by actin polymerization II: the elastic ratchet and tethered filaments. Biophys J. 2003;84(3):1591–1605. doi: 10.1016/S0006-3495(03)74969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jurado LA, Chockalingam PS, Jarrett HW. Apocalmodulin. Physiol Rev. 1999;79(3):661–682. doi: 10.1152/physrev.1999.79.3.661. [DOI] [PubMed] [Google Scholar]
- 105.Oertner TG, Matus A. Calcium regulation of actin dynamics in dendritic spines. Cell Calcium. 2005;37(5):477–482. doi: 10.1016/j.ceca.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 106.Fettiplace R, Hackney CM. The sensory and motor roles of auditory hair cells. Nat Rev Neurosci. 2006;7(1):19–29. doi: 10.1038/nrn1828. [DOI] [PubMed] [Google Scholar]
- 107.Littlewood Evans A, Muller U. Stereocilia defects in the sensory hair cells of the inner ear in mice deficient in integrin alpha8beta1. Nat Genet. 2000;24(4):424–428. doi: 10.1038/74286. [DOI] [PubMed] [Google Scholar]
- 108.Longo-Guess CM, et al. A missense mutation in the previously undescribed gene Tmhs underlies deafness in hurry-scurry (hscy) mice. Proc Natl Acad Sci U S A. 2005;102(22):7894–7899. doi: 10.1073/pnas.0500760102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Su MC, et al. Expression and localization of Tmie in adult rat cochlea. Histochem Cell Biol. 2008;130(1):119–126. doi: 10.1007/s00418-008-0385-z. [DOI] [PubMed] [Google Scholar]
- 110.Mitchem KL, et al. Mutation of the novel gene Tmie results in sensory cell defects in the inner ear of spinner, a mouse model of human hearing loss DFNB6. Hum Mol Genet. 2002;11(16):1887–1898. doi: 10.1093/hmg/11.16.1887. [DOI] [PubMed] [Google Scholar]
- 111.Ernstson S, Smith CA. Stereo-kinociliar bonds in mammalian vestibular organs. Acta Otolaryngol. 1986;101(5–6):395–402. doi: 10.3109/00016488609108624. [DOI] [PubMed] [Google Scholar]
- 112.Li A, Xue J, Peterson EH. Architecture of the mouse utricle: macular organization and hair bundle heights. J Neurophysiol. 2008;99(2):718–733. doi: 10.1152/jn.00831.2007. [DOI] [PubMed] [Google Scholar]
- 113.Bashtanov ME, Goodyear RJ, Richardson GP, Russell IJ. The mechanical properties of chick (Gallus domesticus) sensory hair bundles: relative contributions of structures sensitive to calcium chelation and subtilisin treatment. J Physiol. 2004;559(Pt 1):287–299. doi: 10.1113/jphysiol.2004.065565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brown SD, Hardisty-Hughes RE, Mburu P. Quiet as a mouse: dissecting the molecular and genetic basis of hearing. Nat Rev Genet. 2008;9(4):277–290. doi: 10.1038/nrg2309. [DOI] [PubMed] [Google Scholar]
- 115.Morell RJ, et al. A new locus for late-onset, progressive, hereditary hearing loss DFNA20 maps to 17q25. Genomics. 2000;63(1):1–6. doi: 10.1006/geno.1999.6058. [DOI] [PubMed] [Google Scholar]
- 116.Zhu M, et al. Mutations in the gamma-actin gene (ACTG1) are associated with dominant progressive deafness (DFNA20/26) Am J Hum Genet. 2003;73(5):1082–1091. doi: 10.1086/379286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.van Wijk E, et al. A mutation in the gamma actin 1 (ACTG1) gene causes autosomal dominant hearing loss (DFNA20/26) J Med Genet. 2003;40(12):879–884. doi: 10.1136/jmg.40.12.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rendtorff ND, et al. A novel missense mutation in ACTG1 causes dominant deafness in a Norwegian DFNA20/26 family, but ACTG1 mutations are not frequent among families with hereditary hearing impairment. Eur J Hum Genet. 2006;14(10):1097–1105. doi: 10.1038/sj.ejhg.5201670. [DOI] [PubMed] [Google Scholar]
- 119.Furness DN, Katori Y, Mahendrasingam S, Hackney CM. Differential distribution of beta- and gamma-actin in guinea-pig cochlear sensory and supporting cells. Hear Res. 2005;207(1–2):22–34. doi: 10.1016/j.heares.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 120.Shin JB, et al. Hair bundles are specialized for ATP delivery via creatine kinase. Neuron. 2007;53(3):371–386. doi: 10.1016/j.neuron.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Street VA, McKee-Johnson JW, Fonseca RC, Tempel BL, Noben-Trauth K. Mutations in a plasma membrane Ca2+-ATPase gene cause deafness in deafwaddler mice. Nat Genet. 1998;19(4):390–394. doi: 10.1038/1284. [DOI] [PubMed] [Google Scholar]
- 122.Grati M, et al. Rapid turnover of stereocilia membrane proteins: evidence from the trafficking and mobility of plasma membrane Ca(2+)-ATPase 2. J Neurosci. 2006;26(23):6386–6395. doi: 10.1523/JNEUROSCI.1215-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]