Abstract
In laboratory studies, hamsters (Mesocricetus spp.) exhibit intense male-male aggression, thus making them an excellent model system for studies of the functional and mechanistic bases of aggression. In a field study of golden hamsters (M. auratus) in the wild, however, the few documented male-male interactions were not highly aggressive. Thus, we tested the hypothesis that familiarity modulates aggression in hamsters. Previous investigations of the effects of familiarity on aggression have mostly involved familiarization of unfamiliar individuals through agonistic interactions. Here we allowed male Turkish hamsters (M. brandti) to become familiar with each other by housing them together but separated by a wire-mesh partition (thus ‘non-agonistic’ familiarity). We found that although non-agonistic familiarity did not decrease investigation of the familiar male, it did decrease the occurrence of fights, the number of fights, and the percentage of time fighting; it also increased the latency to fight. These results are consistent with the ‘dear enemy’ hypothesis, which proposes that males are less aggressive toward familiar neighbors than to unfamiliar conspecifics because previous interactions have provided enough information about the other individual to render severe aggression unnecessary. Most importantly, our results suggest that information gained about other individuals through non-agonistic interactions decrease the frequency and intensity of fights with those individuals. We conclude that results from laboratory studies on aggression that do not consider the kind of social interactions that individuals have in nature should be interpreted with caution.
Keywords: aggression, chemical communication, familiarity, individual recognition, Mesocricetus brandti, odours, social memory, Turkish hamster
Given the potential costs of aggression, selection should favor mechanisms that allow animals to gain information about one another, remember that information, and recognize previously encountered individuals (O'Connor et al. 2000), thus decreasing the need for repeated aggressive interactions. In many territorial species, disputes between familiar animals are less intense than those between unfamiliar animals (Chase 1985; Ydenberg et al. 1988). Previous non-agonistic or moderately agonistic interactions may be enough to establish memories of individuals and/or dominant-subordinate relationships. Thus, intense fights may be unnecessary (Ydenberg et al. 1988; Jensen & Yngvesson 1998; Petrulis et al. 2004). Such exchanges of information may occur when juvenile males disperse to their new locations and interact with other juveniles and established adult males. If juvenile males do not elicit high levels of aggression from adult males, familiarity via non-agonistic interactions may occur. Thus, when juvenile males become agonistic, aggression between them and the established adult males may be buffered by previous familiarity.
For this strategy to work, however, males need to remember individuals from previous encounters (O'Connor et al. 2000; Trigosso-Venario et al. 2002), and this ability has been convincingly shown in the golden hamster (Mesocricetus auratus). After laboratory male hamsters engaged in aggressive interactions, the losers recognized the winner after short-term and long-term intervals (30 min and one week, respectively; Lai & Johnston 2002). In another study, male hamsters that lost a fight showed more locomotion and escape behaviours in response to familiar winners than to unfamiliar winners, indicating recognition of the male to which they had lost. The losers also scent marked less in response to the odours of a familiar winner than to those of an unfamiliar winner (Petrulis et al. 2004); given that scent marking is an agonistic behavior, losers were less agonistic toward familiar than toward unfamiliar conspecifics (Johnston 1975a, b, c, 1977). However, in all of these studies the information about a familiar individual was gained by fighting with a previously unfamiliar individual. Whether relevant information leading to familiarity with another male may be obtained without agonistic interactions is still unclear.
We hypothesize that non-agonistic familiarity modulates the level of aggression between males. We tested this hypothesis by conducting two experiments with male Turkish hamsters (M. brandti). In experiment 1 we examined whether familiarity obtained during non-agonistic interactions with an individual decreased later investigation of that individual's odours. We predicted that an individual would spend less time investigating cues from familiar individuals than those from unfamiliar individuals. To test this prediction we measured the time that individuals investigated the odours of familiar and unfamiliar males. In experiment 2 we examined whether familiarity obtained in a non-agonistic context between two individuals would affect aggression in later interactions. We predicted that non-agonistic familiarity would increase the latency to fight, and would decrease the frequency and duration of fights. To test this prediction we placed two familiar or two unfamiliar males in the same arena and measured agonistic behaviour.
Hamsters (Mesocricetus spp.) are commonly used to investigate the functional and mechanistic bases of aggression. Several researchers have shown that male laboratory hamsters (M.auratus) that are unfamiliar with one another engage in high intensity fights until one of the pair leaves the arena (Lai & Johnston 2002; Petrulis et al. 2004; Albers et al. 2006; Bath & Johnston 2007). These fights can result in injury; in subsequent encounters between the same males, the loser actively avoids the winner (Lai & Johnston 2002; Lai et al. 2005; Bath & Johnston 2007). In the wild, highly aggressive interactions can be very costly because such encounters use large amounts of energy, increase exposure to predators, and potentially result in injury or death (King 1973; Jakobsson et al. 1995; O'Connor et al. 2000). Thus, avoiding fights may be beneficial for both participants and, in the wild, intense fights that continue until one or both males are severely injured may be more rare than lab studies suggest. In fact, in a field study of wild golden hamsters in south central Turkey, we rarely saw two males interact with one another, and the few interactions we did observe did not involve prolonged fighting; indeed, actual contact rarely occurred, and when it did occur, contact was very brief, from less than a second to three seconds (R. E. Johnston, Z. Song, S. Larimer, & J. E. Johnston, unpublished data).
Experiment 1
Methods
All animals were born and raised in captivity at Cornell University, Ithaca, NY. We weaned hamsters at 30 days of age and housed them singly in solid bottom polycarbonate cages (45 × 24 × 14.5 cm) with sani-chip bedding material and constant access to food (Prolab 1000, Agway, Syracuse, NY) and water. We maintained the colony on a 16L:8D light-dark schedule with lights off between 10:00 and 18:00 hours (Eastern Standard Time). We run experiments within the first three hours of the dark phase. Pairs of males were not closely related to each other, nor had they had any experience with each other prior to the start of the experiment. Experimenters wore clean latex gloves to avoid the transfer of human scents.
We used 10 male Turkish hamsters divided into five pairs. They were 273.4 ± 17.71 (mean ± SE) days old and weighed 136.8 ± 4.09 g. We matched the five pairs for weight (4.4 ± 0.68 g difference) and age (27.6 ± 7.85 d difference). We placed each pair of males in a clean plexiglas cage (45 × 24 × 14.5 cm) separated into two areas by a wire-mesh screen (24 × 14.5 cm; 1.25 × 1.25 cm holes) in the center of the cage. The two males were thus able to see, hear, smell and touch one another but they were unable to fight. Investigation across this type of wire-mesh barrier has been shown to be sufficient for hamsters to learn individually distinctive odours of other individuals (Johnston & Peng 2008). These familiarization trials lasted 10 min and were repeated for 10 days (with a two-day break between days 5 and 6). In all of these familiarization trials both males spent most of the time by the wire-mesh screen investigating each other. After each of the familiarization trials, we placed each male by himself into an arena (60 × 60 × 17.8 cm) for a 10-min period to habituate him to this arena. The arena was divided into four quadrants (interior dimensions of each quadrant, 29.6 × 29.6 × 17.8 cm) by a cross-shaped, polycarbonate divider. In the middle of each arm of the cross there was an opening (6.4 × 6.4 cm) that allowed subjects to move freely throughout the four quadrants of the arena. We had two of these arenas, so that both males within a pair were familiarized with this type of arena simultaneously. After the familiarization trial and the arena habituation period, each male was returned to his own home cage.
We tested males in one of these arenas on day 11, the day after the tenth day of familiarization trials. We tested each male twice, once in the familiar context and once in the unfamiliar context (see below). We assigned the first test for each male at random (we tested four males in the familiar context first, whereas we tested the other six males in the unfamiliar context first). In both contexts, we attached four glass plates (17.8 × 7.6 cm) per quadrant to the arena walls using metal spring clips. Two glass plates per quadrant were unscented and the other two were scented. In each trial, the ten scented glass plates contained the odours of the same male donor. In the familiar context, the odour donor for the trial of a given male was the male used during the 10 familiarization trials in the other side of the partition (i.e., the familiar male). In the unfamiliar context, the odour donors were males with which the subjects had had no prior experience. Odours consisted of flank-gland secretions, which have a major role in chemical communication in hamsters (Johnston et al. 1993). To place the flank gland odours of a male on the glass plate, we rubbed the plate 10 times against the flank gland area of the donor male. The scented area ran horizontally across the glass plate approximately 2 cm from a short edge of the plate. This short edge was the one closer to the ground. In each trial, we measured the number of seconds the subject investigated each glass plate. An investigation began when the animal's nose was oriented toward the scent and was within 1 cm of the scented portion of the glass plate. In all cases, the animal was visibly sniffing the plate. Following each trial, we removed all of the plates, cleaned them with hot water and soap, air-dried them, and rubbed them with 50% ethanol. We cleaned the arena with 50% ethanol.
We used paired-sample t-tests to compare investigation time of the scented and unscented plates within trials and a General Linear Model with two factors to compare investigation time of the scented plates between the familiar and unfamiliar contexts (one factor was condition, i.e., familiar or unfamiliar context; the other factor was order, i.e., whether the subject male was exposed first to the odours of a familiar male and second to the odours of an unfamiliar male, or vice versa). We usedSPSS 14.0 for Windows for statistical analyses. We tested normality using the Kolmogorov-Smirnov test (all variables had a normal distribution, P > 0.05).
Results
Males investigated scented locations more than unscented locations regardless of whether the odours were from a familiar male (scented plates, 64.47 ± 5.69 s, 95% confidence interval (CI) = 51.61-77.34; unscented plates, 16.97 ± 2.11 s, 95% CI = 10.47-18.65; paired t-test: t9 = -8.58, P < 0.0005) or unfamiliar male (scented plates, 58.12 ± 6.39 s, 95% CI = 43.67-72.57; unscented plates, 14.56 ± 1.81 s, 95% CI = 10.47-18.65; t9 = -8.11, P < 0.0005; Fig. 1). However, we did not find any significant differences between the time that focal males spent investigating the odours of familiar and unfamiliar males (F1,16 = 2.21, P = 0.16). Similarly, total investigation time (adding together the investigation time of unscented and scented locations) was similar in the familiar and unfamiliar conditions (F1,16 = 2.72, P = 0.12). There was an order effect (F1,16 = 9.58, P = 0.007), with males investigating scented locations for a longer time during the first test (familiar odours, 76.83 ± 8.97 s, 95% CI = 48.28-105.37; unfamiliar odours, 67.98 ± 7.72 s, 95% CI = 48.13-87.83) than during the second test (familiar odours, 56.23 ± 5.56 s, 95% CI = 41.94-70.53; unfamiliar odours, 43.33 ± 5.93 s, 95% CI = 24.46-62.19).
Fig. 1.
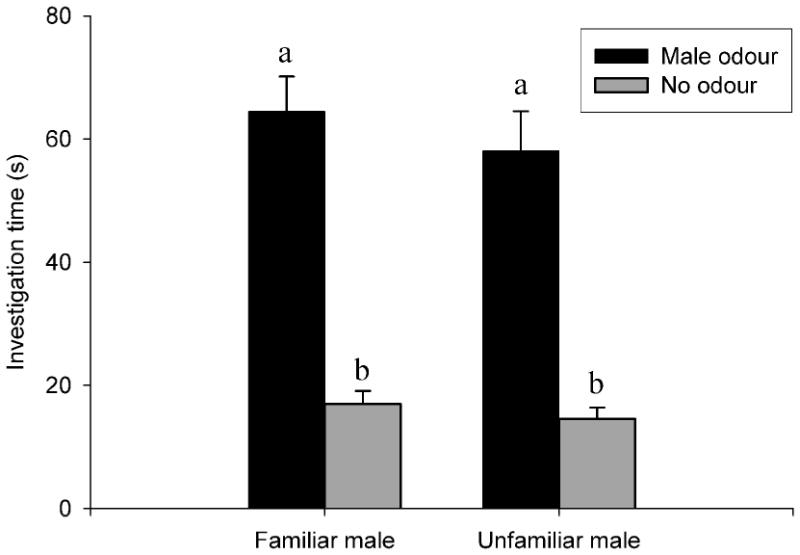
Number of seconds that male hamsters investigated scented (‘Male odour’) versus unscented (‘No odour’) locations, depending on whether the odour donor was a familiar or an unfamiliar individual. All values are show as mean ± SE. A different letter indicates a statistically significant difference.
Experiment 2
In this experiment we investigated whether familiarity in a non-agonistic context between two males affects latency to fight, number of fights, or fighting duration, when those two males are placed in the same arena.
Methods
Rearing conditions were similar to those described in experiment 1. We run trials approximately two hours into the dark phase of the daily cycle. We tested 44 Turkish hamsters (average age, 211.05 ± 9.17 d; average weight, 132.43 ± 2.56 g) in either a familiar or an unfamiliar condition, i.e., each male was tested only once. We used two males per trial (N = 11 per condition). Pairs of subjects were not closely related nor had they had any experience with each other prior to the start of the experiment. We matched all pairs of males tested together for weight (5.05 ± 1.48 g difference) and age (20.27 ± 5.66 d difference). We housed pairs of males for two days in the same cage (45 × 48 × 14.5 cm) but separated from each other by a wire-mesh barrier (1.25 × 2.5 cm mesh; each cage section was 45 × 24 × 14.5 cm). This barrier allowed the two males to hear, see, smell, and touch each other but prevented fighting. In the test trial for the familiar condition, we tested in an arena a pair of males that had cohabited continuously for 48 h across the wire mesh. The arena (60 × 60 × 29.5 cm) was partitioned into four quadrants (interior dimensions, 28 × 28 cm) by a cross-shaped divider. The arena and the divider were made of wood, sealed with a waterproof latex paint. Each arm of the divider had an opening (6 × 6 cm) in the middle that allowed subjects to move freely throughout the four quadrants of the arena. We placed the two males simultaneously in opposite quadrants in the arena. For identification purposes, we placed a small piece of tape on the back of one male. The trial lasted for four minutes and was filmed (Sony Handycam DCR-HC38) for later measurement of agonistic interactions. After the trial, we transferred the two males to separate, clean cages, and we cleaned the arena with soap and warm water followed by 50% ethanol to remove any scents deposited by the subjects. In the test trial for the unfamiliar condition, we also used pairs of males that had cohabited for 48 h across the wire mesh from another male. We used two pairs at a time. That is, we tested each male of a pair with a male chosen at random from the other pair. Thus, even though those four males had cohabited with one male for 48 hours, we tested them with an unfamiliar male. Testing procedures in the arena were the same as for the familiar condition.
We measured four variables. “Latency to first fight” was the time from the first interaction to the beginning of the first fight. The first interaction occurred when the two animals investigated each other for the first time. The criterion for a ‘fight’ was that it was a rolling fight, in which both males wrap themselves around one another perpendicularly and roll while biting or attempting to bite each other on the flanks (Siegel 1985). If no fight occurred, we measured the amount of time from the first interaction to the end of the trial. We calculated “Fights per minute” by dividing the total number of fights by the number of minutes from the first interaction to the end of the trial. We considered two fights separate from each other when there was at least a delay of four seconds between them. If no fight occurred, the value was zero. We also calculated the percentage of time (from the first interaction to the end of the trial) that the two males spent fighting. “Interaction percentage” was the percentage of time (from the first interaction to the end of the trial) that the two males spent interacting with each other, i.e., smelling, following, touching, or fighting with each other.
We used two-tailed independent t-tests to compare the four variables between the familiar and unfamiliar conditions. We tested normality using the Kolmogorov-Smirnov test. We log-transformed “Fights per minute” and “Fighting percentage” prior to statistical analysis to normalize those two variables. We used SPSS 14.0 for Windows for statistical analyses unless otherwise noticed.
Results
Unfamiliar males engaged in more fights than did familiar males, and fights were also longer in duration when males were unfamiliar with each other. The latency to the first fight was longer in the familiar condition (149.91 ± 27.55 s, 95% CI = 88.52-211.29) than in the unfamiliar condition (74.73 ± 16.15 s, 95% CI = 38.74-110.71; independent t-test: t16 = 2.35, P = 0.032; Fig. 2a). There were fewer fights per minute in the familiar condition (0.27 ± 0.08 %, 95% CI = 0.09-0.45) than in the unfamiliar condition (0.6 ± 0.15 %, 95% CI = 0.27-0.93; t20 = -2.24, P = 0.036; Fig. 2b). The percentage of time spent fighting was lower in the familiar condition (4.8 ± 1.72 %, 95% CI = 0.97-8.64) than in the unfamiliar condition (16.44 ± 5.58%, 95% CI = 4.01-28.87; t17 = -2.32, P = 0.033; Fig. 2c).
Fig. 2.
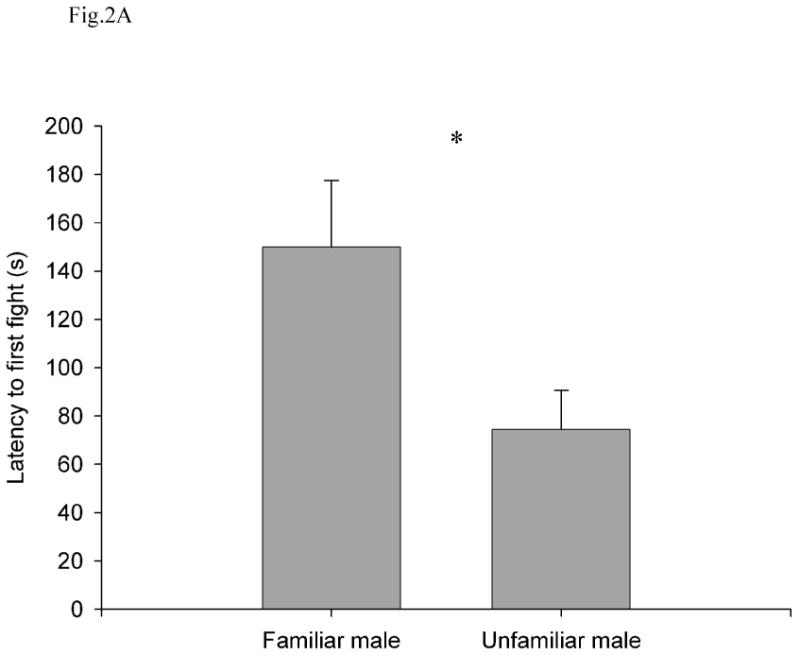
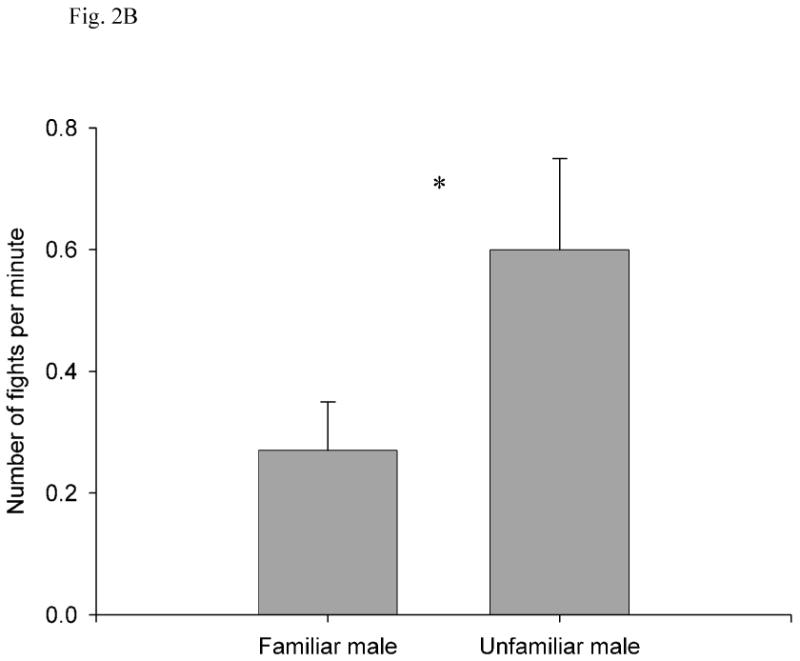
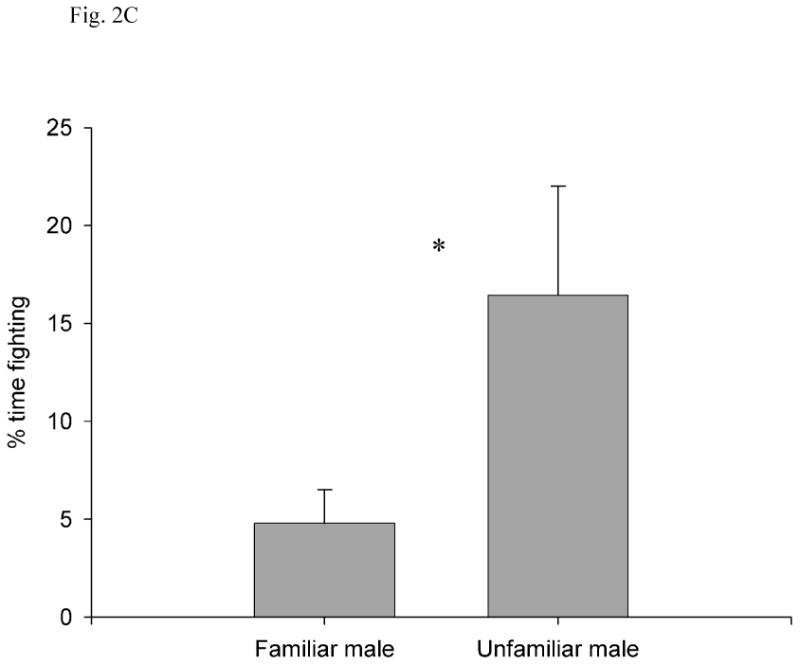
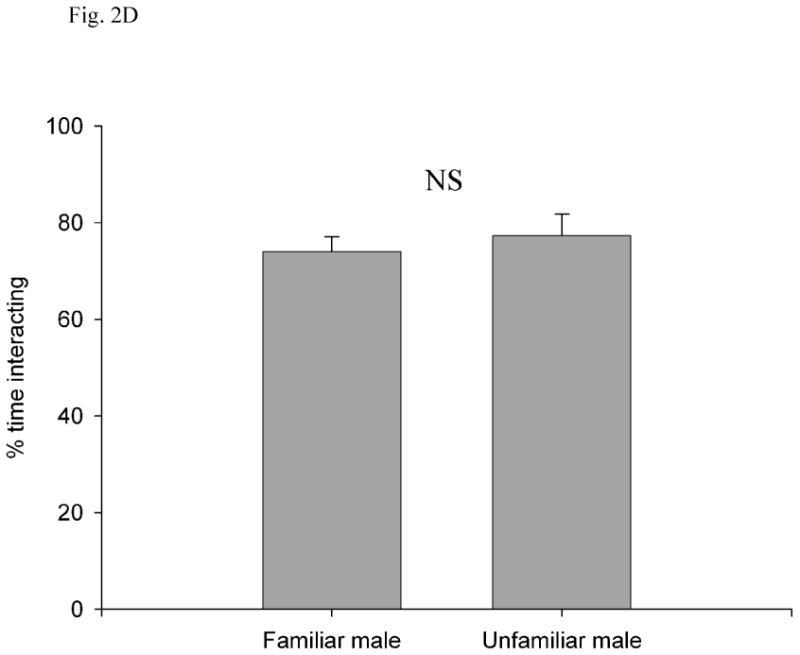
(A) Latency to first fight, (B) number of fights per minute, (C) percentage of time fighting, and (D) percentage of time interacting as a function of familiarity between the two subject males. All values are show as mean ± SE. *, P < 0.05; NS, P > 0.05.
The percentage of time spent interacting did not differ significantly between the familiar (74.05 ± 3.06, 95% CI = 67.22-80.88) and unfamiliar (77.34 ± 4.39, 95% CI = 67.56-87.12; t20 = -0.61, P = 0.55) conditions (Fig. 2d). Thus, the higher percentage of time spent fighting observed between unfamiliar males cannot be explained by longer interactions between them. In fact, there was no relationship between percentage of time spent interacting and percentage of time spent fighting (Spearman's correlation: coefficient = 0.25, N = 22, P = 0.27).
Fights occurred in all the trials (11/11) in the unfamiliar condition. The males in the familiar condition fought in only seven of the 11 trials (63.6%). However, the difference in the percentage of trials with fights for the familiar and unfamiliar conditions was not statistically significant (Fisher's Exact Test for Count Data, using R (R-Development-Core-Team 2006): d.f. = 1, P = 0.09).
Discussion
In this study, we tested the hypothesis that familiarity obtained during non-agonistic interactions reduces aggression between male Turkish hamsters. We predicted that time spent investigating odours from a familiar male would be less than time spent investigating odours from an unfamiliar male due to habituation and a decreased interest to investigate the familiar individual. We also predicted that familiarity gained during non-agonistic interactions would enable animals to determine relative social status without aggression, resulting in fewer fights between familiar males compared to males that were unfamiliar with one another.
We found that male Turkish hamsters investigated the odours of familiar and unfamiliar males equally (experiment 1), and familiar and unfamiliar pairs of males interacted for most of the time they were together in an arena (experiment 2). That is, familiarity obtained in a non-agonistic context did not seem to decrease motivation to interact with the familiar male. Non-agonistic familiarity did, however, decrease the number of fights and the percentage of time fighting. Familiarity also increased the latency to fight. Our results thus are consistent with the ‘dear enemy’ hypothesis, which predicts less aggression toward familiar neighbors than toward unfamiliar conspecifics (Ydenberg et al. 1988). This hypothesis further proposes that, after two individuals have fought and established a social relationship, the information gained makes future aggression less likely, whereas a lack of information about unfamiliar conspecifics constitutes more of a threat and may lead to agonistic interactions (Ydenberg et al. 1988). Our results add to the ‘dear enemy’ hypothesis the notion that familiarity with neighbors may be gained in the absence of aggressive interactions.
A decrease in aggressive behaviour due to non-agonistic familiarity may be explained in two ways. First, males able to investigate each other through a wire-mesh barrier may be able to determine their relative quality, so that when they interact later on, they can use that knowledge to determine whether or not fighting is necessary or likely to have a negative outcome. Second, males familiarized with each other may become mutually habituated to the point that each becomes less of a motivating stimulus to the other. If so, when placed together, there should be less interest in interacting with each other in any way, including fighting. Our results (similar investigation of or interaction with a familiar male or an unfamiliar male) do not support the second explanation, i.e. loss of interest due to habituation. The decrease in fighting toward familiar males strongly suggests that information gained in previous non-agonistic encounters may diminish the value of highly aggressive interactions as a way of obtaining information about competing conspecifics.
Several studies in other species using females as subjects have established that familiarity may reduce antagonistic behaviours toward males. For example, female red-backed salamanders (Plethodon cinereus) were more submissive toward familiar versus unfamiliar males in controlled dyadic interactions (Guffey et al. 1998). This type of reduction in aggressive behaviour is, however, related to sexual behaviour and not to the establishment of dominant-subordinate relationships between same-sex conspecifics.
Similar to our results, male lizards (Liolaemus tenuis) showed delayed aggressive behaviour toward another male with whom they had shared a cage as compared to animals with whom they had had no prior exposure, although the frequency of attacks was not affected by familiarity (Trigosso-Venario et al. 2002). However, in this and similar studies, familiarity occurred by placing the subjects together in the same enclosure, so fighting interactions may have been part of the familiarity process. In contrast, in our study we investigated the effect of familiarity in the absence of any agonistic interactions prior to testing for aggressive interactions.
In a study of meadow voles (Microtus pennsylvanicus), two males were paired in a clear plastic tube and agonistic behaviours measured (Ferkin 1988). In half of the trials, the pair had been exposed to nesting material from the other male's home cage over a six-day period. Contrary to what we found with Turkish hamsters, male voles that had been exposed to their opponent's bedding showed more agonistic behaviours than males that had not been exposed to each other's odours. Interestingly, the opposite result was found for females, i.e., odour familiarity reduced agonistic behaviour. As in the current study, investigative behaviour was not affected in either sex by familiarity. Similarly to the study with meadow voles, Zhang et al. (2001) found that when allowed to interact in a glass arena, male rat-like hamsters (Cricetulus triton) that were considered familiar with one another (i.e., trapped within 5 m of each other) were more aggressive than those considered unfamiliar (i.e., animals trapped > 1 km away from each other or trapped in locations separated by a barrier such as a river or highway). As in Ferkin's (1988) study, familiarity decreased aggression between females. More information is needed about the natural history of Turkish hamsters to explain why familiarity decreases aggression in male Turkish hamsters, but increases aggression in male meadow voles and male rat-like hamsters. Given that the potential costs of aggression may be similar in these species, there may be different selection pressures leading to opposite aggressive responses toward familiar individuals in different species.
We propose that laboratory hamsters may be highly aggressive because in laboratory experiments they are forced to interact with unfamiliar males about whom they have no prior information. Our results indicate that information obtained via visual, auditory, olfactory, and tactile cues is sufficient to decrease levels of aggression between male hamsters. More support for the idea that aggression may be less intense in the wild than in laboratory conditions comes from a study in which male golden hamsters that interacted with a non-aggressive male three times per week for three weeks prior to testing were much less aggressive than males reared in social isolation (Albers et al. 2006). Those socially experienced individuals may have been more similar to males in the wild in that animals are unlikely to be socially isolated for weeks, even in a solitary species like the Turkish hamster. Our results indicate that male-male social experience influenced the level and intensity of aggression in laboratory tests. We suggest that studies designed to understand typical or natural aspects of aggression in the laboratory should further investigate the effects of various types of social interaction and social knowledge on the level, intensity and types of aggression observed.
Acknowledgments
We thank Kay Simmons for help running experiment 1. This work was supported by NIH Grant RO1 MH-58001 to R.E.J.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethical Note
Experiment 2 involved fights between males. Some males were superficially injured, but did not seem distressed and completely recovered. All research was conducted with approval from Cornell University's Institutional Animal Care and Use Committee (protocol #1993-0120).
References
- Albers HE, Dean A, Karom MC, Smith D, Huhman KL. Role of V1a vasopressin receptors in the control of aggression in Syrian hamsters. Brain Research. 2006;1073-1074:425–430. doi: 10.1016/j.brainres.2005.12.081. [DOI] [PubMed] [Google Scholar]
- Bath KG, Johnston RE. Dominant-subordinate relationships in hamsters: Sex differences in reactions to familiar opponents. Hormones and Behavior. 2007;51:258–264. doi: 10.1016/j.yhbeh.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase ID. The sequential analysis of aggressive acts during hierarchy formation: an application of the ‘jigsaw puzzle’ approach. Animal Behaviour. 1985;33:86–100. doi: 10.1016/S0003-3472(85)80122-6. [DOI] [Google Scholar]
- Ferkin MH. The effect of familiarity on social interactions in meadow voles, Microtus pennsylvanicus: a laboratory and field study. Animal Behaviour. 1988;36:1816–1822. doi: 10.1016/S0003-3472(88)80121-0. [DOI] [Google Scholar]
- Guffey C, MaKinster JG, Jaeger RG. Familiarity affects interactions between potentially courting territorial salamanders. Copeia. 1998;1998:205–208. [Google Scholar]
- Jakobsson S, Brick O, Kullberg C. Escalated fighting behaviour incurs increased predation risk. Animal Behaviour. 1995;49:235–239. doi: 10.1016/0003-3472(95)80172-3. [DOI] [Google Scholar]
- Jensen P, Yngvesson J. Aggression between unacquainted pigs--sequential assessment and effects of familiarity and weight. Applied Animal Behaviour Science. 1998;58:49–61. [Google Scholar]
- Johnston RE. Scent marking by male golden hamsters (Mesocricetus auratus). I. Effects of odors and social encounters. Zeitschrift fur Tierpsychologie. 1975a;37:75–98. doi: 10.1111/j.1439-0310.1975.tb01128.x. [DOI] [PubMed] [Google Scholar]
- Johnston RE. Scent marking by male golden hamsters (Mesocricetus auratus). II. The role of the flank gland scent in the causation of marking. Zeitschrift fur Tierpsychologie. 1975b;37:138–144. [PubMed] [Google Scholar]
- Johnston RE. Scent marking by male golden hamsters (Mesocricetus auratus). III. Behavior in a seminatural environment. Zeitschrift fur Tierpsychologie. 1975c;37:213–221. [PubMed] [Google Scholar]
- Johnston RE. The causation of two scent marking behavior patterns in female hamsters (Mesocricetus auratus) Animal Behaviour. 1977;25:317–327. doi: 10.1016/0003-3472(77)90007-0. [DOI] [PubMed] [Google Scholar]
- Johnston RE, Derzie A, Chiang G, Jernigan P, Lee HC. Individual scent signatures in golden hamsters: Evidence for specialization of function. Animal Behaviour. 1993;45:1061–1070. [Google Scholar]
- Johnston RE, Peng A. Memory for individuals: Hamsters (Mesocricetus auratus) require contact to develop multicomponent representations (concepts) of others. Journal of Comparative Psychology. 2008;122:121–131. doi: 10.1037/0735-7036.122.2.121. [DOI] [PubMed] [Google Scholar]
- King JA. The ecology of aggressive behavior. Annual Review of Ecology and Systematics. 1973;4:117–138. [Google Scholar]
- Lai WS, Johnston RE. Individual recognition after fighting by golden hamsters: A new method. Physiology and Behavior. 2002;76:225–239. doi: 10.1016/s0031-9384(02)00721-7. [DOI] [PubMed] [Google Scholar]
- Lai WS, Ramiro LLR, Yu HA, Johnston RE. Recognition of familiar individuals in golden hamsters: a new method and functional neuroanatomy. Journal of Neuroscience. 2005;25:11239–11247. doi: 10.1523/jneurosci.2124-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor KI, Metcalfe NB, Taylor AC. Familiarity influences body darkening in territorial disputes between juvenile salmon. Animal Behaviour. 2000;59:1095–1101. doi: 10.1006/anbe.2000.1401. [DOI] [PubMed] [Google Scholar]
- Petrulis A, Weidner M, Johnston RE. Recognition of competitors by male golden hamsters. Physiology & Behavior. 2004;81:629–638. doi: 10.1016/j.physbeh.2004.03.001. [DOI] [PubMed] [Google Scholar]
- R-Development-Core-Team. Vienna, Austria: 2006. R: A language and environment for statistical computing. R Foundation for Statistical Computing. URL http://www.R-project.org. [Google Scholar]
- Siegel HI. Aggressive behavior. In: Siegel HI, editor. The Hamster: Reproduction and Behavior. New York: Plenum Press; 1985. pp. 261–286. [Google Scholar]
- Trigosso-Venario R, Labra A, Niemeyer HM. Interactions between males of the lizard Liolaemus tenuis: roles of familiarity and memory. Ethology. 2002;108:1057–1064. [Google Scholar]
- Ydenberg RC, Giraldeau LA, Falls JB. Neighbours, strangers, and the asymmetric war of attrition. Animal Behaviour. 1988;36:343–347. doi: 10.1016/S0003-3472(88)80004-6. [DOI] [Google Scholar]
- Zhang JX, Zhang ZB, Wang ZW. Seasonal changes in and effects of familiarity on agonistic behaviors of rat-like hamsters (Cricetulus triton) Ecological Research. 2001;16:309–317. [Google Scholar]


