Abstract
Background
Gray matter lesions are known to be common in multiple sclerosis (MS), and are suspected to play an important role in disease progression and clinical disability. A combination of MRI techniques, double-inversion recovery (DIR) and phase-sensitive inversion recovery (PSIR), has been used for detection and classification of cortical lesions. We now demonstrate that high-resolution three-dimensional (3D) magnetization-prepared rapid acquisition with gradient echo (MPRAGE) improves the classification of cortical lesions by allowing more accurate anatomic localization of lesion morphology.
Methods
11 MS patients with previously identified cortical lesions were scanned using DIR, PSIR, and 3D MPRAGE. Lesions were identified on DIR and PSIR and classified as purely intracortical or mixed. MPRAGE images were then examined and lesions were re-classified based on the new information.
Results
The high signal-to-noise ratio, fine anatomic detail, and clear gray-white matter tissue contrast seen in the MPRAGE images provided superior delineation of lesion borders and surrounding gray-white matter junction, improving classification. 119 lesions were identified as either intracortical or mixed on DIR/PSIR. In 89 cases, MPRAGE confirmed the classification by DIR/PSIR. In 30 cases, MPRAGE overturned the original classification.
Conclusion
Improved classification of cortical lesions was realized by inclusion of high-spatial resolution 3D MPRAGE. This sequence provides unique detail into lesion morphology that is necessary for accurate classification.
Keywords: Cortical lesions, MRI, Demyelination, Multiple sclerosis
Introduction
Accurate classification of multiple sclerosis (MS) lesions originating in the cerebral cortex is important for understanding their role in disease progression and impact on clinical manifestations of the disease. Gray matter lesions are not uncommon in MS [1-3], and may play an important role in disease progression and clinical disability [4]. They may also contribute to cognitive impairment and seizures as manifestations of the disease [5,6,7,8]. Until recently, limitations in cortical lesion detection by MRI compromised our ability to understand their in vivo behavior and clinical correlations. Accurate identification of cortical lesions on conventional MRI remains challenging, due to their small size and poor lesion-to-tissue contrast [9]. Recently improved detection sensitivity of cortical lesions was demonstrated using 3D dual-inversion recovery (DIR) sequences [10,11]. However, DIR suffers from inherently low signal-to-noise ratio (SNR), requiring slice/slab thicknesses on the order of 2-3 mm. Due to the small size of the cortical lesions, partial-volume averaging effects prevent clear delineation of lesion boundaries with respect to the gray/white matter junction, impeding accurate classification of cortical lesions as purely intracortical or otherwise. In addition, the DIR technique is susceptible to flow-related artifacts [12] and has regional variations in GM signal intensity [11] which may lead to false-positive lesion detections [13].
Improved accuracy in identification and classification of cortical lesions was demonstrated in a study that combined DIR with T1-weighted phase-sensitive inversion recovery (PSIR) [13,14]. However, the spatial resolution and SNR with these sequences is constrained by acquisition time and patient motion. Therefore, the use of combined DIR with PSIR for classification of cortical lesions to differentiate purely intracortical lesions from those with some extension past the gray-white matter junction requires refinement.
Reliable cortical lesion classification demands a technique with high spatial resolution and contrast for clear delineation of gray-white matter boundaries. One compelling candidate that satisfies these requirements is three-dimensional (3D) magnetization-prepared rapid acquisition with gradient echo (MPRAGE) imaging [15]. MPRAGE had previously been applied to MS for detection of enhancing lesions [16,17] on a 1.5T MRI system. The purpose of this study was to implement a high spatial resolution, high-SNR, 3D MPRAGE protocol on a 3T scanner with parallel imaging and clinically acceptable acquisition time, and to evaluate its potential for improving cortical lesion classification in MS.
Methods
Eleven MS patients (2 male, 9 female, median age 55 years) with previously identified cortical lesions were included in this study. Twelve subjects from our original cohort of 16 patients [13] were rescanned, but one scan session had to be discarded due to significant motion artifacts. Demographic data is summarized in Table 1. These patients were scanned on a Philips 3T Intera scanner with Quasar Plus gradient systems (maximum gradient amplitude 80 mT/m, slew rate less than 200 mT/m/s) and a six channel SENSE-compatible head coil. The scan protocol included axial DIR and PSIR imaging, each with 0.94 mm × 0.94 mm in-plane resolution (256 × 256 matrix and 24 cm FOV) and 44 slices of 3.0 mm thickness. Coronal 3D MPRAGE images were also acquired with an isotropic voxel size of 0.94 mm × 0.94 mm × 0.94 mm (TI = 1123 msec, excitation RF flip angle 6°), reformatted in the axial plane. SENSE encoding was employed along the A/P direction for DIR and PSIR with a SENSE factor (R) of 2.0. (R = 2.0) and two-dimensional SENSE encoding along R/L (R = 2) and A/P (R = 2.5) for MPRAGE. Acquisition of 3D MPRAGE in the coronal plane allows the use of two dimensional SENSE for reduced scan time and fewer artifacts. Scan times were 7.5 minutes for DIR, 4.2 minutes for PSIR, and 6.4 minutes for MPRAGE. Additional acquisition protocol details are listed in Table 2. All sequences were obtained during the same scanning session. The DIR, PSIR, and MPRAGE images were not spatially registered prior to image analysis. The slice locations for DIR and PSIR were identical as verified by careful visual inspection after the scan acquisition.
Table 1.
Patient demographics
| AGE | GENDER | TYPE | DURATION (y) | EDSS |
|---|---|---|---|---|
| 52 | M | SP | 11 | 6.5 |
| 62 | F | RR | 12 | 2.5 |
| 52 | F | RR | 3 | 0 |
| 57 | F | RR | 22 | 2 |
| 55 | F | RR | 12 | 3 |
| 51 | F | RR | 27 | 5 |
| 26 | F | RR | 7 | 2 |
| 48 | F | RR | 24 | 2.5 |
| 37 | F | RR | 3 | 2 |
| 70 | M | PP | 26 | 4 |
| 41 | F | RR | 13 | 2 |
RR = relapsing remitting multiple sclerosis
SP = secondary progressive multiple sclerosis
PP = primary progressive multiple sclerosis
EDSS = expanded disability status scale
Table 2.
Acquisition protocols
| SEQUENCE | TYPE | VOXEL SIZE (mm) | TR/TE (ms) | TI (ms) | SENSE | ACQ (min) |
|---|---|---|---|---|---|---|
| DIR | 2DTSE | 0.94 × 0.94 × 3.0 | 15000/32 | 3400/325 | 2 | 7.5 |
| PSIR | 2DTSE | 0.94 × 0.94 × 3.0 | 4300/8 | 400 | - | 4.2 |
| MPRAGE | 3DTFE | 0.94 × 0.94 × 0.94 | 8.5/4 | 1123 | 2.0/2.5 | 6.4 |
DIR = Dual Inversion-Recovery
PSIR = Phase-Sensitive Inversion Recovery
MPRAGE = Magnetization-Prepared Rapid Acquisition Gradient Echo
In our original study, cortical lesion detection by PSIR/DIR was by consensus by 3 experts as described [13]. In the current analysis cortical lesions previously identified in each patient, were re-identified on the new DIR/PSIR sequences as either (a) purely intracortical (near total confinement within the cortical ribbon), (b) mixed gray-white (originating in the cortex but with some subcortical extension), or (c) as juxtacortical (primarily subcortical with some involvement of the gray matter). Juxtacortical lesions were not included in this analysis. Once lesion presence and previous classification were confirmed, each lesion was carefully evaluated on the 3D MPRAGE images and compared with the DIR/PSIR classification. Based on the new information provided by the high resolution images a qualitative assessment was made as to whether the MPRAGE sequence confirmed or overturned the prior classification. This was done by one of the experts involved in the original study and one of the physicists involved in the development of the MPRAGE sequence.
Results
Upon initial assessment of the DIR and PSIR images from the eleven patients, a total of 119 cortical lesions were identified, of which 54 were classified as purely intracortical and 65 as mixed. After reviewing the MPRAGE images at the same anatomic locations, 30 of these lesions were re-classified. The majority of the reclassification was found to be from originally mixed to purely intracortical based on details provided by the MPRAGE images. Details on reclassification are summarized in Table 3. A few examples of lesion classifications being either confirmed or overturned by the MPRAGE images are shown in Figures 1 - 4. Only one out of 119 lesions identified on DIR/PSIR was not visualized on the MPRAGE sequences. Detection capabilities of the MPRAGE sequence alone were not evaluated as part of this analysis.
Table 3.
Summary of classification changes
| INITIAL | FINAL | NUMBER |
|---|---|---|
| IC | None† | 1 |
| IC | MX | 7 |
| IC | JX | 2 |
| MX | IC | 13 |
| MX | JX | 7 |
False-positive on DIR/PSIR, unseen on MPRAGE
IC = intracortical, MX = mixed, JX = juxtacortical
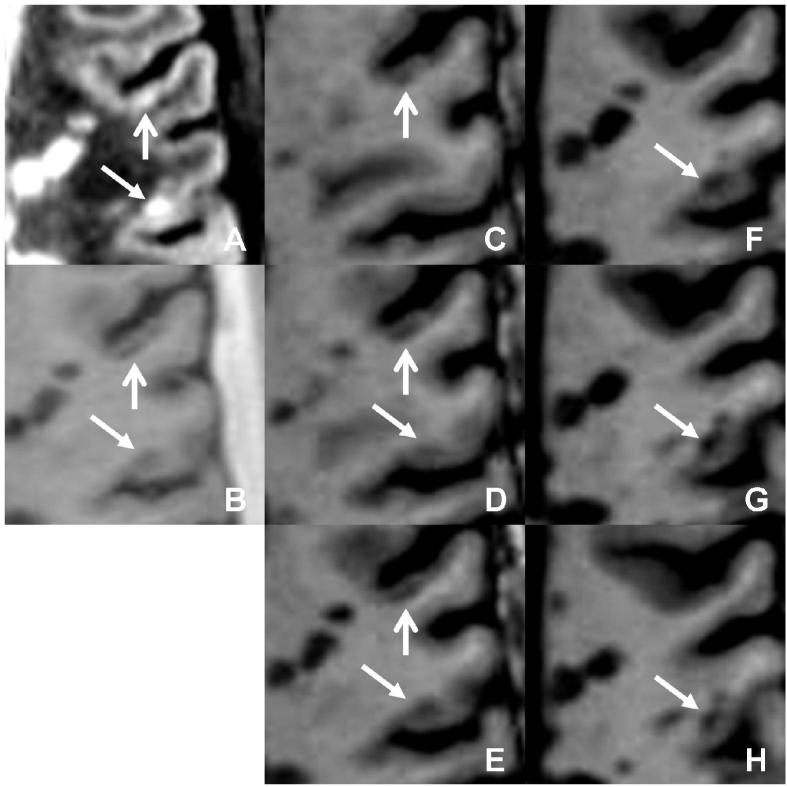
Two intracortical lesions are seen on (a) DIR and (b) PSIR on the same image slice. MPRAGE (c □ h) confirms the classification of the first lesion (vertical arrow) as purely intracortical. However, the classification of the second lesion (oblique arrow) is overturned to juxtacortical.
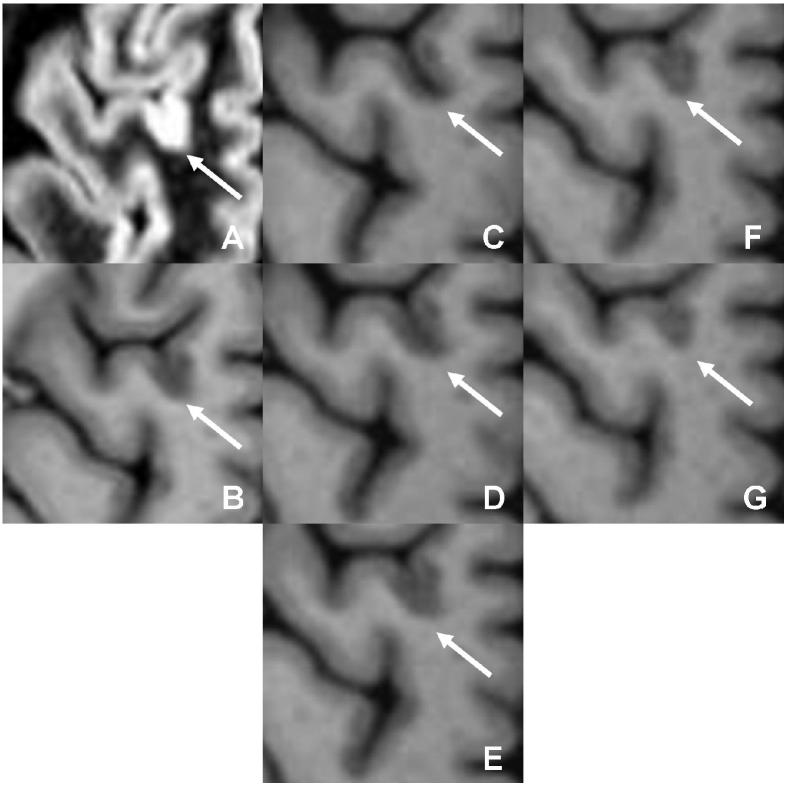
A lesion initially classified as mixed on (a) DIR and (b) PSIR is seen to actually be purely intracortical after inclusion of MPRAGE (c □ g).
Discussion
The pathogenesis of lesions originating in the cerebral cortex remains unclear. Histological findings described in these lesions such as lack of significant inflammatory cell infiltrates [1,18], or complement activation [19] and evidence of neuronal apoptosis [20] differ from the typical findings seen in white matter plaques. These as well as other issues like the lack of correlation between cortical lesions presence with that of white matter plaques [21,22] have contributed to recent acknowledgement of the importance in understanding their role and in vivo behavior. Despite this, most attempts to detect cortical lesions on MRI remain suboptimal. This is especially evident when attempting to determine whether a lesion remains entirely contained within the gray matter or may have originated within the subcortical white matter. This limits one from improving understanding of the heterogeneity of the disease and compromises the ability to establish correlations between the presence and precise characterization of cortical lesions and disease manifestations like cognitive impairment and seizure activity.
An implication of this study is that some cortical lesions described in the MS imaging literature may not be purely cortical. The term “cortical lesion” has at times been used to describe purely intracortical, mixed and even juxtacortical lesions. The more detailed descriptions and classification schemes in the literature are derived from histology [1,21,23], and are beyond present clinical imaging resolution to rigorously utilize. The broadest level of generalization, lumping lesions into the terms “cortical” or “juxtacortical”, has limited clinical utility, but until now has been the only meaningful imaging classification that could be applied to most gray matter lesions described in the literature. The less specific purely intracortical/mixed/juxtacortical classification scheme used in this study was initially proposed as a better match for imaging capabilities [13] but as the present study demonstrates the combination of DIR and PSIR remains suboptimal in some cases where lesions may have minimal extension into the white matter.
This analysis shows that, although a combination of sequences was previously shown to be sensitive and specific for lesion classification (PSIR with DIR), inclusion of 3D MPRAGE images improves the accuracy of cortical lesion classification. Terms such as mixed, purely intracortical and juxtacortical require precise depiction of the gray-white matter junction, as well as clear delineation of the lesion boundaries. The improved spatial resolution and SNR of MPRAGE permit these boundaries to be visualized more clearly than possible with the coarser resolution of DIR and PSIR. The degree of subjectivity in the classification is substantially reduced by inclusion of the anatomic detail provided by the higher resolution scan.
In these preliminary studies, MPRAGE provided better characterization of cortical lesions. We have not investigated if all the cortical lesions can be visualized on MPRAGE without the use of DIR and PSIR images. Convincing demonstration that cortical lesions can be visualized without the aid of DIR and PSIR would have considerable practical clinical importance as both DIR and PSIR sequences could then be eliminated from the protocol to reduce overall scan time. We are addressing this with formal quantitative studies that are underway. Until then, combination DIR/PSIR remains the best option for detection and validation of cortical lesions. However, MPRAGE should be used when accurate differentiation between purely intracortical and mixed lesions is required.
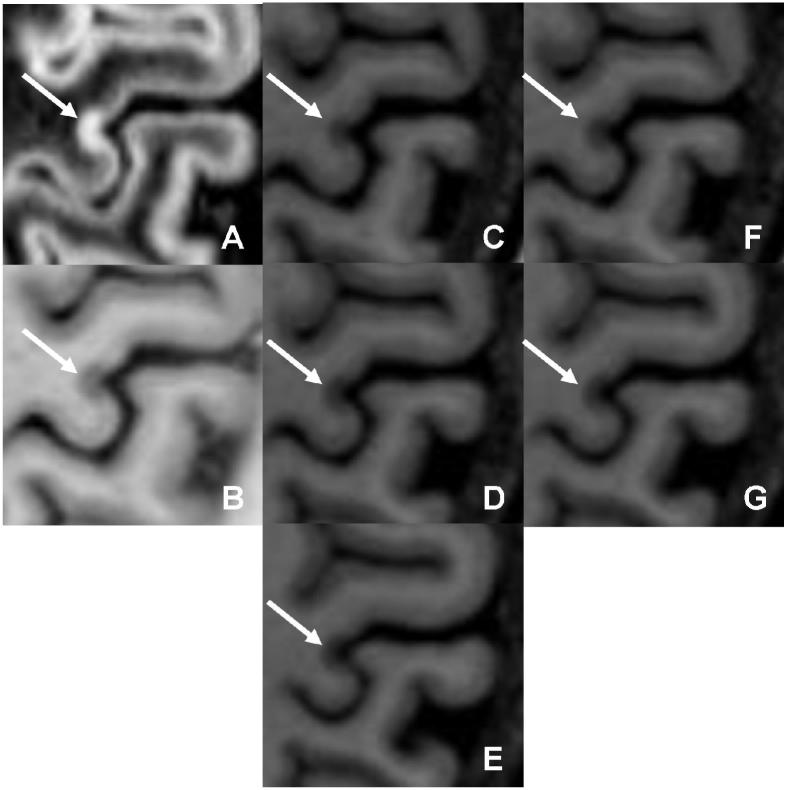
The classification of a lesion as purely intracortical on (a) DIR and (b) PSIR is convincingly confirmed by inclusion of MPRAGE (c □ h).
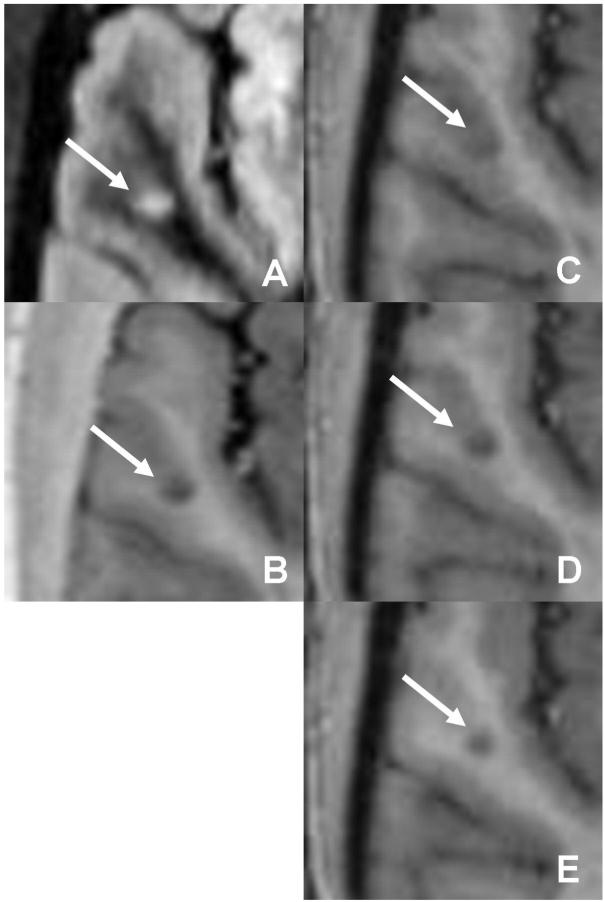
A lesion initially classified as purely intracortical on (a) DIR and (b) PSIR is seen to actually be mixed after inclusion of MPRAGE (c □ e).
Acknowledgements
This work was primarily supported by NIH grants R01 EB02095 (PAN) and S10 RR19186 (PAN) and in part by the Clayton Foundation for Research (FN). We also would like to acknowledge Vipulkumar Patel for invaluable assistance with image scanning and protocol optimization.
References
- 1.Kidd D, Barkhof F, McConnell R, Algra PR, Allen IV, Revesz T. Cortical lesions in multiple sclerosis. Brain. 1999;122:17–26. doi: 10.1093/brain/122.1.17. [DOI] [PubMed] [Google Scholar]
- 2.Bö L, Geurts JJ, Mork SJ, van der Valk P. Grey matter pathology in multiple sclerosis. Acta Neurol Scand. 2006;183(SX):48–50. doi: 10.1111/j.1600-0404.2006.00615.x. [DOI] [PubMed] [Google Scholar]
- 3.Wegner C, Esiri MM, Chance SA, Palace J, Matthews PM. Neocortical neuronal, synaptic, and glial loss in multiple sclerosis. Neurology. 2006;67:960–967. doi: 10.1212/01.wnl.0000237551.26858.39. [DOI] [PubMed] [Google Scholar]
- 4.Bjartmar C, Trapp BD. Axonal and neuronal degeneration in multiple sclerosis, mechanisms and functional consequences. Current opinion in neurology. 2001;14:271–278. doi: 10.1097/00019052-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Rovaris M, Filippi M, Minicucci L, et al. Cortical/subcortical disease burden and cognitive impairment in patients with multiple sclerosis. AJNR. 2000;21:402–408. [PMC free article] [PubMed] [Google Scholar]
- 6.Lazeron RH, Langdon DW, Filippi M, van Waesberghe JH, Stevenson VL, Boringa JB, Origgi D, Thompson AJ, Falautano M, Polman CH, Barkhof F. Neuropsychological impairment in multiple sclerosis patients: the role of (juxta)cortical lesion on FLAIR. Mult Scler. 2000;6:280–285. doi: 10.1177/135245850000600410. [DOI] [PubMed] [Google Scholar]
- 7.Moriarty DM, Blackshaw AJ, Talbot PR, Griffiths HL, Snowden JS, Hillier VF, Capener S, Laitt RD, Jackson A. Memory dysfunction in multiple sclerosis corresponds to juxtacortical lesion load on fast fluid-attenuated inversion-recovery MR images. AJNR. 1999;20:1956–1962. [PMC free article] [PubMed] [Google Scholar]
- 8.Sokic DV, Stojsavljevic N, Drulovic J, Dujmovic I, Mesaros S, Ercegovac M, Peric V, Dragutinovic G, Levic Z. Seizures in multiple sclerosis. Epilepsia. 2001;42:72–79. doi: 10.1046/j.1528-1157.2001.48699.x. [DOI] [PubMed] [Google Scholar]
- 9.Geurts JJ, Bo L, Pouwels PJ, Castelijns JA, Polman CH, Barkhof F. Cortical lesions in multiple sclerosis: combined postmortem MR imaging and histopathology. AJNR. 2005;26:572–577. [PMC free article] [PubMed] [Google Scholar]
- 10.Geurts JJ, Pouwels PJ, Uitdehaag BM, Polman CH, Barkhof F, Castelijns JA. Intracortical lesions in multiple sclerosis: improved detection with 3D double inversion-recovery MR imaging. Radiology. 2005;236:254–260. doi: 10.1148/radiol.2361040450. [DOI] [PubMed] [Google Scholar]
- 11.Pouwels PJ, Kuijer JP, Mugler JP, 3rd, Guttmann CR, Barkhof F. Human gray matter: feasibility of single-slab 3D double inversion-recovery high-spatial-resolution MR imaging. Radiology. 2006;241:873–879. doi: 10.1148/radiol.2413051182. [DOI] [PubMed] [Google Scholar]
- 12.Turetschek K, Wunderbaldinger P, Bankier AA, et al. Double inversion recovery imaging of the brain: initial experience and comparison with fluid attenuated inversion recovery imaging. Magn Reson Imaging. 1998;16:127–135. doi: 10.1016/s0730-725x(97)00254-3. [DOI] [PubMed] [Google Scholar]
- 13.Nelson F, Poonawalla AH, Hou P, Huang F, Wolinsky JS, Narayana P. Improved visualization of intracortical lesions in multiple sclerosis by phase-sensitive inversion recovery in combination with fast double inversion recovery MR imaging. AJNR. 2007;28:1645–9. doi: 10.3174/ajnr.A0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou P, Hasan KM, Sitton CW, Wolinsky JS, Narayana PA. Phase-sensitive T1 inversion recovery imaging: a time-efficient interleaved technique for improved tissue contrast in neuroimaging. AJNR. 2005;26:1432–1438. [PMC free article] [PubMed] [Google Scholar]
- 15.Mugler JP, 3rd, Brookeman JR. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE) Magn Reson Med. 1990;15:152–157. doi: 10.1002/mrm.1910150117. [DOI] [PubMed] [Google Scholar]
- 16.Filippi M, Yousry T, Horsfield MA, et al. A high-resolution three-dimensional T1-weighted gradient echo sequence improves the detection of disease activity in multiple sclerosis. Ann Neurol. 1996;40:901–907. doi: 10.1002/ana.410400612. [DOI] [PubMed] [Google Scholar]
- 17.Filippi M, Rocca MA, Horsfield MA, et al. Increased spatial resolution using a three-dimensional T1-weighted gradient-echo MR sequence results in greater hypointense lesion volumes in multiple sclerosis. AJNR. 1998;19:235–238. [PMC free article] [PubMed] [Google Scholar]
- 18.Bo L, Vedeler CA, Nyland H, Trapp BD, Mork SJ. Intracortical multiple sclerosis lesions are not associated with increased lymphocyte infiltration. Mult Scler. 2003;9:323–331. doi: 10.1191/1352458503ms917oa. [DOI] [PubMed] [Google Scholar]
- 19.Brink BP, Veerhuis R, Breij EC, van der Valk P, Dijkstra CD, Bo L. The pathology of multiple sclerosis is location-dependent: no significant complement activation is detected in purely cortical lesions. J Neuropathol Exp Neurol. 2005;64:147–155. doi: 10.1093/jnen/64.2.147. [DOI] [PubMed] [Google Scholar]
- 20.Peterson JW, Bo L, Mork S, Chang A, Trapp BD, et al. Transected neurites, apoptotic neurons and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol. 2001;50:389–400. doi: 10.1002/ana.1123. [DOI] [PubMed] [Google Scholar]
- 21.Bo L, Geurts JJ, van der Valk P, Polman C, Barkhof F. Lack of correlation between cortical demyelination and white matter pathologic changes in multiple sclerosis. Arch Neurol. 2007;64:76–80. doi: 10.1001/archneur.64.1.76. [DOI] [PubMed] [Google Scholar]
- 22.Kutzelnigg A, Lucchinetti CF, Stadelmann C, Bruck W, Rauschka H, Bergmann M, Schmidbauer M, Parisi JE, Lassmann H. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128:2705–2712. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- 23.Bo L, Vedeler CA, Nyland HI, Trapp BD, Mork SJ. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J Neuropathol Exp Neurol. 2003;62:723–732. doi: 10.1093/jnen/62.7.723. [DOI] [PubMed] [Google Scholar]