Abstract
Animal models indicate that the neuroactive steroids 3α,5α-THP (allopregnanolone) and 3α,5α-THDOC (allotetrahydroDOC) are stress responsive, serving as homeostatic mechanisms in restoring normal GABAergic and hypothalamic-pituitary-adrenal (HPA) function following stress. While neurosteroid increases to stress are adaptive in the short term, animal models of chronic stress and depression find lower brain and plasma neurosteroid concentrations and alterations in neurosteroid responses to acute stressors. It has been suggested that disruption in this homeostatic mechanism may play a pathogenic role in some psychiatric disorders related to stress. In humans, neurosteroid depletion is consistently documented in patients with current depression and may reflect their greater chronic stress. Women with the depressive disorder, premenstrual dysphoric disorder (PMDD), have greater daily stress and a greater rate of traumatic stress. While results on baseline concentrations of neuroactive steroids in PMDD are mixed, PMDD women have diminished functional sensitivity of GABAA receptors and our laboratory has found blunted allopregnanolone responses to mental stress relative to non-PMDD controls. Similarly, euthymic women with histories of clinical depression, which may represent a large proportion of PMDD women, show more severe dysphoric mood symptoms and blunted allopregnanolone responses to stress versus never-depressed women. It is suggested that failure to mount an appropriate allopregnanolone response to stress may reflect the price of repeated biological adaptations to the increased life stress that is well documented in depressive disorders and altered allopregnanolone stress responsivity may also contribute to the dysregulation seen in HPA axis function in depression.
Keywords: Neurosteroids, Allopregnanolone, Stress, Premenstrual dysphoric disorder, Depressive disorders
1. Introduction
The concept of stress is as old as medical history itself, dating back at least to the time of Hippocrates who referred both to the suffering associated with disease (pathos) and to the toil (ponos) — the fight of the body to restore itself to normalcy (Hippocrates, 1923). In more recent history, both Walter Cannon (Cannon, 1939) and Claude Bernard (Bernard, 1949) described the ability of all organisms to maintain a constancy of their internal milieu or homeostasis, and 70 years ago Hans Selye, the pioneer of contemporary stress research, first described the General Adaptation Syndrome (GAS) as a chronological development of the response to stressors when their action is prolonged (Selye, 1936). While we will return to the concept of generalized biological responses to stress and stress response adaptation as a context to understand pathophysiological processes for neurosteroids in depressive disorders, it is of historical interest to point out here that Selye was the first to document the relatively immediate (within minutes) anesthetic and anticonvulsant properties of progesterone and related compounds administered itraperitoneally to rats (Selye, 1941, 1956). This provided the first evidence that endogenous steroid hormones could influence neuronal excitability on a time scale inconsistent with the classic genomic mechanisms of steroid action. The central nervous system effects of steroid hormones documented by Selye led him to speculate on whether the somnolence of pregnancy, premenstrual tension, and other clinical conditions accompanied by increased steroid-hormone production may find their explanation in that phenomenon (Selye, 1956).
In keeping with the early observations of Selye (1941, 1956) that steroid hormone metabolites could exert central effects within minutes of administration, Purdy and colleagues (Purdy et al., 1991; Barbaccia et al., 1996, 1997) were among the first to demonstrate in rat models that acute stress results in significant increases in both plasma and CNS concentrations of the 3α-hydroxy ring A-reduced steroid metabolites, 3α,5α-THP (allopregnanolone) and 3α,5α-THDOC (allotetrahydroDOC), in physiologic ranges known to enhance GABA receptor-activated Cl- currents (Purdy et al., 1991; Reddy, 2006).
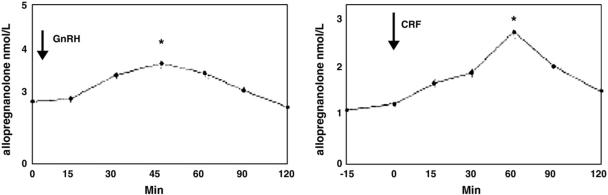
The neuroactive steroids, allopregnanolone and allotetrahydroDOC, are among the most potent allosteric modulators of the GABAA receptors (nanomolar concentrations) via dose-dependent enhancement of GABA-induced Cl- ion channels (Morrow et al., 1987), and it is through this mechanism that they exert profound anxiolytic effects (Bitran et al., 1995; Brot et al., 1997; Bitran et al., 1999, 2000). Allopregnanolone is the A-ring reduced metabolite of progesterone and is synthesized not only in ovary and adrenals but also de novo in brain (Paul & Purdy, 1992). AllotetrahydroDOC, on the other hand, is derived exclusively from adrenal sources since its mineralocorticoid precursor, deoxycorticosterone (DOC), is synthesized in the adrenal zona fasciculate under the control of ACTH, with no evidence for de novo synthesis of allotetrahydroDOC in brain (Reddy, 2006). Both allopregnanolone and allotetrahydroDOC are highly lipophillic, and animal studies using adrenalectomized versus control rats have demonstrated that not only do peripherally produced concentrations of these neuroactive steroids following stress readily cross the blood–brain barrier, contributing significantly to stress-induced increases in central concentrations of both allopregnanolone and allotetrahydroDOC, but that the major proportion of brain allopregnanolone and allotetrahydroDOC increased by acute stress is produced by peripheral tissues (Purdy et al., 1991). The time course of peripheral versus central neurosteroid responses to stress in rats is quite distinct, however, with the peripheral increase in allopregnanolone being more delayed, peaking between 30 and 70 min following the onset of acute swim stress (Paul and Purdy, 1992; Barbaccia et al., 1998; see Fig. 1). It should be noted that, in contrast to this initial animal work on stress-induced allopregnanolone that relied on high performance liquid chromatography (HPLC) combined with radioimmunoassay (RIA) to measure neurosteroids, more recent studies by Purdy and colleagues (Vallee et al., 2000) using more sensitive and specific gas chromatography/mass spectrometry (GC/MS) methods found lower cortex concentrations of allopregnanolone in response to stress, though plasma allopregnanolone concentrations following acute swim stress remained equivalent to those measured by HPLC/RIA (Fig. 1). The greater central concentrations of allopregnanolone in the earlier studies using HPLC/RIA methods was presumably due to the detection of other related compounds (i.e., pregnenolone) in the brain (Vallee et al., 2000). Nonetheless, the central versus peripheral difference in time course to peak allopregnanolone concentrations in rat following stress remains.
Fig. 1.
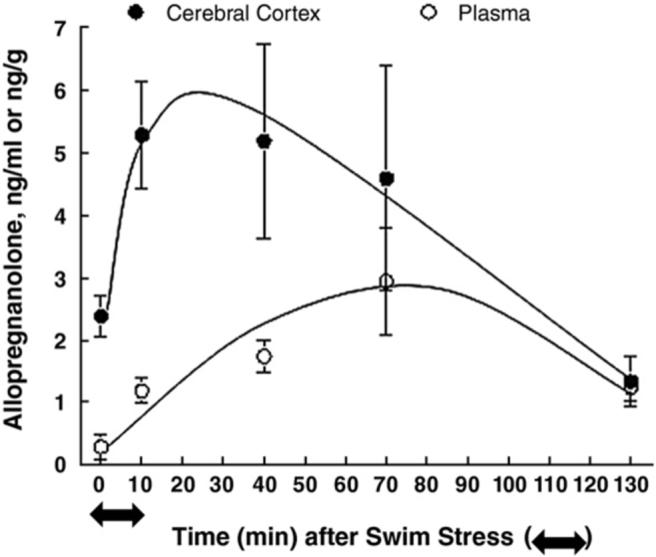
Allopregnanolone levels in plasma and brain of adult Sprague-Dawley male rats after acute swim stress. Rats were subjected to acute stress by swimming for 10 min in ambient temperature water. Allopregnanolone levels were measured by RIA after purification of the steroid by HPLC. The data represent the mean±SEM for 3 separate experiments (n=12 rats per time point) for the cerebral cortex and plasma. Stress-induced increases of allopregnanolone in the cerebral cortex and plasma were statistically significant at 10, 40, and 70 min after the initiation of swim stress compared with nonstressed levels at 0 time [analysis of variance (ANOVA), P<0.05; Tukey's honestly significant difference procedure (HSD), P<0.05]. Adapted from Purdy et al. (1991).
2. Neuroactive steroid responses to stress: behavioral and endocrine adaptations
In rodents, the behavioral effects seen following administration of certain A-ring reduced steroid derivatives, particularly allopregnanolone and allotetrahydroDOC, including anxiolytic (Bitran et al., 1995; Akwa et al., 1999), anti-conflict (Perche et al., 2001; Pinna et al., 2003; Pibiri et al., 2006), antiseizure (Frye, 1995), and antinociceptive effects (Kavaliers & Wiebe, 1987; Wiebe & Kavaliers, 1988; Frye & Duncan, 1994), are consistent with an integrated and adaptive response to stress (Purdy et al., 1991). The pretreatment of animals with the 5α-reductase inhibitor, finasteride (an inhibitor of the rate limiting step in the conversion of progesterone and DOC to their metabolite precursors of allopregnanolone and allotetrahydroDOC) or the administration of GABA antagonists, bicuculline, or picrotoxin (Kavaliers & Wiebe, 1987; Wiebe & Kavaliers, 1988) eliminates many of these behavioral responses to stress, suggesting that these behavioral adaptations to acute stressors are mediated by the 5α-reduced neurosteroids (Reddy, 2003).
In addition to the adaptive short-term behavioral effects associated with increases in allopregnanolone and allotetrahydroDOC summarized above, animal models provide strong and consistent evidence that the A-ring reduced steroid metabolites also act as endogenous suppressors of the endocrine response to stress (Purdy et al., 1991). Specifically, neurosteroids appear to serve as endogenous homeostatic mechanisms restoring both normal GABAergic and hypothalamic-pituitary-adrenal (HPA) function following acute stress (Guo et al., 1995; Patchev et al., 1996; Barbaccia et al., 1998; Strous et al., 2006). Animal models indicate that acute stress results in a rapid decrease in GABAergic neurotransmission and that this reduction in inhibitory neurotransmission with stress is mediated by the GABAA receptor (Concas et al., 1988; Drugan et al., 1989; Biggio et al., 1990). While the decrease in GABAergic tone occurs immediately with the onset of acute stress, the increase in both plasma and brain allopregnanolone concentrations occur subsequent to both the stressor onset and the reduction in GABAergic neurotransmission, resulting in a functional correlation between endogenous allopregnanolone concentration in brain, the recovery of GABAergic transmission tone, and the disappearance of conflict behavior (i.e. anxiety) (Barbaccia et al., 1998).
Stress-induced increases in allopregnanolone and allotetrahydroDOC also serve to negatively modulate HPA axis activation, thereby facilitating the recovery of physiologic homeostasis following stressful stimuli. For example, using animal preparations, allopregnanolone, dose-dependently suppresses the hypothalamic release of GnRH in vitro. The effect is blocked by bicuculline, a GABAA receptor antagonist (Calogero et al., 1998), suggesting that hypothalamic suppression by allopregnanolone involves the GABAA receptor. Additionally, allopregnanolone and allotetrahydroDOC counteract the anxiogenic activity of corticotrophin-releasing factor (CRF) and attenuate methoxamine-stimulated CRF release in vitro in a dose-dependent fashion (Patchev et al., 1994). Several laboratories have also shown that the pretreatment of rats with allopregnanolone, allotetrahydroDOC, or progesterone significantly attenuates stress-induced increases in plasma ACTH and cortisol (Owens et al., 1992; Patchev et al., 1996) and that allopregnanolone affects the gene transcription of arginine vasopressin (AVP) in the paraventricular nucleus (PVN) of the hypothalamus in a pattern similar to that seen with glucocorticoids (Patchev et al., 1996). Increased GABAergic tone also inhibits hypophysiotropic CRF neurons (Owens et al., 1992). The significance of GABA modulation of CRF neurons is underscored by the evidence that CRF neurons in the locus coeruleus and throughout the CNS integrate the autonomic and behavioral responses to stress (Chrousos and Gold, 1992; Owens et al., 1992). Thus, endogenous allopregnanolone and allotetrahydroDOC represent homeostatic mechanisms in the context of adaptation to stress by limiting the extent and duration of reduction in GABAergic inhibitory transmission and activation of the HPA axis. Within this context, it has been suggested that disruption in this homeostatic mechanism may play an etiopathogenetic role in some psychiatric disorders related to stress and often associated with increased adrenal glucocorticoid output (Barbaccia et al., 1998), the prototypical profile seen in human depression.
In addition to their role in restoring GABAergic tone and suppressing the acute endocrine stress response, animal models also indicate that the A-ring reduced neurosteroids provide long-term protection against adverse effects of early life stressful events. For example, in the validated animal paradigm of early life stress involving the repeated separation of ratpupsfromtheir mothersduring early post-natal life, administration of allotetrahydroDOC not only suppresses the stress vocalizations in maternally deprived rats following separation, but it also abolishes the anxiogenic behavioral after-affects of neonatal stress seen in deprived pups exposed to the plus-maze test (Patchev et al., 1997). Pretreatment with allopregnanolone or allotetrahydroDOC in rat pups also exerts long-term protection against HPA axis responses to stress since it abolishes the greater corticosterone response to subsequent acute stressors seen in neonatally deprived animals relative to never-deprived animals (Patchev et al., 1997; Mitev et al., 2003). Thus, allopregnanolone and allotetrahydroDOC may protect the developing brain against adverse emotional challenges, indicating a role for neurosteroids in the long-term control of behavioral and endocrine responsiveness to stress.
3. Stress dysregulation in neuroactive steroids: implications for depressive disorders
While neurohormonal activation in response to stress is adaptive in the short-term, long-term activation of such responses due to repeated or chronic stress may lead to persistent dysregulation in these stress-responsive factors and set the stage for subsequent illness. This was first documented by Selye in rats, when he described the chronologic development of the nonspecific response to stressors when their action is prolonged. He termed this the GAS (Selye, 1936, 1951, 1955, 1976), which involved a triphasic response: (i) the alarm reaction, in which adaptation has not yet been acquired; (ii) the stage of resistance, in which adaptation is optimum; and (iii) the stage of exhaustion, in which the acquired adaptation is lost (Selye, 1955). Selye subsequently described diseases of adaptation, including psychosomatic diseases as well as immunologic and inflammatory diseases, which depended primarily on excessive or inappropriate responses to stressors, where an essentially useful defensive reaction (e.g., HPA axis activation or emotional arousal in preparation for fight) can be the major cause of disease if the defense is inappropriate under the circumstances (Selye, 1976). While Selye's GAS was first described 70 years ago (Selye, 1936), it is briefly reviewed above because it provides a cornerstone to current conceptualizations on the development of stress-related illness in humans.
In humans, the price of repeated biological adaptations to stress has been termed allostatic load and refers to the long-term effect of physiologic responses to stress (McEwen, 1998). Allostatic load may be expressed as repeated elevations of neurohormonal stress mediators (e.g., cortisol, norepinephrine) over long periods, as a failure to adapt to the same stressor, as a failure to shut off the normal stress response, or as an inadequate hormonal response to stress that may allow other systems that are normally counter-regulated to become overactive (e.g., inadequate secretion of glucocorticoids, resulting in increased levels of inflammatory factors that are normally regulated by the glucocorticoids; McEwen and Seeman, 1999). It has been suggested that such hypoactivation of stress responses may result from a wearing out or exhaustion of the stress-responsive system due to long-term allostatic load (McEwen, 1998). Both animal and human studies indicate that chronic or severe stress exposure, especially early in life (Coplan et al., 1996), can result in persistent alterations in neurobiological systems that are stress responsive (Bremner & Vermetten, 2001; Heim et al., 2001). There is particularly compelling evidence that such long-term adaptation to either early life stress or to chronic on-going stress occurs for HPA axis regulation (Lemieux & Coe, 1995; Resnick et al., 1995; Coplan et al., 1996; Ladd et al., 1996; Kaufman et al., 1997, Heim et al., 2001; Szikszay & Benedek, 1989; D'Amore et al., 1993; Heim et al., 2002; Bennett et al., 2004), and there is also evidence that it occurs for noradrenergic regulation (Bremner et al., 1996; Young & Breslau, 2004).
Similarly for neuroactive steroid responses to stress, it has been suggested that while such responses may be protective against the damaging effects of stress, dysregulation in the neurosteroid stress response may predispose individuals to the negative effects of stress (Poromaa et al., 2003), perhaps forming the basis for the development of mood disorders (Heim et al., 2001). This is supported, in part, by evidence from animals demonstrating that stressors that produce behavioral depression and anxiety not only alter neurosteroid levels but also regulate GABAA receptor expression and result in insensitivity to benzodiazepines and neurosteroids (Deutsch et al., 1994; Serra et al., 2000; Dong et al., 2001). Such alterations in GABAA receptor function, which we will return to later, could set the stage for dysphoric mood potentially resulting from chronic stress.
Indeed, and consistent with a greater allostatic load model (McEwen, 1998), it has been suggested that the neurosteroid depletion consistently documented in patients with depression is likely associated with chronic stress (Reddy, 2003). When compared with non-depressed controls, depressed patients have lower plasma and CSF concentrations of allopregnanolone (Romeo et al., 1998; Uzunova et al., 1998; Strohle et al., 1999, 2000; Nappi et al., 2001; Eser et al., 2006, Uzunova et al., 2006) but higher allotetrahydroDOC concentrations (Strohle et al., 1999, 2000), which may reflect differential alteration in the biosynthesis of deoxycorticosterone or its metabolites in depression (Strohle et al., 1999, 2000). The pathophysiological relevance of these altered neurosteroid concentrations in humans comes from studies showing inverse relationships between allopregnanolone concentrations and severity of the depressive illness (Uzunova et al., 1998; Nappi et al., 2001) and from other studies showing that clinically efficacious treatment with antidepressants is associated with increases in allopregnanolone (Uzunova et al., 1998; Romeo et al., 1998; Strohle et al., 1999, 2000) and decreases in allotetrahydroDOC (Strohle et al., 2000). The antidepressant-like effect of allopregnanolone is also well established in animal models (Khisti and Chopde, 2000; Khisti et al., 2000; Frye & Walf, 2002; Uzunova et al., 2003, 2004).
Although no studies to date have specifically examined the association of chronic stress with neuroactive steroid function in depressed patients, it could be argued that clinical depression is itself a chronic stressor; the link between chronic stress and neurosteroid depletion is supported in part by animal models showing that protracted long-term adaptation to the stress of social isolation, a characteristic feature of human depression (Prince et al., 1997; Roberts et al., 1997), is associated with anxiety, aggression, and decreased response to GABAmimetic drugs and with significantly lower brain and plasma neuroactive steroid concentrations, including allopregnanolone and allotetrahydroDOC (Serra et al., 2000; Guidoti et al., 2001; Dong et al., 2001). The finding that the expression of 5α-reductase (mRNA and protein) in mouse brain, the rate limiting enzyme in the conversion of progesterone to allopregnanolone, is down-regulated during protracted social isolation supports the view that chronic stress could alter neuroactive steroid synthesis (Reddy, 2006). Moreover, the persistent decreases in the concentrations of neuroactive steroids in the brain of rats exposed to long-term social isolation stress is also associated with a decrease in the function of brain GABAA receptors and with persistent alterations in neuroactive steroid responses to subsequent acute stressors (Serra et al., 2000). Similar to depressed patients, socially isolated animals respond to the anti-depressant fluoxetine with a normalization of allopregnanolone brain content (Guidoti et al., 2001).
In contrast to the diminished neuroactive steroid concentrations seen in chronically stressed rats, and consistent with the role of the neuroactive steroids as endogenous negative modulators of the HPA axis (Guo et al., 1995; Patchev et al., 1996), plasma corticosterone concentrations are elevated in isolated animals compared with controls (Serra et al., 2000). Thus, this profile of diminished neurosteroid concentrations coupled with elevated glucocorticoids in socially isolated animals is strikingly similar to that seen in patients with melancholic depression, since many depressed patients are also hypercortisolimic relative to non-depressed controls (e.g., Nemeroff, 1998; Young et al., 2000a, 2000b). Chronic activation of the HPA axis, including greater CRF concentrations and gene expression in depressed patients (Roy et al., 1987; Raadsheer et al., 1995; Nemeroff, 1998), is clearly implicated in the pathophysiology of depressive disorders (Nemeroff, 1998). While numerous mechanisms may contribute to HPA axis hyperactivation in depression, including dysregulation in glucocorticoid negative feedback mechanisms or altered circadian rhythm (von Bardeleben and Holsboer, 1988; Young and Veldhuis, 2006), diminished neuroactive function represents an additional candidate.
Both clinical and empirical evidence clearly indicates that stress plays a major, independent role in contributing to the onset and exacerbation of depressive disorders (Kendler et al., 1993; Brown et al., 1994; Frank et al., 1994). Consistent with the concept of allostatic load, the possibility exists that an inadequate neuroactive steroid response to stress may allow the HPA axis, which is normally counter-regulated by neurosteroids, to become overactive. Research on neuroactive steroid responses to stress in humans is scant. Genazzani and colleagues (1998) initially demonstrated in healthy men and women that both GnRH and CRF administration increased serum progesterone and allopregnanolone concentrations. In their study, plasma was sampled every 15 min for 120 min, with peak allopregnanolone responses to the endocrine GnRH and CRF challenge occurring at 45 and 60 min, respectively, post-challenge (see Fig. 2). Suppression of adrenal steroidogenesis with dexamethasone markedly reduced allopregnanolone, indicating that in humans both ovary and adrenal cortex are major sources of circulating allopregnanolone. Subsequent work by this group has yielded a similar time course to peak plasma allopregnanolone response to the endocrine CRF challenge in healthy male and female controls, although not in women with hypothalamic amenorrhea who fail to show any increase in allopregnanolone (Meczekalski et al., 2000) or in obese men and women who show an exaggerated allopregnanolone response to CRF infusions with an earlier peak (Menozzi et al., 2002). Taken together, the results of these endocrine challenge studies suggest that neuroactive steroids may increase in response to stress in healthy humans as they do in animals. If so, any alterations in neuroactive steroid responses to stress may play a pathophysiological role in the development or maintenance of stress-related disorders, including depressive disorders.
Fig. 2.
Mean±SEM allopregnanolone levels in response to the GnRH test (*, P<0.01 vs. 0 min) and in response to the CRF test (*, P<0.01 vs. 0 min). Adapted from Genazzani et al. (1998).
4. Baseline neuroactive steroids concentrations in premenstrual dysphoric disorder
Our laboratory has had the exciting opportunity to conduct some of the initial research on neuroactive steroid responses to mental stressors, particularly allopregnanolone, in women with depressive disorders. Our earliest work examined allopregnanolone concentrations and reactivity to laboratory stressors in women with premenstrual dysphoric disorder (PMDD). PMDD, a depressive disorder, is characterized by the cyclic recurrence during the luteal phase of the menstrual cycle of a variety of emotional and physical symptoms of sufficient severity to interfere with function. Importantly, in order to meet PMDD criteria as outlined in the Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV, 1994), there must be clear evidence of complete symptom remission shortly after the onset of menses. Consequently, PMDD is differentiated from the premenstrual exacerbation of a chronic depression, dysthymia, or other mood disturbance. Finally, PMDD must be confirmed by prospective daily symptoms records over a minimum of 2 months (DSM-IV, 1994). PMDD afflicts 5−10% of women in their reproductive years (Cohen et al., 2002), and although the symptoms of PMDD are of shorter duration than those of other depressive disorders, the impact of PMDD symptoms on quality of life during the premenstrual luteal phase is equivalent to that seen with major depression, post-traumatic stress disorder, and panic disorder (Freeman & Sondheimer, 2003).
Due to the cyclical nature of the mood disturbance in PMDD, much early attention was paid to the pathophysiological role of the gonadal steroid hormones, particularly progesterone since it is elevated during the symptomatic luteal phase only and at very low concentrations during the follicular and ovulatory phases of the cycle. However, those studies found little evidence to support the view that either an excess or deficiency in progesterone or estradiol concentrations are etiologically relevant to the disorder (Rubinow & Schmidt, 1992), and the majority of controlled trials have failed to find that progesterone administration is efficacious in PMDD (Freeman et al., 1995; see Freeman, 2004, for review). Nonetheless, there is clearly evidence for an obligatory role of the gonadal steroid hormones in PMDD, since PMDD is evident only during the reproductive years (DSM-IV, 1994) and its associated behavioral and emotional symptoms are alleviated with ovarian suppression (Schmidt et al., 1998) or during spontaneous anovulatory cycles (Hammarback et al., 1991). Consequently, investigations into diagnosis-related differences in neuroactive metabolites of progesterone have been undertaken.
Alterations in allopregnanolone concentrations have received the greatest attention in PMDD research, since plasma levels of allopregnanolone follow closely those of progesterone during the symptomatic luteal phase. The anxiogenic GABAA receptor antagonists or partial agonists, such as dehydroepiadrosterone (DHEA), DHEA sulfate, pregnenolone, and pregnenolone sulfate have also been examined in PMDD, as well as the potential imbalance between these positive versus negative GABAA receptor modulators. Since retrospective reporting of premenstrual symptoms is associated with an overestimation of severity and with a high rate of false positive PMDD diagnoses (Marvan & Cortes-Iniestra, 2001), this review will be confined to the 6 studies to date that have examined neuroactive steroid concentrations in prospectively confirmed women with PMDD (see Table 1).
Table 1.
Summary of baseline neuroactive steroid findings in women with prospectively confirmed PMDD (P) compared with non-PMDD controls (C)
| Study | Sample size | DSM criteria for PMDD | Current psych Dx | Prior psych Dx | Cycle daysa | Number of cycles assessed | Results |
|---|---|---|---|---|---|---|---|
| Schmidt et al., 1994 | P: 15 | No | P: No | P: Yes | L: 20−24 | 1 | L Allo: P=C |
| C: 12 | C: NA | C: NA | L Preg: P=C | ||||
| Wang et al., 1996 | P: 12 | Yes | P: NA | P: NA | F: 1−4 | 2 | F&L Allo: P=C |
| C: 8 | C: NA | C: NA | L: 10−27 | F&L PE: P=C | |||
| F&L PS: P=C | |||||||
| Rapkin et al., 1997 | P: 35 | Yes | P: No | P: No | L: 19 & 26 | 1 | L Allo day 19: P=C |
| C: 36 | C: No | C: No | L Allo day 26: P<C | ||||
| Monteleone et al., 2000 | P: 28 | No | P: No | P: NA | F: 5−8 | 3 | F Allo: P=C |
| C: 28 | C: No | C: NA | L: 22−26 | L Allo: P<C | |||
| Girdler et al., 2001 | P: 25 | Yes | P: No | P: Yes | L: 21−25 | 1 | L Allo: P>C |
| C: 13 | C: No | C: Yes | |||||
| Lombardi et al., 2004 | P: 20 | No | P: No | P: No | F: 5−7 | 3 | F Allo: P=C |
| C: 20 | C: No | C: No | L: 22−26 | L Allo: P<C | |||
| F&L: DHEA: P>C | |||||||
| F&L: DHEAS, ANDR, PE: P=C |
NA, not assessed; L, luteal phase; F, follicular phase; Psych Dx, psychiatric diagnosis; Allo, allopregnanolone; Preg, pregnanolone; PE, pregnenolone; PS, pregnenolone sulfate; DHEAS, dehydroepiandrosterone sulfate; ANDR, androstenedione.
Reflects days adjusted to idealized 28-day cycle.
The earliest studies by Schmidt et al. (1994) and Wang et al. (1996), based on small samples of PMDD and control women reported no differences between patients and controls in either follicular or luteal phase plasma concentrations of the 3α-hydroxysteroid metabolites of progesterone, allopregnanolone, and pregnanolone or in pregnenolone sulfate, an anxiogenic inverse agonist at GABAA receptors. Furthermore, the Schmidt et al. (1994) study found no diagnosis-related differences in the allopregnanolone/pregnanolone ratio or in the ratios of allopregnanolone or pregnanolone to progesterone and found no association of neuroactive steroid concentrations with symptoms of depression or anxiety in PMDD women. In contrast to the study of Schmidt et al. (1994) that assessed neuroactive steroids at only 1 time point in a single luteal phase, in the study of Wang et al. (1996), that assessed plasma concentrations of neurosteroids and symptoms daily during the luteal phase of 2 consecutive cycles, more negative symptoms occurred in cycles higher in pregnenolone and pregnenolone sulfate, while more positive symptoms occurred in cycles higher in allopregnanolone and its precursor (5α-DHP).
In a larger sample of 35 PMDD women and 38 controls, Rapkin et al. (1997) reported that PMDD patients had lower luteal phase plasma concentrations of allopregnanolone and lower allopregnanolone/progesterone ratio than controls. Thus, the study of Rapkin and colleagues also suggested possible diagnosis-related differences in the conversion of progesterone to allopregnanolone. Lower allopregnanolone concentrations in PMDD women were also found in 2 subsequent studies. Monteleone et al. (2000) found lower plasma allopregnanolone in 28 women seeking help from a PMS clinic compared with non-help seeking controls during the luteal but not in the follicular phase, and Lombardi et al. (2004) found that PMS women had lower baseline allopregnanolone levels in the luteal phase only, though higher testosterone, DHEA and a DHEA/allopregnanolone ratio in both cycle phases. In contrast to the studies reviewed above, the first study from our laboratory to assess plasma allopregnanolone concentrations in 25 PMDD women compared with 13 non-PMDD controls found that PMDD women had higher luteal phase plasma allopregnanolone concentrations and a greater allopregnanolone/progesterone ratio than controls.
Why might such discrepancies exist in the literature on neuroactive steroids in PMDD? Some methodological differences across studies that may, either independently or in combination, influence findings are also summarized in Table 1. One methodological factor involves differences in the diagnostic criteria employed for PMDD since only some of the existing studies relied on DSM criteria (Wang et al., 1996; Rapkin et al., 1997; Girdler et al., 2001), while others did not (Schmidt et al., 1994; Monteleone et al., 2000; Lombardi et al., 2004). Differences across studies in the assessment and inclusion or exclusion of women with current or past psychiatric illness might also impact on findings since histories of depression are more prevalent in PMDD populations (Pearlstein et al., 1990; Cohen et al., 2002), since reduced allopregnanolone concentrations are consistently found in patients with current depression as summarized above, and since our own work suggests that histories of depressive disorders impact on allopregnanolone concentrations, at least during stress (see below). Additionally, since changes in allopregnanolone concentrations parallel changes in progesterone concentrations, and since progesterone concentrations can vary markedly and change rapidly day-to-day in the luteal phase, study differences in the luteal phase sampling period may also contribute to discrepancies in the literature. This is supported by the study of Rapkin et al. (1997) where diagnosis-related differences in allopregnanolone concentration were examined at 2 time points in the luteal phase, days 19 and 26 of an idealized 28-day cycle. In that study, PMDD and non-PMDD groups were equivalent in their allopregnanolone concentrations on day 19 but not on day 26 of the cycle when PMDD women had significantly lower concentrations than non-PMDD controls. Finally, to the extent that allopregnanolone concentrations are responsive to venipuncture stress, then study differences in blood sampling procedures and/or other nonspecific effects associated with novelty of the laboratory or clinic environment may impact on diagnosis-related differences in allopregnanolone concentrations as our own work reviewed later in this paper suggests.
While our initial study (Girdler et al., 2001) that found higher, and not lower, luteal phase allopregnanolone concentrations in PMDD women was limited by the relatively small sample of non-PMDD control women, we did employed strict DSM diagnostic criteria to confirm PMDD status and used structured clinical interview to exclude both PMDD and non-PMDD women with any current Axis I diagnosis. To the best of our knowledge, our study was the only one to sample allopregnanolone using an indwelling intravenous line following a 25-min adaptation rest period, while the other studies appeared to have relied on single stick venipuncture. Although women with histories of Axis I disorders, particularly depression, were enrolled in our study, we did not control for potential diagnosis-related differences in the prevalence of histories of depression, nor did the majority of the studies summarized in Table 1. Our subsequent work, summarized below, suggests that histories of depression may impact on allopregnanolone concentrations in women.
5. HPA axis and GABAA receptor function in PMDD
With these procedural caveats in mind, it is important to point out that our findings for higher, and not lower, allopregnanolone concentrations in PMDD women is consistent with a number of other observations in PMDD populations. First, as reviewed above, allopregnanolone negatively modulates the HPA axis in animal models, and although null findings have been reported (Su et al., 1997; Bloch et al., 2000; Lombardi et al., 2004) where diagnosis-related differences exist, they suggest blunted HPA axis function in PMDD. For example, a number of studies have reported lower circulating ACTH or cortisol concentrations in PMDD women (Rabin et al., 1990; Redei & Freeman, 1993; Girdler et al., 1998), increased ACTH response to ovine CRF (consistent with lower endogenous CRF; Rabin et al., 1990), a delayed (Steiner et al., 1999) or blunted HPA axis response to serotonergic agents (Bancroft et al., 1991; Su et al., 1997), and the absence of the normal plasma cortisol and ACTH response to exercise stress in the luteal phase that is seen in non-PMDD women (Roca et al., 2003). More compelling evidence that the greater allopregnanolone concentrations that we observed in PMDD women might negatively modulate HPA axis function in that population comes from our own results reported in Girdler et al. (2001), documenting significantly lower plasma cortisol concentrations in the PMDD women than the controls (5.8±0.6 vs. 7.7±0.7 μg/dL, respectively; P<0.05). Moreover, for the entire sample of women, higher luteal phase allopregnanolone tended to be associated with lower luteal phase cortisol concentrations (r=−0.32, P=0.05). Although it should be pointed out that higher allopregnanolone concentrations in obese subjects in response to a CRF challenge test was not found to be related to plasma cortisol concentrations (Menozzi et al., 2002).
Higher circulating concentrations of allopregnanolone are also consistent with the evidence for alterations in GABAA receptor function in PMDD as assessed via saccadic eye velocity (SEV) responses to benzodiazepines or GABAA agonists. Since benzodiazepines, acting via the GABAA receptor, are known to reduce SEV in a dose-dependent fashion (Hommer et al., 1986), SEV is believed to be a sensitive measure of benzodiazepine/GABAA receptor sensitivity in humans. Using placebo-controlled designs, Sundstrom and colleagues have shown in a series of studies that PMDD women show less of a reduction in SEV to intravenous diazepam (Sundstrom et al., 1997a), midazolam (Sundstrom et al., 1997b), and pregnanolone (Sundstrom et al., 1998) than non-PMDD controls. Behaviorally, PMDD women also generally report less sedation in response to the i.v. benzodiazepines (Sundstrom et al., 1997a, 1997b). The clinical significance of these results comes from their findings that when PMDD women are divided into lower versus higher symptom severity groups, the more severe PMDD symptom groups respond with less of a reduction in SEV (Sundstrom et al., 1997b, 1998) and with lower sedation ratings (Sundstrom et al., 1998) in response to benzodiazepines. Since there is no evidence that PMDD women differ in the density or affinity of peripheral benzodiazepine receptors, at least as measured on lymphocytes (Daly et al., 2001), the findings of Sundstrom and colleagues indicate diminished functional sensitivity of GABAA receptors in PMDD.
This is consistent with studies finding modest or no clinical efficacy with benzodiazepine treatment for PMDD (Schmidt et al., 1993; Freeman et al., 1995; Evans et al., 1998), though exceptions exist (Harrison et al., 1987), and with lack of clinical benefit associated with a progesterone intervention or with progesterone induction of supraphysiologic levels of 5α- and 5β-progesterone metabolites (Freeman et al., 1995; Vanselow et al., 1996; see Wyatt et al., 2001 for review). It is also consistent with the results of a placebo-controlled clinical trial of antidepressants that found that PMDD women who were classified as “improved” with treatment had significantly lower, and not higher, plasma allopregnanolone concentrations at post-treatment than PMDD women who did not improve (Freeman et al., 2002).
6. The neurosteroid withdrawal hypothesis in PMDD
The animal literature suggests that high concentrations of allopregnanolone may change the characteristics and function of the GABAA receptor. For example, studies in rats have shown that both in vitro and in vivo long-term exposure to high concentrations of positive allosteric modulators acting at various sites of the GABAA receptor results in a down-regulation of the receptor through a reduction in the abundance of specific receptor subunit mRNAs (see Follesa et al., 2001, for review). Moreover, the changes in the abundance of the subunit mRNAs are associated with changes in GABAA function, since long-term exposure to progesterone and its metabolites results in reduced sensitivity of GABAA receptors to the administration of diazepam or allopregnanolone (Follesa et al., 1998; Concas et al., 1999; Follesa et al., 2001). If similar effects occur in human brain, then this mechanism could account for the reduced sensitivity to positive GABAA allosteric modulators documented in PMDD as summarized above.
Relatedly, because any potentially dysphoric effects of allopregnanolone in the luteal phase would occur following a period of relative allopregnanolone withdrawal in the follicular phase, animal models of allopregnanolone withdrawal have been developed to study neurosteroid mechanisms in PMDD (Gallo & Smith, 1993; Smith et al., 2006). The work of Smith and her colleagues has demonstrated that neurosteroid withdrawal results in a state of anxiety and to a relative insensitivity to the anxiolytic effects of benzodiazepines and that these effects appear to be mediated by an increase in the expression of the α4-containing GABAA receptor subunit in brain (Smith et al., 1998a, 1998b, 2006). Recently, Smith et al. (2006), examined withdrawal from naturally occurring high nocturnal concentrations of allopregnanolone in mice via the administration of finasteride for 3 days. In that study, only vehicle control mice showed the expected anxiolytic effects of allopregnanolone administration in the elevated plus maze paradigm, and this was true whether mice were tested in the presence or absence of an aversive auditory stimulus. In mice treated with finasteride, however, and in the presence of the aversive auditory stimulus, allopregnanolone administration had the opposite effect, eliciting significant decreases in open arm time and number of entries in the plus maze test, indicative of increased anxiety. These mice were also insensitive to benzodiazepine administration. In the absence of the aversive stimulus, allopregnanolone administration had no significant effect on behavior of finasteride-treated animals. These results indicate that both withdrawal from high concentrations of allopregnanolone, and aversive, stressful stimuli are necessary to reverse the normally anxiolytic effect of allopregnanolone to an anxiogenic action, and that this may come about through changes in the agonism properties of GABAA receptors following exposure to and withdrawal from allopregnanolone (Smith et al., 2006). That such a phenomenon might occur in PMDD women is supported by the work of Le Melledo et al. (2000) showing that exposure to flumazenil, a benzodiazepine receptor antagonist, in the luteal phase elicits marked increases in panic symptoms in PMDD women but not in non-PMDD controls. This effect is consistent with a shift in benzodiazepine sensitivity toward inverse agonism, as is seen with panic patients exposed to flumazenil (Nutt et al., 1990).
While speculative, our finding for increased luteal phase plasma allopregnanolone concentrations in PMDD women relative to non-PMDD women (Girdler et al., 2001), the work of Freeman et al. (2001), suggesting that clinical improvement with antidepressant treatment is associated with lower, and not higher, allopregnanolone concentrations in PMDD, the observation for positive correlations between luteal phase progesterone concentrations and premenstrual symptom severity in PMDD women but not controls (Redei & Freeman, 1995), and the work of Schmidt et al. (1998) demonstrating that administration of the parent compound, progesterone, precipitates the return of dysphoric symptoms in PMDD women whose symptoms had been minimized following ovarian suppression, an effect not seen in non-PMDD women, is consistent with the animal studies summarized above suggesting that allopregnanolone may actually reverse to increase anxiety rather than to act as anxiolytic under some conditions (i.e., in the context of stress) and in certain vulnerable individuals (Smith et al., 2006).
7. Allopregnanolone responses to stress in PMDD
To the extent that stress exposure modifies the effects of allopregnanolone on GABAA receptor function and behavior, as the animal work of Smith et al. (2006) suggests, then women who develop PMDD may be at increased vulnerability for dysregulation in allopregnanolone function. PMDD women report more stressful life events and that daily stressors have a greater impact on their lives than non-PMDD women (Girdler et al., 1993; Woods et al., 1997). PMDD women also report more traumatic life stress, including sexual and physical abuse histories (Paddison et al., 1990; Golding & Taylor, 1996, 2000; Girdler et al., 2003, 2007). Since allopregnanolone is stress responsive, at least in animal models (Purdy et al., 1991; Barbaccia et al., 1996, 1997), greater allostatic load in PMDD resulting from more severe life stress could contribute to the elevated allopregnanolone concentrations that we have seen in PMDD women (Girdler et al., 2001) and result in the documented alterations in GABAA receptor function in PMDD (Sundstrom et al., 1997a, 1997b, 1998), including alterations in the agonism properties of the GABAA receptor as animal models (Smith et al., 2006) and human studies of PMDD suggest (Le Melledo et al., 2000). This could then explain the seemingly paradoxical association between increasing allopregnanolone concentrations in the luteal phase and increased dysphoric mood states in PMDD. This is supported by a recent study (Andreen et al., 2006) in postmenopausal women given increasing sequential doses of oral micronized progesterone, where the corresponding increases in allopregnanolone concentrations were associated with the development of negative mood states, at least within physiologic ranges corresponding to the increase in allopregnanolone seen from the follicular to the early–mid luteal phase in normally cycling women (Genazzani et al., 1998).
Further evidence that alterations in allopregnanolone concentrations or mechanisms may reflect increased allostatic load in PMDD also comes from our first study of allopregnanolone in PMDD (Girdler et al., 2001), where we had the exciting opportunity to be the first laboratory to assess allopregnanolone reactivity to mental stress in humans. In the luteal phase, we sampled plasma allopregnanolone via indwelling i.v. at 2 time points, once following an extended baseline rest period (yielding the group differences in allopregnanolone concentrations and inverse relationship to plasma cortisol described earlier) and again ∼ 17 min after the onset of a mental stressor battery involving speech preparation, delivery of a speech and a serial addition task. Allopregnanolone reactivity to mental stress was analyzed as change scores, subtracting baseline concentrations from stress concentrations. In the 24 PMDD women and 13 non-PMDD controls, we found a tendency (P<0.010) for the groups to differ in the direction of their allopregnanolone response to mental stress since only the controls showed the expected stress-induced increase in allopregnanolone (see Fig. 3). In fact, a full 83% of controls showed a stress-induced increase in allopregnanolone compared with only 42% of PMDD women (P<0.05). Moreover, lack of allopregnanolone responsiveness to stress in PMDD women was related to their greater baseline allopregnanolone concentrations since allopregnanolone reactivity and baseline concentrations were negatively correlated in the PMDD group (r=−0.59, P<0.001) but not in the controls (r=−0.23, P=0.46).
Fig. 3.
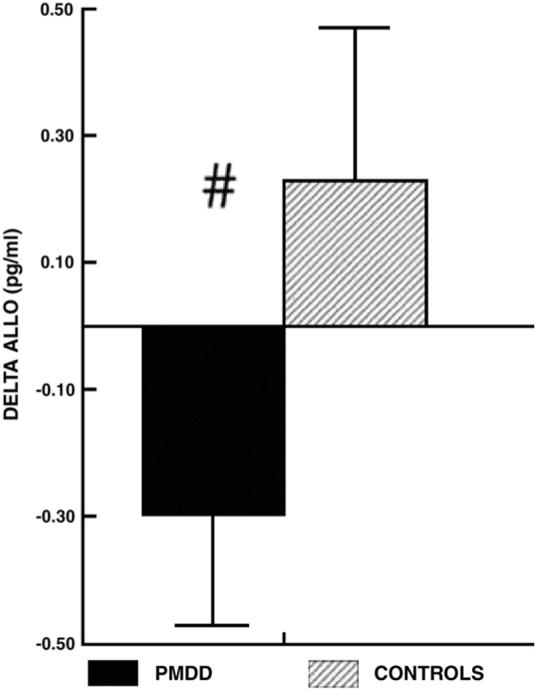
Mean (± SEM) change in luteal phase plasma allopregnanolone (ALLO) during stress in PMDD women and control subjects (#, P<0.010). From Girdler et al. (2001).
Our finding for dysregulation in the allopregnanolone response to mental stress in PMDD is consistent with earlier work from our laboratory, showing alterations in cardiovascular reactivity and plasma norepinephrine and cortisol concentrations at rest and during mental stressors in PMDD women relative to non-PMDD controls (Girdler et al., 1993, 1998). It is also consistent with the endocrine challenge work of Monteleone et al. (2000) and Lombardi et al. (2004) in PMDD women. Despite the fact that those investigators reported lower, and not higher, baseline allopregnanolone in PMDD (Table 1), in response to an ACTH stimulation test following dexamethasone suppression, PMDD women had a significantly blunted adrenal allopregnanolone response compared with controls during the luteal phase (see Fig. 4; Lombardi et al., 2004), and a blunted ovarian allopregnanolone response to a GnRH challenge relative to controls (Monteleone et al., 2000). Taken together, these results indicate an impaired anxiolytic GABAA-mediated response during stress in PMDD (Monteleone et al., 2000) that we suggest may be related to a greater allostatic load resulting from repeated biological adaptations to the greater daily stress and/or traumatic stress documented in women with PMDD. As described earlier (McEwen, 1998; McEwen & Seeman, 1999), increased allostatic load may be expressed as elevations of stress mediators over long periods, such as the greater baseline allopregnanolone that we documented or as an inadequate hormonal response to stress as documented in the blunted allopregnanolone responses to mental and pharmacological challenges in PMDD women.
Fig. 4.
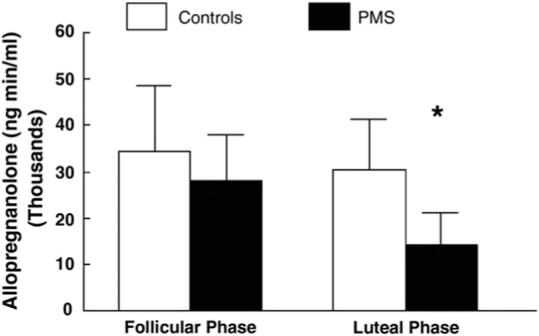
Allopregnanolone response to the adrenocorticotropic hormone (ACTH) test in patients with premenstrual syndrome (shaded bars) and in the control group (white bars) expressed as area under the curve (*, P<0.05). Adapted from Lombardi et al. (2004).
8. Histories of depression and allopregnanolone stress responsivity
While our first study generated interesting preliminary data suggesting dysregulation in allopregnanolone reactivity to stress in PMDD, one significant limitation to our work and to all the work on allopregnanolone in PMDD that preceded it involves the failure to either assess or to control for group differences in prior histories of depression. Histories of depression, which are more prevalent in PMDD women (Cohen et al., 2002), may provide a context of vulnerability for the dysphoric effects associated with steroid hormones (Rubinow, 2005). For example, Bloch et al. (2000) demonstrated that euthymic women with a past history of postpartum depression experienced significant depression when exposed to high doses of hormone add-back and withdrawal, effects not seen in women with no prior history of depression. Divergent evidence suggests that in euthymic women, histories of depression are associated with persistent disturbance in HPA axis function (Young et al., 2000a, 2000b) as well as thyroid axis function (Pedersen et al., 1993) relative to never-depressed women.
Consequently, in our subsequent study (Klatzkin et al., 2006a), we set out to address the issue of whether the discrepancies that exist in the literature on diagnosis-related differences in allopregnanolone concentrations seen between PMDD and non-PMDD women may relate, in part, to a greater likelihood of prior depression in PMDD samples. Thus, we specifically recruited non-PMDD women with or without histories of depression, requiring greater than 7 months in full remission from depression for both PMDD and non-PMDD women. We compared 26 PMDD women with 39 non-PMDD controls; and based on structured interview, we classified 14 PMDD women (54%) and 17 non-PMDD women (44%) as having a history of a depressive disorder (69% with prior major depression, 19% with prior minor depression, 12% with prior adjustment disorder with depressed mood). No subject was taking any prescription medication and for both groups it had been, on average, 60 months since their last depressive episode. In confirmed luteal phases, we sampled plasma allopregnanolone immediately after venipuncture stress associated with the i.v. placement, again 25 min later after an extended baseline rest, and at 30 and 60 min following the onset of the Trier Social Stress Test (TSST) (Kirschbaum et al., 1993), a stressor battery involving speech and mental arithmetic lasting ∼ 25 min. The TSST reliably induces large and consistent HPA axis responses (Kirschbaum et al., 1993, 1995a, 1995b). This time, by characterizing women based on prior depression, we found that all women with depression histories, regardless of PMDD diagnosis, showed alterations in their allopregnanolone responses to stress. Women with prior depression showed significant decreases in allopregnanolone from baseline to post-stress at both the 30- and 60-min time points (P<0.005) while women with no prior depression showed modest, though nonsignificant increases in allopregnanolone from baseline to post-stress (see Fig. 5). Nonetheless, the directional difference in allopregnanolone response to mental stress between the 2 groups was statistically different (P<0.005).
Fig. 5.

Change (stress−baseline) in allopregnanolone (ALLO) concentrations at 30 and 60 min post-stress in women as a function of histories of depression (DEP). From Klatzkin et al. (2006a).
The lack of a significant increase in post-stress allopregnanolone in the never-depressed women is worth comment. While this study sampled for stress allopregnanolone 30 and 60 min following the onset of the mental stressor battery, modeling the sampling intervals on animal studies (Purdy et al., 1991) and human endocrine challenge studies (Genazzani et al., 1998), the possibility exists that these intervals missed the peak allopregnanolone response to mental stress in human females. This is supported by the comparison of the stress-induced increase in allopregnanolone in the never-depressed women in the present study with the non-PMDD controls in our first study (Fig. 3), since the former group showed a response that was only 20% of what we observed in the non-PMDD controls when allopregnanolone was sampled earlier, at 17 min post-stress onset. This is also supported by recent preliminary data from our laboratory indicating that in healthy, non-PMDD females with no history of depression, peak allopregnanolone responses occur ∼ 15 min after stress onset (unpublished data). Consequently, failure to capture peak allopregnanolone responses to stress may have contributed to our inability to detect PMDD-related differences in allopregnanolone stress responsivity in this second study (Klatzkin et al., 2006a). An alternative, though not mutually exclusive possibility, is that as part of the dysregulation in allopregnanolone stress responsivity, women with depressive disorders show a different time course of the allopregnanolone response to stress and this may contribute to the stress-induced decreases in allopregnanolone that we documented both in PMDD women (Fig. 3) and in women with histories of depression (Fig. 5).
In our second study (Klatzkin et al., 2006b), women with prior depression also failed to show the expected recovery in allopregnanolone from venipuncture stress to extended rest that was evident in never-depressed women (i.e., never-depressed women showed a decrease in allopregnanolone from venipuncture to extended baseline rest). Also, women with prior depression did not show the expected positive correlation between luteal phase progesterone and luteal phase allopregnanolone concentrations (r=0.16) that was seen in never-depressed women (r=0.37, P<0.005). This finding is similar to the absence of a relationship between progesterone and allopregnanolone documented in women with current post-partum dysphoria (Nappi et al., 2001) and documented in PMDD women relative to controls in the luteal phase (Monteleone et al., 2000), although that study did not assess histories of depression. Thus, what our initial study indicated was an effect of PMDD diagnosis on allopregnanolone responsivity to mental stress, our more recent work suggests may have reflected, at least in part, the greater prevalence rates of histories of depression in PMDD women.
One remarkable feature of these findings is that, on average, women had been in full remission from their prior depressive episode for ∼ 5 years. Thus, these data suggest that histories of depression may be associated with persistent, long-term effects on allopregnanolone responsivity to stress and that the greater likelihood of prior depression in PMDD women may have contributed to previous findings for lower allopregnanolone concentrations or blunted allopregnanolone responsivity to challenge in PMDD women (Rapkin et al., 1997; Monteleone et al., 2000; Girdler et al., 2001; Lombardi et al., 2004). Moreover, to the extent that allopregnanolone negatively modulates the HPA axis following stress, then our results are also consistent with HPA axis abnormalities seen in depression, since it is well established that a large proportion of patients with melancholy depression are hypercortisolimic (Nemeroff, 1998) and show exaggerated HPA axis responses to mental stress (Heim et al., 2000; Young and Breslau, 2004). More relevant, euthymic women with a history of major depression show elevated diurnal salivary cortisol relative to never-depressed women (Young et al., 2000a, 2000b). Thus, the possibility exists that the hypercortisolimia frequently documented in depression may be an associated feature of the depleted allopregnanolone concentrations seen in patients with depression or the altered allopregnanolone response to stressors seen in our sample of women with depression histories.
9. Alterations in allopregnanolone responses to stress in prior depression: relevance to PMDD
Our findings for differences in allopregnanolone responsivity as a function of prior depression and not PMDD in our second study is not to say that depression-related alterations in allopregnanolone responsivity to challenge do not have special clinical relevance for PMDD women. First, 45−60% of PMDD women have a history of major depression (Pearlstein et al., 1990; Cohen et al., 2002). Second, in our study we also documented that women with histories of depression had more severe premenstrual symptoms than never-depressed women, including greater premenstrual depression (P<0.005), irritability (P<0.001), labile mood (P<0.005), and fatigue (P<0.07). Using multiple regression techniques, we then examined the degree to which allopregnanolone measures predicted premenstrual symptom severity in PMDD women with and without prior depression. Greater allopregnanolone levels at extended baseline rest (reflecting a failure to recover from venipuncture stress) was a significant predictor of greater premenstrual depression, irritability, and labile mood, while greater decreases in allopregnanolone at 30 min following stress was a significant predictor of greater premenstrual depression. Together these measures of allopregnanolone reactivity to stress accounted for a significant 30−54% of the variance in symptoms. But this was true only for PMDD women with depression histories. Allopregnanolone responsivity to stress did not predict premenstrual symptoms in PMDD women with no prior history of depression. Thus, the possibility exists that failure to recover from stressors or failure to mount an appropriate allopregnanolone response to stress reflects the price of repeated biological adaptations to the increased life stress that is well documented in PMDD women and may contribute to the greater emotional and behavioral impact of daily stressors in the large subgroup (45−60%) of PMDD with histories of depression. If confirmed, these results are consistent with more recent conceptualizations of PMDD as a heterogeneous disorder with respect to pathophysiological mechanisms (Halbreich, 2003).
10. Conclusions and future directions
In humans, as in animal models, it appears that allopregnanolone is responsive to HPA axis activation induced by pharmacological challenge or by mental stress. While animal models provide compelling evidence for the adaptive role of stress-induced allopregnanolone, in terms of both behavioral and neuroendocrine responses to acute stress, these studies also demonstrate that chronic stress, such as occurs in animal models of depression, results in persistent alterations in GABAA receptor function and neuroactive steroid responses to subsequent acute stress. The evidence for alterations in baseline neuroactive steroid concentrations in depressive disorders, combined with our preliminary work suggesting altered allopregnanolone reactivity to stress in women with depressive disorders, suggest that investigations into the role of neuroactive steroid stress responsivity and the regulatory role of such responses with regard to the HPA axis in the pathophysiology of depressive disorders in humans is indicated. While this represents an exciting and innovative approach to understanding causal factors in depressive illness, it will be important to proceed cautiously by first establishing reliable studies in healthy human males and females on the time course of the allopregnanolone response to stress, and to investigate other neuroactive steroids that are detectable in human plasma, are stress responsive, and are potent modulators of the GABAA receptor, including both positive modulators such as allotetrahydroDOC and negative modulators such as pregnenolone sulfate. The nature of the stressful stimuli in terms of duration, controllability and predictability, as well as gender and ethnicity, may be important modulators of neurosteroid responses to stress, since each of these factors has been shown to alter the magnitude and time course of other stress-responsive neuroendocrine factors. Some of these initial studies are currently underway in our laboratory (e.g., Girdler et al., 2006).
The establishment of a reliable neuroactive stress response profile in healthy humans, as has been done for the HPA axis response to social stress (e.g., Kirschbaum et al., 1993, 1995a, 1995b; Young et al., 2000a, 2000b), will be needed to address questions suggested by some of the recent work from our laboratory. For example, whether depressive illness results in long-term alterations in neurosteroid responsivity, persisting beyond remission of the depressive episode, and whether such dysregulation results in altered stress responsiveness to even mild stressors later, either directly or indirectly via homeostatic forces affecting the HPA axis, setting the stage for the recurrence of a depressive episode with stress. Such studies may have direct implications for treatment of depressive illness since selective serotonin reuptake inhibitors (SSRI), while the first line of pharmacotherapy for depressive disorders, do not show uniform efficacy for all depressive illness. This is especially true for PMDD where there is a 40% non-response rate to SSRI (e.g., Steiner et al., 1995; Pearlstein et al., 2000).
“Stress is the salt of life — few people would like to live an existence of no runs, not hits, no errors” (Selye, 1976). Thus, the role of the stress researcher may not be to focus on ways of eliminating stress from individuals’ lives but to identify ways of maintaining adaptive biological and cognitive responses to life's stressors.
Aknowledgments
This work was supported by NIH grants MH051246 and GCRC RR00046.
Abbreviations
- 3α,5α-THP
allopregnanolone
- 3α,5α-THDOC
allotetrahydroDOC
- HPA
hypothalamic-pituitary-adrenal
- PMDD
premenstrual dysphoric disorder
References
- Akwa Y, Purdy RH, Koob GF, Britton KT. The amygdale mediates the anxiolytic-like effect of the neurosteroid allopregnanolone in rat. Behav Brain Res. 1999;106:119–125. doi: 10.1016/s0166-4328(99)00101-1. [DOI] [PubMed] [Google Scholar]
- Andreen L, Sundstrom-Poromaa I, Bixo M, Nyberg S, Backstrom T. Allopregnanolone concentration and mood-a bimodal association in postmenopausal women treated with oral progesterone. Psychopharmacology. 2006;187:209–221. doi: 10.1007/s00213-006-0417-0. [DOI] [PubMed] [Google Scholar]
- Bancroft J, Cook A, Davidson D, Bennie J, Goodwin G. Blunting of neuroendocrine responses to infusion of L-tryptophan in women with perimenstrual mood change. Psychol Med. 1991;21:305–312. doi: 10.1017/s0033291700020407. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Mostallino MC, Concas A, Purdy RH, et al. Time-dependent changes in rat brain neuroactive steroid concentrations and GABA A receptor function after acute stress. Neuroendocrinology. 1996;63:166–172. doi: 10.1159/000126953. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Purdy RH, Mostallino MC, Concas A, et al. The effects of inhibitors of GABAergic transmission and stress on brain and serum allopregnanolone concentrations. Br J Pharmacol. 1997;120:1582–1588. doi: 10.1038/sj.bjp.0701046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaccia ML, Concas A, Serra M, Biggio G. Stress and neurosteroids in adult and aged rats. Exp Gerontol. 1998;33:697–712. doi: 10.1016/s0531-5565(98)00042-4. [DOI] [PubMed] [Google Scholar]
- Bennett GG, Merritt MM, Wolin KY. Ethnicity, education, and the cortisol response to awakening: a preliminary investigation. Ethn Health. 2004;9:337–347. doi: 10.1080/1355785042000285366. [DOI] [PubMed] [Google Scholar]
- Bernard C. An introduction to the study of experimental medicine, 1927. 2nd Ed. H.C. Greene; New York: 1949. [Google Scholar]
- Biggio G, Concas A, Corda MG, Giorgi O, Sanna E, Serra M. GABAergic and dopaminergic transmission in the rat cerebral cortex: effect of stress, anxiolytic and anxiogenic drugs. Pharmacol Ther. 1990;48:121–142. doi: 10.1016/0163-7258(90)90077-f. [DOI] [PubMed] [Google Scholar]
- Bitran D, Shiekh M, McLeod M. Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABAA receptors. J Neuroendocrinol. 1995;7:171–177. doi: 10.1111/j.1365-2826.1995.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Bitran D, Dugan M, Renda P, Ellis R, Foley M. Anxiolytic effects of the neuroactive steroid pregnanolone (3alpha-OH-5beta-pregnan-20-one) after microinjection in the dorsal hippocampus and luteal septum. Brain Res. 1999;850:217–224. doi: 10.1016/s0006-8993(99)02150-2. [DOI] [PubMed] [Google Scholar]
- Bitran D, Klibansky DA, Martin GA. The neurosteroid pregnanolone prevents the anxiogenic-like effect of inescapable shock in the rat. Psychopharmacol (Berl) 2000;151:31–37. doi: 10.1007/s002130000472. [DOI] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. 2000;157:924–930. doi: 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse. 1996;23:39–51. doi: 10.1002/(SICI)1098-2396(199605)23:1<39::AID-SYN5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E. Stress and development: behavioral and biological consequences. Dev Psychopathol. 2001;13:473–489. doi: 10.1017/s0954579401003042. [DOI] [PubMed] [Google Scholar]
- Brot MD, Akwa Y, Purdy RH, Koob GF, Britton KT. The anxiolytic-like effects of the neurosteroid allopregnanolone: interactions with GABAA receptors. Eur J Pharmacol. 1997;315:1–7. doi: 10.1016/s0014-2999(97)00096-4. [DOI] [PubMed] [Google Scholar]
- Brown GW, Harris TO, Hepworth C. Life events and endogenous depression. A puzzle reexamined. Arch Gen Psychiatry. 1994;51:525–534. doi: 10.1001/archpsyc.1994.03950070017006. [DOI] [PubMed] [Google Scholar]
- Calogero AE, Palumbo MA, Bosboom AMJ, Burrello N, Ferrara E, Palumbo G, et al. The neuroactive steroid allopregnanolone suppresses hypothalamic gonadotropin-releasing hormone release through a mechanism mediated by the gamma-aminobutyric acidA receptor. J Endocrinol. 1998;158:121–125. doi: 10.1677/joe.0.1580121. [DOI] [PubMed] [Google Scholar]
- Cannon WB. The Wisdom of the Body. W.W. Nortyon and Co.; New York: 1939. [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Cohen LS, Soares CN, Otto MW, Sweeney BH, Liberman RF, Harlow BL. Prevalence and predictors of premenstrual dysphoric disorder (PMDD) in older premenopausal women — the Harvard study of moods and cycles. J Affect Disord. 2002;70:125–132. doi: 10.1016/s0165-0327(01)00458-x. [DOI] [PubMed] [Google Scholar]
- Concas A, Pepitoni S, Atsoggiu T, Toffano G, Biggio G. Aging reduces the GABA-dependent 36CI-flux in rat brain membrane vesicles. Life Sci. 1988;43:1761–1771. doi: 10.1016/0024-3205(88)90275-5. [DOI] [PubMed] [Google Scholar]
- Concas A, Follesa P, Barbaccia ML, Purdy RH, Biggio G. Physiological modulation of GABAA receptor plasticity by progesterone metabolites. Eur J Pharmacol. 1999;375:225–235. doi: 10.1016/s0014-2999(99)00232-0. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Andrews MW, Rosenblum LA, Owens MJ, Friedman S, Gorman JM, et al. Persistent elevations of cerebrospinal fluid concentrations of corticotrophin-releasing factor in adult nonhuman primates exposed to early-life stressors: implications for the pathophysiology of mood and anxiety disorders. Proc Natl Acad Sci. 1996;93:1619–1623. doi: 10.1073/pnas.93.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly RC, Schmidt PJ, Davis CL, Danaceau MA, Rubinow DR. Effects of gonadal steroids on peripheral benzodiazepine receptor density in women with PMS and controls. Psychoneuroendocrinology. 2001;26:539–549. doi: 10.1016/s0306-4530(01)00005-1. [DOI] [PubMed] [Google Scholar]
- D'Amore A, Marano G, Loizzo A. Reduced antinociceptive response to beta-endorphin in adult mice after chronic neonatal handling. Physiol Behav. 1993;53:1025–1027. doi: 10.1016/0031-9384(93)90286-o. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Park CH, Hitri A. Allosteric effects of a GABA receptor-active steroid are altered by stress. Pharmacol Biochem Behav. 1994;47:913–917. doi: 10.1016/0091-3057(94)90296-8. [DOI] [PubMed] [Google Scholar]
- Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) 4th ed. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Dong E, Matsumoto K, Uzunova V, Sugaya I, Takahata H, Nomura H, et al. Brain 5α-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. PNAS. 2001;98:2849–2854. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drugan RC, Morrow AL, Weizman R, Weizman A, Deutsch SI, Crawley JN, et al. Stress-induced behavioral depression in the rat is associated with a decrease in GABA receptor-mediated chloride ion flux and brain benzodiazepine receptor occupancy. Brain Res. 1989;487:45–51. doi: 10.1016/0006-8993(89)90938-4. [DOI] [PubMed] [Google Scholar]
- Eser D, Romeo E, Baghai TC, di Michele F, Schule C, Pasini A, et al. Neuroactive steroids as modulators of depression and anxiety. Neuroscience. 2006;138:1041–1048. doi: 10.1016/j.neuroscience.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Levin FR, Foltin RW, Fischman MW. Mood and performance changes in women with premenstrual dysphoric disorder: acute effects of alprazolam. Neuropsychopharmacology. 1998;19:499–516. doi: 10.1016/S0893-133X(98)00064-5. [DOI] [PubMed] [Google Scholar]
- Follesa P, Floris S, Tuligi G, Mostallino MC, Concas A, Biggio G. Molecular and functional adaptation of the GABAA receptor complex during pregnancy and after delivery in the rat brain. Eur J Neurosci. 1998;10:2905–2912. doi: 10.1111/j.1460-9568.1998.00300.x. [DOI] [PubMed] [Google Scholar]
- Follesa P, Concas A, Porcu P, Sanna E, Serra M, Mostallino MC, et al. Role of allopregnanolone in regulation of GABAA receptor plasticity during long-term exposure to and withdrawal from progesterone. Brain Res Rev. 2001;37:81–90. doi: 10.1016/s0165-0173(01)00125-4. [DOI] [PubMed] [Google Scholar]
- Frank E, Anderson B, Reynolds CF, Ritenour A, Kupfer DJ. Life events and the research diagnostic criteria endogenous subtype. A confirmation of the distinction using the Bedford College methods. Arch Gen Psychiatry. 1994;51:519–524. doi: 10.1001/archpsyc.1994.03950070011005. [DOI] [PubMed] [Google Scholar]
- Freeman EW. Luteal phase administration of agents for the treatment of premenstrual dysphoric disorder. CNS Drugs. 2004;18(7):453–468. doi: 10.2165/00023210-200418070-00004. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Sondheimer SJ. Premenstrual dysphoric disorder: recognition and treatment. Prim Care Companion J Clin Psychiat. 2003;5:30–39. doi: 10.4088/pcc.v05n0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman EW, Rickels K, Sondheimer SJ, Polansky M. A double-blind trial of oral progesterone, alprazolam, and placebo in treatment of severe premenstrual syndrome. JAMA. 1995;274:51–57. [PubMed] [Google Scholar]
- Freeman EW, Frye CA, Rickels K, Martin PAG, Smith SS. Allopregnanolone levels and symptom improvement in severe premenstrual syndrome. J Clin Psychopharmacol. 2002;22:516–520. doi: 10.1097/00004714-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Frye CA. The neurosteroid 3α,5α-THP has antiseizure and possible neuroprotective effects in an animal model of epilepsy. Brain Res. 1995;696:113–120. doi: 10.1016/0006-8993(95)00793-p. [DOI] [PubMed] [Google Scholar]
- Frye CA, Duncan JE. Progesterone metabolites, effective at the GABAA receptor complex, attenuate pain sensitivity in rats. Brain Res. 1994;643:194–203. doi: 10.1016/0006-8993(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Changes in progesterone metabolities in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav. 2002;41:306–315. doi: 10.1006/hbeh.2002.1763. [DOI] [PubMed] [Google Scholar]
- Gallo MA, Smith SS. Progesterone withdrawal decreases latency to and increases duration of electrified prod burial: a possible rat model of PMS anxiety. Pharmacol Biochem Behav. 1993;46:897–904. doi: 10.1016/0091-3057(93)90219-j. [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Petraglia F, Bernardi F, Casarosa E, Salvestroni C, Tonetti A, et al. Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab. 1998;83:2099–2103. doi: 10.1210/jcem.83.6.4905. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Pedersen CA, Stern RA, Light KC. Menstrual cycle and premenstrual syndrome: modifiers of cardiovascular reactivity in women. Health Psychol. 1993;12:180–192. doi: 10.1037//0278-6133.12.3.180. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Pedersen C, Straneva A, Leserman PA, Stanwyck J, Benjamin CL, et al. Dysregulation of cardiovascular and neuroendocrine responses to stress in premenstrual dysphoric disorder. Psychiatry Res. 1998;81:163–178. doi: 10.1016/s0165-1781(98)00074-2. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49:788–797. doi: 10.1016/s0006-3223(00)01044-1. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Sherwood A, Hinderliter AL, Leserman J, Costello NL, Straneva PA, et al. Biological correlates of abuse in women with premenstrual dysphoric disorder and healthy controls. Psychosom Med. 2003;65:849–856. doi: 10.1097/01.psy.0000088593.38201.cd. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Mechlin MB, Light KC, Morrow AL. Ethnic differences in allopregnanolone concentrations in women during rest and following mental stress. Psychophysiology. 2006;43:331–336. doi: 10.1111/j.1469-8986.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Leserman J, Bunevicius R, Klatzkin R, Pedersen CA, Light KC. Persistent alterations in cardiovascular and neuroendocrine profiles in women with abuse histories: influence of premenstrual dysphoric disorder. Health Psychol. 2007;26:201–213. doi: 10.1037/0278-6133.26.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding JM, Taylor DL. Sexual assault history and premenstrual distress in two general population samples. J Womens Health. 1996;5:143–152. [Google Scholar]
- Golding JM, Taylor DL, Menard L, King MJ. Prevalence of sexual abuse history in a sample of women seeking treatment for premenstrual syndrome. J Psychosom Obstet Gynaecol. 2000;21:69–80. doi: 10.3109/01674820009075612. [DOI] [PubMed] [Google Scholar]
- Guidoti A, Dong E, Matsumoto K, Pinna G, Rasmusson AM, Costa E. The socially-isolated mouse: a model to study the putative role of allopregnanolone and 5α-dihydroprogesterone in psychiatric disorders. Brain Res Rev. 2001;37:110–115. doi: 10.1016/s0165-0173(01)00129-1. [DOI] [PubMed] [Google Scholar]
- Guo AL, Petraglia F, Criscuolo M, Ficarra G, Nappie RE, Palumbo MA, et al. Evidence for a role of neurosteroids in modulation of diurnal changes and acute stress-induced corticosterone secretion in rats. Gynecol Endocrinol. 1995;9:1–7. doi: 10.3109/09513599509160184. [DOI] [PubMed] [Google Scholar]
- Halbreich U. The etiology, biology, and evolving pathology of premenstrual syndromes. Psychoneuroendocrinology. 2003;28:55–99. doi: 10.1016/s0306-4530(03)00097-0. [DOI] [PubMed] [Google Scholar]
- Hammarback St., Ekholm U-B, Backstrom T. Spontaneous anovulation causing disappearance of cyclical symptoms in women with the premenstrual syndrome. Acta Endocrinol (Copenh) 1991;125:132–137. doi: 10.1530/acta.0.1250132. [DOI] [PubMed] [Google Scholar]
- Harrison WM, Endicott J, Rabkin JG, Nee JC, Sandberg D. Treatment of premenstrual dysphoria with alprazolam and placebo. Psychopharmacol Bull. 1987;23:150–153. [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Wagner D, Wilcox MM, Miller AH, Nemeroff CB. The role of early adverse experience and adulthood stress in the prediction of neuroendocrine stress reactivity in women: a multiple regression analysis. Depress Anxiety. 2002;15:117–125. doi: 10.1002/da.10015. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Hippocrates . On airs, waters, and places (translated by WHS Jones, New York) W. Heinmann; New York: 1923. [Google Scholar]
- Hommer DW, Matsuo V, Wolkowitz O, Chrousos G, Greenblatt DJ, Weingater H, et al. Benzodiazepine sensitivity in normal human subjects. Arch Gen Psychiatry. 1986;43:542–551. doi: 10.1001/archpsyc.1986.01800060032005. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaer B, Perel J, Dahl RE, Moreci P, Nelson B, et al. The corticotrophin-releasing hormone challenge in depressed abused, depressed nonabused, and normal control children. Biol Psychiatry. 1997;42:669–679. doi: 10.1016/s0006-3223(96)00470-2. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Wiebe JP. Analgesic effects of the progesterone metabolite, 3α-hydroxy-5α-pregnan-20-one, and possible modes of action in mice. Brain Res. 1987;415:393–398. doi: 10.1016/0006-8993(87)90228-9. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kessler RC, Neale MC, Heath AC, Eaves LJ. The prediction of major depression in women: toward an integrated etiologic model. Am J Psychiatry. 1993;150:1139–1148. doi: 10.1176/ajp.150.8.1139. [DOI] [PubMed] [Google Scholar]
- Khisti RT, Chopde CT. Serotonergic agents modulate antidepressant-like effect of the neurosteroid 3α-hydroxy-5α-pregnan-20-0ne in mice. Brain Res. 2000;865:291–300. doi: 10.1016/s0006-8993(00)02373-8. [DOI] [PubMed] [Google Scholar]
- Khisti RT, Chopde CT, Jain SP. Antidepressant-like effect of the neurosteroid 3α-hydroxy-5α-pregnan-20-0ne in mice forced swim test. Pharmacol Biochem Behav. 2000;67:137–143. doi: 10.1016/s0091-3057(00)00300-2. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’ — a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kirshbaum C, Klauer T, Filip SH, Hellhammer DS. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosom Med. 1995a;57:23–31. doi: 10.1097/00006842-199501000-00004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Prussner JC, Stone AA, Federenko I, Gaab J, Lintz D, et al. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosom Med. 1995b;57:468–474. doi: 10.1097/00006842-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Klatzkin RR, Morrow AL, Light KC, Pedersen CA, Girdler SS. Histories of depression, allopregnanolone responses to stress, and premenstrual symptoms in women. Biol Psychol. 2006a;71:2–11. doi: 10.1016/j.biopsycho.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Klatzkin RR, Morrow AL, Light KC, Pedersen CA, Girdler SS. Associations of histories of depression and PMDD diagnosis with alloprepnanolone concentrations following the oral administration of micronized progesterone. Psychoneuroendocrinology. 2006b;31:1208–1219. doi: 10.1016/j.psyneuen.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Owens MJ, Nemeroff CB. Persistent changes in corticotrophin-releasing factor neuronal systems induced by maternal deprivation. Endocrinology. 1996;137:1212–1218. doi: 10.1210/endo.137.4.8625891. [DOI] [PubMed] [Google Scholar]
- Le Melledo J-M, Van Driel M, Coupland NJ, Lott P, Jhangri GS. Response to flumazenil in women with premenstrual dysphoric disorder. Am J Psychiatry. 2000;157:821–823. doi: 10.1176/appi.ajp.157.5.821. [DOI] [PubMed] [Google Scholar]
- Lemieux AM, Coe CL. Abuse-related posttraumatic stress disorder: evidence for chronic neuroendocrine activation in women. Psychosom Med. 1995;57:105–115. doi: 10.1097/00006842-199503000-00002. [DOI] [PubMed] [Google Scholar]
- Lombardi I, Luisi S, Quirici B, Monteleone P, Benardi F, Liut M, et al. Adrenal response to adrenocorticotropic hormone stimulation in patients with premenstrual syndrome. Gynecol Endocrinol. 2004;18:79–87. doi: 10.1080/09513590310001652955. [DOI] [PubMed] [Google Scholar]
- Marvan ML, Cortes-Iniestra S. Women's beliefs about the prevalence of premenstrual syndrome and biases in recall of premenstrual changes. Health Psychol. 2001;20:276–280. doi: 10.1037//0278-6133.20.4.276. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. Review article. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostatis and allostatic load. Ann N Y Acad Sci. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- Meczekalski B, Tonetti A, Monteleone P, Bernardi F, Luisi S, Stomati M, et al. Hypothalamic amenorrhea with normal body weight: ACTH, allopregnanolone and cortisol responses to corticotrophin-releasing hormone tests. Eur J Endocrinol. 2000;142:280–285. doi: 10.1530/eje.0.1420280. [DOI] [PubMed] [Google Scholar]
- Menozzi R, Florio P, Bondi M, Luisi T, Cobellis L, Genazzani AR, et al. Increased response of plasma allopregnanolone to corticotrophin-releasing hormone in obese patients. Neuroendocrinology. 2002;75:124–129. doi: 10.1159/000048228. [DOI] [PubMed] [Google Scholar]
- Mitev YA, Darwish M, Wolf SS, Holsboer F, Almeida OFX, Patchev VK. Gender differences in the regulation of 3α-sydroxysteroid dehydrogenase in rat brain and sensitivity to neurosteroid-mediated stress protection. Neuroscience. 2003;120:541–549. doi: 10.1016/s0306-4522(03)00287-2. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Luisi S, Tonetti A, Bernardi F, Genazzani AD, Luisi M, et al. Allopregnanolone concentrations and premenstrual syndrome. Eur J Endocrinol. 2000;142:269–273. doi: 10.1530/eje.0.1420269. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Suzdak PD, Paul SM. Steroid hormone metabolities potentiate GABA receptor-mediated chloride ion flux with nanomolar potency. Eur J Pharmacol. 1987;142:483–485. doi: 10.1016/0014-2999(87)90094-x. [DOI] [PubMed] [Google Scholar]
- Nappi RE, Petraglia F, Luisi S, Polatti F, Farina C, Genazzani AR. Serum allopregnanolone in women with postpartum “blues”. Obstet Gynecol. 2001;97:77–80. doi: 10.1016/s0029-7844(00)01112-1. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. The neurobiology of depression. Sci Am. 1998:42–49. doi: 10.1038/scientificamerican0698-42. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, Glue P, Lawson CW, Wilson S. Flumazenil provocation of panic attacks: evidence for altered benzodiazepine sensitivity in panic disorder. Arch Gen Psychiatry. 1990;47:917–925. doi: 10.1001/archpsyc.1990.01810220033004. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Ritchie JC, Nemeroff CB. 5α-Pregnane-3α,21-diol-20-one (THDOC) attenuates mild stress-induced increases in plasma corticosterone via a non-glucocorticoid mechanism: comparison with alprazolam. Brain Res. 1992;573:353–355. doi: 10.1016/0006-8993(92)90788-b. [DOI] [PubMed] [Google Scholar]
- Paddison PL, Gise LH, Lebovits A, Strain JJ, Cirasole DM, Levine JP. Sexual abuse and premenstrual syndrome: comparison between a lower and higher socioeconomic group. Psychosomatics. 1990;31:265–272. doi: 10.1016/S0033-3182(90)72162-7. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Shoaib M, Holsboer F, Almeida OF. The neurosteroid tetrahydroprogesterone counteracts corticotrophin-releasing hormone-induced anxiety and alters the release and gene expression of corticotrophin-releasing hormone in the rat hypothalamus. Neuroscience. 1994;62:265–271. doi: 10.1016/0306-4522(94)90330-1. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Hassan AHS, Holsboer F, Almeida OFX. The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress an exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology. 1996;15:533–540. doi: 10.1016/S0893-133X(96)00096-6. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Montkowski A, Rouskova D, Korany L, Holsboer F, Almeida OFX. Neonata treatment of rats with the neuroactive steroid tetahydrodeoxycorticosterone (THDOC) abolishes the behavioral and neuroendocrine consequences of adverse early life events. J Clin Invest. 1997;99:962–966. doi: 10.1172/JCI119261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Pearlstein TB, Frank E, Rivera-Tovar A, Thoft JS, Jacobs E, Mieczkowski TA. Prevalence of axis I and axis II disorders in women with late luteal phase dysphoric disorder. J Affect Disord. 1990;20:129–134. doi: 10.1016/0165-0327(90)90126-s. [DOI] [PubMed] [Google Scholar]
- Pearlstein TB, Halbreich U, Batzar ED, Brown CS, Endicott J, Frank E, et al. Psychosocial functioning in women with premenstrual dysphoric disorder before and after treatment with sertraline or placebo. J Clin Psychiatry. 2000;61:101–109. doi: 10.4088/jcp.v61n0205. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Stern RA, Pate J, Senger MA, Bowes WA, Mason GA. Thyroid and adrenal measures during late pregnancy and the puerperium in women who have been major depressed or who become dysphoric postpartum. J Affect Disord. 1993;29:201–211. doi: 10.1016/0165-0327(93)90034-h. [DOI] [PubMed] [Google Scholar]
- Perche F, Young J, Robel P, Simon NG, Haug M. Prenatal testosterone treatment potentiates the aggression-inhibiting effect of the neurosteroid dehydroepiandrosterone in female mice. Aggress Behav. 2001;27:130–138. [Google Scholar]
- Pibiri F, Marianela N, Carboni G, Pinna G. Neurosteroids regulate mouse aggression induced by anabolic androgenic steroids. NeuroReport. 2006;17:1537–1541. doi: 10.1097/01.wnr.0000234752.03808.b2. [DOI] [PubMed] [Google Scholar]
- Pinna G, Dong E, Matsumoto K, Costa E, Guidotti A. In socially isolated mice, the reversal of brain allopregnanolone down-regulation mediates the anti-aggressive action of fluoxetine. PINAS. 2003;100:2035–2049. doi: 10.1073/pnas.0337642100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poromaa IS, Smith S, Gulinello M. GABA receptors, progesterone and premenstrual dysphoric disorder. Arch Women Ment Health. 2003;6:23–41. doi: 10.1007/s00737-002-0147-1. [DOI] [PubMed] [Google Scholar]
- Prince MJ, Harwood RH, Blizard RA, Thomas A, Mann AH. Social support deficits, loneliness and life events as risk factors for depression in old age. The Gospel Oak Project IV. Psychol Med. 1997;27:323–332. doi: 10.1017/s0033291796004485. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr., Paul SM. Stress-induced elevations of γ-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raadsheer FC, van Heerikhuize JJ, Lucassen PJ, Hoogendijk WJ, Tilders FJ, Swaab DF. Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer's disease and depression. Am J Psychiatry. 1995;152:1372–1376. doi: 10.1176/ajp.152.9.1372. [DOI] [PubMed] [Google Scholar]
- Rabin DS, Schmidt PJ, Campbell G, Gold PW, Jensvold M, Rubinow DR, et al. Hypothalamic-pituitary-adrenal function in patients with the premenstrual syndrome. J Clin Endocrinol Metab. 1990;71:1158–1162. doi: 10.1210/jcem-71-5-1158. [DOI] [PubMed] [Google Scholar]
- Rapkin AJ, Morgan M, Goldman L, Brann DW, Simone D, Mahesh VB. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol. 1997;90:709–714. doi: 10.1016/S0029-7844(97)00417-1. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Is there a physiological role for the neurosteroid THDOC in stress-sensitive conditions? Trends Pharmacol Sci. 2003;24:103–106. doi: 10.1016/S0165-6147(03)00023-3. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Physiological role of adrenal deoxycorticosterone-derived neuroactive steroids in stress-sensitive conditions. Neuroscience. 2006;138:911–920. doi: 10.1016/j.neuroscience.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Redei E, Freeman EW. Preliminary evidence for plasma adrenocorticotropin levels as biological correlates of premenstrual symptoms. Acta Endocrinol. 1993;128:536–542. doi: 10.1530/acta.0.1280536. [DOI] [PubMed] [Google Scholar]
- Redei E, Freeman EW. Daily plasma estradiol and progesterone levels over the menstrual cycle and their relation to premenstrual symptoms. Psychoneuroendocrinology. 1995;20:259–267. doi: 10.1016/0306-4530(94)00057-h. [DOI] [PubMed] [Google Scholar]
- Resnick HS, Yehuda R, Pitman RK, Foy DW. Effect of previous trauma on acute plasma cortisol level following rape. Am J Psychiatry. 1995;152:1675–1677. doi: 10.1176/ajp.152.11.1675. [DOI] [PubMed] [Google Scholar]
- Roca CA, Schmidt PJ, Altemus M, Deuster P, Danaceau MA, Putnam K, et al. Differential menstrual cycle regulation of hypothalamic-pituitary-adrenal axis in women with premenstrual syndrome and controls. J Clin Endocrinol Metab. 2003;88:3057–3063. doi: 10.1210/jc.2002-021570. [DOI] [PubMed] [Google Scholar]
- Romeo E, Strohle A, Spalletta G, di Michele F, Hermann B, Holsboer F, et al. Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatry. 1998;155:910–913. doi: 10.1176/ajp.155.7.910. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Kaplan GA, Shema SJ, Strawbridge WJ. Does growing old increase the risk for depression? Am J Psychiatry. 1997;154:1384–1390. doi: 10.1176/ajp.154.10.1384. [DOI] [PubMed] [Google Scholar]
- Roy A, Pickar D, Paul S, Doran A, Chrousos GP, Gold PW. CSF corticotrophin-releasing hormone in depressed patients and normal control subjects. Am J Psychiatry. 1987;144(5):641–645. doi: 10.1176/ajp.144.5.641. [DOI] [PubMed] [Google Scholar]
- Rubinow DR. Reproductive steroids in context. Invited editorial. Arch Women Ment Health. 2005;8:1–5. doi: 10.1007/s00737-005-0065-0. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Schmidt PJ. Premenstrual syndrome: a review of endocrine studies. The Endocrinologist. 1992;2:47–54. [Google Scholar]
- Schmidt PJ, Grover GN, Rubinow DR. Alprazolam in the treatment of premenstrual syndrome: a double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1993;50:467–473. doi: 10.1001/archpsyc.1993.01820180069007. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Purdy RH, Moore PH, Jr., Paul SM, Rubinow DR. Circulating levels of anxiolytic steroids in the luteal phase in women with premenstrual syndrome and in control subjects. J Clin Endocrinol Metab. 1994;79:1256–1260. doi: 10.1210/jcem.79.5.7962316. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. 1998;338:209–216. doi: 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]
- Selye H. A syndrome produced by diverse nocuous agents. Nature. 1936;138:230–231. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- Selye H. The anesthetic effect of steroid hormones. Proc Soc Exp Biol Med. 1941;46:116–121. [Google Scholar]
- Selye H. The general-adaptation-syndrome and the diseases of adaptation. South Med J. 1951;113(10):315–323. [PubMed] [Google Scholar]
- Selye H. Stress and disease. Science. 1955;122:625–631. doi: 10.1126/science.122.3171.625. [DOI] [PubMed] [Google Scholar]
- Selye H. Stress and psychiatry. Am J Psychiatry. 1956;113(5):423–427. doi: 10.1176/ajp.113.5.423. [DOI] [PubMed] [Google Scholar]
- Selye H. Forty years of stress research: principal remaining problems and misconceptions. CMA J. 1976;115:53–56. [PMC free article] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Littera M, Papi G, Sanna E, Tuveri F, et al. Social isolation-induced decreases in both the abundance of neuroactive steroids and GABAA receptor function in rat brain. J Neurochem. 2000;75:732–740. doi: 10.1046/j.1471-4159.2000.0750732.x. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, et al. Withdrawal from 3α-OH-5α-pregnan-20-one using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor α4 subunit in association with increased anxiety. J Neurosci. 1998a;18:5275–5284. doi: 10.1523/JNEUROSCI.18-14-05275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Gone QH, Hsu F-C, Markowitz RS, ffrench-Mullen JMH, Li X. GABAA receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998b;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Smith SS, Ruderman Y, Frye C, Homanics G, Yuan M. Steroid withdrawal in the mouse results in anxiogenic effects of 3α,5α-THP: A\kern-3pta possible model of premenstrual dysphoric disorder. Psychopharmacology. 2006;186:323–333. doi: 10.1007/s00213-005-0168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner M, Steinberg S, Stewart D, Carter D, Berger C, Reid R, et al. Fluoxetine in the treatment of premenstrual dysphoria. N Engl J Med. 1995;332:1529–1534. doi: 10.1056/NEJM199506083322301. [DOI] [PubMed] [Google Scholar]
- Steiner M, Yatham LN, Coote M, Wilkins A, Lepage P. Serotonergic dysfunction in women with pure premenstrual dysphoric disorder: is the fenfluramine challenge test still relevant? Psychiatry Res. 1999;87:107–115. doi: 10.1016/s0165-1781(99)00062-1. [DOI] [PubMed] [Google Scholar]
- Strohle A, Romeo E, Hermann B, Pasini A, Spalletta G, di Michele F, et al. Concentrations of 3α-reduced neuroactive steroids and their precursors in plasma of patients with major depression and after clinical recovery. Biol Psychiatry. 1999;45:274–277. doi: 10.1016/s0006-3223(98)00328-x. [DOI] [PubMed] [Google Scholar]
- Strohle A, Pasini A, Romeo E, Hermann B, Spalletta G, di Michele F, et al. Fluoxetine decreases concentrations of 3α,5α-tetrahydrodeoxycorticosterone (THDOC) in major depression. J Psychiatr Res. 2000;34:183–186. doi: 10.1016/s0022-3956(00)00006-6. [DOI] [PubMed] [Google Scholar]
- Strous RD, Maayan R, Weizman A. The relevance of neurosteroids to clinical psychiatry: from the laboratory to the bedside. Eur Neuropsychopharmacol. 2006;16:155–169. doi: 10.1016/j.euroneuro.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Su T-P, Schmidt PJ, Danaceua M, Murphy DL, Rubinow DR. Effect of menstrual cycle phase on neuroendocrine and behavioral responses to the serotonin agonist m-chlorophenylpiperazine in women with premenstrual syndrome and controls. J Clin Endocrinol Metab. 1997;83:1220–1228. doi: 10.1210/jcem.82.4.3905. [DOI] [PubMed] [Google Scholar]
- Sundstrom I, Ashbrook D, Backstrom T. Reduced benzodiazepine sensitivity in patients with premenstrual syndrome: a pilot study. Psychoneruoendocrinology. 1997a;22:25–38. doi: 10.1016/s0306-4530(96)00035-2. [DOI] [PubMed] [Google Scholar]
- Sundstrom I, Nyberg S, Backstrom T. Patients with premenstrual syndrome have reduced sensitivity to midazolam compared to control subjects. Neuropsychopharmacology. 1997b;17:370–381. doi: 10.1016/S0893-133X(97)00086-9. [DOI] [PubMed] [Google Scholar]
- Sundstrom I, Andersson A, Nybery S, Ashbrook D, Purdy RH, Backstrom T. Patients with premenstrual syndrome have a different sensitivity to a neuroactive steroid during the menstrual cycle compared to control subjects. Neuroendocrinology. 1998;67:126–138. doi: 10.1159/000054307. [DOI] [PubMed] [Google Scholar]
- Szikszay M, Benedek G. Tolerance to morphine induced by chronic mild environmental stressors. Acta Physiol Hung. 1989;73:447–453. [PubMed] [Google Scholar]
- Uzunova V, Ceci M, Kohler C, Uzunov DP, Wrynn AS. Region-specific dysregulation of allopregnanolone brain content in the olfactory bulbectomized rat model of depression. Brain Res. 2003;976:1–8. doi: 10.1016/s0006-8993(03)02577-0. [DOI] [PubMed] [Google Scholar]
- Uzunova V, Ceci M, Kohler C, Uzunov DP, Wrynn AS. Chronic antidepressants reverse cerebrocortical allopregnanolone decline in the olfactory-bulbectomized rat. Eur J Pharmacol. 2004;486:31–34. doi: 10.1016/j.ejphar.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Uzunova V, Sampson L, Uzunov DP. Relevance of endogenous 3α-reduced neurosteroids to depression and antidepressant action. Psychopharmacology. 2006;186:351–361. doi: 10.1007/s00213-005-0201-6. [DOI] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, et al. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee M, Rivera JD, Koob GF, Purdy RH, Fitzgerald RL. Quantification of neurosteroids in rat plasma and brain following swim stress and allopregnanolone administration using negative chemical ionization gas chromatography/mass spectrometry. Anal Biochem. 2000;287:153–166. doi: 10.1006/abio.2000.4841. [DOI] [PubMed] [Google Scholar]
- Vanselow W, Dennerstein L, Greenwood KM, de Lignieres B. Effect of progesterone and its 5α and 5β metabolites on symptoms of premenstrual syndrome according to route of administration. J Psychosom Obstet Gynaecol. 1996;17:29–38. doi: 10.3109/01674829609025661. [DOI] [PubMed] [Google Scholar]
- von Bardeleben U, Holsboer F. Human corticotropin releasing hormone: clinical studies in patients with affective disorders, alcoholism, panic disorder and in normal controls. Prog Neuropsychopharmacol Biol Psychiat. 1988;13:S165–S187. doi: 10.1016/0278-5846(88)90079-6. [DOI] [PubMed] [Google Scholar]
- Wang M, Seippel L, Purdy RH, Backstrom T. Relationship between symptom severity and steroid variation in women with premenstrual syndrome: Study on serum pregnenolone, pregnenolone sulfate, 5α-pregnane-3,20-dione and 3α-hydroxy-5α-pregnan-20-one. J Clin Endocrinol Metab. 1996;81:1076–1082. doi: 10.1210/jcem.81.3.8772579. [DOI] [PubMed] [Google Scholar]
- Wiebe JP, Kavaliers M. Analgesic effects of the putative FSH-suppressing gonadal steroid, 3α-hydroxy-4-pregnen-20-one: possible modes of action. Brain Res. 1988;461:150–157. doi: 10.1016/0006-8993(88)90733-0. [DOI] [PubMed] [Google Scholar]
- Woods NF, Lentz M, Mitchell ES, Heitkemper M, Shaver J. PMS after 40: persistence of a stress-related symptom pattern. Res Nurs Health. 1997;20:319–340. doi: 10.1002/(sici)1098-240x(199708)20:4<329::aid-nur6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Wyatt K, Dimmock P, Jones P, Obhrai M, O'Brien S. Efficacy of progesterone and progestogens in management of premenstrual syndrome: systematic review. BMJ. 2001;323:776–780. doi: 10.1136/bmj.323.7316.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EA, Breslau N. Cortisol and catecholamines in posttraumatic stress disorder. Arch Gen Psychiatry. 2004;61:394–401. doi: 10.1001/archpsyc.61.4.394. [DOI] [PubMed] [Google Scholar]
- Young EA, Veldhuis JD. Disordered adrenocorticotropin secretion in women with major depression. J Clin Endocrinol Metab. 2006;91:1924–1928. doi: 10.1210/jc.2005-2397. [DOI] [PubMed] [Google Scholar]
- Young EA, Aggen SH, Prescott CA, Kendler KS. Similarity in saliva cortisol measures in monozygotic twins and the influence of past major depression. Biol Psychiatry. 2000a;48:70–74. doi: 10.1016/s0006-3223(00)00842-8. [DOI] [PubMed] [Google Scholar]
- Young EA, Lopez JF, Murphy-Weinberg V, Watson SJ, Akil H. Hormonal evidence for altered responsiveness to social stress in major depression. Neuropsychopharmacology. 2000b;23:411–418. doi: 10.1016/S0893-133X(00)00129-9. [DOI] [PubMed] [Google Scholar]