Summary
Ca2+/calmodulin-dependent protein kinase II (CaMKII) is central to synaptic transmission. Here we show that synaptic CaMKIIα binds to the third intracellular loop of the limbic dopamine D3 receptor (D3R). This binding is Ca2+-sensitive and is sustained by autophosphorylation of CaMKII, providing an unrecognized route for the Ca2+-mediated regulation of D3Rs. The interaction of CaMKIIα with D3Rs transforms D3Rs into a biochemical substrate of the kinase and promotes the kinase to phosphorylate D3Rs at a selective serine site (S229). In accumbal neurons in vivo, CaMKIIα is recruited to D3Rs by rising Ca2+ to increase the CaMKIIα-mediated phosphorylation of D3Rs, thereby transiently inhibiting D3R efficacy. Notably, the D3R inhibition is critical for integrating dopamine signaling to control behavioral sensitivity to the psychostimulant cocaine. Our data identify CaMKIIα as a recruitable regulator of dopamine receptor function. By binding and phosphorylating limbic D3Rs, CaMKIIα modulates dopamine signaling and psychomotor function in an activity-dependent manner.
Keywords: striatum, nucleus accumbens, calcium, calmodulin, adenylyl cyclase, cAMP, CREB, locomotion, cocaine
Introduction
Dopamine is a major neurotransmitter in the mammalian brain. This transmitter exerts its pleiotropic actions through interactions with two classes of dopamine receptors: the D1 class (D1 and D5 subtypes) and the D2 class (D2, D3, and D4 subtypes). The former is coupled to Gαs-proteins, whereas the latter is generally coupled to Gαi/o-proteins. Through Gαi/o-proteins, the D2 class of receptors inhibits adenylyl cyclase and the downstream formation of cAMP (Neve et al., 2004). Remarkably, among all dopamine receptor subtypes, the dopamine D3 receptor (D3R), one of the lesser-investigated subtypes, shows a unique distribution pattern in the forebrain. D3Rs are preferentially expressed in the mesolimbic dopaminergic projection areas, including the nucleus accumbens (NAc) (Sokoloff et al., 1990; Bouthenet et al., 1991). This specific localization of D3Rs suggests a significant role of the receptor in the control of reward, motivation, cognition, emotion, and extrapyramidal motor functions. Moreover, malfunction of D3Rs has been frequently associated with the pathogenesis of various mental illnesses such as schizophrenia, depression, and substance addiction (reviewed in Heidbreder et al., 2005; Sokoloff et al., 2006; Richtand, 2006).
Ca2+/calmodulin-dependent protein kinase II (CaMKII) is an enzyme that is abundant in brain cells, especially at synaptic sites (Kelly et al., 1984). The enzyme is removed from its autoinhibitory state and activated by the binding of Ca2+ and calmodulin (CaM). Activated CaMKII accesses and phosphorylates not only exogenous substrates but also its own autophosphorylation site (T286 in the α isoform) within the autoregulatory domain. The autophosphorylation renders a Ca2+/CaM-independent (autonomous) kinase activity even after the initial Ca2+ stimulus subsides (Hudmon and Schulman, 2002; Colbran and Brown, 2004; Griffith, 2004). As such, CaMKII integrates and prolongs the information conveyed by diverse forms of Ca2+ transients and serves as a dynamic regulator that decodes activity-dependent Ca2+ signals into various forms of synaptic activity and plasticity (reviewed in Hudmon and Schulman, 2002; Lisman et al., 2002; Colbran and Brown, 2004).
The multifunctional nature of CaMKII also stems from its interactions with various binding partners in defined subcellular regions (Colbran, 2004). Through direct protein-protein interactions, CaMKII vigorously regulates expression and function of interacting partners and vice versa. In searching for new targets of CaMKII at synaptic sites, we identify the limbic D3R as a direct substrate of CaMKII. We show that CaMKIIα interacts directly with the N-terminal region of the third intracellular loop of D3Rs in a Ca2+- and autophosphorylation-sensitive manner in vitro. This interaction allows CaMKII to phosphorylate D3Rs at a selective serine residue. In accumbal neurons in vivo, Ca2+ stimulates the association of CaMKII with D3Rs and increases serine phosphorylation of D3Rs, resulting in suppression of D3R function. The CaMKII-mediated inhibition of D3Rs plays a significant role in regulating behavioral responsiveness to cocaine. Collectively, our results demonstrate a new synaptic model for the activity-dependent regulation of limbic D3Rs by CaMKII.
Results
CaMKIIα directly binds to D3Rs in vitro
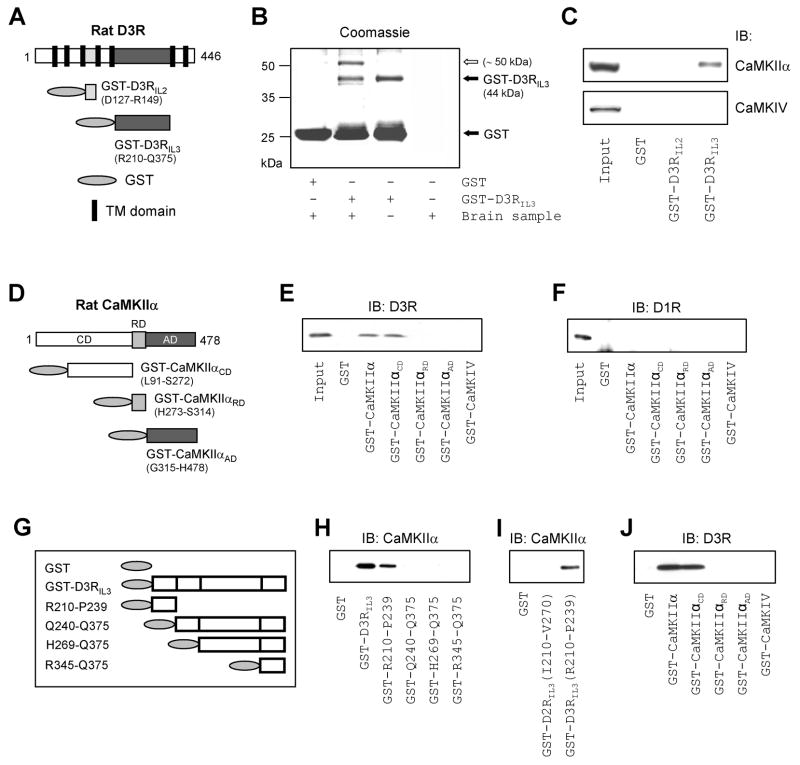
D3Rs contain a large third intracellular domain that is a main site for protein-protein interactions (Fig. S1). To search for new proteins that bind to this domain, we prepared glutathione-S-transferase (GST)-fusion proteins containing the second or third intracellular loop of rat D3Rs, D3RIL2 or D3RIL3 (Fig. 1A), and used them as immobilized (GST-bound) baits to screen NAc lysates in pull-down assays. We found a protein (~50 kDa) that was specifically precipitated by GST-D3RIL3, but not by GST alone (Fig. 1B) or GST-D3RIL2 (data not shown). This protein was subsequently identified as CaMKIIα by mass spectrometry. Immunoblot analysis confirmed that GST-D3RIL3, but not GST-D3RIL2 or GST alone, precipitated CaMKIIα (Fig. 1C). Neither the GST-fusion proteins nor GST precipitated CaMKIV, another isoform of CaMK (Fig. 1C). This indicates the selectivity of D3Rs in the interaction with CaMKII. To identify the specific CaMKIIα region responsible for the interaction with D3Rs, GST-fusion proteins were prepared from three major structures of CaMKIIα: the N-terminal catalytic domain (GST-CaMKIIαCD), the regulatory domain (GST-CaMKIIαRD), and the C-terminal association domain (GST-CaMKIIαAD) (Fig. 1D). We found that GST-CaMKIIαCD precipitated D3Rs in accumbal lysates, whereas GST-CaMKIIαRD, GST-CaMKIIαAD, and GST-CaMKIV did not (Fig. 1E). None of the GST-fusion proteins precipitated dopamine D1 receptors (D1Rs) (Fig. 1F). Thus, the primary CaMKIIα region for the interaction with D3Rs is restricted to CaMKIIαCD. All blots were probed in parallel with a GST antibody to ensure equivalent protein loading (data not shown).
Figure 1. Association of CaMKIIα with D3Rs.
(A) GST-fusion proteins derived from rat D3Rs with 7 transmembrane (TM) domains. (B) Coomassie staining of a gel resolved from pull-down assays with rat accumbal lysates. An open arrow indicates a protein (~50 kDa) pulled down by GST-D3RIL3. (C) GST-D3RIL3 precipitated CaMKIIα from rat accumbal lysates. (D) GST-fusion proteins derived from rat CaMKIIα. (E and F) GST-CaMKIIα and GST-CaMKIIαCD precipitated D3Rs (E), but not D1Rs (F), from rat accumbal lysates. (G) GST-fusion proteins derived from D3RIL3. (H–J) Results from in vitro binding assays with GST-fusion proteins and purified CaMKIIα (H and I) or D3RIL3 (J). Precipitated proteins from pull-down assays (C, E, and F) and proteins bound to CaMKIIα or D3RIL3 from in vitro binding assays (H–J) were visualized with immunoblots (IB) using the specific antibodies as indicated.
To define whether the CaMKIIα-D3R interaction is direct, and to reveal the accurate region of D3RIL3 involved in the interaction, we conducted in vitro binding assays with purified proteins (Fig. 1G-J). We found that CaMKIIα bound to immobilized GST-D3RIL3 and GST-D3RIL3(R210-P239), but not to other GST-D3RIL3 fragments (Fig. 1H). No CaMKIIα bound to GST-D2RIL3(I210-V270) (Fig. 1I). In the reverse experiment, D3RIL3 bound to immobilized GST-CaMKIIα and GST-CaMKIIαCD, but not to GST-CaMKIIαRD, GST-CaMKIIαAD, and GST-CaMKIV (Fig. 1J). These results demonstrate that CaMKIIα directly binds to D3Rs in vitro. The R210-P239 fragment, a 30-amino acid region at the N-terminus of D3RIL3, contains a site critical for binding to the catalytic domain of CaMKIIα.
Ca2+ enhances the binding of CaMKIIα to D3Rs
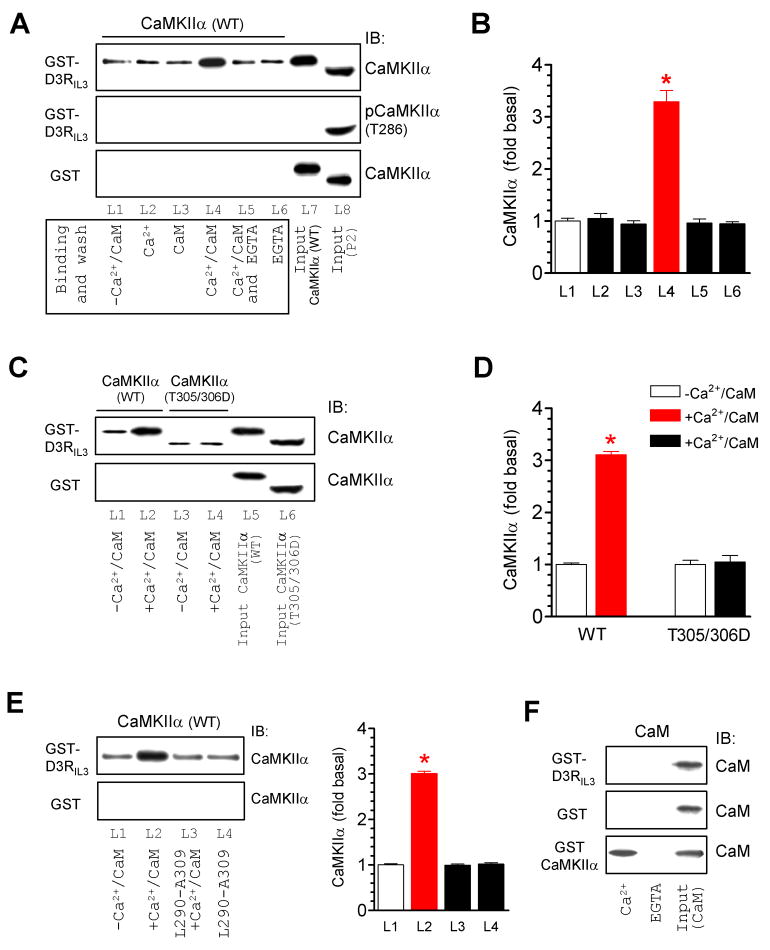
Binding of Ca2+/CaM to the regulatory domain of CaMKII is an essential step for releasing autoinhibition and activating the enzyme. Thus, Ca2+ usually regulates the binding properties of CaMKII (Bayer et al., 2001; Colbran et al., 2004). To determine if Ca2+ affects the CaMKIIα association with D3Rs, we tested the capacity of CaMKIIα to bind to GST-D3RIL3 in the presence of Ca2+, CaM, or both. Similar to the results from the above in vitro binding assays performed in the absence of exogenously added Ca2+/CaM, CaMKIIα showed a low level of constitutive binding to GST-D3RIL3 (L1 in Fig. 2A). Interestingly, the addition of Ca2+ (0.5 mM) and CaM (1 μM) together, but not Ca2+ or CaM alone, significantly increased the amount of CaMKIIα bound to D3RIL3 (L4 vs. L2 and L3 in Fig. 2A and 2B). This increase was prevented by adding the Ca2+ chelator EGTA at 1 mM (L5 in Fig. 2A), and could occur to CaMKIIα that was not autophosphorylated at T286 as evidenced by the fact that no T286-autophosphorylated CaMKIIα (pCaMKIIα) was detected in these binding assays with a phospho-specific antibody due to the absence of ATP throughout the reaction (the middle panel of Fig. 2A). These results demonstrate that activation of CaMKIIα by Ca2+/CaM was sufficient to enhance its affinity for D3Rs. Consistent with this, an increase in the CaMKIIα-D3RIL3 binding was observed only with the wild-type (WT) CaMKIIα, but not with the Ca2+/CaM binding-defective CaMKIIα mutant T305/306D (Fig. 2C and 2D) (Colbran and Soderling, 1990). Moreover, a peptide fragment (L290-A309), that corresponds to the CaM-binding domain of CaMKII and thereby antagonizes the CaM-CaMKII binding, blocked the Ca2+/CaM-induced enhancement of the CaMKIIα binding to D3Rs (Fig. 2E). To determine if CaM itself binds to D3RIL3, we conducted in vitro binding assays with purified CaM. No binding of CaM to GST-D3RIL3 or GST was observed, whereas CaM bound to GST-CaMKIIα in a Ca2+-dependent fashion (Fig. 2F).
Figure 2. Ca2+/CaM-regulated CaMKIIα binding to D3RIL3.
(A and B) Addition of Ca2+/CaM increased the CaMKIIα binding to GST-D3RIL3. Note that, in the middle panel of Fig. 2A, CaMKIIα was not autophosphorylated at T286 in binding assays lacking ATP (L1-L6) as opposed to T286-autophosphorylated CaMKIIα detected in the synaptosomal fraction (P2) from the rat NAc (L8) with a phospho-specific antibody. (C and D) Addition of Ca2+/CaM did not alter the binding of CaMKIIα T305/306D mutant to GST-D3RIL3. (E) Co-addition of an inhibitory peptide (L290-A309) prevented an increase in the CaMKIIα-D3RIL3 binding induced by Ca2+/CaM. (F) CaM did not bind to GST-D3RIL3 or GST whereas it bound to GST-CaMKIIα in a Ca2+-dependent manner. Binding assays were performed between His-tagged WT CaMKIIα (~57 kDa), T305/306D mutant (~50 kDa), or CaM proteins and immobilized GST, GST-D3RIL3, or GST-CaMKIIα in the presence or absence of CaCl2 (0.5 mM), CaM (1 μM), EGTA (1 mM), or L290-A309 (5 μM). Data are presented as means ± SEM for 3–6 experiments per group. *p < 0.05 versus L1.
Autophosphorylation of CaMKIIα further enhances the binding of CaMKIIα to D3Rs
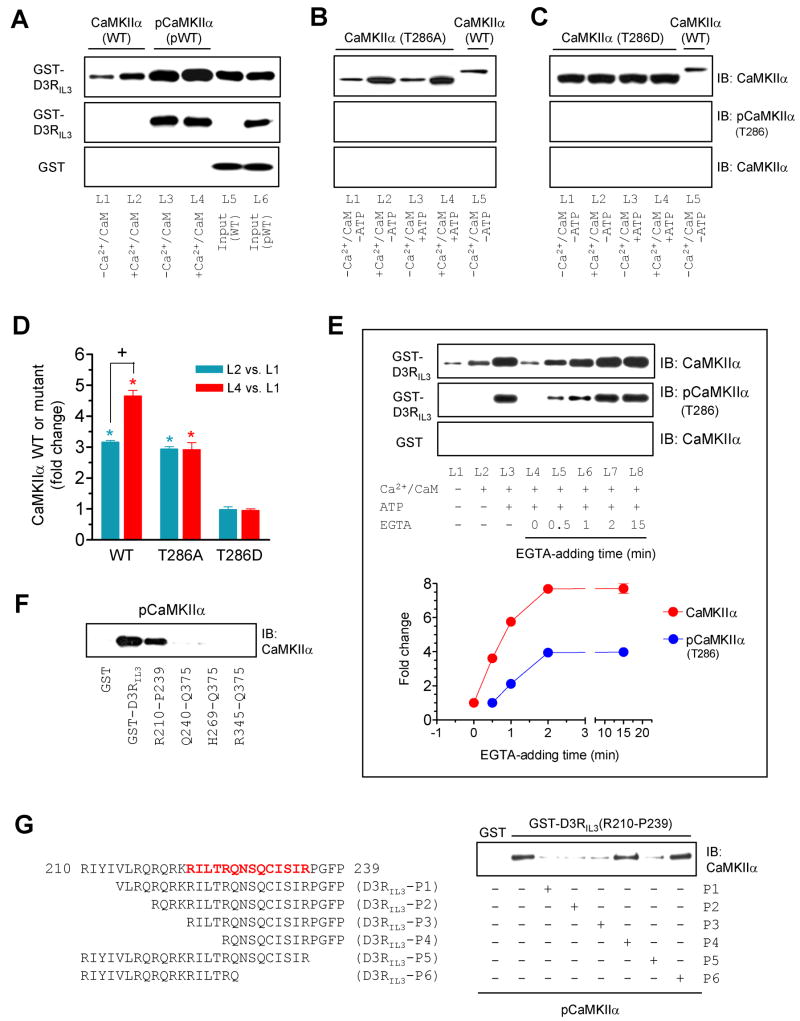
Activation of CaMKIIα by Ca2+/CaM results in autophosphorylation at T286 in the regulatory domain of adjacent CaMKIIα subunits, which ‘traps’ CaM and keeps the enzyme in a catalytically active conformation, an autonomous (Ca2+/CaM-independent) activity, even after the initial Ca2+/CaM signal subsides (Meyer et al., 1992). A series of experiments was carried out to determine the influence of T286 autophosphorylation on the CaMKIIα-D3R binding. The unphosphorylated WT kinase exhibited constitutive and enhanced binding to D3RIL3 in the absence and presence of Ca2+/CaM, respectively (Fig. 3A). Interestingly, WT CaMKIIα, that was activated by Ca2+/CaM and autophosphorylated at T286 prior to the binding assays, showed a further enhanced level of binding (L3 and L4 in Fig. 3A) as compared to unphosphorylated WT, regardless of the presence or absence of Ca2+/CaM in the binding assays (Fig. 3A and 3D). An autophosphorylation-defective mutant, T286A, showed the same level of binding to D3Rs in the presence or absence of ATP (Fig. 3B and 3D). In contrast, a constitutively active (Ca2+/CaM-independent) mutant, T286D that shares the property of autophosphorylated CaMKIIα, showed a high level of binding to D3Rs, which was not affected by the addition of Ca2+/CaM or Ca2+/CaM/ATP (Fig. 3C and 3D). These data indicate that, while the basal (unstimulated) and Ca2+/CaM-stimulated binding to D3Rs requires no autophosphorylation, autophosphorylation of T286 further increases the capacity of CaMKIIα to bind to D3Rs.
Figure 3. Influence of T286 autophosphorylation on the CaMKIIα-D3RIL3 binding.
(A–C) Binding of WT CaMKIIα/pCaMKIIα (A), mutant T286A (B), or mutant T286D (C) to GST-D3RIL3. (D) A graph of the data from A–C. Blue and red bars represent changes in lane 2 (L2) and lane 4 (L4), respectively, over lane 1 (L1). *p < 0.05 versus L1. +p < 0.05 versus L2. (E) Effects of EGTA added at different times on the CaMKIIα binding to GST-D3RIL3. EGTA was added 0, 0.5, 1, 2, or 15 min after the onset of the binding reaction. Representative immunoblots are shown above the quantified data. (F) pCaMKIIα bound to GST-D3RIL3 and the GST-D3RIL3(R210-P239) fragment. (G) Effects of six peptides derived from the R210-P239 fragment on the binding of pCaMKIIα to GST-D3RIL3(R210-P239). The first 30-amino acid sequence of D3RIL3 is shown. Bold letters (in red) indicate the potential CaMKIIα binding motif. Binding assays were performed between His-tagged WT CaMKIIα (~57 kDa), WT pCaMKIIα (~57 kDa), T286A mutant (~50 kDa), or T286D mutant (~50 kDa) proteins and immobilized GST-D3RIL3 or GST-D3RIL3(R210-P239) in the presence or absence of CaCl2 (0.5 mM), CaM (1 μM), ATP (50 μM), or EGTA (1 mM) as indicated. Bound CaMKIIα, pCaMKIIα, or mutants were visualized by immunoblots. Data are presented as means ± SEM for 4–6 experiments per group.
The binding properties of autophosphorylated CaMKIIα were further analyzed in binding assays with EGTA (1 mM) added at different times. As before, an increased amount of D3RIL3-bound CaMKIIα was detected when Ca2+/CaM and ATP were added for 20 min (L3 in Fig. 3E). The addition of EGTA substantially inhibited the CaMKIIα binding. However, this inhibition occurred only when EGTA was added in the early phase (< 2 min, L4-L6 in Fig. 3E). When the binding reaction lasted long enough (2–15 min), EGTA was no longer effective (L7 and L8 in Fig. 3E). The D3RIL3-bound enzyme from the ATP-containing assays was autophosphorylated at T286, and the level of this autophosphorylation paralleled well with the capacity of the enzyme in binding to D3Rs at different EGTA-adding times (Fig. 3E). These data suggest that the CaMKIIα binding to D3Rs and T286-autophosphorylation are initially reversible, but become Ca2+/CaM-independent when the kinase transforms to an autonomous state.
Like the unphosphorylated enzyme, autophosphorylated CaMKIIα bound to the same N-terminal region (R210-P239) of D3RIL3 (Fig. 3F). This region was therefore further analyzed to identify a sufficient binding motif. A panel of peptides derived from this region was used to compete with the R210-P239 fragment for binding to CaMKIIα. It was found that four out of six peptides (P1, P2, P3, and P5) blocked the binding of GST-D3RIL3(R210-P239) to active CaMKIIα (Fig. S2A) or autophosphorylated CaMKIIα (Fig. 3G). These four peptides, when used in a biotinylated form, pulled down CaMKIIα (Fig. S2B). Thus, a 14-residue motif (RILTRQNSQCISIR) contained by all four peptides constitutes a core motif for CaMKIIα binding. Notably, this motif is highly conserved among mammals.
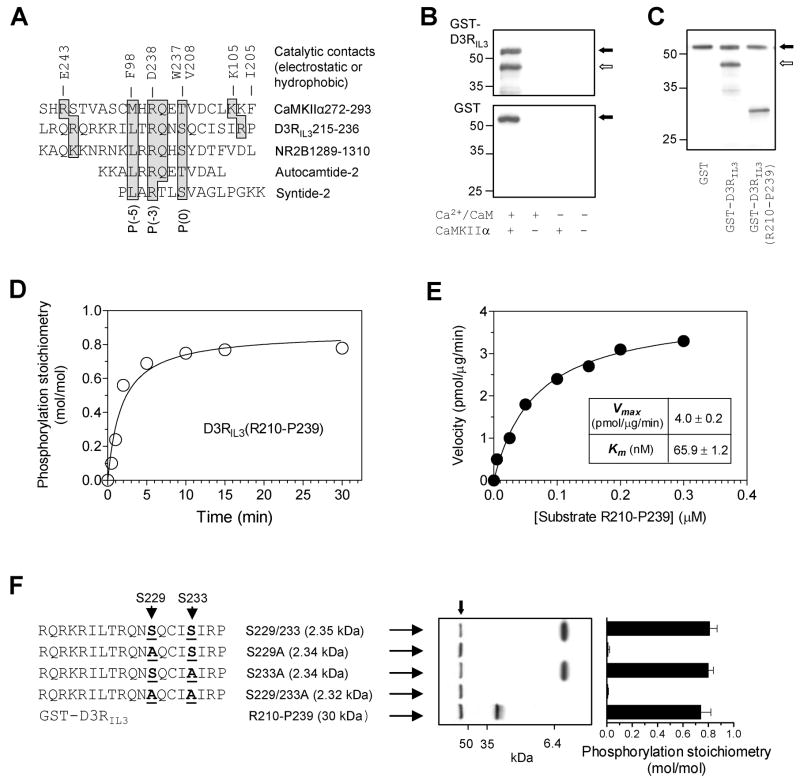
CaMKII phosphorylates S229 in D3RIL3
A comparison of the amino acid sequences revealed a remarkable homology between the CaMKIIα-binding region in D3RIL3 and the sequences spanning a T286 pseudosubstrate region in CaMKIIα (Yang and Schulman, 1999), S1303 in NR2B (Strack et al., 2000), and an S/T site in two classical CaMKII substrates, autocamtide-2 and syntide-2 (Fig. 4A). This suggested that D3RIL3 might serve as a phosphorylation substrate of CaMKII. Indeed, CaMKIIα phosphorylated GST-D3RIL3, but not GST, in the presence of Ca2+/CaM (Fig. 4B). Similarly, CaMKIIα phosphorylated the GST-D3RIL3(R210-P239) fragment (Fig. 4C). This phosphorylation was rapid (Fig. 4D) and comparable to the phosphorylation of an NR2B fragment (Fig. S3A). The kinetic parameter, Km, was notably low, 65.9 ± 1.2 nM (Fig. 4E), suggesting that D3RIL3 like NR2B (Omkumar et al., 1996) acts as a high-affinity substrate. This high-affinity nature implies that phosphorylation of D3RIL3 could occur under physiological conditions. In support of this, GST-D3RIL3(R210-P239) was phosphorylated at two physiological Ca2+ concentrations (Fig. S3B).
Figure 4. Phosphorylation of D3RIL3 by CaMKIIα in vitro.
(A) Alignment of CaMKII phosphorylation sequences from different substrates. The key residues shaded in gray are contacted electrostatically or hydrophobically by the residues (E243, F98, D238, W237, V208, K105, and I205) from the catalytic domain of CaMKII. (B) Autoradiographs illustrating phosphorylation of GST-D3RIL3 (upper) but not GST (lower) in the presence of Ca2+/CaM. (C) An autoradiograph illustrating phosphorylation of GST-D3RIL3 and GST-D3RIL3(R210-P239). (D) Time course of D3RIL3(R210-P239) phosphorylation. (E) Kinetic analysis of D3RIL3(R210-P239) phosphorylation. The inset table shows the kinetic parameters. (F) Phosphorylation of synthetic peptides (WT or mutants). An autoradiograph shows phosphorylation of three peptides. Phosphorylation stoichiometry (means ± SEM) was calculated from the radioactivity measured on Coomassie-stained bands by liquid scintillation counting. Substrate proteins at 0.1 μM (B, C, D, and F) or increased concentrations (E) were incubated at 30°C for 1 min (E), 10 min (B, C, and F), or indicated times (D) with [γ-32P]ATP and CaMKIIα in the presence of Ca2+ (0.5 mM)/CaM (1 μM) (C, D, E, and F) or as indicated (B). The solid arrows (B, C, and F) indicate autophosphorylated CaMKIIα whereas open arrows (B and C) indicate phosphorylated GST-D3RIL3.
To identify accurate phosphorylation site(s) within the R210-P239 fragment, we used a series of synthetic peptides with or without mutations of two known serine sites in this fragment (S229 and S233). Figure 4F shows that the WT S229/233 peptide substrate was phosphorylated by CaMKIIα to a considerable stoichiometry (0.81 mol of phosphate/mol of substrate). Mutation of S233 to Ala (S233A) did not affect the phosphorylation. In contrast, mutation of S229 to Ala (S229A) or S229/233 to Ala (S229/233A) completely abolished the phosphorylation. Thus, S229 rather than S233 or T225 is the site of phosphorylation. The fact that S229 lies within the CaMKII binding motif in D3RIL3 and aligns with a phospho-S/T site predicted from the consensus CaMKII phosphorylation sequence, (I/L)XRXX(S/T) (White et al., 1998), is noteworthy. The results from tandem mass spectrometry analysis of tryptic digests of CaMKII-phosphorylated GST-D3RIL3 conclusively identified S229 as a phosphorylation site (data not shown).
Although the CaMKIIα binding domain in D3RIL3 aligns with the substrate recognition motif and contains a high affinity phosphorylation site (S229), a classical enzyme-substrate binding mechanism does not appear to be involved in the CaMKIIα binding to D3RIL3. This is supported by the finding that syntide-2 (a model peptide substrate for CaMKII) at concentrations up to 400 μM (>30 times the Km for phosphorylation; Hashimoto and Soderling, 1987) failed to compete for the CaMKIIα binding to D3RIL3 (Fig. S3C).
CaMKIIα interacts with D3Rs in vivo
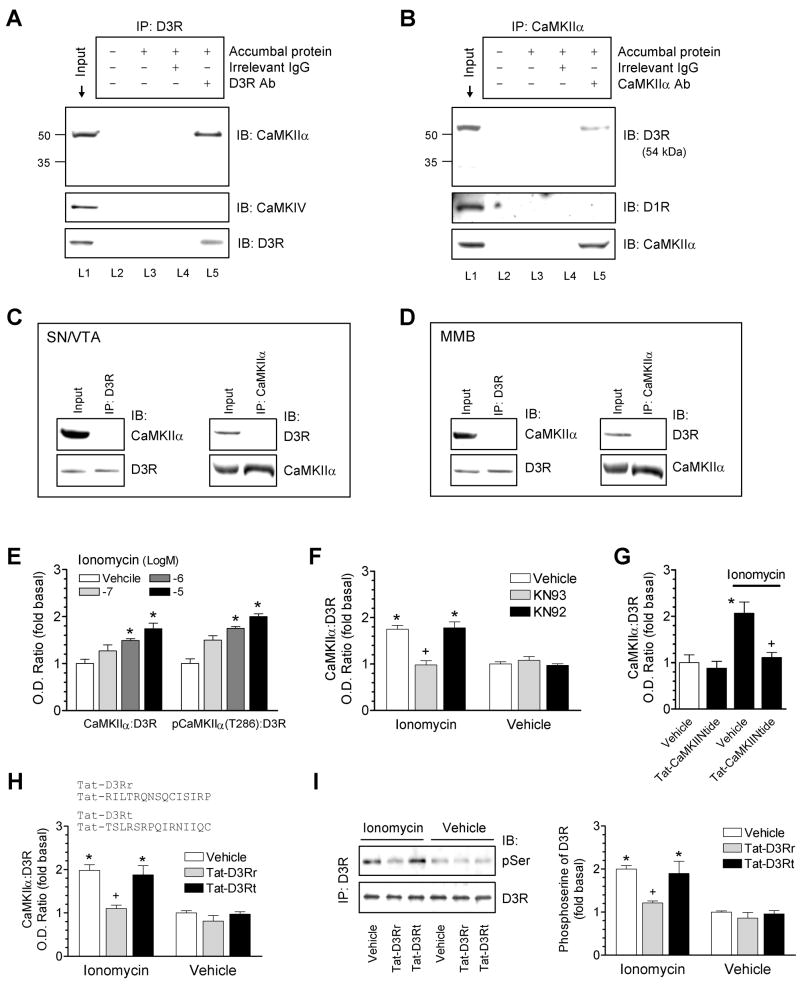
D3Rs are enriched in accumbal neurons. To determine whether native CaMKIIα and D3Rs interact with each other in these neurons, coimmunoprecipitation was performed using the solubilized synaptosomal fraction (P2) from the rat NAc. A clear and specific band of CaMKIIα, but not CaMKIV, was shown in the D3R precipitates (Fig. 5A). No immunoreactivity of other key synaptic proteins, including postsynaptic density (PSD)-93, PSD-95, synapse-associated protein (SAP) 97, and SAP102, was detected in the D3R precipitates (data not shown). In a reverse coimmunoprecipitation assay, a D3R-specific band was seen in the CaMKIIα precipitates (Fig. 5B). CaMKIIα in D3R-expressing neurons of the rat midbrain structures (substantia nigra and ventral tegmental area) and the rat medial mammillary bodies showed no interaction with D3Rs because no coimmunoprecipitation between the two proteins was seen in the P2 fraction from these regions (Fig. 5C and 5D). In the NAc, the coimmunoprecipitation was seen in WT mice, but not in D3R mutant mice (Fig. S4A). Furthermore, the coimmunoprecipitation occurred in the purified PSD fraction of rat accumbal neurons (Fig. S4B). These data indicate a region-specific interaction between CaMKIIα and D3Rs in the NAc in vivo and an interaction that can occur in the PSD. Further evidence supporting the interaction of the two proteins in vivo was obtained from yeast two-hybrid assays. Using D3RIL3 as bait in the yeast two-hybrid system, we found a prominent interaction between D3RIL3 and CaMKIIα (Fig. S4C). The strong interaction with CaMKIIα was also seen for the D3RIL3(R210-P239), but not D3RIL3(Q240-Q375), fragment (Fig. S4C).
Figure 5. Association of CaMKIIα with D3Rs in accumbal neurons in vivo.
(A and B) Coimmunoprecipitation (IP) of CaMKIIα and D3Rs in the synaptosomal fraction from the rat NAc. Lanes 3 and 4 showed no specific bands due to the lack of any antibody (L3) and the use of an irrelevant IgG (L4). (C and D) No coimmunoprecipitation of CaMKIIα and D3Rs was detected in the synaptosomal fraction from the rat substantia nigra (SN)/ventral tegmental area (VTA) and medial mammillary bodies (MMB). (E) Ionomycin (15 min) concentration-dependently increased the association of CaMKIIα and pCaMKIIα (T286) with D3Rs. (F) KN93 blocked the effect of ionomycin. Ionomycin (10 μM) was co-treated with KN93 or KN92 (20 μM) for 15 min. (G) Tat-CaMKIINtide blocked the effect of ionomycin. Tat-CaMKIINtide (5 μM) was applied 1 h prior to ionomycin (10 μM, 15 min). (H) Tat-D3Rr blocked the ionomycin-induced association of CaMKIIα with D3Rs. (I) Tat-D3Rr blocked the ionomycin-stimulated serine phosphorylation of D3Rs. Tat peptides (5-10 μM) were applied 1 h prior to ionomycin (10 μM, 15 min). Immunoblots (IB) of CaMKIIα, pCaMKIIα (T286), phosphoserine, or D3Rs were performed on D3R precipitates from drug-treated accumbal slices (E–G). Data are presented as means ± SEM for 3–5 experiments per group. *p < 0.05 versus vehicle or vehicle + vehicle. +p < 0.05 versus vehicle + ionomycin.
To determine whether the CaMKIIα-D3R interaction is subject to the Ca2+ regulation as was observed in vitro, we tested the interaction in the presence of the Ca2+ ionophore, ionomycin, in rat striatal/NAc slices. Ionomycin induced a concentration-dependent increase in the amounts of CaMKIIα and pCaMKIIα (T286) in the D3R precipitates (Fig. 5E). The CaMK inhibitor KN93 (20 μM), which inhibits CaMK by preventing the CaM binding to the enzyme, blocked the ionomycin-induced binding of CaMKIIα (Fig. 5F) and pCaMKIIα (T286) (Fig. S5) to D3Rs. The inactive analog of KN93, KN92, had no effect (Fig. 5F and Fig. S5). Like KN93, the highly selective CaMKII inhibitory peptide Tat-CaMKIINtide (Chang et al., 1998; Vest et al., 2007) at 5 μM (1 h before ionomycin) blocked the effect of ionomycin (Fig. 5G). These results demonstrate a Ca2+-sensitive nature of CaMKIIα-D3R interactions in vivo.
To evaluate the role of CaMKIIα-D3RIL3 binding in vivo, we synthesized a Tat-fusion peptide (Tat-D3Rr). The peptide contains a Tat domain (YGRKKRRQRRR) from the human immunodeficiency virus-type 1 (HIV-1) and a CaMKIIα-binding motif (RILTRQNSQCISIRP) from D3RIL3 (Fig. 5H). The arginine-enriched Tat domain renders cell permeability (Aarts et al., 2002; Mao et al., 2005; Liu et al., 2006) and the CaMKIIα-binding motif competes with D3Rs for CaMKII binding in living neurons. In pull-down assays with biotinylated Tat-fusion peptides, Tat-D3Rr but not Tat-D3Rt (a sequence-scrambled control) pulled down recombinant and endogenous CaMKIIα (Fig. S6A), indicating the ability of Tat-D3Rr to bind to CaMKIIα. Tat-D3Rr (5-10μM, 1 h before ionomycin) substantially reduced the ionomycin-stimulated association of the two proteins in striatal/NAc slices, while Tat-D3Rt did not (Fig. 5H). Thus,active CaMKIIα was directly recruited to D3RIL3 in response to Ca2+ rises. Ionomycin was found to increase serine phosphorylation of D3Rs as detected by an antibody selective for phosphoserine (Fig. 5I). This increase no longer occurred in slices pretreated with Tat-D3Rr (Fig. 5I) or KN93 (Fig. S6B), indicating that the interaction of active CaMKIIα with D3RIL3 is required for the ionomycin-stimulated phosphorylation of the receptor. The selectivity of Tat-D3Rr in disrupting the CaMKIIα-D3R interaction was confirmed by the lack of its effect in interfering with the ionomycin-stimulated CaMKIIα interaction with a number of other CaMKII substrates, including NR2B, the cyclin-dependent kinase 5 activator p35, or α-actinin-1 (Fig. S7).
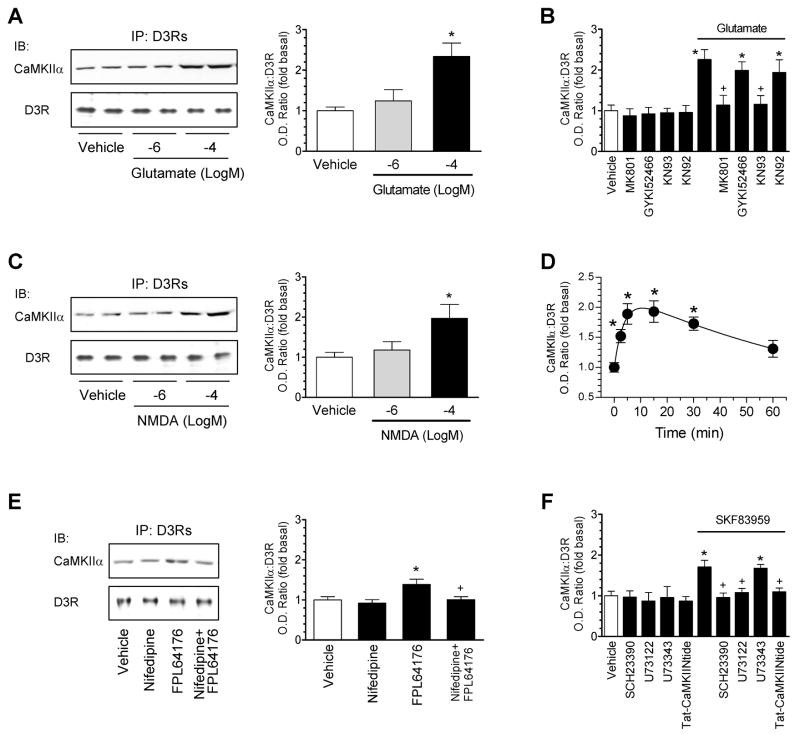
To identify the receptors that might provide a Ca2+ entry to trigger the CaMKIIα association to D3Rs, we tested the effect of glutamate receptor activation on the interaction in NAc slices. Glutamate mimicked the effect of ionomycin in stimulating the CaMKIIα-D3R association (Fig. 6A). The effect of glutamate was blocked by KN93 and the NMDA receptor antagonist MK801, but not by KN92 and the AMPA receptor antagonist GYKI52466 (Fig. 6B). Thus, glutamate, through activating NMDA receptors, elevates the CaMKIIα-D3R association. In support of this, NMDA itself induced a concentration-dependent and rapid increase in the association of the two proteins (Fig. 6C and 6D). FPL64176, an activator of L-type voltage-operated Ca2+ channels (VOCCs), slightly enhanced the CaMKIIα-D3R interaction, which was blocked by the antagonist nifedipine (Fig. 6E). Together, VOCCs and especially NMDA receptors can provide a Ca2+ entry to trigger the CaMKIIα-D3R association.
Figure 6. Regulation of CaMKIIα-D3R interactions by glutamate and dopamine receptors and VOCCs.
(A) Glutamate increased the CaMKIIα-D3R interaction. (B) MK801 and KN93 but not GYKI52466 and KN92 blocked the CaMKIIα-D3R interaction induced by glutamate. (C) NMDA increased the CaMKIIα-D3R interaction. (D) The time course of the NMDA-stimulated CaMKIIα-D3R interaction. NMDA (100 μM/glycine 10 μM) was applied for 2.5, 5, 15, 30, or 60 min (D). (E) The VOCC activator FPL64176 induced a nifedipine-sensitive increase in the CaMKIIα-D3R interaction. (F) SKF83959 elevated the CaMKIIα-D3R interaction. Rat accumbal slices were treated with glutamate (1 or 100 μM/glycine 10 μM), NMDA (1 or 100 μM/glycine 10 μM), FPL64176 (20 μM), or SKF83959 (10 μM) alone or with MK801 (10 μM), GYKI52466 (10 μM), KN93 (20 μM), KN92 (20 μM), nifedipine (20 μM), SCH23390 (1 μM), U73122 (20 μM), or U73343 (20 μM) for 15 min. Tat-CaMKIINtide peptides (5μM) were applied 1 h prior to SKF83959 (10 μM, 15 min). Immunoblots (IB) of CaMKIIα or D3Rs were performed on D3R precipitates from drug-treated accumbal slices. Data are presented as means ± SEM for 4–5 experiments per group. *p < 0.05 versus vehicle or vehicle + vehicle. +p < 0.05 versus glutamate (B), FPL64176 (E), or SKF83959 (F).
Activation of phosphatidylinositol (PI)-linked D1Rs (Gαq-coupled) rather than classical cAMP-linked D1Rs (Gαs-coupled) elicits the Ca2+ release from intracellular stores through a phospholipase Cβ (PLCβ)-dependent and adenylyl cyclase-independent mechanism (Felder et al., 1989; Undie and Friedman, 1990). To determine if the Ca2+ release induced by activating PI-linked D1Rs affects the CaMKIIα-D3R interaction, we examined the effect of activating these receptors on the interaction of the two proteins in NAc slices. Pharmacological activation of PI-linked D1Rs with a selective agonist SKF83959 (Jin et al., 2003) increased the interaction of CaMKIIα with D3Rs (Fig. 6F). This increase was blocked by the D1R antagonist SCH23390, the amino steroid PLCβ inhibitor U73122 (but not its inactive analog U73343), and Tat-CaMKIINtide (Fig. 6F). Thus, the Ca2+ release by PI-linked D1Rs possesses the ability to upregulate the CaMKIIα-D3R interaction. The previous data showing the selective activation of CaMKII by PI- but not cAMP-linked D1Rs (Zhen et al., 2004) support this notion.
CaMKIIα-D3RIL3 interactions downregulate D3R function
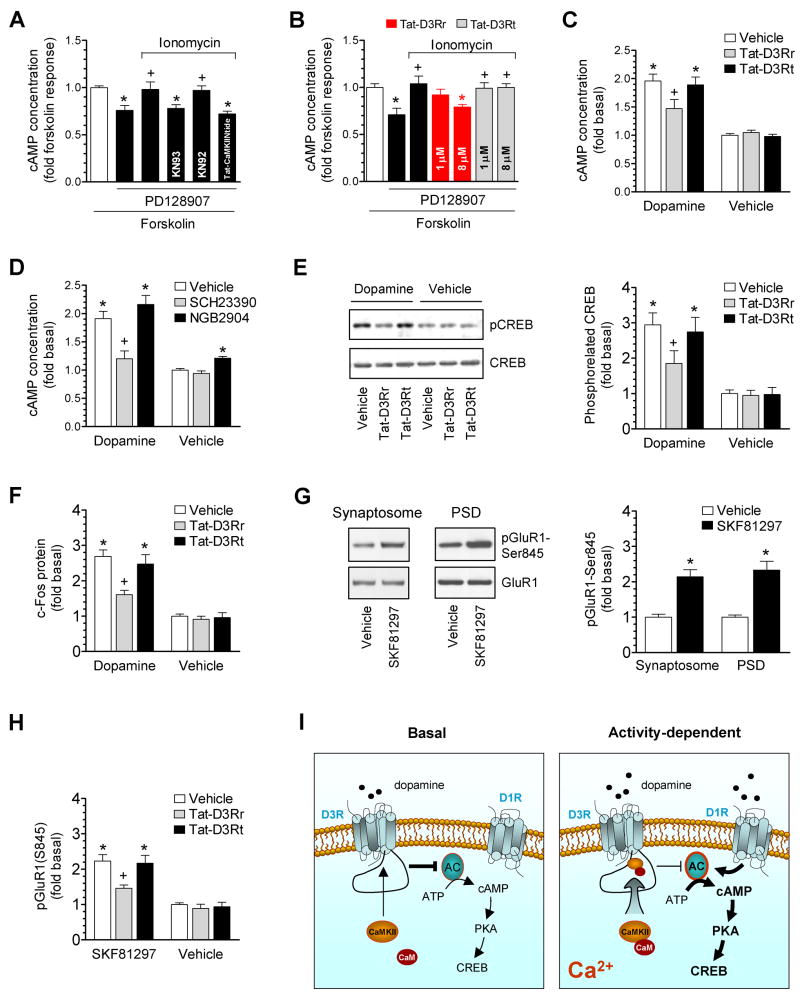
Activation of D3Rs inhibits the adenylyl cyclase-mediated cAMP formation (Ahlgren-Beckendorf and Levant, 2004; Jeanneteau et al., 2006). We next investigated whether the binding of CaMKII to D3RIL3 alters the D3R function in inhibiting cAMP formation. In rat accumbal slices, the adenylyl cyclase activator forskolin (1 μM, 20 min) markedly elevated cAMP levels above a baseline of 4.1 ± 0.3 pmol/ml. A D3R selective agonist PD128907 at low concentrations (1–3 nM) significantly reduced the forskolin-activated cAMP accumulation (Fig. 7A). The selectivity of PD128907 was demonstrated by the observation that the agonist when applied at these sufficiently low concentrations did not alter the forskolin effect in slices from D3R mutant mice (Fig. S8). Co-treatment with ionomycin (10 μM) antagonized the effect of PD128907 (Fig. 7A). The effect of ionomycin was sensitive to KN93 and Tat-CaMKIINtide but not KN92 (Fig. 7A). Furthermore, Tat-D3Rr (8μM, 1 h prior to ionomycin/PD128907) attenuated the effect of ionomycin while Tat-D3Rt did not (Fig. 7B). Neither Tat-D3Rr nor Tat-D3Rt had any effect on the forskolin-stimulated cAMP formation or on the inhibition of forskolin-stimulated cAMP formation by PD128907 (data not shown). These data indicate that Ca2+-activated CaMKII associates with D3Rs and reduces D3R efficacy in inhibiting cAMP production.
Figure 7. Inhibition of D3R function by CaMKII in rat accumbal slices.
(A) Ionomycin antagonized the D3R-mediated inhibition of forskolin-stimulated cAMP accumulation. (B) Tat-D3Rr reversed the effect of ionomycin. (C) Effects of Tat-fusion peptides on dopamine-stimulated cAMP accumulation. (D) Effects of the D1R or D3R antagonist on dopamine-stimulated cAMP accumulation. (E) Effects of Tat-fusion peptides on dopamine-stimulated CREB phosphorylation. (F) Effects of Tat-fusion peptides on dopamine-stimulated c-Fos expression. (G) SKF81297 increased pGluR1-S845 levels. (H) Effects of Tat-fusion peptides on SKF81297-stimulated GluR1 phosphorylation. (I) A model of the Ca2+-dependent regulation of D3Rs by CaMKII. AC, adenylyl cyclase. PD128907 (1–3 nM), ionomycin (10 μM with 1 mM CaCl2), KN93 (20 μM), KN92 (20 μM), SCH23390 (1 μM), and/or NGB2904 (50 nM) were co-treated with forskolin (1 μM, 20 min) or dopamine (5 μM, 20 min) in A–D. In the experiments in which the Tat-fusion peptide (Tat-CaMKIINtide, Tat-D3Rr, or Tat-D3Rt) was employed, the peptide at 5 μM (A), 1 and 8 μM (B), or 8 μM (C, E, F, and H) was applied 1 h prior to forskolin (A and B), dopamine (C, E, and F), or SKF81297 (H). SKF81297 was incubated at 10 μM for 5–10 min (G and H). Data are presented as means ± SEM for 5–7 experiments per group. *p < 0.05 versus forskolin alone (A and B), vehicle (G), or vehicle + vehicle (C–F and H). +p < 0.05 versus PD128907 + forskolin (A and B), vehicle + dopamine (C–F), or vehicle + SKF81297 (H).
More experiments were performed in accumbal slices to evaluate the role of CaMKII in regulating dopamine-mediated cAMP signaling. We found that dopamine (5 μM) consistently elevated cAMP levels. This elevation was reduced by Tat-D3Rr (Fig. 7C). Thus, CaMKII-D3R interactions inhibit the cAMP response to dopamine. Tat-D3Rr alone did not alter basal cAMP levels (Fig. 7C), suggesting a minimal impact of CaMKIIα-D3RIL3 interactions to D3R function under basal conditions, in which relatively lower levels of basal CaMKIIα-D3RIL3 binding would be anticipated. It is noteworthy that the dopamine concentration (5 μM) applied here is within the physiological range of evoked dopamine release in striatal slice preparations (Jones et al., 1998). In separate experiments, the D1R antagonist SCH23390 blocked the dopamine-stimulated cAMP response (Fig. 7D), indicating that D1R activation largely mediates the positive cAMP response to dopamine. In contrast to the D1R antagonist, the highly selective D3R antagonist NGB2904, when incubated at 50 nM (EC50 of 14.4 nM and 1280.0 nM for D3 and D2 receptors, respectively; Yuan et al., 1998; Grundt et al., 2005), slightly increased the cAMP response to dopamine (although P > 0.05, Fig. 7D). NGB2904 alone increased basal cAMP concentrations, indicating a tonic D3R activity in inhibiting basal cAMP formation.
CaMKII-D3R interactions further impact downstream effectors of cAMP/protein kinase A (PKA). In accumbal slices, as shown in Fig. 7E, Tat-D3Rr markedly reduced the dopamine-stimulated phosphorylation of cAMP-responsive element binding protein (CREB), a nuclear transcription factor downstream to PKA activation. Tat-D3Rs also reduced the dopamine-stimulated expression of c-Fos, an immediate early gene downstream to CREB (Fig. 7F). In addition to CREB and c-Fos, we examined another target of PKA at excitatory synapses. PKA phosphorylated AMPA receptor GluR1 subunits at serine 845 in mouse striatal slices treated with the D1R agonist SKF81297 (Snyder et al., 2000). Similarly, we observed that SKF81297 increased protein levels of phospho-GluR1 at serine 845 (pGluR1-S845) in synaptosomal fractions from rat accumbal slices (Fig. 7G). A higher level of pGluR1-S845 was also seen in the PSD fraction (Fig. 7G), confirming that the event occurred at synaptic sites. Tat-D3Rr was able to reduce the SKF81297-stimulated GluR1 phosphorylation in synaptosomes (Fig. 7H), indicating that D3Rs are restored to inhibit the PKA-mediated GluR1 phosphorylation. Together, our observations demonstrate that CaMKII inhibits D3R function. As illustrated in Fig. 7I, under basal conditions, CaMKII interacts with D3Rs at a low level. D3Rs, due to their higher affinity for dopamine than D1/D2 receptors (Sokoloff et al., 1992), are activated by basal levels of dopamine to inhibit adenylyl cyclase. When dopamine release is enhanced, the inhibitory tone of D3Rs is suppressed by the Ca2+-induced interaction of CaMKII with D3RIL3, which, in concert with D1R stimulation, augments the cAMP/PKA response to dopamine.
A further attempt was made to explore a possible role of our CaMKIIα-D3R interaction model in modulating another key downstream effector of D3Rs. D3Rs expressed in heterologous cells activate extracellular signal-regulated protein kinases 1 and 2 (ERK1/2 or p44/p42) via the Gβγ pathway (Cussac et al., 1999; Beom et al., 2004). In HEK293 cells transiently transfected with D3Rs, we found a similar transient increase in ERK1/2 phosphorylation following dopamine application (Fig. S9A). This was blocked by NGB2904, confirming the role of D3Rs in mediating this event (Fig. S9B). Noticeably, in HEK293 cells co-transfected with D3Rs and CaMKIIα, ionomycin (2 μM) reduced the dopamine-stimulated ERK2 phosphorylation (Fig. S9C). Tat-D3Rr but not Tat-D3Rt reversed the effect of ionomycin (Fig. S9C). In accumbal slices, activation of D3Rs with PD128907 increased ERK2 phosphorylation, which was reduced by ionomycin (Fig. S9D). Tat-D3Rr antagonized the effect of ionomycin, while Tat-D3Rt did not (Fig. S9D). These data indicate that the interaction of CaMKIIα with D3Rs suppresses the ability of D3Rs to phosphorylate ERK1/2 in heterologous cells and neurons.
CaMKIIα-D3RIL3 interactions suppress the D3R-mediated inhibition of motor activity
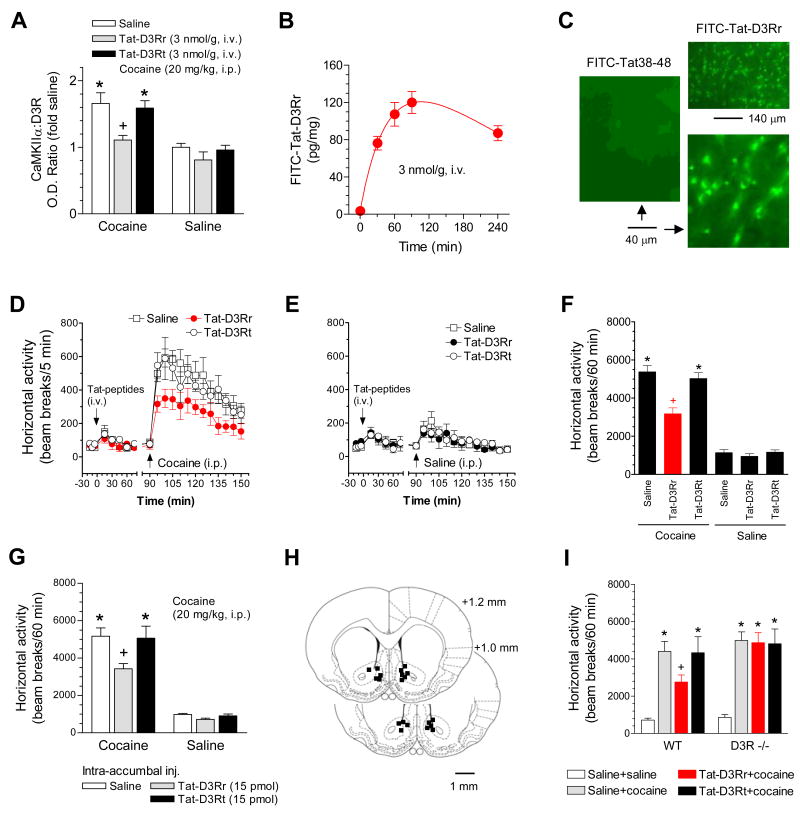
We extended our molecular observations to behavioral experiments to investigate whether regulated CaMKIIα-D3RIL3 interactions modulate behavioral responsiveness to dopamine stimulation. Cocaine is known to stimulate motor activity through a mechanism that results in elevated synaptic dopamine levels. Given that postsynaptic D3Rs are generally inhibitory to stimulant-induced motor activity (Xu et al., 1997; Bahi et al., 2005; Richtand, 2006; Pritchard et al., 2007), we speculated that cocaine would suppress D3Rs by enhancing the interaction of CaMKIIα with D3Rs, which participates in the regulation of the motor response to cocaine. Consistent with this model, cocaine at 20 mg/kg markedly increased the formation of CaMKIIα-D3RIL3 complexes in the rat NAc (Fig. 8A). A slight increase in this complex formation was induced by cocaine at 5 mg/kg (19.3 ± 0.03% over control). The effect of cocaine (20 mg/kg) was blocked by pretreatment with an intravenous (i.v.) injection of the interfering peptide Tat-D3Rr (3 nmol/g, 1.5 h before cocaine). The control peptide (Tat-D3Rt) had no effect. Using fluorescently tagged peptides (FITC-Tat-D3Rr), the dynamic presence of these systemically active and cell-permeable peptides in striatal tissues was demonstrated after a single injection (Fig. 8B). Intracellular uptake of a FITC-Tat-D3Rr peptide, but not a control peptide containing HIV-1 Tat residues 38 to 48 (KALGISYGRKK; Tat38-48) that lie outside the transduction domain, was evident in accumbal neurons following an i.v. injection (Fig. 8C). Interestingly, in open field locomotor activity assays, Tat-D3Rr but not Tat-D3Rt reduced the horizontal activity induced by cocaine (20 mg/kg) while neither peptide had any effect on spontaneous locomotion (Fig. 8D-F). To determine whether the effect of systemic peptides primarily occurs in the local NAc, a microinjection study targeting the NAc was performed in freely moving rats. A local injection of Tat-D3Rr (15 pmol, 1 h prior to cocaine) produced the same effect as that observed following a systemic injection (Fig. 8G and H). Additionally, an intra-accumbal injection of KN93 but not KN92 (10 nmol) reduced the effect of cocaine on the formation of CaMKIIα-D3R complexes (data not shown). In WT mice, Tat-D3Rr reduced the cocaine-stimulated locomotion whereas no such effect was observed in D3R mutant mice (Fig. 8I). Together, these results support a model in which cocaine recruits CaMKIIα to D3Rs to downregulate the D3R function in inhibiting motor responses to cocaine, which ultimately enhances behavioral sensitivity to cocaine stimulation.
Figure 8. Disruption of CaMKIIα-D3R interactions altered cocaine-stimulated motor responses.
(A) Effects of Tat-fusion peptides on the cocaine-stimulated CaMKIIα-D3R interaction. Rats were sacrificed 20 min after cocaine injection for conducting coimmunoprecipitation with the P2 fraction from the rat NAc. (B) Measurements of Tat-D3Rr concentrations in rat striatal tissue. Rats injected with FITC-Tat-D3Rr (3 nmol/g, i.v.) were sacrificed at indicated times. (C) Neuronal transduction of FITC-Tat-D3Rr but not FITC-Tat38–48 (3 nmol/g, i..v, 1.5 h) in the NAc shell region of rat accumbal sections. (D and E) Systemic Tat-D3Rr attenuated cocaine-stimulated locomotion (D) and had no effect on spontaneous motor activity (E). (F) A graph of the data from D and E obtained during 60 min after i.p. injection of drugs. (G) Intra-accumbal injection of Tat-D3Rr attenuated the cocaine-stimulated locomotion. (H) Location of microinjection sites within the NAc. Representative sites are illustrated on only one side for the bilateral injections of Tat-D3Rr (right) or Tat-D3Rt (left). (I) Tat-D3Rr attenuated the cocaine-stimulated locomotion in WT but not in D3R mutant mice. Cocaine (20 mg/kg, i.p.) was injected 60 or 90 min after an intra-accumbal injection (15 pmol/0.4 μl) or a systemic injection (3 nmol/g, i.v. for rats and 10 nmol/g, i.p. for mice) of Tat peptides, respectively. Data are presented as means ± SEM (n = 4–14 per group). *p < 0.05 versus saline + saline. +p < 0.05 versus saline + cocaine.
Discussion
A new discovery in this study is the protein-protein interaction between CaMKIIα and D3Rs in accumbal neurons. The interaction is region-selective since it did not occur in other brain areas, including the substantia nigra and the ventral tegmental area. The interaction involves the consensus CaMKII-binding sequence (I/L)XRXX(S/T) (White et al., 1998) located in the third intracellular loop of D3Rs. Another important characteristic of the CaMKIIα-D3R interaction is its Ca2+ sensitivity. Activation of CaMKIIα by Ca2+/CaM enhanced the binding affinity of the kinase for D3Rs in vitro. In accumbal neurons in vivo, activation of NMDA receptors, VOCCs, and PI-linked D1Rs that elicit the Ca2+ release from intracellular stores increased the CaMKIIα-D3R interaction, providing the linkage of these Ca2+ routes to D3R physiology. Moreover, autophosphorylation of CaMKIIα regulates the CaMKIIα-D3R interaction. This was shown by the findings that the autophosphorylation at T286 further enhanced the affinity of the kinase for D3Rs and sustained the kinase association with D3Rs after Ca2+ was chelated with EGTA. Thus, the autophosphorylated CaMKIIα is believed to still have an influence on D3R function even after a transient Ca2+ rise recovers to the original level.
The NMDA receptor was the first receptor that was identified to be bound by CaMKIIα at synaptic sites (Gardoni et al., 1998; Leonard et al, 1999). In response to Ca2+ influx, CaMKIIα is recruited to the PSD of excitatory synapses by its increased association with the NMDA receptor NR2B subunit (Colbrain, 2004; Bayer et al., 2006). The Ca2+-evoked CaMKIIα-D3R interaction identified in the current work suggests a new recruiter of CaMKII to the PSD. This is supported by the distribution of abundant D3Rs in the purified PSD (this study). The fact that multiple synaptic receptors interact with CaMKII and recruit it to presumably distinct submembranous microdomains is in good accordance with the multivalent and multifunctional nature of holoenzymes and with the high abundance of the enzyme at synaptic sites.
The D3RIL3 is a critical region for Gαi/o-coupling (Ilani et al., 2002), and activation of D3Rs inhibits adenylyl cyclase in heterologous cells (Ahlgren-Beck and Levant, 2004; Sokoloff et al., 2006). In accumbal neurons, the D3R-mediated inhibition of forskolin-stimulated cAMP accumulation was established in this study. Notably, the D3R efficacy was subject to the regulation by Ca2+ because the cytoplasmic Ca2+ rise induced by an ionophore reversed the effect of a D3R agonist. This Ca2+ regulation relies on the CaMKII activation and the subsequent association of activated CaMKII with D3Rs as demonstrated by the findings that both the CaMKII activation inhibitors (KN93 and CaMKIINtide) and Tat-D3Rr, which disrupted the CaMKIIα-D3RIL3 binding, antagonized the effect of the Ca2+ ionophore. Together, the Ca2+-triggered recruitment of CaMKII to D3Rs suppresses the D3R efficacy and thus relieves the inhibitory tone of D3Rs on the adenylyl cyclase/cAMP cascade. In this model, a state-dependent nature of CaMKII regulation is recognized (see Fig. 7I). Under basal conditions, the regulation is less significant due to a lower level of CaMKII binding to D3Rs. As a result, D3Rs become tonically active in inhibiting cAMP production. Of note, the D3R is the only subtype with an affinity (Ki = 30 nM; Sokoloff et al., 1992) within the nanomolar range of basal dopamine concentrations at synapses (~ 50 nM; Ross, 1991). This allows D3Rs to respond to baseline dopamine. When transformed to a stimulated state in which the synaptic dopamine level is increased to the micromolar range, CaMKII is recruited to D3Rs by Ca2+ to depress the D3R efficacy. This, in concert with the simultaneously activated D1 efficacy (Surmeier et al., 1996; Ridray et al., 1998), would produce a net upregulation of the cAMP-PKA pathway and its downstream targets, including CREB phosphorylation, c-Fos expression, and AMPA receptor phosphorylation (Snyder et al., 2000; Zhang et al., 2004; Jiao et al., 2007). Together, the CaMKII-D3R coupling discovered here suggests a new model of the integration of limbic dopamine signals under basal and stimulated conditions.
D1R activation contributes to cocaine-stimulated motor activity. In response to cocaine, synaptic dopamine levels are increased, which in turn activates D1Rs on striatonigral neurons. Through cAMP/PKA, D1Rs enhance excitability of these neurons to glutamate, thereby increasing outflow of the direct striatonigral pathway and motor activity (Surmeier et al., 2007). In contrast to D1Rs, D3Rs inhibit the cAMP-PKA pathway. Thus, D3Rs are believed to exert an inhibitory effect on dopamine-mediated motor activity. Indeed, D3R mutant mice displayed hyperactivity in a novel environment (Accili et al., 1996; Xu et al., 1997), indicating an inhibitory tone of postsynaptic D3Rs on normal motor activity (Koeltzow et al., 1998). D3Rs also suppress motor responsiveness to cocaine as evidenced by the fact that cocaine at a low dose (5 mg/kg) induced a significantly greater motor response in D3R mutant mice than in WT mice (Xu et al., 1997). Other behavioral studies utilizing D3R mutant mice or D3R-preferring agents generally support this view (Bahi et al., 2005; Richtand, 2006; Pritchard et al., 2007). However, the D3R-mediated inhibition is subject to downregulation in response to increasing doses of cocaine because cocaine at higher doses (20–40 mg/kg) induced similar increases in motor responses in D3R mutant mice as compared to WT mice (Xu et al., 1997). Indeed, in this study, we observed a suppression of D3R function by cocaine via a mechanism involving CaMKII. This was demonstrated by the findings that 1) cocaine dose-dependently increased the association of CaMKII with D3Rs, and 2) the disruption of this association by Tat-D3Rr restored the ability of D3Rs to suppress the motor response to cocaine (20 mg/kg) in rats and WT mice but not in D3R mutant mice. Tat-D3Rr also restored the efficiency of D3Rs to inhibit the D1R-mediated phosphorylation of synaptic AMPA receptors at a PKA-sensitive site (serine 845) in accumbal neurons. Together, through a specific protein-protein interaction between CaMKII and D3Rs, cocaine transiently releases the D3R-mediated ‘brake’ on behavioral responses to the stimulant. This dynamic downregulation of D3R function could enhance behavioral sensitivity to cocaine stimulation.
Experimental Procedures
Animals
Adult male Wistar rats weighting 200–225 g (Charles River, New York, NY), adult WT mice, and mutant mice lacking D3Rs were individually housed at 23°C and humidity of 50 ± 10% with food and water available ad libitum. The animal room was on a 12/12 h light/dark cycle with lights on at 0700. All animal use procedures were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
Protein extraction
Rats were anesthetized with 2.5–3% isoflurane and decapitated. Brains were removed and coronal sections were cut. Bilateral punches of the local NAc (core and shell) or other brain regions were obtained using punch corers. Samples were removed into a microtube containing ice-cold sample buffer (20 mM Tris-HCl, pH 7.4, 1 mM dithiothreitol, 10 mM NaF, 2 mM Na3VO4, 1 mM EDTA, 1 mM EGTA, 5 μM microcystin-LR, and 0.5 mM phenylmethylsulfonyl fluoride), and homogenized by sonication. Homogenates were centrifuged at 700 g for 10 min at 4°C. The supernatant was centrifuged at 10,000 g at 4°C for 30 min to obtain the P2 pellet (synaptosomal fraction). After solubilization (see supplemental experimental procedures), protein concentrations were determined.
Cloning, expression, and purification of GST-fusion proteins
The cDNA fragments encoding the D3RIL2(D127-R149), D3RIL3(R210-Q375), D3RIL3(R210-P239), D3RIL3(Q240-Q375), D3RIL3(H269-Q375), D3RIL3(R345-Q375), D2RIL3(I210-V270), CaMKIIαCD(L91-S272), CaMKIIαRD(H273-S314), or CaMKIIαAD(G315-H478) were generated by PCR amplification from full-length cDNA clones. These fragments were subcloned into BamHI-EcoRI sites of the pGEX4T-3 plasmid (Amersham Biosciences, Arlington Heights, IL) or SpeI-XhoI sites of the pET-41a(+) plasmid (Novagen, Madison, WI). Initiation methionine residues and stop codons were also incorporated where appropriate. To confirm appropriate splice fusion, all constructs were sequenced. GST-fusion proteins were expressed in E. coli BL21 cells (Amersham) or BL21(DE3)pLysE cells (Novagen) and purified from bacterial lysates as described by the manufacturer. GST- or His-tagged full-length CaMKIIα (M1-H478) and GST-tagged full-length CaMKIV (M1-Y473) were expressed and purified via a baculovirus/Sf9 insect cell expression system.
Affinity purification (pull-down) assay
Solubilized accumbal extracts (50–100 μg of protein) were diluted with 1X PBS/1% Triton X-100 and incubated with 50% (v/v) slurry of glutathione-Sepharose 4B beads (Amersham) saturated with GST alone or with the indicated GST-fusion protein (5–10μg) for 2–3 h at 4°C. Beads were washed four times with 1X PBS/1% Triton X-100. Bound proteins were eluted with 2X lithium dodecyl sulfate (LDS) sample buffer, resolved by SDS-PAGE, and immunoblotted with a specific antibody.
In vitro binding assay
GST-fusion proteins (1–5 μg) in PBS were digested with 0.2 NIH unit of thrombin (Sigma, St. Louis, MO) for 2 h at room temperature. The reaction was stopped by adding PMSF (10 μM). GST was removed by glutathione Sepharose (Amersham). The supernatant was equilibrated to binding buffer (200 mM NaCl, 0.2% Triton X-100, 0.1 mg/ml BSA, and 50 mM Tris, pH, 7.5) with or without 0.5 mM CaCl2, 1 μM CaM, 1 mM EGTA, or 50 μM ATP as indicated. In some experiments, His-tagged CaMKIIα WT (~57 kDa), WT pCaMKIIα, CaMKIIα mutants (~50 kDa), or CaM were used. Binding reactions were initiated by adding purified GST-fusion proteins and were remained at 4°C for 2–3 h unless otherwise indicated. GST-fusion proteins were precipitated using 100 μl of 10% glutathione Sepharose. The precipitate was washed three times with binding buffer. Bound proteins were eluted with 2X LDS loading buffer, resolved by SDS-PAGE, and immunoblotted with a specific antibody.
cAMP enzyme immunoassay
After incubation with drugs indicated for 20–30 min at room temperature, accumbal slices were homogenized in 0.1 M HCl solution for 15 min with gentle shaking and spun in the microcentrifuge tubes. cAMP concentration of the supernatant was measured using the Direct cAMP Enzyme Immunoassay (EIA) Kit (Assay Designs, Ann Arbor, MI) following the manufacturer’s instruction. Protein concentrations were determined using a BCA Protein Assay kit (Pierce).
Behavioral assessments
Locomotion in an open field was evaluated as described previously (Liu et al., 2006).
Supplementary Material
The Supplemental Data for this article can be found online.
Acknowledgments
This work was supported by NIH grants DA10355 (J.Q.W.), MH61469 (J.Q.W.), DA14644 (M.X.), and DA17323 (M.X.), and by a grant from Saint Luke’s Hospital Foundation. We thank Dr. Howard Schulman and Dr. K. Ulrich Bayer for providing T286A, T286D, and T305/306 mutant vectors, Dr. Xiaoming Xia for help in preparing plasmids, Dr. Kim A. Neve for critical reading of the manuscript, Ms. Lucy S. Wang, Dr. Xiaoqiang Yu, and Dr. Nilofer Qureshi for technical support and comments, and Dr. Amy Hauck Newman (Medicinal Chemistry Section, NIDA-IRP) and Dr. Zheng-Xiong Xi (NIDA-IRP) for providing NGB2904.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, Wang YT, Salter MW, Tymianski M. Treatment of ischemic brain damage by pertubing NMDA receptor-PSD-95 protein interactions. Science. 2002;298:846–850. doi: 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- Accili D, Fishburn CS, Drago J, Steiner H, Lachowicz JE, Park BH, Gauda EB, Lee EJ, Cool MH, Sibley DR, Gerfen CR, Westphal H, Fuchs S. A targeted mutation of the D3 dopamine receptor gene is associated with hyperactivity in mice. Proc Natl Acad Sci USA. 1996;93:1945–1949. doi: 10.1073/pnas.93.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlgren-Beckendorf JA, Levant B. Signaling mechanisms of the D3 dopamine receptor. J Recept Signal Transduct Res. 2004;24:117–130. doi: 10.1081/rrs-200029953. [DOI] [PubMed] [Google Scholar]
- Bahi A, Boyer F, Bussard G, Dreyer JL. Silencing dopamine D3-receptors in the nucleus accumbens shell in vivo induces changes in cocaine-induced hyperlocomotion. Eur J Neurosci. 2005;21:3415–3426. doi: 10.1111/j.1460-9568.2005.04157.x. [DOI] [PubMed] [Google Scholar]
- Bayer KU, Koninck PD, Leonard AS, Hell JW, Schulman H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411:801–805. doi: 10.1038/35081080. [DOI] [PubMed] [Google Scholar]
- Bayer KU, LeBel E, McDonald GL, O’Leary H, Schulman H, Koninck PD. Transition from reversible to persistent binding of CaMKII to postsynaptic sites and NR2B. J Neurosci. 2006;26:1164–1174. doi: 10.1523/JNEUROSCI.3116-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beom SR, Cheong D, Torres G, Caron MG, Kim KM. Comparative studies of molecular mechanisms of dopamine D2 and D3 receptors for the activation of extracellular signal-regulated kinase. J Biol Chem. 2004;279:28304–28314. doi: 10.1074/jbc.M403899200. [DOI] [PubMed] [Google Scholar]
- Bouthenet ML, Souil E, Martres MP, Sokoloff P, Giros B, Schwartz JC. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res. 1991;564:203–219. doi: 10.1016/0006-8993(91)91456-b. [DOI] [PubMed] [Google Scholar]
- Chang BH, Mukherji S, Soderling TR. Characterization of a calmodulin kinase II inhibitor protein in brain. Proc Natl Acad Sci USA. 1998;95:10890–10895. doi: 10.1073/pnas.95.18.10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbran RJ. Targeting of calcium/calmodulin-dependent protein kinase II. Biochem J. 2004;378:1–16. doi: 10.1042/BJ20031547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbran RJ, Brown AM. Calcium/calmodulin-dependent protein kinase II and synaptic plasticity. Curr Opin Neurobiol. 2004;14:318–327. doi: 10.1016/j.conb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Colbran RJ, Soderling TR. Calcium/calmodulin-independent autophosphorylation sites of calcium/calmodulin-dependent protein kinase II. Studies on the effect of phosphorylation of threonine 305/306 and serine 314 on calmodulin binding using synthetic peptides. J Biol Chem. 1990;265:11213–11219. [PubMed] [Google Scholar]
- Cussac D, Newman-Tancredi A, Pasteau V, Millan MJ. Human dopamine D3 receptors mediate mitogen-activated protein kinase activation via a phosphatidylinositol 3-kinase and an atypical protein kinase C-dependent mechanism. Mol Pharmacol. 1999;56:1025–1030. doi: 10.1124/mol.56.5.1025. [DOI] [PubMed] [Google Scholar]
- Felder CC, Jose PA, Axelrod J. The dopamine-1 agonist, SKF82526, stimulates phospholipase-C activity independent of adenylate cyclase. J Pharmacol Exp Ther. 1989;248:171–175. [PubMed] [Google Scholar]
- Gardoni F, Caputi A, Cimino M, Pastorino L, Cattabeni F, Di Luca M. Calcium/calmodulin-dependent protein kinase II is associated with NR2A/B subunits of NMDA receptor in postsynaptic densities. J Neurochem. 1998;71:1733–1741. doi: 10.1046/j.1471-4159.1998.71041733.x. [DOI] [PubMed] [Google Scholar]
- Griffith LC. Regulation of calcium/calmodulin-dependent protein kinase II activation by intramolecular and intermolecular interactions. J Neurosci. 2004;24:8394–8398. doi: 10.1523/JNEUROSCI.3604-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundt P, Carlson EE, Cao J, Bennett CJ, McElveen E, Taylor M, Luedtke RR, Newman AH. Novel heterocyclic trans olefin analogues of N{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl} arylcarboxamides as selective probes with high affinity for the dopamine D3 receptor. J Med Chem. 2005;48:839–848. doi: 10.1021/jm049465g. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Soderling TR. Calcium · calmodulin-dependent protein kinase II and calcium · phospholipid-dependent protein kinase activities in rat tissues assayed with a synthetic peptide. Arch Biochem Biophy. 1987;252:418–425. doi: 10.1016/0003-9861(87)90048-8. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Gardner EL, Xi ZX, Thanos PK, Mugnaini M, Hagan JJ, Ashby CR., Jr The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain Res Rev. 2005;49:77–105. doi: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudman A, Schulman H. Neuronal Ca2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu Rev Biochem. 2002;71:473–510. doi: 10.1146/annurev.biochem.71.110601.135410. [DOI] [PubMed] [Google Scholar]
- Ilani T, Fishburn CS, Levavi-Sivan B, Carmon S, Raveh L, Fuchs S. Coupling of dopamine receptors to G proteins: studies with chimeric D2/D3 dopamine receptors. Cell Mol Neurobiol. 2002;22:47–56. doi: 10.1023/a:1015341712166. [DOI] [PubMed] [Google Scholar]
- Jeanneteau F, Funalot B, Jankovic J, Deng H, Lagarde JP, Lucotte G, Sokoloff P. A functional variant of the dopamine D3 receptor is associated with risk and age-at-onset of essential tremor. Proc Natl Acad Sci USA. 2006;103:10753–10758. doi: 10.1073/pnas.0508189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao H, Zhang L, Gao F, Lou D, Zhang J, Xu M. Dopamine D1 and D3 receptors oppositely regulate NMDA- and cocaine-induced MARK signaling via NMDA receptor phosphorylation. J Neurochem. 2007;103:840–848. doi: 10.1111/j.1471-4159.2007.04840.x. [DOI] [PubMed] [Google Scholar]
- Jin LQ, Goswami S, Cai G, Zhen X, Friedman E. SKF83959 selectively regulates phosphatidylinositol-linked D1 dopamine receptors in rat brain. J Neurochem. 2003;85:378–386. doi: 10.1046/j.1471-4159.2003.01698.x. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci. 1998;18:1979–1986. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PT, McGuinness TL, Greengard P. Evidence that the major postsynaptic density protein is a component of a Ca2+/calmodulin-dependent protein kinase. Proc Natl Acad Sci USA. 1984;81:945–949. doi: 10.1073/pnas.81.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeltzow TE, Xu M, Cooper DC, Hu XT, Tonegawa S, Wolf ME, White FJ. Alterations in dopamine release but not dopamine autoreceptor function in dopamine D3 receptor mutant mice. J Neurosci. 1998;18:2231–2238. doi: 10.1523/JNEUROSCI.18-06-02231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard AS, Lim IA, Hemsworth DE, Horne MC, Hell JW. Calcium/calmodulin-dependent protein kinase II is associated with the N-methyl-D-aspartate receptor. Proc Natl Acad Sci USA. 1999;96:3239–3244. doi: 10.1073/pnas.96.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Schlman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioral memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Liu XY, Chu XP, Mao LM, Wang M, Lan HX, Li MH, Zhang GC, Parelkar NK, Fibuch EE, Haines M, Neve KA, Liu F, Xiong ZG, Wang JQ. Modulation of D2R-NR2B interactions in response to cocaine. Neuron. 2006;52:897–909. doi: 10.1016/j.neuron.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Mao L, Yang L, Tang Q, Samdani S, Zhang G, Wang JQ. The scaffold protein Homer1b/c links metabotropic glutamate receptor 5 to extracellular signal-regulated protein kinase cascades in neurons. J Neurosci. 2005;25:2741–2742. doi: 10.1523/JNEUROSCI.4360-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T, Hanson PI, Stryer L, Schulman H. Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science. 1992;256:1199–1201. doi: 10.1126/science.256.5060.1199. [DOI] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- Omkumar RV, Kiely MJ, Rosenstein AJ, Min KT, Kennedy MB. Identification of a phosphorylation site for calcium/calmodulin-dependent protein kinase II in the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 1996;271:31670–31678. doi: 10.1074/jbc.271.49.31670. [DOI] [PubMed] [Google Scholar]
- Pritchard LM, Newman AH, McNamara RK, Logue AD, Taylor B, Welge JA, Xu M, Zhang J, Richtand NM. The dopamine D3 receptor antagonist NGB 2904 increases spontaneous and amphetamine-stimulated locomotion. Pharmacol Biochem Behav. 2007;86:718–726. doi: 10.1016/j.pbb.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Richtand NM. Behavioral sensitization, alternative splicing, and D3 dopamine receptor-mediated inhibitory function. Neuropsychopharmacology. 2006;31:2368–2375. doi: 10.1038/sj.npp.1301163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridray S, Griffon N, Mignon V, Souil E, Carboni S, Diaz J, Schwartz JC, Sokoloff P. Coexpression of dopamine D1 and D3 receptors in islands of Calleja and shell of nucleus accumbens of the rat: opposite and synergistic functional interactions. Eur J Neurosci. 1998;10:1676–1686. doi: 10.1046/j.1460-9568.1998.00173.x. [DOI] [PubMed] [Google Scholar]
- Ross SB. Synaptic concentration of dopamine in the mouse striatum in relationship to the kinetic properties of the dopamine receptors and uptake mechanism. J Neurochem. 1991;56:22–29. doi: 10.1111/j.1471-4159.1991.tb02557.x. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Allen PB, Fienberg AA, Valle CG, Huganir RL, Nairn AC, Greengard P. Regulation of phosphorylation of the GluR1 AMPA receptor in the neostriatum by dopamine and psychostimulants in vivo. J Neurosci. 2000;20:4480–4488. doi: 10.1523/JNEUROSCI.20-12-04480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Diaz J, Le Foll B, Guillin O, Leriche L, Bezard E, Gross C. The dopamine D3 receptor: a therapeutic target for the treatment of neuorpsychiatric disorders. CNS Neurol Disord Drug Targets. 2006;5:25–43. doi: 10.2174/187152706784111551. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Martres MP, Giros B, Bouthenet ML, Schwartz JC. The third dopamine receptor (D3) as a novel target for antipsychotics. Biochem Pharmacol. 1992;43:659–666. doi: 10.1016/0006-2952(92)90227-a. [DOI] [PubMed] [Google Scholar]
- Strack S, Choe S, Lovinger DM, Colbran RJ. Translocation of autophosphorylated calcium/calmodulin-dependent protein kinase II to the postsynaptic density. J Biol Chem. 1997;272:13467–13470. doi: 10.1074/jbc.272.21.13467. [DOI] [PubMed] [Google Scholar]
- Strack S, McNeill RB, Colbran RJ. Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 2000;275:23798–23806. doi: 10.1074/jbc.M001471200. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. TINS. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undie AS, Friedman E. Stimulation of a dopamine D1 receptor enhances inositol phosphates formation in rat brain. J Pharmacol Exp Ther. 1990;253:987–992. [PubMed] [Google Scholar]
- Vest RS, Davies KD, O’Leary H, Prot JD, Bayer KU. Dual mechanism of a natural CaMKII inhibitor. Mol Biol Cell. 2007;18:5024–5033. doi: 10.1091/mbc.E07-02-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RR, Kwon YG, Taing M, Lawrence DS, Edelman AM. Definition of optimal substrate recognition motifs of Ca2+-calmodulin-dependent protein kinases IV and II reveals shared and distinctive features. J Biol Chem. 1998;273:3166–3179. doi: 10.1074/jbc.273.6.3166. [DOI] [PubMed] [Google Scholar]
- Xu M, Koeltzow TE, Santiago GT, Moratalla R, Cooper DC, Hu XT, White NM, Graybiel AM, White FJ, Tonegawa S. Dopamine D3 receptor mutant mice exhibit increased behavioral sensitivity to concurrent stimulation of D1 and D2 receptors. Neuron. 1997;19:837–848. doi: 10.1016/s0896-6273(00)80965-4. [DOI] [PubMed] [Google Scholar]
- Yang E, Schulman H. Structural examination of autoregulation of multifunctional calcium/calmodulin-dependent protein kinase II. J Biol Chem. 1999;274:26199–26208. doi: 10.1074/jbc.274.37.26199. [DOI] [PubMed] [Google Scholar]
- Yuan J, Chen X, Brodbeck R, Primus R, Braun J, Wasley JW, Thurkauf A. NGB 2904 and NGB 2849: two highly selective dopamine D3 receptor antagonists. Bioorg Med Chem Lett. 1998;8:2715–2718. doi: 10.1016/s0960-894x(98)00469-7. [DOI] [PubMed] [Google Scholar]
- Zhang L, Lou D, Jiao H, Zhang D, Wang X, Xia Y, Zhang J, Xu M. Cocaine-induced intracellular signaling and gene expression are oppositely regulated by the dopamine D1 and D3 receptors. J Neurosci. 2004;24:3344–3354. doi: 10.1523/JNEUROSCI.0060-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen X, Goswami S, Abdali SA, Gil M, Bakshi K, Friedman E. Regulation of cyclin-dependent kinase 5 and calcium/calmodulin-dependent protein kinase II by phosphatidylinositol-linked dopamine receptor in rat brain. Mol Pharmacol. 2004;66:1500–1507. doi: 10.1124/mol.104.002279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Supplemental Data for this article can be found online.