Abstract
Lung tissue kallikrein (TK) is a serine proteinase that putatively plays a role in the pathophysiology of asthma by generating kallidin and bradykinin, mediators that contribute to airway hyperresponsiveness. In previous studies we observed biphasic increases in TK activity in bronchoalveolar lavage fluid following airway allergen challenge in allergic sheep. Although glandular TK is likely a major source of the initial increase in TK, the sources of the late increases in TK that are associated with the development of airway hyperresponsiveness may be dependent on activated resident and recruited inflammatory cells including alveolar macrophages (AMs) and neutrophils (PMNs). These cells increase concomitantly with the late increases in TK activity. To test this hypothesis, we obtained AMs from bronchoalveolar lavage fluid and PMNs and monocytes (precursors of AMs) from sheep blood and determined whether these cells contained TK and whether these same cells could release TK upon activation. Using confocal microscopy, immunocytochemical techniques, and enzyme activity assays, we found that all three cell types contained and secreted TK. All three cell types demonstrated basal release of TK, which could be increased after stimulation with zymosan. In addition, PMNs also released TK in the presence of phorbol ester, suggesting multiple secretory pathways in these cells. Further-more, we showed that human monocytes also contain and secrete TK. We conclude that in the airways, monocytes, PMNs, and AMs may contribute to increased TK activity. Knowing the sources of TK in the airways could be important in understanding the mechanisms of inflammation that contribute to the pathophysiology of asthma and may help in the development of new therapies to control the disease.
Keywords: inflammation, serine proteases, asthma, airway hyperresponsiveness, neutrophils, macrophages
Tissue kallikreins (TK) (EC 3.4.21.35) are a multiple family of serine proteinases that generate kallidin (lysyl-bradykinin) from low- and high-molecular-weight kininogen. In the lung, the generation of kallidin and its cleavage product bradykinin can contribute to the pathophysiology of asthma (5, 14, 33, 34, 38, 39, 51). Both peptides cause vasodilatation (47) and increases in vascular permeability (6) and bronchoconstriction (2, 42). Furthermore, studies in asthma patients and animal models of asthma indicate that elevated TK and kinin levels in bronchoalveolar lavage fluid (BALF) may mediate airway hyperresponsiveness (AHR), a hallmark of the disease (13, 18, 40, 42). TK has also been shown to be an important activator of type IV collagenase (92-kDa gelatinase) (16), which is involved in the degradation of extracellular matrix components and, therefore, could be important in airway remodeling seen in chronic asthma (7). These links between elevated TK and asthma symptoms suggest that increases in TK levels and/or activity may be critical factors in disease pathogenesis. Therefore, understanding the sources of TK and its regulation in the airways could be important therapeutically.
Airway challenge with antigen is associated with both an acute (<30 min) increase and a late (6–24 h) increase in BALF TK activity (3, 5, 14, 18, 40–42). The source(s) of the immediate increase in TK activity is most likely from submucosal gland cell secretion triggered by secretagogue mediators such as chymase and/or elastase (3, 5, 13, 14, 21, 41, 46) and from previously secreted TK that is bound to hyaluronan on the apical surface of the ciliated epithelium. This bound TK is inactive, but in the presence of oxygen radicals that are generated in the airways following allergen challenge (30), the TK-hyaluronan bond can be broken, thereby activating the TK (22, 23). Although glandular and epithelial-bound TK may contribute to the immediate increase in airway TK following allergen challenge, these may not be the primary sources for the more prolonged increases in TK/kinin levels seen 6–24 h after antigen challenge that are linked to AHR.
Our previous studies indicate that recruited inflammatory cells may be one important source for this late, more sustained increase in airway TK activity. Increases in the numbers of polymorphonuclear leukocytes (PMNs) and alveolar macrophages (AMs) in BALF coincide with the late increases in BALF TK activity, and inhibition of this cellular inflammatory response with adhesion molecule inhibitors prevented the increase in TK activity and the associated AHR (1, 3). Collectively, these studies suggest that PMNs and AMs could be potential sources of the enzyme.
There are data to support the hypothesis that both PMNs and macrophages can generate kinins. PMNs have been studied extensively and have been shown to generate kinins (28). Subsequent findings confirmed the presence of TK mRNA and protein expression in these cells (10, 19, 20), and more recent work indicates that PMN-associated TK can be released at sites of inflammation. Williams and coworkers (51) found that PMNs obtained from the synovial fluid of patients with rheumatoid arthritis had reduced TK activity compared with circulating PMNs from the same patients, which suggests that the PMNs released TK at some point in transit from the vasculature to the inflammatory locus. Such a mechanism would be consistent with PMNs contributing to the late antigen-induced increases in BALF TK activity.
Macrophages may also be a source of TK (15, 44). Macrophages obtained from rat intestine granulomas contain and secrete TK (44), therefore it is possible that AMs also contain and secrete TK. This fact cannot be assumed, however, because tissue-resident macrophages adapt to their local environment to perform specific functions (29). TK mRNA was found to be present in promyelocytic cultures of an HL-60 cell line induced to differentiate toward monocytes (MONOs) (37), indicating that MONOs could also contain and secrete TK. Because these cells eventually migrate to the lung and become AMs, it is possible that both cell types could contribute to lung TK activity.
In this study, then, we formally test the hypotheses that AMs, MONOs, and PMNs contain and secrete TK. To do this, we used specific enzyme activity assays and immunocyto-chemistry to verify the presence of TK in both MONOs and AMs, and we used confocal microscopy to assess the localization of TK in PMNs by double labeling TK and elastase (which is present in the azurophilic granule of PMNs). In addition, we determined the functional aspects of TK release in these cells after stimulation with phorbol ester (PMA) and opsonized zymosan (OZ), two secretagogues that affect the secretory mechanisms of these cells differently (4, 11, 26, 32, 45, 48, 50, 52). Our findings show that AMs and, for the first time, MONOs (obtained from both sheep and human peripheral blood) contain and release TK. We also corroborate studies demonstrating that PMNs secrete TK and described the secretory pathways involved in TK release by these cells. Colocalization studies also revealed that, unlike elastase, TK is not stored in azurophilic granules.
MATERIALS AND METHODS
In this study, the PMNs and MONOs from blood and the AMs from BALF were obtained from sheep. This study was approved by the Mount Sinai Medical Center Animal Research Committee, which is responsible for ensuring the humane care and use of experimental animals. Peripheral blood from human volunteers was also obtained for the isolation of MONOs. This procedure was determined to be exempt from informed consent by the Mount Sinai Medical Center Institutional Review Board.
All chemicals and reagents were obtained from Sigma (St. Louis, MO) unless otherwise indicated. For stimulation studies, we prepared OZ by boiling the zymosan for 15 min in 0.85% saline solution and then washing it twice by centrifugation. We achieved opsonization by incubating the zymosan with 5 mg/ml of sheep serum in a rotator shaker for 1 h at 37°C. After incubation, the solution was centrifuged, and the pellet was washed twice and then resuspended in phosphate-buffered saline (PBS) without Ca2+ and Mg2+ at a concentration of 100 mg/ml. Aliquots were frozen at −70°C until used.
Isolation of MONOs from peripheral blood
Monocytes were isolated from anticoagulated blood (0.5 ml of 4.3% EDTA/ml for sheep blood and 10 units of heparin/ml for human blood) by density gradient centrifugation according to the method of Boyum (9). Briefly, blood was diluted 1:1 with PBS without Ca2+ and Mg2+. Histopaque-1077 was layered at the bottom of the tube, and then the preparation was centrifuged at 450 g for 30 min at room temperature. After centrifugation, a distinct layer at the plasma-histopaque interface was formed, which contained the mononuclear cells. To maximize the collection of MONOs, we collected the entire overlying layer and interface. The collective fraction was centrifuged at 300 g, and the pellet was washed with PBS twice at room temperature. This method has recently been substantiated to increase the yield of MONOs to >60% (36). Cell viability, assessed by trypan blue exclusion, was between 90 and 95%. Cell differentials with Wright-Giemsa stain showed that for the various experiments between 50 and 70% of the cells in the interface were MONOs, with the remainder being lymphocytes. Because lymphocytes are not phagocytic cells and because neither mature lymphocytes (37) nor lymphocytic cell lineages have been shown to contain or express TK (37), further attempts to separate MONOs and lymphocytes were not pursued to avoid cell loss. For each experiment, we resuspended the mononuclear cell preparation (MONOs plus lymphocytes) at a concentration of 3–5 × 106 cells/ml in RPMI 1640 and then adjusted it to a concentration of 106 MONOs/ml by correcting for the cell differential in each experiment. TK values were reported as ng/106 MONOs.
Isolation of PMNs from peripheral blood
PMNs were obtained by the density gradient centrifugation preparation described above. Because of their density, PMNs are found in the bottom layer along with the erythrocytes after centrifugation. PMNs were separated from the red blood cells as previously described (12). Cell viability, assessed by trypan blue exclusion, was between 90 and 99%, and cell differentials were 95–99% PMNs, with eosinophils being the other cell present. PMNs were resuspended at 1 × 107/ml in RPMI 1640, and TK values were reported as ng/106 PMNs.
Isolation of AMs from BALF
Conscious sheep were restrained in a modified shopping cart, and BAL was performed as previously described (12). Briefly, the distal tip of a specially designed 80-cm fiber optic bronchoscope was wedged into a subsegmental bronchus of one lung. BAL was done with 90 ml of PBS without Ca2+ and Mg2+ in three different airways. The BALF was filtered through one single layer of gauze and then centrifuged at room temperature for 15 min at 300 g to collect the cell pellet. The pellets from the three airways were combined, washed two times with PBS, and resuspended in Hanks’ balanced salt solution without Ca2+ and Mg2+ (HBSS). The washed cells were resuspended in 2 ml of HBSS with 100 units of penicillin and 0.1 mg/ml of streptomycin. The cell suspension was then carefully layered on top of two layers of Percoll (densities of 1.04 and 1.06 g/ml, respectively) in sterile, 15-ml polypropylene tubes and was centrifuged at 500 g for 30 min at room temperature. The cells were collected at the interface of 1.06 g/ml, resuspended in PBS, and washed twice by centrifugation at 300 g at room temperature. Cell counts, viability test by 0.5% trypan blue solution, and a cytospin for cell differentiation were performed. Cell viability for these preparations was ~90%, and cell differentiation showed purity between 80 and 90%, with lymphocytes being the main contaminating cell type.
PMN stimulation
To assess TK release from PMNs, we incubated 107 cells/ml at 38°C in a rotator water bath in the presence of 10 ng/ml of PMA or 1 mg/ml of OZ. The doses selected for these experiments were those that gave the maximum response in dose-response curves performed for each agent (data not shown). PMNs incubated with medium alone served as controls. In all experiments, samples were placed on ice for 5 min after incubation to stop the reaction and then centrifuged at 250 g for 10 min at 4°C. Supernatants were separated into aliquots, and in those where neutrophil elastase (NE) was measured (see below), aliquots were adjusted to a final concentration of 0.5MNaCl. Samples were frozen at −70°C until assayed (see below).
Stimulation of AMs and MONOs
One milliliter of 106 AMs/ml or 106 MONOs/ml in RPMI 1640 was incubated in a rotator water bath at 38°C (sheep) and 37°C (human) for 1 h with OZ (1 mg/ml), PMA (10 ng/ml), or medium alone (control). The supernatants were collected, centrifuged at 250 g for 10 min at 4°C, and then frozen at −70°C until assayed.
Enzyme activity assays
Enzyme activities were measured using chromogenic substrates DL Val-Leu-Arg pNA for TK as described (21, 25) and N-methoxysuccynyl-Ala-Ala-Pro-Val pNA for NE as previously described (24, 35). Values were obtained from a standard curve done in parallel with the samples and reported as ng/106 cells. When the release of TK and NE was compared, values were reported in mU/107 PMNs with 1 mU being equal to 1 mmol of substrate degraded per min at 22°C.
Laser confocal microscopy
Double labeling immunofluorescence of PMNs was made from cytospin preparations of suspensions of 106 cells/ml on Vectabond-coated slides (Vector Laboratories, Burlingame, CA). Cells were permeabilized with cold methanol (10 min) and labeled with rabbit antihuman urinary kallikrein (1:500; Calbiochem-Novabiochem, La Jolla, CA) and mouse antihuman elastase (1 µg/ml, Vector Laboratories) overnight at 4°C. After washing with PBS (3 × 10 min), visualization was achieved with rabbit anti-mouse FITC (5 µg/ml) and goat anti-rabbit CY3. Confocal microscopy was performed in an Odyssey XL microscope (Noran Instruments, Middleton, WI) using an Omnichrome laser source. For colocalization experiments (FITC/CY3), a detection channel was used with excitation of 495 nm and an emission of 525 nm. The images were collected using Intervision software (Noran Instruments).
Immunocytochemistry of sheep AM and MONOs
Cytospin preparation of mononuclear cells were prepared on slides coated with Vectabond at a concentration of 106 cells/ml and then fixed with 4% paraformaldehyde. Slides were washed in PBS (5 min), blocked with 20% goat serum in PBS for 1 h, and then incubated with antibodies to human urinary kallikrein (5 µg/ml in 20% goat serum, Calbiochem) at 4°C overnight. After being washed in PBS (three times for 5 min each), slides were incubated with peroxidase-labeled goat anti-rabbit IgG (ABC kit, Vector Laboratories) at room temperature for 30 min and washed with PBS (five times for 5 min each). We achieved visualization by incubating with diaminobenzamidine (DAB, 1 mg/ml) in PBS plus H2O2 0.01% at room temperature for 8 min and counterstained with Harris hematoxylin. We prepared controls by omitting the first antibody (22).
TK immunocytochemistry in human MONOs
Cytospins of isolated MONOs were double labeled with rabbit anti-human urinary kallikrein (1:500) and mouse anti-CD14 (clone M-M42), a marker for MONOs (27, 43) (1:100, Vector Laboratories), both diluted in 20% goat serum or nonimmune rabbit and mouse serum (control) followed by goat anti-rabbit horseradish peroxidase (HRP) and goat anti-mouse alkaline phosphatase (AP), respectively. Color visualization was obtained with HistoMax Red for AP (KLP, Gaithersburg, MD) and DAB for HRP. Nuclear staining was achieved with hematoxylin.
Statistical analysis
Kruskal-Wallis one-way analysis of variance and Mann-Whitney U-test were used to determine statistical significance. P < 0.05 was considered significant using a two-tailed test. Values in the text and figures are presented as means ± SE.
RESULTS
Stimulated sheep AMs, MONOs, and PMNs release TK
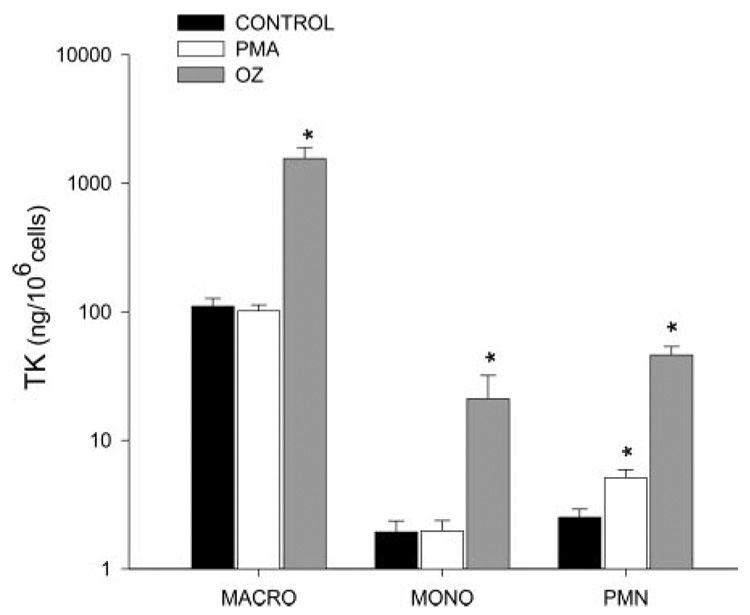
Under control conditions, all sheep cells released TK. AMs showed the largest basal release per cell, followed by PMNs and MONOs. Stimulation of all three cell types with OZ resulted in significant increases in TK activity. As illustrated in Fig. 1, TK activity increased in OZ-stimulated PMNs from a control value of 2.5 ± 0.3 ng/106 cells (n = 6) to 46 ± 7.7 ng/106 cells (n = 4, P < 0.01). MONOs stimulated with OZ increased TK activity from 1.7 ± 0.4 ng/106 cells to 18.4 ± 8.2 ng/106 cells (n = 4, P < 0.03), and TK activity in OZ-stimulated AMs rose from a control value of 110 ± 19 ng/106 cells to 1,558 ± 340 ng/106 cells (n = 4, P < 0.025). In contrast to OZ, PMA only significantly stimulated TK release in PMNs: control 2.5 ± 0.3 ng/106 cells (n = 6) to 5.1 ± 0.8 (ng/106 cells n = 5) after PMA stimulation.
Fig. 1.
Tissue kallikrein (TK) values in ng/106 cells are shown for alveolar macrophages (macro), monocytes (MONOs), and polymorphonuclear leukocytes (PMNs). Cells were incubated for 60 min without stimulation (closed bars), stimulated with 1 mg of opsonized zymosan (OZ, gray bars), and with PMA (open bars). OZ significantly increased TK activity in all cell types, whereas only PMNs responded to PMA. Values are means ± SE for 4–6 separate experiments. *P < 0.05 vs. control.
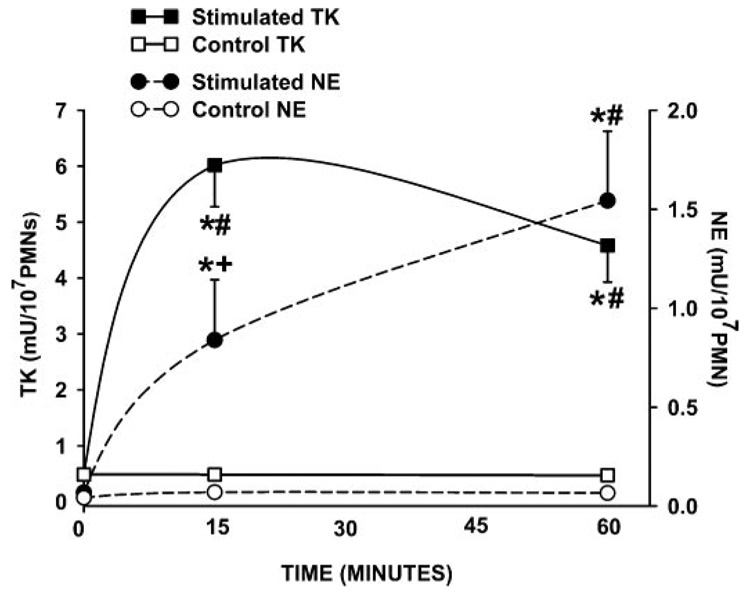
The release of granule constituents from PMNs is known to occur sequentially, with the contents of the specific granules being released before the contents of the azurophilic granules, which contain primarily NE (52). Because OZ causes exocytosis of both granule types, following the time course of TK and NE release from OZ-stimulated PMNs should give an indication as to the potential location of TK in these cells (8). Figure 2 shows that in supernatants from OZ-stimulated PMNs, TK activity reaches a maximum after 15 min, whereas NE activity is low at this time. NE activity then continues to increase during the 60-min observation period. These data, in conjunction with the fact that PMA only causes the release of specific granule (53) contents, suggest that TK is not likely contained in azurophilic granules.
Fig. 2.
Time course of TK and neutrophil elastase (NE) release from PMNs stimulated with OZ. TK activity (■) reaches its highest value at 15 min after which it remains constant. In contrast, NE activity (●) lags behind TK activity at 15 min but continues to increase throughout 60 min. Controls (unstimulated cells) showed no changes in TK (□) or NE (○) activity over 60 min. *P < 0.02 vs. control, #P < 0.02 vs. baseline, and +P < 0.02 vs. 60 min. Left axis: TK activity; right axis: NE activity. Values are means ± SE and expressed as mU/107 cells for 5–8 separate experiments.
Confocal microscopy demonstrates the presence of TK in sheep PMNs
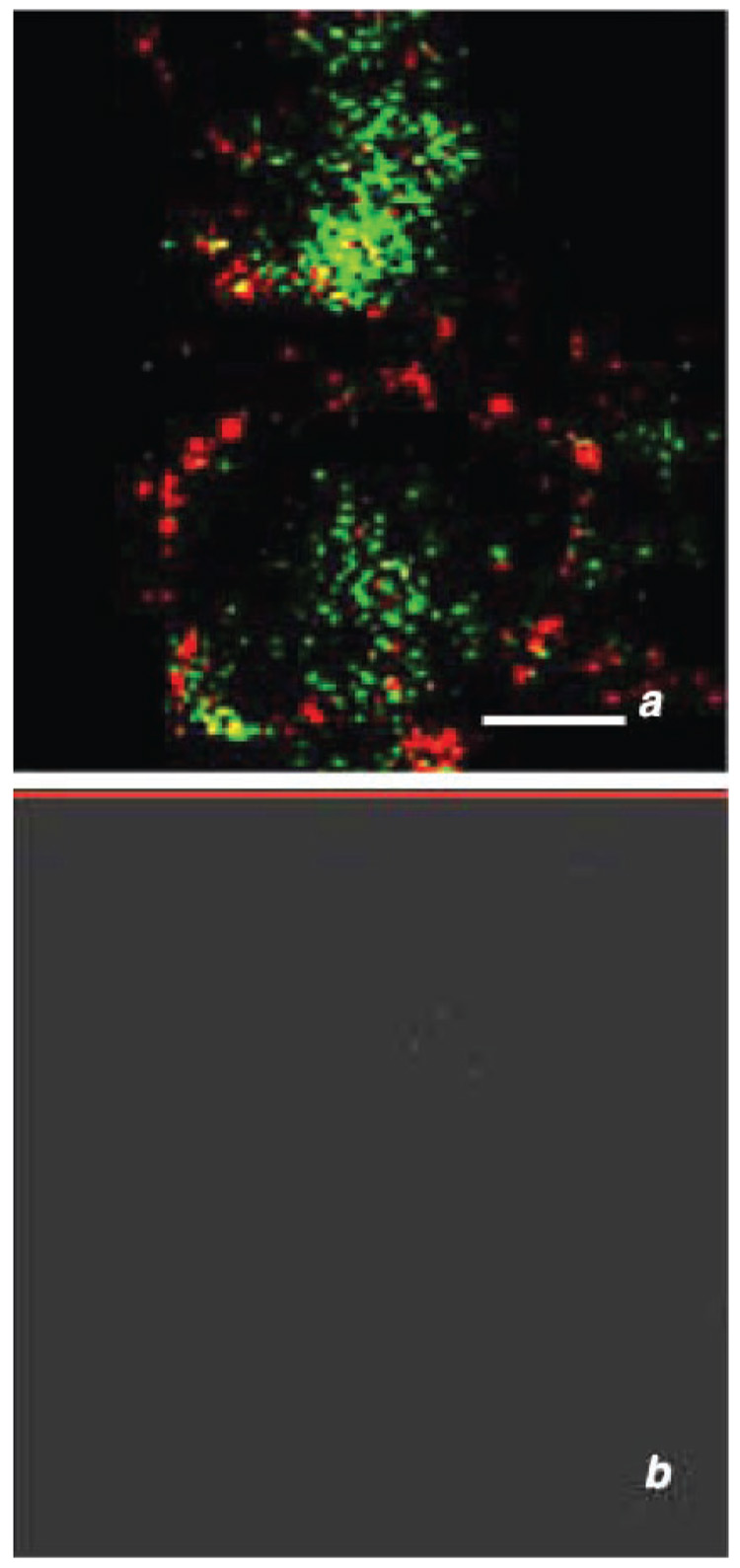
Visualization of TK (red fluorescence) and NE (green fluorescence) supports the functional release studies, which indicate that NE and TK are stored in different granule populations (Fig. 3A). There is clear definition in the intracellular granules between red and green fluorescence with little colocalization. Control slides where the primary antibody was omitted show no fluorescence (Fig. 3B).
Fig. 3.
Confocal microscopy immunofluorescence using double labeling for elastase and TK in permeabilized purified PMNs as described in materials and methods. A: visualization of TK (red) and elastase (green) confirmed that these proteases are stored in different granule populations. B: control where first antibodies have been omitted. Bar = 10 µm.
Sheep AMs and peripheral blood MONOs contain TK
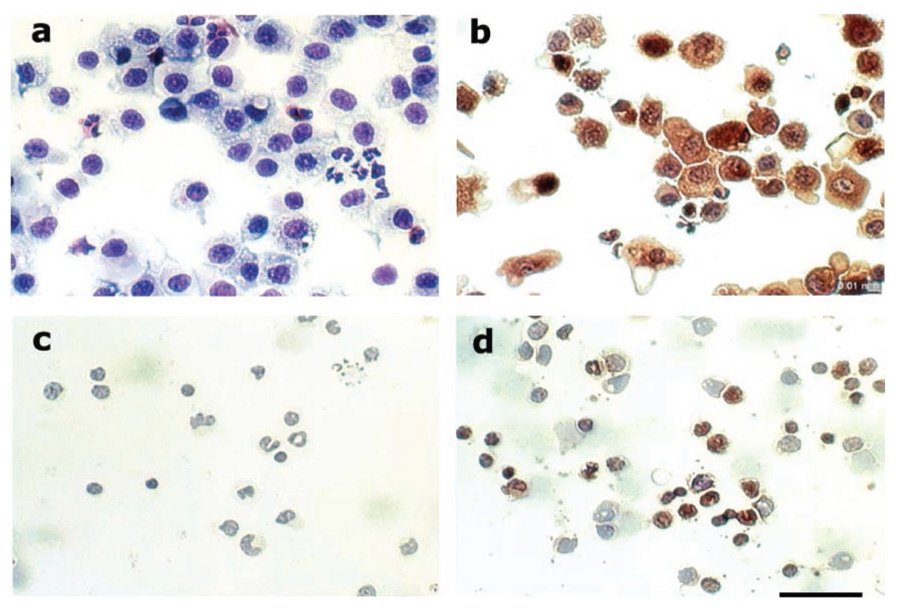
Immunoperoxidase staining of AMs and MONOs indicate that both cell types contain TK. TK is present in AMs (Fig. 4B) as evidenced by positive labeling (brown). There is no staining in the control cells, where the primary antibody was omitted (Fig. 4A). Peripheral blood MONOs (Fig. 4D) show staining characteristics similar to the AMs. Likewise, controls where the primary antibody were omitted show no labeling (Fig. 4C), confirming the specificity of TK staining. These data support the enzyme assays and functional release studies, demonstrating that both AMs and MONOs release TK.
Fig. 4.
Alveolar macrophages (A, B) and blood MONOs (C, D) were fixed, permeabilized, and labeled with anti-human urinary kallikrein antiserum (B, D) to demonstrate the presence of TK. Controls (A, C) had the primary antibody omitted. TK (brown stain) was visualized with horseradish peroxidase (HRP)-conjugated secondary antibodies, followed by diaminobenzamidine (DAB) as a substrate. Slides were counterstained with hematoxylin. Bar = 50 µm.
Human peripheral blood MONOs contain and secrete TK
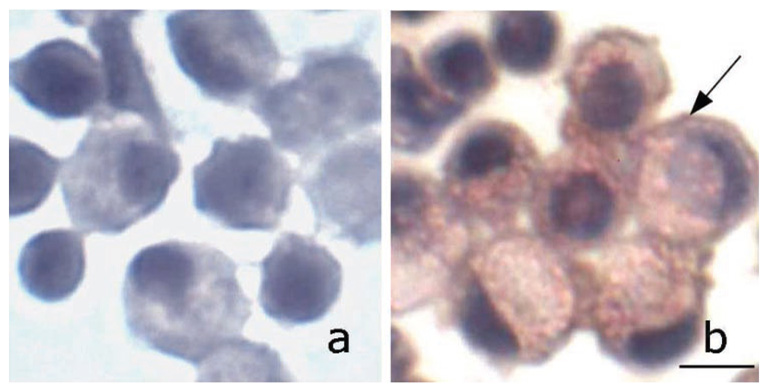
In two separate experiments from two different volunteers, human MONOs obtained from peripheral blood were found to secrete TK under basal and stimulated conditions. Basal TK release was 7.6 ± 3.5 ng/106 MONOs and increased to 15.2 ± 5.4 ng/106 MONOs after stimulation with OZ. As was observed with sheep MONOs, PMA failed to stimulate TK release. That the cells that contain and secrete TK are MONOs is supported by the positive immunostaining seen in Fig. 5B. The identity of these cells was confirmed by the presence of CD14 by immunocytochemistry (Fig. 5B), a marker for MONOs (27, 43). Similar to the sheep studies, control cells (where nonimmune rabbit serum was used instead of the first antibody) were negative (Fig. 5A).
Fig. 5.
Presence of TK in human blood MONOs. Cytospins of isolated mononuclear cells were double labeled with rabbit anti-human urinary kallikrein (1:500) and mouse anti-CD14 (clone M-M42), a marker for MONOs (1:100, Vector Laboratories), both diluted in 20% goat serum or nonimmune rabbit and mouse serum (control) followed by goat anti-rabbit HRP and goat anti-mouse alkaline phosphatase (AP), respectively. Color visualization was obtained with Histo-Max Red for AP (KLP) and DAB for HRP. Nuclear staining was achieved with hematoxylin. TK (brown) and CD14 (red) are clearly visible in MONOs; maroon (arrow) is likely due to colocalization of TK and CD14 in the cell membrane. Bar = 20 µm.
DISCUSSION
The results of this study show that TK is present in and can be released from sheep PMNs, MONOs, and AMs. In addition we provide novel evidence that human MONOs contain and secrete TK. All three cell types show basal release of TK, and this release is increased following stimulation with OZ. In addition, PMNs stimulated with PMA also show increased TK release. Because these cell types are increased in the lung in various inflammatory conditions such as asthma, our findings support the hypothesis that these cells may act as a source of TK in the lungs and, in doing so, may contribute to the functional consequences (e.g., AHR) associated with increased TK activity as shown by us previously (3, 21, 41, 42).
We used two different agonists, i.e., OZ and PMA, to better understand the cellular pathways involved in stimulated TK release from these three cell types (4, 11, 26, 31, 32, 45, 48–50, 52). We found that OZ, a particulate stimulus of phagocytosis in granulocytes, stimulates the release of TK in all cells studied, whereas PMA, a protein kinase C stimulator, only stimulates release from PMNs. As discussed below, the difference in release patterns between OZ- and PMA-stimulated PMNs, AMs, and MONOs is important for identifying the possible cellular location of TK in these three cells types.
Human PMNs have four distinct granules: azurophilic, specific, tertiary, and secretory vesicles, whereas the ruminant PMNs used in this study contain only three. Two of the ruminant granules resemble the azurophilic and specific granules of human PMNs, whereas the third granule, termed a “large granule,” is unique to ruminants (4, 11, 26). Exocytosis of the distinct granule populations may occur independently (48), since discharge of the different granule types may be dependent on the stimulus and/or surfaces the PMNs contact during migration. Unlike azurophilic granules, which are hardly mobilized, specific and tertiary granules are readily exocytosed on cell activation (52). This may explain why the specific and tertiary granules contain many of the components involved in neutrophil adhesion and extravasations including adhesion molecules, extracellular matrix proteases, and enzymes implicated in the generation of soluble mediators of inflammation (17). On the basis of the differential secretory response of PMNs to OZ and PMA (both time course and stimulus specificity) and the confocal microscopy data, our findings indicate that TK and NE are contained in different PMN granules. Previous studies have shown that PMA induces the exocytosis of specific and large granules but not azurophilic granules (4, 11, 26, 52), whereas OZ causes the release of all granule types, but with specific granules being released before the azurophilic granules (8). Our functional data showing the selective release of TK in PMA-stimulated PMNs and TK release preceding NE release in OZ-stimulated PMNs suggest that TK is likely localized in the specific and/or large granules and not azurophilic granules. These functional results are consistent with the confocal microscopy images, which show clear separation of TK- and NE-containing organelles.
We also demonstrate that AMs contain and release TK. Unlike PMNs, however, only OZ stimulated TK release in these cells. Because PMA is a poor stimulator of macrophage lysosome secretion (50), our findings would suggest that TK is contained in AM lysosomes. Our demonstration of TK in AM is consistent with findings of Stadnicki and coworkers (44), who reported TK activity in macrophages present in submucosal intestinal granulomas. These investigators also provided evidence that these macrophages secrete TK and that this TK contributes to the intestinal inflammatory response in a rat model of experimental enterocolitis. The results of our study confirm and extend these observations to include not only AMs, but also peripheral blood MONOs, the cellular precursors of AMs. Our data clearly show that peripheral blood MONOs from both sheep and humans contain and secrete TK. Our findings that TK is present in and can be secreted by MONOs (both sheep and human) as well as AMs, in addition to those of Stadnicki et al. (44), differ from those of Figueroa et al. (19), who report that TK was present only in peripheral blood PMNs and not other blood leukocytes. Differences in the immunocytochemical methods used by these investigators may account for this discordance. Our immunocytochemical and functional data, in addition to the fact that TK mRNA is present in promyelocytes, argue strongly that MONOs do contain and secrete TK. This observation has important implications for inflammatory responses in the lung, where AMs increase in inflammatory conditions, e.g., resulting from allergen exposure. Thus the inflammatory-induced migration of peripheral blood MONOs to the lung provides another source of TK.
Although we did not specifically examine the relationship between increased TK activity and AHR in this study, we have extensively described this association in earlier studies, and, in fact, these previous findings were the impetus for us to search for additional sources of the enzyme (1, 3). The results of the present study indicate that cells that migrate to the airways following an inflammatory insult, such as allergen challenge, have the potential to perpetuate the inflammatory state and its pathophysiological consequences, e.g., AHR, by providing a continued localized source of TK. The importance of this cycle is further highlighted by our previous studies showing that interrupting this cascade with inhibitors of cell migration, e.g., selectin inhibitors, blocks the leukocyte recruitment to the lung, the increase in TK activity, and the AHR (1).
In conclusion, we showed that PMNs, AMs, and MONOs contain and secrete TK. Therefore, these cells, which are increased in inflamed lungs, may be likely sources contributing to the sustained increases in lung TK activity observed in airway disease.
ACKNOWLEDGMENTS
The authors thank Dr. Pedro Salas for help with confocal microscopy and Dr. Marina Casalino-Matsuda for the immunocytochemistry staining of TK in human monocytes.
GRANTS
This work was supported in part by National Institutes of Health Grants HL-R01068992 and K0103534 (R. M. Forteza) and ES-10594 (W. M. Abraham).
REFERENCES
- 1.Abraham WM, Ahmed A, Sabater JR, Lauredo IT, Botvinnikova Y, Bjercke RJ, Hu X, Revelle BM, Kogan TP, Scott IL, Dixon RAF, Yeh ETH, Beck PJ. Selectin blockade prevents antigen-induced late bronchial responses and airway hyperresponsiveness in allergic sheep. Am J Respir Crit Care Med. 1999;159:1205–1214. doi: 10.1164/ajrccm.159.4.9806002. [DOI] [PubMed] [Google Scholar]
- 2.Abraham WM, Burch RM, Farmer SG, Sielczak MW, Ahmed A, Cortes A. A bradykinin antagonist modifies allergen-induced mediator release and late bronchial responses in sheep. Am Rev Respir Dis. 1991;143:787–796. doi: 10.1164/ajrccm/143.4_Pt_1.787. [DOI] [PubMed] [Google Scholar]
- 3.Abraham WM, Gill A, Ahmed A, Sielczak MW, Lauredo IT, Botvinnikova Y, Lin KC, Pepinsky B, Leone DR, Lobb RR, Adams SP. A small-molecule, tight binding inhibitor of the integrin alpha(4)beta(1) blocks antigen-induced airway responses and inflammation in experimental asthma in sheep. Am J Respir Crit Care Med. 2000;162:603–611. doi: 10.1164/ajrccm.162.2.9911061. [DOI] [PubMed] [Google Scholar]
- 4.Baggiolini M, Horisberger U, Gennaro R, Dewald B. Identification of three types of granules in neutrophils of ruminants. Ultrastructure of circulating and maturing cells. Lab Invest. 1985;52:151–158. [PubMed] [Google Scholar]
- 5.Baumgarten CR, Nichols RC, Naclerio RM, Proud D. Concentrations of glandular kallikrein in human nasal secretions during experimentally induced allergic rhinitis. J Immunol. 1986;137:1323–1328. [PubMed] [Google Scholar]
- 6.Bell RD, Wainer BS. Effects of bradykinin on renal lymph flow and composition. Lymphology. 1983;16:38–42. [PubMed] [Google Scholar]
- 7.Belleguic C, Corbel M, Germain N, Lena H, Boichot E, Delaval PH, Lagente V. Increased release of matrix metalloproteinase-9 in the plasma of acute severe asthmatic patients. Clin Exp Allergy. 2002;32:217–223. doi: 10.1046/j.1365-2222.2002.01219.x. [DOI] [PubMed] [Google Scholar]
- 8.Bentwood BJ, Henson PM. The sequential release of granule constituents from human neutrophils. J Immunol. 1980;124:855–862. [PubMed] [Google Scholar]
- 9.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest. 1968;97:77–89. [PubMed] [Google Scholar]
- 10.Bruner HD, Schmidt CF. Blood flow in the bronchial artery of anesthetized dog. Am J Physiol. 1947;148:643–666. doi: 10.1152/ajplegacy.1947.148.3.648. [DOI] [PubMed] [Google Scholar]
- 11.Buchta R. Functional and biochemical properties of ovine neutrophils. Vet Immunol Immunopathol. 1990;24:97–112. doi: 10.1016/0165-2427(90)90013-i. [DOI] [PubMed] [Google Scholar]
- 12.Chapman GA, Signoretti F, Sielczak MW, Ahmed A, Lauredo IT, Seybold SV, Abraham WM. Cellular LTD4 production differs in allergic sheep with and without late airway airway responses. Am J Physiol Lung Cell Mol Physiol. 1990;259:L136–L143. doi: 10.1152/ajplung.1990.259.2.L136. [DOI] [PubMed] [Google Scholar]
- 13.Christiansen SC, Proud D, Cochrane CG. Detection of tissue kallikrein in the bronchoalveolar lavage fluid of asthmatic subjects. J Clin Invest. 1987;79:188–197. doi: 10.1172/JCI112782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christiansen SC, Proud D, Sarnoff RB, Juergens U, Cochrane CG, Zuraw BL. Elevation of tissue kallikrein and kinin in the airways of asthmatic subjects after endobronchial allergen challenge. Am Rev Respir Dis. 1992;145:900–905. doi: 10.1164/ajrccm/145.4_Pt_1.900. [DOI] [PubMed] [Google Scholar]
- 15.Desmazes C, Galineau L, Gauthier F, Bromme D, Lalmanach G. Kininogen-derived peptides for investigating the putative vasoactive properties of human cathepsins K and L. Eur J Biochem. 2003;270:171–178. doi: 10.1046/j.1432-1033.2003.03382.x. [DOI] [PubMed] [Google Scholar]
- 16.Desrivieres S, Lu H, Peyri N, Soria C, Legrand Y, Menashi S. Activation of the 92 kDa type IV collagenase by tissue kallikrein. J Cell Physiol. 1993;157:587–593. doi: 10.1002/jcp.1041570319. [DOI] [PubMed] [Google Scholar]
- 17.Falloon J, Gallin JI. Neutrophil granules in health and disease. J Allergy Clin Immunol. 1986;77:653–662. doi: 10.1016/0091-6749(86)90404-5. [DOI] [PubMed] [Google Scholar]
- 18.Featherstone RL, Parry JE, Church MK. Kallikrein-like activity in the bronchoalveolar lavage (BAL) fluid of sensitized, challenged guineapigs. Agents Actions Suppl. 1992;38:462–466. [PubMed] [Google Scholar]
- 19.Figueroa CD, MacIver AG, Bhoola KD. Identification of a tissue kallikrein in human polymorphonuclear leucocytes. Br J Haematol. 1989;72:321–328. doi: 10.1111/j.1365-2141.1989.tb07711.x. [DOI] [PubMed] [Google Scholar]
- 20.Figueroa CD, MacIver AG, Dieppe P, Mackenzie JC, Bhoola KD. Presence of immunoreactive tissue kallikrein in human polymorphonuclear (PMN) leukocytes. Adv Exp Med Biol. 1989;247B:207–210. doi: 10.1007/978-1-4615-9546-5_34. [DOI] [PubMed] [Google Scholar]
- 21.Forteza R, Botvinnikova Y, Ahmed A, Cortes A, Gundel RH, Wanner A, Abraham WM. The interaction of α1-proteinase inhibitor and tissue kallikrein in controlling allergic ovine airway hyperresponsiveness. Am J Respir Crit Care Med. 1996;154:36–42. doi: 10.1164/ajrccm.154.1.8680696. [DOI] [PubMed] [Google Scholar]
- 22.Forteza R, Lauredo I, Abraham WM, Conner GE. Bronchial tissue kallikrein activity is regulated by hyaluronic acid binding. Am J Respir Cell Mol Biol. 1999;21:666–674. doi: 10.1165/ajrcmb.21.6.3651. [DOI] [PubMed] [Google Scholar]
- 23.Forteza R, Lieb T, Aoki T, Savani RC, Conner GE, Salathe M. Hyaluronan serves a novel role in airway mucosal host defense. FASEB J. 2001;15:2179–2186. doi: 10.1096/fj.01-0036com. [DOI] [PubMed] [Google Scholar]
- 24.Franck C, Byrjalsen I. A simple and sensitive chromogen assay for the measurement of elastase-type enzyme activity in human plasma. Biol Chem Hoppe Seyler. 1988;369:677–682. doi: 10.1515/bchm3.1988.369.2.677. [DOI] [PubMed] [Google Scholar]
- 25.Geiger R, Miska W. Human tissue kallikrein. Methods Enzymol. 1988;163:102–115. doi: 10.1016/0076-6879(88)63012-6. [DOI] [PubMed] [Google Scholar]
- 26.Gennaro R, Dewald B, Horisberger U, Gubler HU, Baggiolini M. A novel type of cytoplasmic granule in bovine neutrophils. J Cell Biol. 1983;96:1651–1661. doi: 10.1083/jcb.96.6.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grange-Griebenow E, Flad HD, Ernst M. Heterogeneity of human peripheral blood monocyte subsets. J Leukoc Biol. 2001;69:11–20. [PubMed] [Google Scholar]
- 28.Greenbaum LM, Kim KS. The kinin-forming and kininase activities of rabbit polymorphonuclear leukocytes. Br J Pharmacol. 1967;29:238–247. doi: 10.1111/j.1476-5381.1967.tb01956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hume DA, Ross IL, Himes SR, Sasmono RT, Wells CA, Ravasi T. The mononuclear phagocyte system revisited. J Leukoc Biol. 2002;72:621–627. [PubMed] [Google Scholar]
- 30.Lansing MW, Ahmed A, Cortes A, Sielczak MW, Wanner A, Abraham WM. Oxygen radicals contribute to antigen-induced airway hyperresponsiveness in conscious sheep. Am Rev Respir Dis. 1993;147:321–326. doi: 10.1164/ajrccm/147.2.321. [DOI] [PubMed] [Google Scholar]
- 31.Leoni P, Dean RT. Mechanisms of lysosomal enzyme secretion by uman monocytes. Biochim Biophys Acta. 1983;762:378–389. doi: 10.1016/0167-4889(83)90002-2. [DOI] [PubMed] [Google Scholar]
- 32.McCarthy K, Musson RA, Henson PM. Protein synthesis-dependent nd protein synthesis-independent secretion of lysosomal hydrolases rom rabbit and human macrophages. J Reticuloendothel Soc. 1982;31:131–144. [PubMed] [Google Scholar]
- 33.Naicker S, Naidoo S, Ramsaroop M, Moodley D, Bhoola K. Tissue allikrein and kinins in renal disease. Immunopharmacology. 1999;44:183–192. doi: 10.1016/s0162-3109(99)00089-2. [DOI] [PubMed] [Google Scholar]
- 34.Naidoo S, Ramsaroop R, Bhoola R, Bhoola KD. The evaluation of issue kallikrein in helicobacter pylori-associated gastric ulcer disease. Immunopharmacology. 1997;36:263–269. doi: 10.1016/s0162-3109(97)00031-3. [DOI] [PubMed] [Google Scholar]
- 35.O’Riordan TG, Otero R, Mao YM, Lauredo I, Abraham WM. Elastase contributes to antigen-induced mucociliary dysfunction in ovine airways. Am J Respir Crit Care Med. 1997;155:1522–1528. doi: 10.1164/ajrccm.155.5.9154852. [DOI] [PubMed] [Google Scholar]
- 36.Plesner A. Increasing the yield of human mononuclear cells and low serum conditions for in vitro generation of macrophages with M-CSF. J Immunol Methods. 2003;279:287–295. doi: 10.1016/s0022-1759(03)00234-5. [DOI] [PubMed] [Google Scholar]
- 37.Podlich D, Kemme M. Expression of human tissue kallikrein in differentiated HL-60 cells. Biol Chem. 2000;381:629–632. doi: 10.1515/BC.2000.082. [DOI] [PubMed] [Google Scholar]
- 38.Proud D, Kaplan AP. Kinin formation: mechanisms and role in inflammatory disorders. Annu Rev Immunol. 1988;6:49–83. doi: 10.1146/annurev.iy.06.040188.000405. [DOI] [PubMed] [Google Scholar]
- 39.Raidoo DM, Ramsaroop R, Naidoo S, Muller-Esterl W, Bhoola KD. Kinin receptors in human vascular tissue: their role in atheromatous disease. Immunopharmacology. 1997;36:153–160. doi: 10.1016/s0162-3109(97)00015-5. [DOI] [PubMed] [Google Scholar]
- 40.Scuri M, Abraham WM, Botvinnikova Y, Forteza R. Hyaluronic acid blocks porcine pancreatic elastase (PPE)-induced bronchoconstriction in sheep. Am J Respir Crit Care Med. 2001;164:1855–1859. doi: 10.1164/ajrccm.164.10.2011115. [DOI] [PubMed] [Google Scholar]
- 41.Scuri M, Botvinnikova Y, Lauredo IT, Abraham WM. Recombinant α1-proteinase inhibitor blocks antigen- and mediator-induced airway responses in sheep. J Appl Physiol. 2002;93:1900–1906. doi: 10.1152/japplphysiol.00400.2002. [DOI] [PubMed] [Google Scholar]
- 42.Scuri M, Forteza R, Lauredo I, Sabater JR, Botvinnikova Y, Allegra L, Abraham WM. Inhaled porcine pancreatic elastase causes bron-choconstriction via a bradykinin-mediated mechanism. J Appl Physiol. 2000;89:1397–1402. doi: 10.1152/jappl.2000.89.4.1397. [DOI] [PubMed] [Google Scholar]
- 43.Smith WB, Holt PG. Professional antigen-presenting cells. In: Busse WW, Holgate ST, editors. Asthma and Rhinitis. Oxford: Blackwell Science; 2003. [Google Scholar]
- 44.Stadnicki A, Chao J, Stadnicka I, Val Tol E, Lin KF, Li F, Sartor RB, Colman RW. Localization and secretion of tissue kallikrein in peptidoglycan-induced enterocolitis in Lewis rats. Am J Physiol Gastrointest Liver Physiol. 1998;275:G854–G861. doi: 10.1152/ajpgi.1998.275.4.G854. [DOI] [PubMed] [Google Scholar]
- 45.Sundler R. Lysosomal and cytosolic pH as regulators of exocytosis in mouse macrophages. Acta Physiol Scand. 1997;161:553–556. doi: 10.1046/j.1365-201X.1997.00262.x. [DOI] [PubMed] [Google Scholar]
- 46.Szelke M, Evans DM, Jones DM, Fawcett L, Ashworth D, Olsson H, Featherstone RL, Church MK. Synthetic inhibitors of tissue kallikrein: effects in vivo in a model of allergic inflammation. Braz J Med Biol Res. 1994;27:1943–1947. [PubMed] [Google Scholar]
- 47.Tadjkarimi S, O’Neil GS, Luu TN, Allen SP, Schyns CJ, Chester AH, Yacoub MH. Comparison of cyclic GMP in human internal mammary artery and saphenous vein: implications for coronary artery bypass graft patency. Cardiovasc Res. 1992;26:297–300. doi: 10.1093/cvr/26.3.297. [DOI] [PubMed] [Google Scholar]
- 48.Tapper H. The secretion of preformed granules by macrophages and neutrophils. J Leukoc Biol. 1996;59:613–622. doi: 10.1002/jlb.59.5.613. [DOI] [PubMed] [Google Scholar]
- 49.Tapper H, Sundler R. Glucan receptor and zymosan-induced lysosomal enzyme secretion in macrophages. Biochem J. 1995;306:829–835. doi: 10.1042/bj3060829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tapper H, Sundler R. Protein kinase C and intracellular pH regulate zymosan-induced lysosomal enzyme secretion in macrophages. J Leukoc Biol. 1995;58:485–494. doi: 10.1002/jlb.58.4.485. [DOI] [PubMed] [Google Scholar]
- 51.Williams RJ, Henderson LM, Naidoo Y, Cassim B, Elson CJ, Bhoola KD. Immunocytochemical analysis of tissue kallikrein and the kinin moiety in rheumatoid synovial fluid neutrophils. Br J Rheumatol. 1997;36:420–425. doi: 10.1093/rheumatology/36.4.420. [DOI] [PubMed] [Google Scholar]
- 52.Wright DG, Bralove DA, Gallin JI. The differential mobilization of human neutrophil granules. Effects of phorbol myristate acetate and ionophore. Am J Pathol. 1977;87:237–284. [PMC free article] [PubMed] [Google Scholar]
- 53.Yurewicz EC, Zimmerman M. Cytochalasin B-dependent release of azurophil granule enzymes from human polymorphonuclear leukocytes. Inflammation. 1977;2:259–264. doi: 10.1007/BF00921005. [DOI] [PubMed] [Google Scholar]