Abstract
Human motor control systems orchestrate complex scale-invariant patterns of activity over a wide range of time scales (minutes to hours). The neural mechanisms underlying scale-invariance are unknown in humans. In rats, the master circadian pacemaker [suprachiasmatic nucleus (SCN)] is crucially involved in scale-invariant activity fluctuations over multiple time scales from minutes to 24 h. Aging and Alzheimer's disease (AD) are associated with progressive dysfunction of the SCN. Thus, if the SCN is responsible for the scale-invariant activity fluctuations in humans, we predict disturbances of scale-invariant activity fluctuations in elderly humans and even more pronounced disturbances in elderly humans with AD. To test these hypotheses, we studied spontaneous daytime activity patterns in 13 young adults (mean ± SD: 25.5 ± 6.1 y); 13 elderly people with early-stage AD (68.5 ± 6.1 y) matched with 13 elderly controls (68.6 ± 6.1 y); and 14 very old people with late-stage AD (83.9 ± 6.7 y) matched with 12 very old controls (80.8 ± 8.6 y). In young adults, activity exhibited robust scale-invariant correlations across all tested time scales (minutes to 8 h). The scale-invariant correlations at 1.5–8 h declined with age (P = 0.01) and were significantly reduced in the elderly (P = 0.04) and very old controls (P = 0.02). Remarkably, an age-independent AD effect further reduced the scale-invariant correlations at 1.5–8 h (P = 0.04), leading to the greatest reduction of the scale-invariant correlations in very old people with late-stage AD—resembling closely the loss of correlations at large time scales in SCN-lesioned animals. Thus, aging and AD significantly attenuate the scale invariance of activity fluctuations over multiple time scales. This attenuation may reflect functional changes of the SCN.
Keywords: actigraphy, fractal, motor control, sleep–wake rhythm, suprachiasmatic nucleus
Human motor activity displays complex temporal fluctuations characterized by scale-invariant/fractal patterns, i.e., the temporal structure and properties of fluctuations remain similar over a wide range of time scales (1, 2). The scale-invariant patterns are independent from scheduled and environmental influences and identical in humans and rats, suggesting a common intrinsic activity control mechanism in the 2 species (2). Although the underlying neural mechanisms are unknown in humans, lesion studies in rats have shown that the circadian pacemaker [suprachiasmatic nucleus (SCN)] is crucially involved in scale-invariance of activity fluctuations at multiple time scales (minutes to 24 h) with strongest influences at large time scales (greater than ≈4 h) (2). This multiscale influence of the SCN on activity in rats supports the hypothesis of a neuronal network for scale-invariant activity regulation, and suggests that the SCN is a major node in this network (2). Moreover, these data suggest that an index of scale-invariant activity regulation could potentially be used as a noninvasive marker of SCN function.
Neuroanatomical changes in the human SCN, including reduced gene expression of neurotransmitters vasopressin and vasoactive intestinal peptide, have been demonstrated with aging and are even more pronounced in patients with Alzheimer's disease (AD) (3–7). These changes in SCN function are believed to be crucially involved in the disturbances of sleep and daily activity that are frequently observed in the elderly and in AD patients (8–13). We hypothesize that the SCN in humans, as in rats, is crucially involved in the scale-invariant regulation of activity fluctuations. This hypothesis predicts progressive disturbances in the scale-invariance of activity fluctuations in the elderly and in AD patients.
To test the hypothesis, we studied 5 groups of subjects: (i) 13 young adult subjects (mean ± SD: 25.5 ± 6.1 y); (ii) 13 elderly subjects with early-stage AD (68.5 ± 6.1 y); (ii) 13 elderly control subjects (68.6 ± 6.1 y) who were matched for age and living condition with the elderly early-stage AD subjects; (iv) 14 very old subjects with late-stage AD (83.9 ± 6.7 y); and (v) 12 very old control subjects (80.8 ± 8.6 y), who were matched for age and living condition with the very old subjects with late-stage AD. Spontaneous activity data were measured by using a wristwatch-sized activity recorder (Actiwatch; MiniMitter) (14). Data were collected continuously for at least 1 week while subjects maintained their habitual sleep/wake schedules (15). Detrended fluctuation analysis (DFA) was performed to quantify the scale-invariant patterns in activity fluctuations (16). The DFA enables a reliable detection of intrinsic scale-invariance in nonstationary physiological signals including motor activity (1), gait (17), respiration (18), and heart rate fluctuations (16). A major advantage of DFA over conventional analyses (e.g., Hurst analysis and power spectral analysis) is that DFA avoids the spurious detection of scale-invariance caused by an artifact of extrinsic polynomial trends (19). Briefly, DFA derives the amplitude of activity fluctuations, F(n), at different time scales n. Scale invariance is characterized by a power-law form of the fluctuation function, F(n) ≈ nα, with the scaling exponent α indicating correlations in fluctuations (i.e., α = 0.5 indicates white noise with no correlation, α > 0.5 indicates positive correlations; and α = 1 indicates a fluctuation pattern that is associated with the most complex physical and physiological systems) (see Data Collection and Methods).
Results
Daytime Activity Fluctuations of Young Control Subjects Exhibit Scale-Invariant Patterns from Minutes to 8 h.
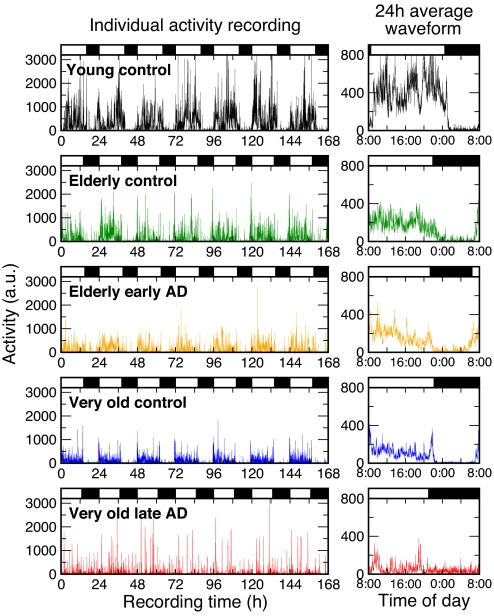
Fig. 1 shows activity recordings and average 24-h profiles for representative individuals from each group. Day–night rhythms and within-day activity fluctuations are clearly visible. We found that activity fluctuations of young controls during the scheduled daytime wake periods are scale-invariant across the entire range of investigated time scales: from minutes up to 8 h (Fig. 2). This scale-invariance is demonstrated as a power-law form of the fluctuation function, F(n) ≈ nα, which can be recognized as a straight line when log–log plotting function F(n) against time scale n (Fig. 2A and B). Young controls had a scaling exponent of α = 0.91 ± 0.02 (mean ± SE) that is much >0.5 and close to 1, indicating strong scale-invariant correlations in activity fluctuations.
Fig. 1.
Seven-day continuous activity recordings and average 24-h waveforms of 5 representative individuals: a young adult, an elderly control, an elderly with early-stage AD, a very old control, and a very old late-stage AD patient. (Left) Shown are the individuals' continuous activity recordings. (Right) Shown are the same individuals' activity recordings averaged over 24 h. The individual data and average waveform of activity is expressed in arbitrary units. Black bars indicate the individual sleep episodes.
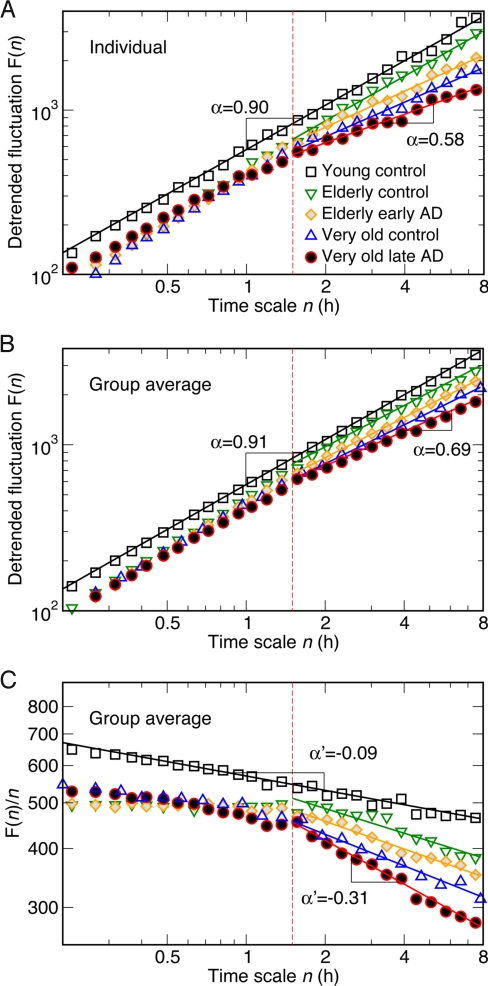
Fig. 2.
Altered scale-invariant correlations of activity fluctuations in elderly and AD subjects. Detrended fluctuation functions were obtained from activity data during the daytime between 11 a.m. and 7 p.m. (A) Representative individuals from each group. (B) Group averages. Data are shown on log–log plots. On the abscissa, n represents the time scale in hours. The detrended fluctuation functions F(n) are vertically shifted for better visualization of differences between groups. F(n) in young controls (squares) exhibits a simple power-law form over the whole range from minutes to 8 h, indicated by a straight line in the log–log plot. In contrast, there is clearly a “break point” in the log–log relationship at a time scale of ≈1.5 h in the elderly controls and in the AD subjects (see dotted vertical lines in each plot, with different scaling behaviors below and above this time scale). (C) The break point can be seen more clearly where group average F(n) divided by time scale n was plotted. The exponent obtained from the power-law fitting of F(n)/n is α′ = α − 1.
Effects of Aging and AD.
The 4 older groups (i.e., elderly early-stage AD with matched elderly control, very old late-stage AD with matched very old control) showed a significantly altered pattern of the scale-invariance compared with young controls (Fig. 2). The difference is best exemplified by different scaling behaviors in 2 time scale regions separated by a break point at ≈1.5 h (Fig. 2) (see Data Collection and Methods for details on the estimation of the break point). The scale-invariant correlations were much weaker at time scales above the break point (Region II: large time scales from 1.5 to 8 h) than below the break point (Region I: small time scales <1.5 h) for the 4 groups (P < 0.0001). The break point and the alteration of the scaling behavior can be better visualized in the derived plots in Fig. 2C, which represented F(n)/n with the slope α′ = α − 1.
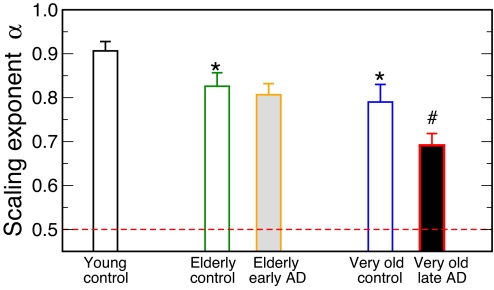
The most significant difference between the 4 older groups and the young control group was the reduced scale-invariant correlations of activity fluctuations in the older groups in Region II (time scales from 1.5 to 8 h) as characterized by a smaller value of the scaling exponent (Fig. 3). In the elderly control and the very old control groups, the scaling exponents in Region II were significantly smaller than that of the young control group (young control: α = 0.91 ± 0.02; elderly control: α = 0.83 ± 0.03, P = 0.04; very old control: α = 0.79 ± 0.04, P = 0.02) (Fig. 3). A regression analysis of data in young, elderly, and very old control subjects confirmed a significant decline in the scaling exponent in Region II with age (slope = −0.0019; r = −0.40; P = 0.01). However, there was no significant difference between the very old controls and the elderly controls in the scaling exponent, possibly related to the relatively small difference in average age (about 12 y) between the 2 groups. The early-stage AD subjects showed a similar reduction in scale-invariant correlations in Region II (α = 0.80 ± 0.03) as the elderly control group, and no significant difference was observed in these 2 age- and living condition-matched elderly groups. The scale-invariant correlations were further reduced in very old late-stage AD subjects, as indicated by the scaling exponent (α = 0.69 ± 0.03) that was significantly smaller than the value of the age- and living condition-matched very old controls (P = 0.04) (Figs. 2 and 3). The smaller exponents of the 4 older groups in Region II indicates a scaling behavior closer to white noise [α = 0.5] at these time scales.
Fig. 3.
Scaling exponent α in all groups obtained from the detrended fluctuation analysis. Because there was no break point in the log–log plot in young control subjects, α was obtained in this group by fitting the detrended fluctuation function F(n) at time scales between 5 min and 8 h. Because of the break-point in the other groups, α was obtained between 90 min and 8 h. α = 0.50 indicates “white noise” or the scaling exponent in activity of experimental animals in the absence of the SCN influence (dashed line) (2). Error bars indicate standard error of the mean. Compared to young controls, the elderly control and very old control subjects had smaller α values, as indicated by *, P < 0.05. Elderly control and very old control subjects showed no significant difference. Early-stage AD subjects and age- and living condition-matched elderly controls showed no significant difference. Late-stage AD subjects have significantly smaller α than the age- and living condition-matched very old controls, as indicated by # (P < 0.05).
There were no significant differences in the scaling exponent in Region I (time scales <1.5 h) among the 4 older groups. Also, the mean values of the scaling exponent in Region I in very old controls (α = 0.96 ± 0.03) and very old late-stage AD subjects (α = 0.94 ± 0.03) were not significantly different from that of young controls. The only notable difference in Region I was that elderly controls and elderly early-stage AD subjects showed slightly stronger scale-invariant activity correlations than young controls, as indicated by a larger value of the scaling exponent (young control: α = 0.91 ± 0.02; elderly control: α = 1.00 ± 0.02, P = 0.003; elderly early-stage AD: α = 1.00 ± 0.02, P = 0.006).
Discussion
We previously reported that activity fluctuations in young adults exhibit a scale-invariant pattern at time scales from minutes to ≈2 h and that the pattern is independent of environmental influences and scheduled events such as sleep/wake cycles (1, 20). In this study, we found that young controls maintain this scale-invariance of daytime activity fluctuations across a wider range of time scales up to 8 h. The exponent that characterizes this scale-invariance (α = 0.91 ± 0.02) is remarkably consistent with the scaling exponent we found in our previous study (α = 0.92 ± 0.01) (1), suggesting the existence of a robust underlying control mechanism of activity regulation in young adults. Moreover, the current study showed that this underlying control mechanism changes significantly in elderly adults and AD subjects, as indicated by reduced scaling exponents, particularly in the time scale region from 1.5 to 8 h (Fig. 2). The reduced correlations at large time scales were most pronounced in the late-stage AD group, probably due to the summation of aging and AD effects. These results suggest that activity control at large time scales is disturbed with aging and further degraded in late-stage AD.
Involvement of the Circadian Pacemaker in Scale-Invariant Activity Control.
As in humans, activity fluctuations in rats display scale-invariant correlations with a scaling exponent (α ≈ 0.90) that is similar to the exponent in young people found in the current study and previous studies (1, 2). Lesioning the SCN in rats led to a breakdown of the scale-invariant patterns at large time scales (greater than ≈4 h) such that activity fluctuations in the SCN-lesioned rats resemble white noise with a scaling exponent α = 0.5 (2). These results clearly indicate that, in rats, the SCN is a major activity control node that not only generates circadian rhythms but also contributes to scale-invariant activity regulation across a wide range of time scales. These data therefore suggest that the α exponent of activity regulation could potentially be used as a noninvasive marker of SCN function.
In humans, the SCN undergoes anatomical and physiological changes with aging and AD (3–7, 21, 22). It is likely that the changes in SCN function contribute to the 24-h activity rhythm disturbances and sleep disturbances (Fig. 1) (8–13). Here, we found that the scale-invariant correlations in activity fluctuations at large time scales are reduced in the elderly and very old controls and in AD subjects, becoming more similar to white noise, as occurs after lesioning the SCN in experimental animals (2). At smaller time scales, the increase of the scaling exponent in the elderly controls and early-stage AD subjects also resembles the changes of scale-invariance after lesioning the SCN (2). These similarities between results in current human study and the previous animal study support the hypothesis that, as in rats, the SCN in humans also imparts the scale-invariant activity control across a wide range of time scales.
There are certain differences between the present findings in humans and findings in animal studies (2). First, the scaling exponent α at large time scales was close to but still >0.5 in the elderly and AD subjects even for the most affected AD subjects (Fig. 3), indicating a certain degree of preservation of scale-invariant correlations in activity fluctuations of humans with aging and in AD. In contrast, α was ≈0.5 for SCN-lesioned animals, indicating complete breakdown of scale-invariance (2). This difference is not surprising because in the experimental animals, the SCN was completely ablated, whereas the physiological and anatomical changes in the human SCN with aging and AD may only lead to an attenuated functionality rather than complete dysfunction. Future studies in animals with incomplete SCN lesions are required to determine how a partially functional SCN contributes to scale-invariant activity control. Second, the break point in the log–log plot of activity fluctuations against time scale that separates 2 regions of different scaling behaviors in the elderly and very old controls and in both AD groups was positioned at ≈1.5 h (Fig. 2), whereas the breakdown of scale-invariant correlations was observed above ≈4 h in the SCN-lesioned rats (2). This difference might be caused by differences in activity control mechanisms between these species as suggested by: (i) the average SCN neuronal activity has opposing influences on activity levels in the 2 species (23–28); and (ii) intact rats exhibit well-pronounced ultradian rhythms in activity that are not as obvious or systematic in human (19).
Influences of Aging and AD.
Generally, aging is associated with decreased mean activity levels and increased naps during the daytime [supporting information (SI) Text], and age and AD are associated with sleep disturbances and increased activity during the night, leading to blunted day/night rhythms of activity (8, 29). In addition to these conventional measures of motor activity, this study showed that the elderly and very old controls displayed alterations in the temporal organization of activity fluctuations as characterized by reduced scale-invariant correlations at multiple time scales from ≈1.5 to 8 h. More interestingly, in the same range of large time scales (≈1.5–8 h), the late-stage AD subjects had a larger reduction in scale-invariant correlations of activity fluctuations than the other older groups, including the very old control group that had age, living condition, and mean activity level matched (Fig. 3, and Fig. S1). Furthermore, we found that these differences in scale-invariant correlations between the groups were unlikely to be caused by differences in the amount of daytime naps (inactive bouts) or differences in mean activity levels (see details in SI Text and Fig. S2). These findings have 3 implications: (i) aging affects scale-invariant activity correlations; (ii) late-stage AD has a separate influence on scale-invariant activity fluctuations that is independent of aging effects; and (iii) scale-invariant correlations provide additional information of activity control that is complementary to traditional measures of activity patterns. In this study, we did not observe the effect of early-stage AD on the scaling exponent when comparing with the age- and living condition-matched elderly control group. Studies with larger populations are needed to determine the exact relationship between the stage of AD and the degree of changes in scale-invariant correlations of activity fluctuations.
It is notable that the break point in the log–log plot of activity fluctuations against time scale that separates 2 regions of different scaling behaviors in the elderly controls, very old controls, and in both AD groups occurred consistently at ≈1.5 h (Fig. 2). Thus, aging and AD appear to affect the same neuronal controls node(s) or their feedback interactions that influence scale-invariant activity control specifically at larger time scales (e.g., the SCN). In contrast, aging and AD appear to have little influence on the putative control nodes that influence scale-invariant activity control at small time scales. In addition to the SCN, there are numerous candidates that may be also involved in the scale-invariant activity control including: (i) subparaventircular nucleus (SPVN), dorsomedial hypothalamic nucleus (DMH) and lateral hypothalamic area (LHA) that together help to convey the SCN influences on locomotor activity (30); (ii) humoral and hormonal signals such as glucose, leptin, and ghrelin that play important roles in regulating behaviors such as food-seeking behavior and thus locomotor activity (31); and (iii) the spinal cord that may be a major control node of the neural network responsible for the scale-invariant patterns at small time scales. Revealing the underlying cause of the break point in scale-invariance at the specific time scales requires a better understanding of whole neuronal network with interacting control nodes that are responsible for the full range of scale-invariant activity fluctuations.
Significance of the Alteration in Scale-Invariant Activity Pattern.
In physical systems, scale-invariant patterns indicate a complex temporal organization in fluctuations that requires an integrated network of control nodes with feedback interactions (32, 33). Similar scale-invariant patterns have been observed in many physiological fluctuations, including motor activity (1), gait (17), respiration (18), and heart rate fluctuations (16). Scale-invariant control may be beneficial for the adaptability of physiological systems in an ever-changing environment, as evidenced by alterations of the scale-invariant correlations in heart rate fluctuations under pathological conditions and their predictive value for survival (34, 35).
In this study, we report that, in humans, the robust scale-invariant correlation in activity fluctuations is perturbed with aging and with AD. The reduced scale-invariant activity correlation is not simply related to the altered average daytime level of activity in the elderly and AD subjects. Scale-invariant correlations reflect a dynamic property in the fluctuations that is not affected by differences in the mean or the standard deviation. For example, scale-invariant correlations remain unchanged under different study conditions despite large changes in absolute level of activity (1). Additionally, this study showed that scale-invariant correlations were more reduced in the late-stage AD than in the age- and living condition-matched nondemented controls, whereas the 2 groups had similar mean activity levels (see SI Text).
We hypothesize that the changes in scale-invariance of activity fluctuations with aging and AD are caused by functional disturbances of the SCN. Further studies are required to determine whether the scale-invariance of activity fluctuations has predictive or diagnostic value for AD or other neurodegenerative diseases such as Parkinson's disease, in which the ability for long term monitoring of activity and tremor has recently become feasible (36, 37). A further testable prediction of the SCN-related hypothesis is that therapeutic strategies aimed to improve SCN functioning, including bright daytime light and nighttime melatonin (12), may also change the pattern of scale-invariance toward that of healthy controls. Scale-invariance metrics might turn out to be more sensitive measures of SCN control of activity rhythms than any presently available phase and amplitude measures of activity.
Data Collection and Methods
Subjects.
We studied: (i) 13 young adult subjects (8 males and 5 females; mean ± SD: 25.5 ± 6.1 y); (ii) 13 elderly control subjects (9 males and 4 females; 68.6 ± 6.1 y); (iii) 13 elderly subjects (9 males and 4 females; 68.5 ± 6.1 y) with early-stage AD who were carefully matched for age, gender, and living condition with the elderly control subjects; (iv) 12 very old control subjects (all females, 80.8 ± 8.6 y); and (v) 14 very old subjects (2 males and 12 females; 83.9 ± 6.7 y) with late-stage AD who were likewise carefully matched with the very old control subjects. National Institute of Neurological and Communications Disorders and Stroke–Alzheimer's Disease and Related Disorders Association criteria were used for the clinical diagnosis of AD stage (38). Data were collected while subjects maintained their habitual sleep/wake schedules for at least 1 week. The study was approved by the relevant institutional human subjects Internal Review Boards. All subjects provided written informed consent before participation except for the advanced AD subjects, where relatives signed informed consent.
Data Acquisition.
Motor activity levels were continuously assessed by using an Actiwatch (Mini Mitter/Respironics) worn on the wrist of the nondominant hand. The value of changes in acceleration was sampled at 32 Hz and was integrated every 60 seconds (14).
Scale-Invariant Analysis.
To assess the scale-invariant patterns in activity data, detrended fluctuation analysis (DFA) was performed to calculate the correlations in activity fluctuations at different time scales. This method quantifies the detrended fluctuation function, F(n), of activity fluctuations at different time scales n (16). There are 4 steps in the DFA including: (i) removing the global mean and integrating the time series of an activity signal; (ii) dividing the integrated signal into nonoverlapping windows of the same size equal to a chosen time scale n; (iii) detrending the integrated signal in each window by using polynomial functions to obtain residuals; and (iv) calculating the root mean square of residuals in all windows as detrended fluctuation amplitude F(n). The same 4 steps are repeated for each different time scale n. To eliminate the effect of possible linear trends in original data, here we applied the second-order DFA, i.e., the second-order of polynomial functions were used to detrend data (19). A power-law form of the function indicates self-similarity (scale-invariance) in the fluctuations, yielding F(n) ≈ nα. The parameter α, called the scaling exponent, quantifies the correlation properties in the signal as follows: if α = 0.5, there is no correlation in the fluctuations (white noise); if α > 0.5, there are positive correlations, where large activity values are more likely to be followed by large activity values (and vice versa for small activity values). In a signal generated from a physical system, the exponent α = 1.0 indicates highest complexity in the systems (32, 39, 40). Similar α values (close to 1.0) have been observed in many physiological signals under normal conditions, indicating complex feedback interactions (2, 16, 17).
The sensitivity of the Actiwatch is 0.01 g (where g is the unit of acceleration, matching the earth's gravity), and zeros will be given when activity is below this sensitivity (14). Such threshold influence is equivalent to that of white noise in a signal, which is negligible when activity is relatively high during the scheduled wakefulness of daytime. However, during the night when activity level is usually absent or very low because of sleep, the signal-to-noise ratio is greatly reduced, and a minimum activity threshold effect can significantly influence the performance of the scale-invariant analysis (41). Thus, in this study, we focused on the scale-invariant activity patterns during the daytime. Because the duration and schedule of wakeful periods varied for different groups and different individuals, we standardized the analysis of the data collected during the daytime period from 11 a.m. to 7 p.m., which was at least 1 h after waking and at least 1 h before sleep episode for all days in all subjects.
For a signal with different scaling behaviors at small and large time scales, the break point that separates the 2 regions was estimated as the time scale, which corresponds to the maximum difference between the scaling exponents obtained below and above the time scale (42). Briefly, the procedure includes 2 steps. (i) For a chosen time scale n′, F(n) was fitted by using a power-law function at n < n′ [F(n) ≈ nα1] and at n > n′ [F(n) ≈ nα2], separately; (ii) The absolute difference between 2 exponents obtained from fitting was calculated, i.e., Δα = ∣α1 − α2∣. (iii) We repeated the steps (i and ii) for different time scale n′ from 10 min to 6 h; (iv) the n′ that corresponds to the maximum Δα was chosen as the break point. For the elderly and very old control groups and for both AD groups, the break point was ≈90 (±30) min. For consistency and simplification, we chose 90 min as the time scale of the break point for all individuals.
Statistical Analysis.
ANOVA was performed to evaluate group differences on scaling exponents. The aging effect was assessed by using 2 compatible approaches: (i) the comparisons between young control, elderly control, and very old control groups and (ii) a regression analysis performed on data of young control, elderly control, and very old control subjects using age as the independent variable. The effect of early-stage AD was determined from the comparison between elderly control and age-matched early-stage AD subjects, and the late-stage AD effect was determined from the comparison between very old control and age-matched late-stage AD subjects.
Supplementary Material
Acknowledgments.
Rixt Riemersma-van der Lek and Els Most (Netherlands Institute for Neuroscience) are acknowledged for help with actigraphic assessments. This work was supported by National Institutes of Health/National Heart, Lung, and Blood Institute Grants K24 HL076446 and R01 HL076409 (to S.A.S.) and R21 AT002713 (to F.A.J.L.S.) and Netherlands Organization for Scientific Research Vici Grant 453.07.001 (to E.J.W.V.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806087106/DCSupplemental.
References
- 1.Hu K, et al. Non-random fluctuations and multi-scale dynamics regulation of human activity. Physica A. 2004;337:307–318. doi: 10.1016/j.physa.2004.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu K, Scheer FA, Ivanov PC, Buijs RM, Shea SA. The suprachiasmatic nucleus functions beyond circadian rhythm generation. Neuroscience. 2007;149:508–517. doi: 10.1016/j.neuroscience.2007.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swaab DF, Fliers E, Partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985;342:37–44. doi: 10.1016/0006-8993(85)91350-2. [DOI] [PubMed] [Google Scholar]
- 4.Zhou JN, Hofman MA, Swaab DF. VIP neurons in the human SCN in relation to sex, age, and Alzheimer's disease. Neurobiol Aging. 1995;16:571–576. doi: 10.1016/0197-4580(95)00043-e. [DOI] [PubMed] [Google Scholar]
- 5.Liu RY, et al. Decreased vasopressin gene expression in the biological clock of Alzheimer disease patients with and without depression. J Neuropathol Exp Neurol. 2000;59:314–322. doi: 10.1093/jnen/59.4.314. [DOI] [PubMed] [Google Scholar]
- 6.Harper DG, et al. Dorsomedial SCN neuronal subpopulations subserve different functions in human dementia. Brain. 2008;131:1609–1617. doi: 10.1093/brain/awn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu YH, et al. Pineal clock gene oscillation is disturbed in Alzheimer's disease, due to functional disconnection from the “master clock”. FASEB J. 2006;20:1874–1876. doi: 10.1096/fj.05-4446fje. [DOI] [PubMed] [Google Scholar]
- 8.Huang YL, et al. Age-associated difference in circadian sleep-wake and rest-activity rhythms. Physiol Behav. 2002;76:597–603. doi: 10.1016/s0031-9384(02)00733-3. [DOI] [PubMed] [Google Scholar]
- 9.Scherder EJ, Van Someren EJ, Swaab DF. Transcutaneous electrical nerve stimulation (TENS) improves the rest-activity rhythm in midstage Alzheimer's disease. Behav Brain Res. 1999;101:105–107. doi: 10.1016/s0166-4328(98)00150-8. [DOI] [PubMed] [Google Scholar]
- 10.Hatfield CF, Herbert J, Van Someren EJ, Hodges JR, Hastings MH. Disrupted daily activity/rest cycles in relation to daily cortisol rhythms of home-dwelling patients with early Alzheimer's dementia. Brain. 2004;127:1061–1074. doi: 10.1093/brain/awh129. [DOI] [PubMed] [Google Scholar]
- 11.Carvalho-Bos SS, Riemersma-van der Lek RF, Waterhouse J, Reilly T, Van Someren EJ. Strong association of the rest-activity rhythm with well-being in demented elderly women. Am J Geriatr Psychiatry. 2007;15:92–100. doi: 10.1097/01.JGP.0000236584.03432.dc. [DOI] [PubMed] [Google Scholar]
- 12.Riemersma-van der Lek RF, et al. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: A randomized controlled trial. J Am Med Assoc. 2008;299:2642–2655. doi: 10.1001/jama.299.22.2642. [DOI] [PubMed] [Google Scholar]
- 13.Tate B, et al. Disruption of circadian regulation by brain grafts that overexpress Alzheimer beta/A4 amyloid. Proc Natl Acad Sci USA. 1992;89:7090–7094. doi: 10.1073/pnas.89.15.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jean-Louis G, et al. Sleep estimation from wrist activity in patients with major depression. Physiol Behav. 2000;70:49–53. doi: 10.1016/s0031-9384(00)00228-6. [DOI] [PubMed] [Google Scholar]
- 15.Van Someren EJ. Improving actigraphic sleep estimates in insomnia and dementia: How many nights? J Sleep Res. 2007;16:269–275. doi: 10.1111/j.1365-2869.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- 16.Peng CK, et al. Fractal mechanisms and heart rate dynamics. Long-range correlations and their breakdown with disease. J Electrocardiol. 1995;28(Suppl):59–65. doi: 10.1016/s0022-0736(95)80017-4. [DOI] [PubMed] [Google Scholar]
- 17.Hausdorff JM, et al. Fractal dynamics of human gait: stability of long-range correlations in stride interval fluctuations. J Appl Physiol. 1996;80:1448–1457. doi: 10.1152/jappl.1996.80.5.1448. [DOI] [PubMed] [Google Scholar]
- 18.Peng CK, et al. Quantifying fractal dynamics of human respiration: Age and gender effects. Ann Biomed Eng. 2002;30:683–692. doi: 10.1114/1.1481053. [DOI] [PubMed] [Google Scholar]
- 19.Hu K, Ivanov PC, Chen Z, Carpena P, Stanley HE. Effect of trends on detrended fluctuation analysis. Phys Rev E. 2001;64 doi: 10.1103/PhysRevE.64.011114. 011114. [DOI] [PubMed] [Google Scholar]
- 20.Ivanov PC, Hu K, Hilton MF, Shea SA, Stanley HE. Endogenous circadian rhythm in human motor activity uncoupled from circadian influences on cardiac dynamics. Proc Natl Acad Sci USA. 2007;104:20702–20707. doi: 10.1073/pnas.0709957104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofman MA, Swaab DF. Alterations in circadian rhythmicity of the vasopressin-producing neurons of the human suprachiasmatic nucleus (SCN) with aging. Brain Res. 1994;651:134–142. doi: 10.1016/0006-8993(94)90689-0. [DOI] [PubMed] [Google Scholar]
- 22.Wu YH, Zhou JN, van Heerikhuize J, Jockers R, Swaab DF. Decreased MT1 melatonin receptor expression in the suprachiasmatic nucleus in aging and Alzheimer's disease. Neurobiol Aging. 2007;28:1239–1247. doi: 10.1016/j.neurobiolaging.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Shibata S, Oomura Y, Kita H, Hattori K. Circadian rhythmic changes of neuronal activity in the suprachiasmatic nucleus of the rat hypothalamic slice. Brain Res. 1982;247:154–158. doi: 10.1016/0006-8993(82)91041-1. [DOI] [PubMed] [Google Scholar]
- 24.Groos G, Hendriks J. Circadian rhythms in electrical discharge of rat suprachiasmatic neurones recorded in vitro. Neurosci Lett. 1982;34:283–288. doi: 10.1016/0304-3940(82)90189-6. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz WJ, Reppert SM, Eagan SM, Moore-Ede MC. In vivo metabolic activity of the suprachiasmatic nuclei: A comparative study. Brain Res. 1983;274:184–187. doi: 10.1016/0006-8993(83)90538-3. [DOI] [PubMed] [Google Scholar]
- 26.Sato T, Kawamura H. Circadian rhythms in multiple unit activity inside and outside the suprachiasmatic nucleus in the diurnal chipmunk (Eutamias sibiricus) Neurosci Res. 1984;1:45–52. doi: 10.1016/0168-0102(84)90029-4. [DOI] [PubMed] [Google Scholar]
- 27.Meijer JH, Schaap J, Watanabe K, Albus H. Multiunit activity recordings in the suprachiasmatic nuclei: In vivo versus in vitro models. Brain Res. 1997;753:322–327. doi: 10.1016/s0006-8993(97)00150-9. [DOI] [PubMed] [Google Scholar]
- 28.Scheer FA, Kalsbeek A, Buijs RM. Cardiovascular control by the suprachiasmatic nucleus: Neural and neuroendocrine mechanisms in human and rat. Biol Chem. 2003;384:697–709. doi: 10.1515/BC.2003.078. [DOI] [PubMed] [Google Scholar]
- 29.Satlin A, et al. Circadian locomotor activity rhythms in Alzheimer's disease. Neuropsychopharmacology. 1991;5:115–126. [PubMed] [Google Scholar]
- 30.Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci. 2006;9:398–407. doi: 10.1038/nn1651. [DOI] [PubMed] [Google Scholar]
- 31.Buijs RM, Kalsbeek A. Hypothalamic integration of central and peripheral clocks. Nat Rev Neurosci. 2001;2:521–526. doi: 10.1038/35081582. [DOI] [PubMed] [Google Scholar]
- 32.Stanley HE. Introduction to Phase Transitions and Critical Phenomena. London: Oxford Univ Press; 1971. [Google Scholar]
- 33.Basu S, Foufoula-Georgiou E, Porte-Agel F. Synthetic turbulence, fractal interpolation, and large-eddy simulation. Phys Rev E. 2004;70 doi: 10.1103/PhysRevE.70.026310. 026310. [DOI] [PubMed] [Google Scholar]
- 34.Stanley HE, et al. Fractal landscapes in biological systems: Long-range correlations in DNA and interbeat heart intervals. Physica A. 1992;191:1–12. doi: 10.1016/0378-4371(92)90497-e. [DOI] [PubMed] [Google Scholar]
- 35.Goldberger AL, Amaral L, Hausdorff JM, Ivanov PCh. Fractal dynamics in physiology: Alterations with disease and aging. Proc Natl Acad Sci USA. 2002;99:2466–2472. doi: 10.1073/pnas.012579499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Someren EJ, et al. Ambulatory monitoring of tremor and other movements before and after thalamotomy: A new quantitative technique. J Neurol Sci. 1993;117:16–23. doi: 10.1016/0022-510x(93)90148-r. [DOI] [PubMed] [Google Scholar]
- 37.Van Someren EJ, et al. New actigraph for long-term tremor recording. Mov Disord. 2006;21:1136–1143. doi: 10.1002/mds.20900. [DOI] [PubMed] [Google Scholar]
- 38.McKhann G, et al. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 39.Kurths J, et al. Quantitative analysis of heart rate variability. Chaos. 1995;5:88–94. doi: 10.1063/1.166090. [DOI] [PubMed] [Google Scholar]
- 40.Shlesinger MF, West BJ. Random Fluctuations and Pattern Growth: Experiments and Models. Boston: Kluwer Academic; 1998. [Google Scholar]
- 41.Chen Z, Ivanov PC, Hu K, Stanley HE. Effect of nonstationarities on detrended fluctuation analysis. Phys Rev E. 2002;65 doi: 10.1103/PhysRevE.65.041107. 041107. [DOI] [PubMed] [Google Scholar]
- 42.Orr WC, Hoffman HJ, Hegge FW. The assessment of time-dependent changes in human performance. Chronobiologia. 1976;3:293–305. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.