Abstract
The SLP-76 (Src homology 2 domain-containing leukocyte protein of 76 kDa) adapter protein is expressed in T cells and myeloid cells, whereas its homologue BLNK (B cell linker protein) is expressed in B cells. SLP-76 and BLNK link immunoreceptor tyrosine-based activation motif-containing receptors to signaling molecules that include phospholipase C-γ, mitogen-activated protein kinases, and the GTPases Ras and Rho. SLP-76 plays a critical role in T cell receptor, FcɛRI and gpVI collagen receptor signaling, and participates in signaling via FcγR and killer cell inhibitory receptors. BLNK plays a critical role in B cell receptor signaling. We show that murine bone marrow-derived macrophages express both SLP-76 and BLNK. Selective ligation of FcγRI and FcγRII/III resulted in tyrosine phosphorylation of both SLP-76 and BLNK. SLP-76−/− bone marrow-derived macrophages display FcγR-mediated tyrosine phosphorylation of Syk, phospholipase C-γ2, and extracellular signal regulated kinases 1 and 2, and normal FcγR-dependent phagocytosis. These data suggest that both SLP-76 and BLNK are coupled to FcγR signaling in murine macrophages.
Keywords: monocytes, phagocytosis
Immunoreceptor tyrosine-based activation motif-containing receptors (1), which include the T cell receptor (TCR)-CD3 complex, the B cell receptor, Ig Fc receptors (FcR), the platelet gpVI collagen receptor (2), and killer-cell inhibitory receptors (3), all signal via sequential activation of src and syk family tyrosine kinases, phosphatases, and a host of adapter proteins that serve as molecular links to downstream signaling pathways (4).
SLP-76 [Src homology 2 (SH2) domain-containing leukocyte protein of 76 kDa] (5) is an adapter molecule crucial in TCR signaling. SLP-76 has numerous direct and indirect associations, including Grb2 (5), the Grb2-related adapter downstream of Shc (Gads) that links SLP-76 to LAT (linker for activation of T cells) (6), phospholipase C (PLC)-γ (7), Vav (8), and the Fyn binding protein (9), also known as SLP-76-associated protein of 130 kDa (SLAP-130) (10), the SH2 domain-containing phosphatase-1 (11), and Nck (12). PLC-γ activation leads to release of intracellular calcium and stimulation of calcium-dependent pathways. Grb2 and Sos recruit the Ras GTPase to activate the mitogen-activated kinase/extracellular signal regulated kinase (ERK) pathway (13). Vav is a guanine nucleotide exchange factor for the Rac-1 GTPase that controls the Jun amino-terminal kinase pathway (14). These molecular chains link SLP-76 to alterations in gene transcription such as induction of nuclear factor of activated T cells and IL-2 promoter activity (15). Nck provides a connection to the regulation of cytoskeletal actin polymerization (12).
SLP-76 is expressed in murine T cells, myeloid cells (Gr-1+ and Mac-1+ bone marrow cells) (16), and bone marrow-derived mast cells (BMMC) (17), as well as in human monocytic cell lines (5), where it is tyrosine-phosphorylated upon FcγRI crosslinking (18). T cell development in SLP-76−/− mice is arrested at the double-negative stage (19, 20), indicating a critical role for SLP-76 in the TCR-dependent thymocyte transition from double-negative to double-positive. SLP-76−/− mice display peritoneal hemorrhage and defective platelet activation via the gpVI collagen receptor (21). SLP-76−/− mice are resistant to IgE-mediated anaphylaxis, and their BMMC fail to degranulate and synthesize cytokines in response to FcɛRI crosslinking (17). FcγR signaling in SLP-76−/− macrophages has not been studied.
B cells express the SLP-76 homologue B cell linker protein (BLNK) (22), also known as SLP-65 (Src homology 2 domain-containing leukocyte protein of 65 kDa) (23). BLNK is tyrosine-phosphorylated after B cell receptor crosslinking. BLNK associates with PLC-γ1 and -2, Vav, Grb2, and Nck. Thus, it appears to function similarly to SLP-76 in T cells (23). Targeted disruption of BLNK in a chicken B cell line abolished PLC-γ2 phosphorylation, calcium flux, and Jun amino-terminal kinase activation after B cell receptor ligation (24). ERK phosphorylation was reduced, but detectable. Human BLNK is restricted to B cells; no expression was found in T or monocytic cell lines (22). Murine BLNK was found only in B cells and not in T cell lines or fibroblasts (23). Macrophage expression of BLNK has not been reported.
There are three types of FcγR, all of which can be expressed on murine macrophages. FcγRI and FcγRIII are activating receptors containing the immunoreceptor tyrosine-based activation motif-bearing FcR γ chain. In mice, FcγRII is an inhibitory receptor containing tyrosine-based inhibitory motifs (25). We show that both SLP-76 and BLNK are expressed in bone marrow-derived macrophages (BMM) and both are tyrosine-phosphorylated upon crosslinking of FcγRI or FcγRII/III in murine BMM. Tyrosine phosphorylation of total cytoplasmic proteins (Syk, PLC-γ2, ERK-1, and ERK-2) are detected in SLP-76−/− BMM after stimulation via FcγR. In addition, FcγR-mediated phagocytosis proceeds normally. These findings suggest that both SLP-76 and BLNK are coupled to FcγR signaling in murine macrophages.
Methods
Animals.
The derivation of the SLP-76-deficient mice has been described (19). All mice were housed under specific pathogen-free conditions; their use was conducted according to protocols approved by the Institutional Animal Care and Use Committee. Control mice used in these experiments were C57BL/6 × 129/Sv F1 or heterozygous SLP-76+/− littermate controls.
Antibodies.
Commercial reagents used were: goat anti-rat IgG (Cappel/ICN); biotin-conjugated rat anti-mouse CD11b mAb, purified or fluorescein-conjugated rat anti-mouse CD16/32 mAb 2.4G2, and streptavidin cytochrome c (PharMingen/Becton-Dickinson); rabbit anti-rat ERK-1 peptide (K-23), mouse anti-human phospho-ERK-1/2 peptide mAb (E-4), rabbit anti-human PLC-γ2 peptide (Q-20), and goat anti-human SLP-76 peptide (C-20) (Santa Cruz Biotechnology); mouse antiphosphotyrosine mAb 4G10 (Upstate Biotechnology, Lake Placid, NY); and purified mouse IgG2a myeloma UPC10 (Sigma). The BLNK antiserum is from rabbits hyperimmunized with a glutathione S-transferase fusion protein with human BLNK residues 4–205. The Syk antiserum is from rabbits hyperimmunized with a keyhole limpet hemocyanin conjugate of human Syk residues 294–312. The mouse anti-human SLP-76 Src homology 2 domain mAb H3 was generously provided by Paul Findell, FibroGen, San Francisco. Affinity-purified UPC-10 mAb was used as a source of monomeric IgG2a; analysis by nonreducing PAGE established the absence of Ig aggregates. F(ab′)2 fragments of the mAb 2.4G2 were prepared by pepsin digestion with the ImmunoPure F(ab′)2 preparation kit (Pierce) according to the manufacturer's instructions. Digested antibody was subjected to SDS/PAGE followed by silver staining to verify complete digestion.
BMM Culture.
BMM were obtained by standard methods (26). Briefly, bone marrow cells were aspirated from femurs and tibias of euthanized mice. Bone marrow cells were cultured overnight in RPMI medium (JRH Bioscience, Lenexa, KS) supplemented with Hepes, penicillin, streptomycin, l-glutamine, pyruvate, nonessential amino acids, and β-mercaptoethanol (all from GIBCO). L cell-conditioned medium was used at 20%. Nonadherent cells subsequently were transferred to fresh plates and cultured at 106 cells/ml. BMM were studied after a minimum of 5 days in culture.
Flow Cytometry.
BMM were harvested from plates by elution with cold PBS with 0.02% EDTA. Staining and flow cytometry were performed according to standard methods (26) by using a FACSCalibur flow cytometer and cellquest software (Becton–Dickinson).
Stimulation of BMM, Lysis, and Immunoprecipitation.
BMM were incubated in medium without L cell supernatant for 2–4 hr, then at 4°C for 30 min with the primary antibody (2.4G2 or UPC10) at 5 μg/ml [10 μg/ml for 2.4G2 F(ab′)2]. Plates were washed; secondary antibody [goat anti-rat IgG 10 μg/ml or F(ab′)2 goat anti-mouse IgG 10 μg/ml or F(ab′)2 goat anti-rat IgG 20 μg/ml] in medium at 37°C was added; plates then were placed in a 37°C incubator. At time zero or 10 min medium was removed, and cells were scraped from plates in buffer (50 mM Tris, pH 7.4/1 mM EDTA/1 mM EGTA/150 mM sodium chloride) with protease inhibitors (complete protease inhibitor tablet, Boehringer-Mannheim) and phosphatase inhibitors (10 mM sodium fluoride, 10 mM sodium pyrophosphate, 0.4 mM sodium orthovanadate) without detergent or glycerol. Cell pellets then were detergent-lysed (Triton X-100, Calbiochem, 1%) in buffer containing 30% glycerol with protease and phosphatase inhibitors; lysates were precleared with rabbit serum or purified rabbit gamma globulin bound to protein G Sepharose 4B (Amersham Pharmacia) and then incubated with specific immunoprecipitating antibodies bound to protein G Sepharose.
Western Blotting.
Immunoprecipitates were subjected to standard SDS/PAGE under reducing conditions. Electro-transfer and hybridization were carried out by using standard methods. Bound antibodies were detected with protein G-horseradish peroxidase conjugate (Bio-Rad), followed by enhanced chemiluminescence using substrate from Amersham Pharmacia (ECL) or Pierce (SuperSignal Ultra) according to the manufacturers' instructions.
Spleen Adherent Cells.
Spleen cells were dispersed and cultured at 5 × 106 cells/ml in medium as described for BMM, without L cell supernatant. After 4 days, plates were washed to remove nonadherent cells and used in phagocytosis experiments. Virtually all cells were able to bind and ingest IgG-sensitized sheep red blood cells (SRBC).
SRBC Binding and Phagocytosis Assays.
SRBC (PMC Microbiologicals, Richmond, BC, Canada) were labeled with 51Cr (New England Nuclear/DuPont) and coated with rabbit anti-SRBC IgG (Cappel/ICN), 1:800 dilution, by standard methods (26). Labeled SRBC were added in medium at 4°C to BMM in 24-well plates at 2 × 107/well. Plates were spun in a tabletop centrifuge, and SRBC were allowed to bind at 4°C for 15 min. For binding assays, wells were rinsed thoroughly with PBS at 4°C and inspected in an inverted microscope, then 0.5% SDS was added. Bound radioactivity was measured in a gamma counter. For phagocytosis assays, plates were placed in a 37°C, 5% CO2 incubator at time zero. At each time point, SRBC were aspirated, bound cells were lysed with distilled water, and wells were rinsed with PBS, then ingested radioactivity was measured after SDS lysis as described above.
Results
SLP-76 and BLNK Both Are Expressed in Murine BMM and Tyrosine-Phosphorylated After FcγR Crosslinking.
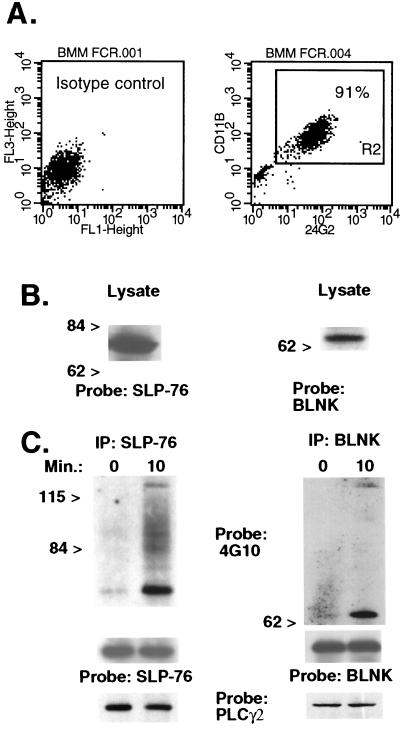
Because relatively few adherent macrophages can be recovered from mouse spleen (107 or less) we performed our biochemical studies in BMM. More than 90% of wild-type bone marrow cells cultured in L cell-conditioned medium express FcγRII/III (CD16/32) and Mac-1 (CD11b) (Fig. 1A) and have macrophage morphology (not shown). We used Western blot analysis to ascertain expression of SLP-76 and its homologue BLNK in these cultured BMM. Fig. 1B shows that goat anti-SLP-76 antiserum detects a 76-kDa band in BMM lysates. In addition, a rabbit anti-BLNK antiserum bound to a 65-kDa band. To examine whether SLP-76 and/or BLNK is tyrosine-phosphorylated in murine BMM after FcγR ligation, we incubated BMM with the anti-FcγRII/III mAb 2.4G2 followed by crosslinking with goat anti-rat IgG. Immunoprecipitates from lysates of stimulated cells were analyzed in Western blots. Fig. 1C shows that both SLP-76 and BLNK are tyrosine-phosphorylated in murine BMM after FcγR crosslinking. These results suggest that both SLP-76 and BLNK are linked to pathways of FcγR signaling in murine macrophages.
Figure 1.
SLP-76 and BLNK are expressed in BMM and tyrosine-phosphorylated after FcγR crosslinking. (A) BMM were incubated with labeled isotype control antibodies (Left) or FITC-conjugated 2.4G2 (anti-FcγRII/III) and biotinylated rat anti-mouse CD11b followed by streptavidin-cytochrome c (Right). The percentage of double-positive cells ranged from 90% to 95% in three separate experiments using different mice. (B) A Western blot of BMM lysates was probed with purified goat anti-SLP-76 or rabbit anti-BLNK antiserum. (C) BMM were exposed to 2.4G2 followed by goat anti-rat IgG for 0 or 10 min. Lysates were immunoprecipitated (IP) by using the anti-SLP-76 H3 murine mAb or rabbit anti-BLNK antiserum. A Western blot was probed initially with antiphosphotyrosine (4G10). The membrane was stripped and reprobed with goat anti-SLP76 or rabbit anti-BLNK to verify equal protein loading. In separate experiments, Western blots of SLP-76 and BLNK immunoprecipitates were hybridized with rabbit anti-human PLC-γ2. Results were identical in three separate experiments using different mice.
A tyrosine-phosphorylated protein band of approximately 120 kDa was found to coimmunoprecipitate with both SLP-76 and BLNK after FcγR ligation. This protein was identified as PLC-γ2 by reprobing these membranes with a specific rabbit antiserum. Surprisingly, PLC-γ2 protein coimmunoprecipitated with both SLP-76 and BLNK also in unstimulated cells (Fig. 1C Lower). This coprecipitation was specific: PLC-γ2 was not detected in Western blots of immunoprecipitates of the Wiskott–Aldrich syndrome protein-interacting protein (27) (not shown).
SLP-76 and BLNK Both Are Linked to FcγRI and FcγRIII.
FcγR crosslinking in murine macrophages leads to tyrosine phosphorylation of Lyn, Syk, ERK, and Jun amino-terminal kinases, and PLC-γ (25). There are functional differences between the immunoreceptor tyrosine-based activation motif-containing FcγRs, FcγRI and FcγRIII. For example, different patterns of cytokines are produced when these ligands are selectively stimulated (28). The mAb 2.4G2 that we used, the only available mAb against murine FcγRIII, also binds to FcγRII. However, it is likely that activating signals after 2.4G2 FcγR crosslinking are generated by the FcRγ-containing FcγRIII, because murine FcγRII is an inhibitory receptor that recruits protein tyrosine-phosphatases via an inhibitory motif in its intracytoplasmic domain (25). It is possible that 2.4G2 interacts with FcRγ-containing FcγRI via its own Fc. Both of the activating receptors, FcγRI and FcγRIII, could be separately coupled to either SLP-76 or BLNK. Alternatively, one or both receptors could be coupled to both SLP-76 and BLNK. To distinguish between these possibilities, we examined tyrosine phosphorylation of SLP-76 and BLNK after selective ligation of FcγRI and FcγRII/III in BMM.
FcγRI is the only FcγR with high affinity for monomeric IgG (25), and it interacts preferentially with IgG2a in comparison to FcγRII and III (29). To selectively ligate FcγRI, normal (SLP-76+/−) BMM were incubated with purified monomeric mouse IgG2a followed by washing and treatment with F(ab′)2 of goat anti-mouse IgG. Immunoprecipitates of stimulated cells were analyzed in Western blots. The effectiveness of the crosslinking strategy was shown by tyrosine phosphorylation of the FcRγ-associated kinase Syk (Fig. 2A). Tyrosine phosphorylation of both SLP-76 and BLNK also was seen. These results suggest that FcγRI is coupled to both SLP-76 and BLNK.
Figure 2.
Tyrosine phosphorylation in BMM after selective ligation of FcγRI or FCγRII/III. (A) BMM were stimulated with purified mouse IgG2a (UPC10 myeloma protein) followed by F(ab′)2 of goat anti-mouse IgG. Cells were harvested at 0 or 10 min, and lysates were immunoprecipitated (IP) with the indicated antibodies. Western blots were probed initially with 4G10, then stripped and reprobed with the immunoprecipitating antibody. (B) BMM were stimulated with F(ab′)2 of 2.4G2 followed by F(ab′)2 of goat anti-rat IgG. Immunoprecipitation and Western blots were as described in A. Data were replicated in three separate experiments using different mice.
To selectively ligate FcγRII/III, we stimulated BMM with F(ab′)2 fragments of 2.4G2 followed by crosslinking with F(ab′)2 of goat anti-rat IgG. With these reagents, there is no opportunity for engagement of FcγRI by antibody Fc. In Fig. 2B, Syk phosphorylation again shows the effectiveness of crosslinking. Selective ligation of FcγRII/III in BMM resulted in tyrosine phosphorylation of both SLP-76 and BLNK. These results suggest that FcγRII/III also is coupled to both SLP-76 and BLNK.
FcγR Signaling in SLP-76-Deficient BMM.
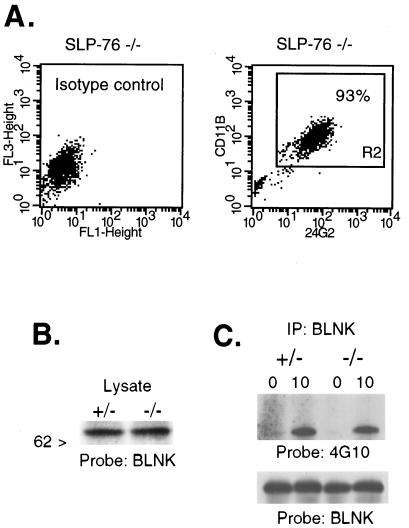
SLP-76−/− mice possess normal numbers of splenic macrophages. However, they have less than 10% of the number of peritoneal macrophages found in wild-type mice. We observed normal development of macrophages from SLP-76−/− bone marrow cells under the influence of L cell-conditioned medium. Fig. 3A shows that SLP-76−/− BMM express FcγRII/III (CD32/16) and Mac-1 (CD11b) in a proportion similar to SLP-76+/− controls (>90%), and with equivalent surface density. The morphology of the BMM and their density in culture are also equivalent in SLP-76−/− and heterozygous control mice (data not shown). These results suggest that SLP-76 is not essential for the development of BMM.
Figure 3.
BMM from SLP-76−/− mice. (A) BMM from SLP-76-deficient mice were analyzed by flow cytometry as in Fig. 1. (B) A Western blot of cell lysates from SLP-76−/− or SLP-76+/− control mice were probed with BLNK antiserum. (C) BMM were stimulated with 2.4G2 and goat anti-rat IgG. Lysates were immunoprecipitated (IP) with BLNK antiserum; a Western blot was probed initially with 4G10. The blot then was stripped and reprobed with BLNK antiserum. Data were replicated in a separate experiment using different mice.
Fig. 3B shows that BLNK is expressed equivalently in SLP-76+/− and SLP-76−/− BMM. More importantly, BLNK is tyrosine-phosphorylated in SLP-76−/− BMM after crosslinking FcγR with 2.4G2 (Fig. 3C). These results suggest that BLNK activation in FcγR signaling is independent of SLP-76 and raise the possibility that downstream signaling elements may be activated through BLNK in the absence of SLP-76.
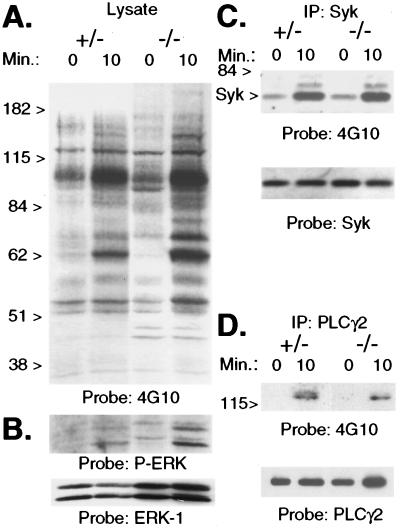
Fig. 4A shows that the pattern of total protein tyrosine phosphorylation after FcγR crosslinking with mAb 2.4G2 is not appreciably different between control and SLP-76−/− mice. There were neither additional nor missing bands in the lysates of SLP-76−/− BMM. The fact that tyrosine-phosphorylated bands appear more intense in lysates of SLP-76−/− BMM in the experiment shown is probably the result of a greater amount of protein loaded. When the same membrane was probed with an antibody that binds ERK-1 and ERK-2, there was clearly more ERK in SLP-76−/− lysates than in control BMM lysates (see Fig. 4B Lower).
Figure 4.
Protein tyrosine phosphorylation after FcγR crosslinking. BMM were stimulated with 2.4G2 and goat anti-rat IgG for 0 or 10 min. A Western blot of BMM lysates was probed initially with antiphosphotyrosine mAb 4G10 (A), then stripped and reprobed with antiphospho-ERK and anti-ERK-1 (B). Lysates also were immunoprecipitated (IP) with anti-Syk (C) and anti-PLC-γ2 (D). Western blots of immunoprecipitates were probed initially with 4G10, then blots were stripped and reprobed with the immunoprecipitating antibody. Data were replicated in 2–4 additional experiments using different mice.
In murine macrophages, FcγR crosslinking leads to tyrosine phosphorylation of Lyn, Syk, PLC-γ, ERK, and Jun amino-terminal kinases (25). We next examined tyrosine phosphorylation of specific signaling intermediates placed upstream (Syk) and downstream (PLC-γ and ERK) of SLP-76 in FcγR signaling, according to the well-established sequence of events in T cell receptor signaling (4, 30). SLP-76−/− and control BMM were stimulated with 2.4G2 and goat anti-rat IgG. Syk and PLC-γ2 immunoprecipitates were analyzed in Western blots probed with an antiphosphotyrosine mAb. Tyrosine phosphorylation of Syk was similar in control and SLP-76−/− BMM (Fig. 4C). Phosphorylation of PLC-γ2 was readily detectable in lysates of stimulated SLP-76−/− BMM, but appeared reduced in comparison to the control (Fig. 4D). To examine phosphorylation of ERK, BMM lysates were probed with an antibody that binds phosphorylated ERK-1 (44 kDa) and ERK-2 (42 kDa). Fig. 4B shows induction of ERK phosphorylation in BMM from control and SLP-76−/− mice. The greater intensity of the bands in SLP-76−/− lanes in the experiment shown is probably caused by protein loading (see above). These data demonstrate that substrates placed both upstream and downstream of SLP-76 are phosphorylated on tyrosines after FcγR ligation in SLP-76−/− BMM.
FcγR-Dependent Phagocytosis Proceeds Normally in SLP-76-Deficient BMM.
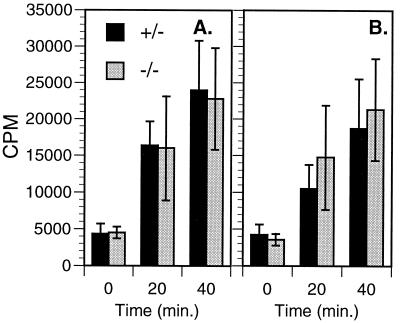
Signaling via FcγRI and FcγRIII is critical for a number of macrophage functions including phagocytosis of antibody-sensitized particles. Phagocytosis of IgG-coated particles involves predominantly FcγRI and FcγRIII (31). To examine whether FcγR-mediated phagocytosis can proceed independently of SLP-76, we examined the capacity of SLP-76−/− BMM to bind and phagocytose rabbit IgG-sensitized SRBC. Binding of IgG-coated SRBC to BMM at 4°C was monitored visually and also was assessed by using radioactively labeled SRBC. BMM from SLP-76−/− mice bound sensitized SRBC equivalently to controls (data not shown). Phagocytosis was evaluated in cultures incubated at 37°C for varying periods of time after SRBC binding at 4°C. Fig. 5A shows that the phagocytic activity of SLP-76−/− BMM was equal to that of controls. Because cytokines in the conditioned culture medium might have permitted SLP-76−/− BMM to overcome a phagocytic defect, we also studied SRBC uptake by freshly isolated spleen macrophages. Fig. 5B shows that primary adherent macrophages from spleens of SLP-76−/− mice also ingest sensitized IgG-coated SRBC normally. These results indicate that SLP-76 is not essential for FcγR-dependent phagocytosis.
Figure 5.
Phagocytosis of IgG-sensitized SRBC. SRBC were labeled with 51Cr and rabbit anti-SRBC IgG, and added to cultured BMM (A) or adherent spleen macrophages (B). Each point represents data from four separate experiments using BMM from different SLP-76−/− and control mice (mean ± SD). Counts per minute (CPM) were adjusted to account for differences in the specific radioactivity of SRBC in each experiment. Ingested CPM with radioactive SRBC in the absence of IgG was <2,000 in all experiments.
Discussion
We have shown that murine BMM express both SLP-76 and BLNK. Furthermore, selective ligation of either FcγRI or FcγRII/III in BMM resulted in tyrosine phosphorylation of both SLP-76 and BLNK. FcγR-mediated tyrosine phosphorylation of Syk and ERK-1/2, and FcγR-dependent phagocytosis were normal in SLP-76-deficient macrophages; tyrosine phosphorylation of PLC-γ2 was slightly reduced. These results suggest that SLP-76 and BLNK both are linked to FcγR signaling pathways in murine BMM.
SLP-76 is expressed and tyrosine-phosphorylated in murine macrophages after FcγR crosslinking. Furthermore, tyrosine-phosphorylated PLC-γ2 coprecipitated with SLP-76 after FcγR ligation (Fig. 1C). The coprecipitation of SLP-76 and PLC-γ2 in stimulated BMM is consistent with the observed coprecipitation of PLC-γ1 and SLP-76 in T cells after TCR/CD3 ligation (5), and in mast cells after FcɛRI ligation (V.I.P. and R.S.G., unpublished observations). These data suggest a role for SLP-76 in FcRγ chain-dependent signaling in murine BMM, as has been reported in a human monocytic cell line (18).
BLNK is a structural and functional homologue of SLP-76. BLNK is expressed in B cells and plays a critical role in signal transduction via the B cell Ig receptor complex (22, 23). Our data show clearly that BLNK is expressed in murine BMM. More importantly, BLNK was tyrosine-phosphorylated after FcγR crosslinking. We also found coimmunoprecipitation of tyrosine-phosphorylated PLC-γ2 with BLNK in BMM after FcγR ligation. Coprecipitation of BLNK with PLC-γ has been observed in activated B cells (22). These results suggest that BLNK is coupled to FcγR signaling in murine BMM.
We observed that PLC-γ2 in its unphosphorylated form coprecipitated with both SLP-76 and BLNK in unstimulated BMM (Fig. 1C). This finding is in contrast to the situation in T cells (5) and B cells (22) where the association of PLC-γ with SLP-76 and BLNK, respectively, is observed only after stimulation. The reason for this discrepancy may relate to the fact that “unstimulated” BMM may not truly be resting, because some signaling pathways may be active after culturing over several days in cytokine-containing medium, and adherence to plastic.
Murine FcγRI and FcγRIII signal via their immunoreceptor tyrosine-based activation motif-containing FcRγ chain, whereas FcγRII transmits inhibitory signals via its tyrosine-based inhibitory motif in the intracytoplasmic domain (25). Because intact anti-FcγRII/III 2.4G2 mAb may interact with FcγRI via its own Fc portion, it may activate macrophages via both FcγRI and FcγRIII. We investigated whether both of these receptors can be linked individually to both SLP-76 and BLNK. We used mouse IgG2a to selectively activate BMM via FcγRI, and 2.4G2 F(ab′)2 to selectively activate BMM via FcγRII/III. Secondary F(ab′)2 reagents were used to avoid recruitment of other receptors via Fc. Our results clearly show that activation via either one of these receptors resulted in simultaneous tyrosine phosphorylation of both SLP-76 and BLNK. It is unlikely that FcγRII or FcγRIII were engaged by murine IgG2a, because these receptors have negligible affinity for monomeric mouse IgG (25) and are selective for IgG1 and IgG2b (29). It is also unlikely that engagement of FcγRII contributed to BLNK and SLP-76 phosphorylation after crosslinking with 2.4G2 F(ab′)2. However, this possibility can be formally eliminated only by examining BMM from mice in which individual FcγR genes are inactivated.
Because both BLNK and SLP-76 are linked to both FcγRI and FcγRIII, it was important to determine whether the simultaneous presence of both adapter molecules is essential for FcγR signaling. We addressed this question by examining FcγR signaling in BMM from SLP-76−/− mice. Although SLP-76−/− mice have normal numbers of splenic macrophages (19), they are severely deficient in peritoneal macrophages. The paucity of peritoneal macrophages in SLP-76−/− mice may reflect an intrinsic defect in the development or function of these cells or may be secondary to another defect, such as the platelet dysfunction and intraperitoneal bleeding observed in these mice. It is unlikely that the paucity of peritoneal macrophages reflects a maturation defect in this cell lineage, because BMM developed normally when SLP-76−/− bone marrow cells were cultured in L cell-conditioned medium. CSF-1 (M-CSF), is an important macrophage differentiation cytokine in this medium (32, 33). Molecules known to associate with SLP-76 in TCR and FcγR signaling pathways such as Grb2 and Cbl (34) are also components of pathways activated by the receptor for CSF-1. However, a role for SLP-76 in CSF-1 signaling has not been reported. Our studies suggest that SLP-76 is not essential for the response of BMM precursors to CSF-1 (Fig. 3A).
Our studies showed that in the absence of SLP-76, several signaling intermediates are activated after FcγR ligation. These include Syk, PLC-γ2 (whose phosphorylation in T cells depends on SLP-76; ref. 30), and the mitogen-activated protein kinases ERK-1/2, which are downstream of SLP-76. Normal tyrosine phosphorylation of Syk in SLP-76−/− mice is not surprising, because it is thought to be upstream of SLP-76. Syk is a substrate of src-family kinases after FcγR crosslinking (25); Syk phosphorylation is intact in SLP-76−/− platelets stimulated via the gpVI collagen receptor (21) and in SLP-76−/− mast cells triggered via FcɛRI (17). Furthermore, normal ZAP-70 phosphorylation has been observed during TCR signaling in a SLP-76-deficient human T cell line (J14-v-29) (30).
Phosphorylation of PLC-γ2 is readily detectable, although slightly reduced in SLP-76−/− BMM. This finding is in contrast to SLP-76−/− platelets (21) and the SLP-76-deficient J14-v-29 line (30), where tyrosine phosphorylation of PLC-γ2 and PLC-γ1, respectively, was not detected upon activation. The TCR is thought to be linked to PLC-γ1 via a SLP-76-Gads-LAT pathway (6, 7). Although Gads is expressed in BMM (6), LAT expression was not seen in the THP-1 cell line (7) or in murine peritoneal macrophages (35). The path linking FcR γ to PLC-γ2 in BMM remains to be determined. Because PLC-γ2 coprecipitates with both SLP-76 and BLNK in BMM, it is possible that both adapters link FcRγ to PLC-γ2 in BMM. The reduced phosphorylation of PLC-γ2 in SLP-76−/− BMM may indicate that BLNK alone is less effective than BLNK and SLP-76 together in linking PLC-γ2 to FcγR.
TCR signaling does not lead to ERK-1 and ERK-2 phosphorylation in the SLP-76-deficient J14-v-29 line (30), suggesting that in T cells SLP-76 is essential for mitogen-activated protein kinase activation. Phosphorylation of ERK-1/2 depends on the Ras-Raf pathway (36). Normal phosphorylation of ERK-1/2 in SLP-76−/− BMM after FcγR ligation suggests that the Grb-2/Sos-Ras connections are intact in these cells, possibly because of BLNK.
Although transfection studies show that all three FcγRs can mediate phagocytosis, macrophages from FcR γ−/− mice cannot phagocytose IgG-coated SRBCs (31). Thus, it appears that FcγRII may not normally be involved in phagocytosis, and that phagocytosis of IgG-coated particles involves predominantly FcγRI and FcγRIII. Phagocytosis of IgG-coated SRBC by SLP-76−/− BMM was completely normal. This finding is consistent with the intact FcγR signaling in these cells as evidenced by phosphorylation of PLC-γ2 and ERK-1/2 and suggests that BLNK may support FcγR-mediated phagocytosis in BMM independently of SLP-76.
In contrast to normal FcγR signaling in macrophages, the biological activities of several cell types are deranged in SLP-76-deficient mice. T cells do not develop past the double-negative stage in the thymus (19, 20), mast cells do not degranulate or flux calcium normally after ligation of FcɛRI (17), and platelets do not aggregate normally after ligation of the gpVI collagen receptor (21). None of these cell types expresses BLNK (refs. 22 and 23; V.I.P. and R.S.G., unpublished data).
To our knowledge, murine BMM are the first cells known to express both SLP-76 and BLNK. Our results strongly suggest that FcγRI and FcγRIII in BMM can couple to both of these adapter molecules. The preservation of FcγR-mediated phagocytosis and activation of signaling molecules in SLP-76−/− BMM suggests that BLNK and SLP-76 may have redundant roles in FcγR signaling. However, we cannot exclude the possibility that SLP-76 phosphorylation after FcγR ligation is not necessary for the biological activities mediated by these receptors, nor that one or more additional SLP-76 homologues are expressed in macrophages. The study of mice deficient in BLNK and in both BLNK and SLP-76 may distinguish between these possibilities.
Acknowledgments
We thank Drs. David I. Beller and Jay C. Unkeless for their helpful advice and Dr. Paul Findell for his generous gift of anti-SLP-76 mAb. This work was supported by National Institutes of Health Grant R01 AI-35714, Baxter Healthcare Corp., Centeon, L.L.C., Olsten Corp., and The Jeffrey Modell Foundation. F.A.B. was supported by National Institutes of Health Grant K08 AI-01426 and the Wolbach Fund. A.C.C. is a Pew Scholar in the Biomedical Sciences and was supported by National Institutes of Health Grant R01 AI-42787.
Abbreviations
- PLC
phospholipase C
- TCR
T cell receptor
- FcR
Fc receptor
- BMM
bone marrow-derived macrophages
- ERK
extracellular signal regulated kinase
- BLNK
B cell linker protein
- SLP-76
Src homology 2 domain-containing leukocyte protein of 76 kDa
- SRBC
sheep red blood cells
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.040543597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.040543597
References
- 1.Cambier J C. J Immunol. 1995;155:3281–3285. [PubMed] [Google Scholar]
- 2.Tsuji M, Ezumi Y, Arai M, Takayama H. J Biol Chem. 1997;272:23528–23531. doi: 10.1074/jbc.272.38.23528. [DOI] [PubMed] [Google Scholar]
- 3.Lanier L L, Corliss B C, Wu J, Leong C, Phillips J H. Nature (London) 1998;391:703–707. doi: 10.1038/35642. [DOI] [PubMed] [Google Scholar]
- 4.Rudd C E. Cell. 1999;96:5–8. doi: 10.1016/s0092-8674(00)80953-8. [DOI] [PubMed] [Google Scholar]
- 5.Jackman J K, Motto D G, Sun Q, Tanemoto M, Turck C W, Peltz G A, Koretzky G A, Findell P R. J Biol Chem. 1995;270:7029–7032. doi: 10.1074/jbc.270.13.7029. [DOI] [PubMed] [Google Scholar]
- 6.Liu S K, Fang N, Koretzky G A. Curr Biol. 1999;9:67–75. doi: 10.1016/s0960-9822(99)80017-7. [DOI] [PubMed] [Google Scholar]
- 7.Zhang W, Sloan-Lancaster J, Kitchen J, Trible R, Samelson L. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 8.Tuosto L, Michel F, Acuto O. J Exp Med. 1996;184:1161–1166. doi: 10.1084/jem.184.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.da Silva A J, Li Z, de Vera C, Canto E, Findell P, Rudd C E. Proc Natl Acad Sci USA. 1997;94:7493–7498. doi: 10.1073/pnas.94.14.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Musci M A, Hendricks-Taylor L R, Motto D G, Paskind M, Kamens J, Turck C W, Koretzky G A. J Biol Chem. 1997;272:11674–11677. doi: 10.1074/jbc.272.18.11674. [DOI] [PubMed] [Google Scholar]
- 11.Mizuno K, Katagiri T, Hasegawa K, Ogimoto M, Yakura H. J Exp Med. 1996;184:457–463. doi: 10.1084/jem.184.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bubeck Wardenburg J, Pappu R, Bu J Y, Mayer B, Chernoff J, Straus D, Chan A C. Immunity. 1998;9:607–616. doi: 10.1016/s1074-7613(00)80658-5. [DOI] [PubMed] [Google Scholar]
- 13.Crompton T, Gilmour K C, Owen M J. Cell. 1996;86:243–251. doi: 10.1016/s0092-8674(00)80096-3. [DOI] [PubMed] [Google Scholar]
- 14.Crespo P, Schuebel K E, Ostrom A A, Gutkind J S, Bustelo X R. Nature (London) 1997;385:169–172. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- 15.Motto D G, Ross S E, Wu J, Hendricks-Taylor L R, Koretzky G A. J Exp Med. 1996;183:1937–1943. doi: 10.1084/jem.183.4.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clements J L, Ross-Barta S E, Tygrett L T, Waldschmidt T J, Koretzky G A. J Immunol. 1998;161:3880–3889. [PubMed] [Google Scholar]
- 17.Pivniouk V I, Martin T R, Lu-Kuo J M, Katz H R, Oettgen H C, Geha R S. J Clin Invest. 1999;103:1737–1743. [PMC free article] [PubMed] [Google Scholar]
- 18.Chu J, Liu Y, Koretzky G A, Duren D L. Blood. 1998;92:1697–1706. [PubMed] [Google Scholar]
- 19.Pivniouk V, Tsitsikov E, Swinton P, Rathbun G, Alt F W, Geha R S. Cell. 1998;94:229–238. doi: 10.1016/s0092-8674(00)81422-1. [DOI] [PubMed] [Google Scholar]
- 20.Clements J L, Yang B, Ross-Barta S E, Eliason S L, Hrstka R F, Williamson R A, Koretzky G A. Science. 1998;281:416–419. doi: 10.1126/science.281.5375.416. [DOI] [PubMed] [Google Scholar]
- 21.Clements J L, Lee J R, Gross B, Yang B, Olson J D, Sandra A, Watson S P, Lentz S R, Koretzky G A. J Clin Invest. 1999;103:19–25. doi: 10.1172/JCI5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu C, Turck C W, Kurosaki T, Chan A C. Immunity. 1998;9:93–103. doi: 10.1016/s1074-7613(00)80591-9. [DOI] [PubMed] [Google Scholar]
- 23.Wienands J, Schweikert J, Wooscheid B, Jumaa H, Nielsen P J, Reth M. J Exp Med. 1998;188:791–795. doi: 10.1084/jem.188.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishiai M, Kurosaki M, Pappu R, Okawa K, Ronko I, Fu C, Shibata M, Iwamatsu A, Chan A C, Kurosaki T. Immunity. 1999;10:117–125. doi: 10.1016/s1074-7613(00)80012-6. [DOI] [PubMed] [Google Scholar]
- 25.Daeron M. Annu Rev Immunol. 1997;15:203–234. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 26.Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current Protocols in Immunology. New York: Wiley; 1998. [Google Scholar]
- 27.Ramesh N, Anton I M, Hartwig J H, Geha R S. Proc Natl Acad Sci USA. 1997;94:14671–14676. doi: 10.1073/pnas.94.26.14671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutterwala F S, Noel G J, Salgame P, Mosser D M. J Exp Med. 1998;188:217–222. doi: 10.1084/jem.188.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinshank R L, Luster A D, Ravetch J V. J Exp Med. 1988;163:1909–1925. doi: 10.1084/jem.167.6.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yablonski D, Kuhne M R, Kadlecek T, Weiss A. Science. 1998;281:413–416. doi: 10.1126/science.281.5375.413. [DOI] [PubMed] [Google Scholar]
- 31.Takai T, Li M, Sylvestre D, Clynes R, Ravetch J V. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka S, Neff L, Baron R, Levy J B. J Biol Chem. 1995;270:14347–14351. doi: 10.1074/jbc.270.24.14347. [DOI] [PubMed] [Google Scholar]
- 33.Park R K, Kyono W T, Liu Y, Durden D L. J Immunol. 1998;160:5018–5027. [PubMed] [Google Scholar]
- 34.Reedquist K A, Fukazawa T, Panchamoorthy G, Langdon W Y, Shoelson S E, Druker B J, Band H. J Biol Chem. 1996;271:8435–8442. doi: 10.1074/jbc.271.14.8435. [DOI] [PubMed] [Google Scholar]
- 35.Weber J R, Orstavik S, Torgerson K M, Danbolt N C, Berg S F, Ryan J C, Tasken K, Imboden J B, Vaage J T. J Exp Med. 1998;187:1157–1161. doi: 10.1084/jem.187.7.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marais R, Marshall C J. Cancer Surv. 1996;27:101–125. [PubMed] [Google Scholar]