Abstract
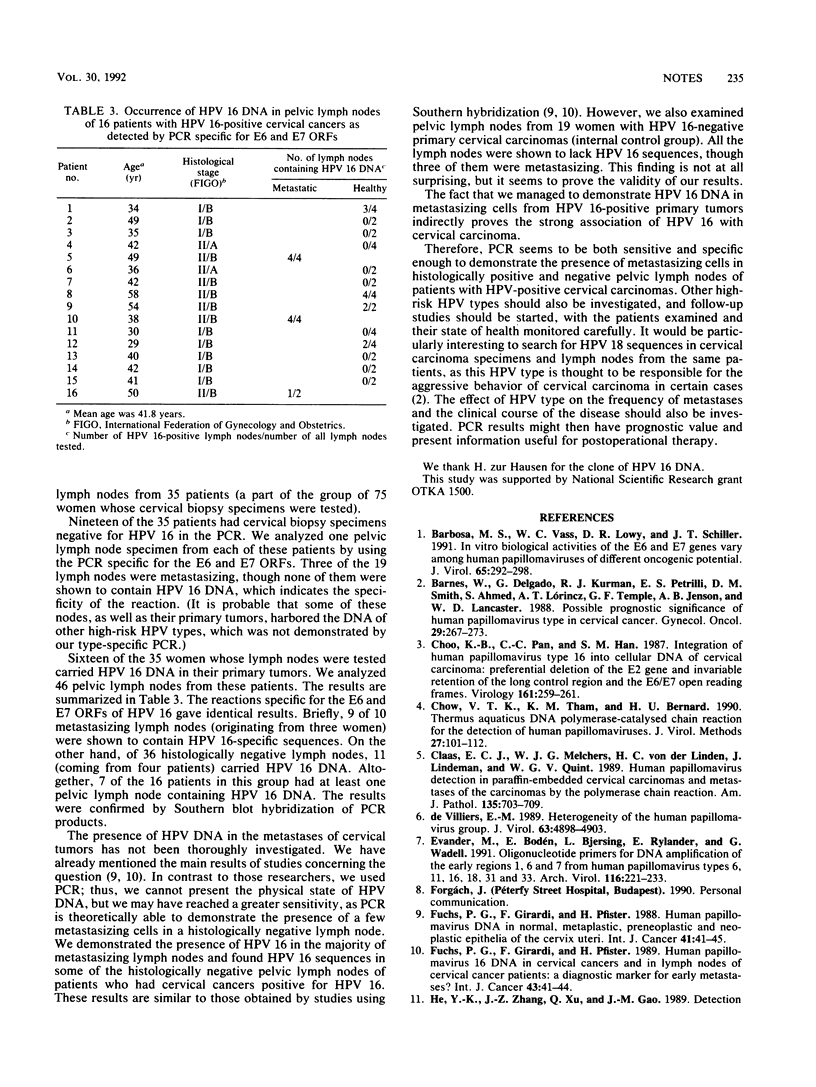
We have used a polymerase chain reaction (PCR) to examine cervical cancer biopsy specimens and pelvic lymph nodes for the presence of human papillomavirus type 16 (HPV 16) DNA. Of the 75 cervical specimens tested, 36 (48%) were positive for HPV 16 in the PCR. A total of 65 pelvic lymph nodes removed during radical surgery on 35 women were also analyzed. Lymph nodes originating from 19 patients whose cervical biopsy specimens were negative for HPV 16 seemed to lack HPV 16 sequences. For 16 women with positive PCR results for cervical biopsy specimens, 9 of 10 lymph node metastases were positive in the PCR, while 11 of their 36 histologically negative lymph nodes were also shown to contain HPV 16 DNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbosa M. S., Vass W. C., Lowy D. R., Schiller J. T. In vitro biological activities of the E6 and E7 genes vary among human papillomaviruses of different oncogenic potential. J Virol. 1991 Jan;65(1):292–298. doi: 10.1128/jvi.65.1.292-298.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes W., Delgado G., Kurman R. J., Petrilli E. S., Smith D. M., Ahmed S., Lorincz A. T., Temple G. F., Jenson A. B., Lancaster W. D. Possible prognostic significance of human papillomavirus type in cervical cancer. Gynecol Oncol. 1988 Mar;29(3):267–273. doi: 10.1016/0090-8258(88)90225-9. [DOI] [PubMed] [Google Scholar]
- Choo K. B., Pan C. C., Han S. H. Integration of human papillomavirus type 16 into cellular DNA of cervical carcinoma: preferential deletion of the E2 gene and invariable retention of the long control region and the E6/E7 open reading frames. Virology. 1987 Nov;161(1):259–261. doi: 10.1016/0042-6822(87)90195-4. [DOI] [PubMed] [Google Scholar]
- Chow V. T., Tham K. M., Bernard H. U. Thermus aquaticus DNA polymerase-catalysed chain reaction for the detection of human papillomaviruses. J Virol Methods. 1990 Jan;27(1):101–112. doi: 10.1016/0166-0934(90)90150-e. [DOI] [PubMed] [Google Scholar]
- Claas E. C., Melchers W. J., van der Linden H. C., Lindeman J., Quint W. G. Human papillomavirus detection in paraffin-embedded cervical carcinomas and metastases of the carcinomas by the polymerase chain reaction. Am J Pathol. 1989 Oct;135(4):703–709. [PMC free article] [PubMed] [Google Scholar]
- Evander M., Bodén E., Bjersing L., Rylander E., Wadell G. Oligonucleotide primers for DNA amplification of the early regions 1, 6, and 7 from human papillomavirus types 6, 11, 16, 18, 31, and 33. Arch Virol. 1991;116(1-4):221–233. doi: 10.1007/BF01319244. [DOI] [PubMed] [Google Scholar]
- Fuchs P. G., Girardi F., Pfister H. Human papillomavirus 16 DNA in cervical cancers and in lymph nodes of cervical cancer patients: a diagnostic marker for early metastases? Int J Cancer. 1989 Jan 15;43(1):41–44. doi: 10.1002/ijc.2910430110. [DOI] [PubMed] [Google Scholar]
- Fuchs P. G., Girardi F., Pfister H. Human papillomavirus DNA in normal, metaplastic, preneoplastic and neoplastic epithelia of the cervix uteri. Int J Cancer. 1988 Jan 15;41(1):41–45. doi: 10.1002/ijc.2910410109. [DOI] [PubMed] [Google Scholar]
- Melchers W., van den Brule A., Walboomers J., de Bruin M., Burger M., Herbrink P., Meijer C., Lindeman J., Quint W. Increased detection rate of human papillomavirus in cervical scrapes by the polymerase chain reaction as compared to modified FISH and southern-blot analysis. J Med Virol. 1989 Apr;27(4):329–335. doi: 10.1002/jmv.1890270413. [DOI] [PubMed] [Google Scholar]
- Münger K., Phelps W. C., Bubb V., Howley P. M., Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989 Oct;63(10):4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman A., Fife K. H. Human papillomaviruses: are we ready to type? Clin Microbiol Rev. 1989 Apr;2(2):166–190. doi: 10.1128/cmr.2.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Takebe N., Tsunokawa Y., Nozawa S., Terada M., Sugimura T. Conservation of E6 and E7 regions of human papillomavirus types 16 and 18 present in cervical cancers. Biochem Biophys Res Commun. 1987 Mar 30;143(3):837–844. doi: 10.1016/0006-291x(87)90325-1. [DOI] [PubMed] [Google Scholar]
- Walboomers J. M., Fokke H. E., Polak M., Volkers H., Houthoff H. J., Barents J., van der Noordaa J., ter Schegget J. In situ localization of human papilloma virus type 16 DNA in a metastasis of an endocervical adenocarcinoma. Intervirology. 1987;27(2):81–85. doi: 10.1159/000149723. [DOI] [PubMed] [Google Scholar]
- Xiao X., Cao M., Miller T. R., Cao Z. Y., Yen T. S. Papillomavirus DNA in cervical carcinoma specimens from central China. Lancet. 1988 Oct 15;2(8616):902–902. doi: 10.1016/s0140-6736(88)92494-4. [DOI] [PubMed] [Google Scholar]
- de Villiers E. M. Heterogeneity of the human papillomavirus group. J Virol. 1989 Nov;63(11):4898–4903. doi: 10.1128/jvi.63.11.4898-4903.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]