Abstract
The physiological significance of the cardiac glycoside-binding site on the Na,K-ATPase remains incompletely understood. This study used a gene-targeted mouse (α2R/R) which expresses a ouabain-insensitive α2 isoform of the Na,K-ATPase to investigate whether the cardiac glycoside-binding site plays any physiological role in active Na+/K+ transport in skeletal muscles or in exercise performance. Skeletal muscles express the Na,K-ATPase α2 isoform at high abundance and regulate its transport over a wide dynamic range under control of muscle activity. Na,K-ATPase active transport in the isolated extensor digitorum longus (EDL) muscle of α2R/R mice was lower at rest and significantly enhanced after muscle contraction, compared with WT. During the first 60 s after a 30-s contraction, the EDL of α2R/R mice transported 70.0 nmol/g·min more 86Rb than WT. Acute sequestration of endogenous ligand(s) in WT mice infused with Digibind to sequester endogenous cardiac glycoside(s) produced similar effects on both resting and contraction-induced 86Rb transport. Additionally, the α2R/R mice exhibit an enhanced ability to perform physical exercise, showing a 2.1- to 2.8-fold lower failure rate than WT within minutes of the onset of moderate-intensity treadmill running. Their enhanced exercise performance is consistent with their enhanced contraction-induced Na,K-ATPase transport in the skeletal muscles. These results demonstrate that the Na,K-ATPase α2 isozyme in skeletal muscle is regulated dynamically by a mechanism that utilizes the cardiac glycoside-binding site and an endogenous ligand(s) and that its cardiac glycoside-binding site can play a physiological role in the dynamic adaptations to exercise.
Keywords: cardiotonic steroids, endogenous cardiac glycosides, ouabain
The cardiac glycoside-binding site on the Na,K-ATPase is a highly conserved domain on the primary α catalytic subunit to which a variety of naturally occurring compounds bind with high specificity. This site is the only known receptor for ouabain and other clinically useful cardiac glycosides and cardiac glycoside antagonists (1, 2). It is also the receptor for endogenous cardiotonic steroids (3) that are present in mammals and whose circulating levels change under meaningful physiological and pathophysiological conditions, including physical exercise, essential hypertension, renal failure and hypertensive pregnancy (4–8).
Four isoforms of the primary catalytic alpha subunit of the Na,K-ATPase have been identified. The ubiquitous α1 isoform is proposed to play a general transport role whereas the α2, α3, and α4 isoforms show distinct tissue-specific patterns of expression and may perform additional regulatory functions. The cardiac glycoside binding site is highly conserved in all alpha isoforms, but its affinity can vary widely in a species and tissue-specific manner (9). This has led to the proposal that the affinity for cardiac glycosides may relate in part to the physiological roles of the different α subunits (10). However, despite extensive research, the physiological significance of the cardiac glycoside binding site remains poorly understood.
The gene-targeted mouse in which the normally high-affinity Na,K-ATPase α2 isoform is made relatively insensitive to ouabain binding (11) has provided a unique model for addressing this question. Ouabain insensitivity is rendered by the substitution of just 2 amino acids that border the binding pocket and are thought to participate directly in binding ouabain (12, 13). This model made it possible to search for possible physiological roles of binding affinity in vivo in tissues that retain normal enzyme content and transport functions, without making any assumptions about the nature of the putative ligand(s). By using this model, it was possible to demonstrate that the cardiac glycoside affinity of the α2 Na,K-ATPase plays a role in blood pressure regulation in response to elevated ACTH (14), a condition that mimics certain hypertensive diseases of humans.
The goal of the present study was to investigate whether the cardiac glycoside-binding site of the Na,K-ATPase α2 isoform can play a regulatory role in active Na+/K+ transport in skeletal muscles or in exercise performance. We expected that skeletal muscle would be an informative tissue for several reasons. The skeletal muscles express the α2 Na,K-ATPase as the major α-isoform and regulate its transport activity over a wide dynamic range. The α2 content of fast-twitch muscles comprises 87% of total α-subunit content [compared with 5–30% in the heart and vasculature (15)], and the skeletal musculature contains 100-fold more ouabain receptors than the heart (16). Na,K-ATPase activity in skeletal muscle responds dynamically to the nearly bipolar demand for Na+/K+ transport as the muscles alternate between periods of rest and activity. Resting skeletal muscles maintain ion gradients and membrane potentials using only 2–6% of their total muscle Na,K-ATPase capacity (17); whereas, muscle contraction rapidly activates up to the 100% of this capacity (18). This acute stimulation occurs within seconds of the onset of contractile activity (19) and is essential for maintaining excitation and contraction.
We compared Na,K-ATPase α-subunit expression, ouabain binding, and 86Rb uptake in the skeletal muscles of WT and α2R/R mice under conditions of rest or contraction in vitro. Additionally, we measured 86Rb uptake under the same conditions in WT mice acutely infused with Digibind before tissue removal. We also compared the ability of WT and α2R/R mice to maintain extracellular potassium homeostasis in skeletal muscles undergoing sustained, evoked contractions in vivo and to perform physical exercise during treadmill running. Our results suggest that the cardiac glycoside-binding site of the α2 Na,K-ATPase and an endogenous ligand(s) participate in the dynamic regulation of Na,K-ATPase transport activity in response to changing conditions of muscle use.
Results
α-Isoform Expression in the Skeletal Muscles of WT and α2R/R Mice.
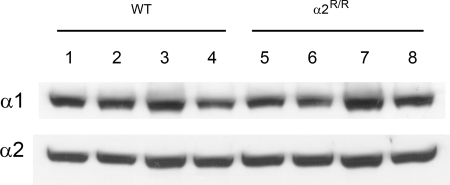
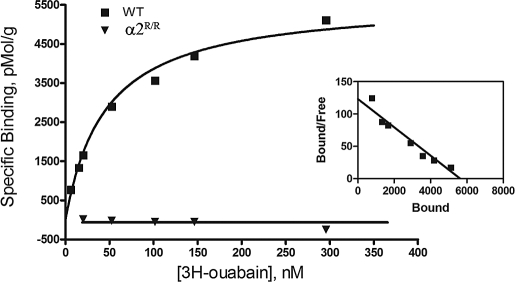
Our previous studies with Na,K-ATPase α2 isoform knockout mice had shown that removal of α2 expression induces compensatory up-regulation of the α1 isoform in skeletal muscle (20). Fig. 1 shows that α-isoform expression is not altered in the skeletal muscles of α2R/R mice. This indicates that ligand affinity to the cardiac glycoside site is not required for expression of the α2 Na,K-ATPase in skeletal muscle; and that its absence from birth does not induce compensatory changes in α1 isoform expression. Importantly, it establishes that any potential differences in Na,K-ATPase function or exercise performance between WT and α2R/R mice cannot be explained by altered α-isoform expression in the skeletal muscles. Fig. 2 shows that cardiac glycoside affinity is not detectable in the skeletal muscles of α2- resistant mice. In contrast, equilibrium 3H-ouabain binding to the skeletal muscles of WT mice is well-fit to a single site, high-affinity model with Bmax = 7,541 ± 841 pmol/g of protein and apparent Kd = 53.3 ± 3.1 nM (4) at 37 °C. This Kd is close to that measured in transverse tubule membranes of rabbit skeletal muscle [≈53 nM (21)] These results demonstrate that the α2 isoform is present at normal abundance in the skeletal muscles of α2R/R mice but is insensitive to ouabain binding at submicromolar concentrations.
Fig. 1.
Na,K-ATPase α1 and α2 isoform expression in adult skeletal muscle from 4 WT mice (lanes 1–4) and 4 α2R/R mice (lanes 5–8). Three similar blots were obtained and averaged for each isoform from 4 WT and 4 α2R/R mice. There was no significant group effect between genotypes for either α1 (P > 0.23) or α2 (P > 0.14) isoform. α-Isoform expression was measured by Western blot analysis using α1- and α2-specific antibodies, as described previously (20, 37). The α3 Na,K-ATPase isoform is not detected in this preparation (20).
Fig. 2.
Saturation curve of 3H-ouabain binding to mouse skeletal muscle membranes of WT (■) and ouabain-resistant αR/R mice (▾). Solid line: fit to 1-site binding model for total binding. Nonspecific binding was 3.1% of total binding at 200 nM ouabain. (Inset) Scatchard representation.
Interstitial Potassium Concentration in the Skeletal Muscles of WT and α2R/R Mice at Rest and During Contraction.
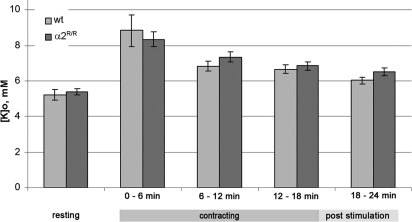
Exercise performance depends critically on the ability of the skeletal muscles to clear the increases in extracellular K+ and intracellular Na+ that accompany membrane excitation. During contraction, extracellular K+ rises to 8 to 20 mM in the muscle interstitial spaces and to 30 mM or greater in the diffusion-limited transverse tubules (19). The Na,K-ATPase is stimulated within seconds of the onset of muscle contraction, to restore the membrane potential and maintain excitation and contraction (22). Fig. 3 compares the interstitial K+ concentration in vivo in the gastrocnemius muscle of WT and α2R/R mice at rest and during evoked muscle contraction (continuous stimulation at 70 Hz for 18 min). The gastrocnemius muscle is representative of the overall mix of fast and slow fibers in the animal, and is a heavily used muscle during exercise. Basal [KISF] was 5.2 ± 0.3 mM (n = 7) in WT, and 5.4 ± 0.2 (n = 7) in α2R/R mice, and not significantly different (P > 0.63). These values are comparable with the plasma potassium concentration in mice (23, 24), which is in equilibrium with muscle interstitial K+ concentrations under resting conditions. This result indicates that the cardiac glycoside affinity of the α2 isoform is not required for maintaining K+ homeostasis in the interstitium of resting skeletal muscles. As expected, [KISF] increased during intense contractile activity. However, there was no difference between genotypes in their ability to clear the steady-state K+ load. During the first 6 min of maintained contraction, [KISF] rose to 8.83 ± 0.87 mM (n = 6) in the WT mice, and 8.34 ± 0.42 mM in α2R/R mice (n = 6) (P > 0.05), and remained elevated in both genotypes after 18 min of sustained contraction. These values are in the range of values for the contraction-related increase in [KISF] in mammalian muscles [7–10 mM (25–27)]. The microdialysis probe reports a time- and space-averaged K+ change along a length of muscle during 6-min intervals. This result indicates that the cardiac glycoside affinity of the α2 isoform is not required for maintaining bulk interstitial K+ homeostasis during intense muscle contraction lasting tens of minutes. This measurement cannot exclude the possibility that the local [K] change in the transverse tubules, where the α2 isoform is localized (28), may differ between genotypes or that earlier, rapid changes may have been missed.
Fig. 3.
Interstitial potassium concentration, [KISF], in the gastrocnemius muscle of WT and α2R/R mice at rest and during maintained contractile activity. [KISF] was measured by microdialysis in anesthetized mice. Evoked isotonic contractions were elicited by stimulation of the sciatic nerve (70 Hz/250-ms tetanus applied at 1 Hz for 18 min). Dialysate was collected for 25 min before stimulation and in 6-min intervals during and after contraction.
Na,K-ATPase Transport in Resting and Contracting Skeletal Muscles of WT, α2R/R, and Digibind-Infused WT Mice.
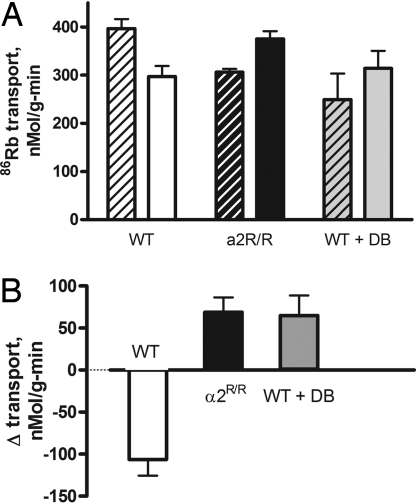
Fig. 4A compares 86Rb uptake in the isolated EDL muscle of WT and α2R/R mice at rest and immediately after a sustained isotonic contraction. Isotonic contraction was used because it is close to the in vivo condition and produces large changes in Na,K-ATPase transport (18). Total resting transport in WT was within the range of reported values for skeletal muscles of rats and mice (29, 30); in contrast, it was significantly lower in the EDL of α2R/R mice. Muscle contraction reversed this relationship. After the contraction, 86Rb uptake decreased in the WT and increased in the α2R/R. Fig. 4B plots the contraction-induced change in 86Rb transport (Δ transport, contraction–resting) between paired muscles of each genotype. 86Rb transport decreased in WT, and increased in the α2R/R. These differences demonstrate that the cardiac glycoside affinity of the Na,K-ATPase α2 isoform participates in the normal regulation of Na,K-ATPase transport activity both at rest and during muscle activity. Because the sole mutation in the α2R/R mouse alters the ability of the α2 isoform to bind ouabain, they further suggest a regulatory mechanism that involves the binding and/or unbinding of an endogenous ligand.
Fig. 4.
Regulation of Na,K-ATPase in the EDL of WT and α2R/R mice at rest and immediately after contraction. (A) Mean total 86Rb uptake at rest (striped bars) and during the first 60 sec after a 30-sec contraction at 90 Hz (solid bars) in WT, α2R/R, and WT infused with Digibind (WT + DB). Resting Rb uptake was 396.5 ± 20.1 (5) in WT, 306.3 ± 6.8 (4) in α2R/R, and 249.1 ± 8.8 (4) in WT + DB. Resting Rb uptake in α2R/R and WT + DB were significantly different from WT, but not significantly different from each other (P = 0.0028; 1-way ANOVA with Tukey's multiple comparison post test). (B) Mean contraction-induced change in 86Rb transport, Δ transport (after contraction–resting), measured in paired (left and right leg) EDL muscles of WT, α2R/R, and WT + DB mice. Δ Transport was −107.3 ± 17.0 (5) in WT, 69.0 ± 17.6 (4) in α2R/R, and 64.9 ± 23.9 (4) in WT + DB. Δ Transport in α2R/R and WT + DB were significantly different from WT, but not significantly different from each other (P = 0.0001; 1-way ANOVA with Tukey's multiple comparison post test). The mean nonspecific 86Rb uptake was 103.3 ± 8.9 (5) in resting muscles and 111.5 ± 7.5 (5) in stimulated muscles. The WT + DB mice were infused with 150–200 ng/g of body weight (equivalent to a plasma concentration of 50–60 nM) via a femoral catheter for 40 min before tissue extraction).
To address this question, we measured Rb uptake in the EDL of WT mice acutely infused with Digibind (WT + DB, Fig. 4B), a Fab antibody that sequesters a range of cardiac glycosides (31). The differences in α2R/R compared with WT are mirrored in the WT + DB, both at rest and after contraction. Thus, either reducing the affinity for cardiac glycoside or sequestering an endogenous ligand(s) produces identical changes in the regulation of Na,K-ATPase transport. This finding suggests that the transport activity of the Na,K-ATPase in skeletal muscle is regulated by a mechanism that involves the binding and/or unbinding of an endogenous ligand of the Na,K-ATPase.
This evidence for the involvement of an endogenous ligand in an isolated muscle without intact circulation might at first seem surprising. However, the skeletal muscles represent a large distribution volume for exogenous cardiac glycosides that are retained after tissue extraction with a half-time of 11 h (32). It is reasonable to expect that endogenous ligand(s) will show a similar distribution volume and that any ligand bound to the skeletal muscles in vivo will remain bound for some time in the isolated muscle at rest. Our experimental time of 30–45 min allows latitude for a wide range of off-rates for unknown ligands.
Exercise Performance of WT and α2R/R Mice During Treadmill Running.
A greater Na,K-ATPase transport rate in contracting skeletal muscles is expected to enhance exercise performance in the animal. Increased transport by the Na,K-ATPase in skeletal muscle consistently leads to enhanced exercise performance (22). Therefore, we compared the ability of WT and α2R/R mice to perform physical exercise.
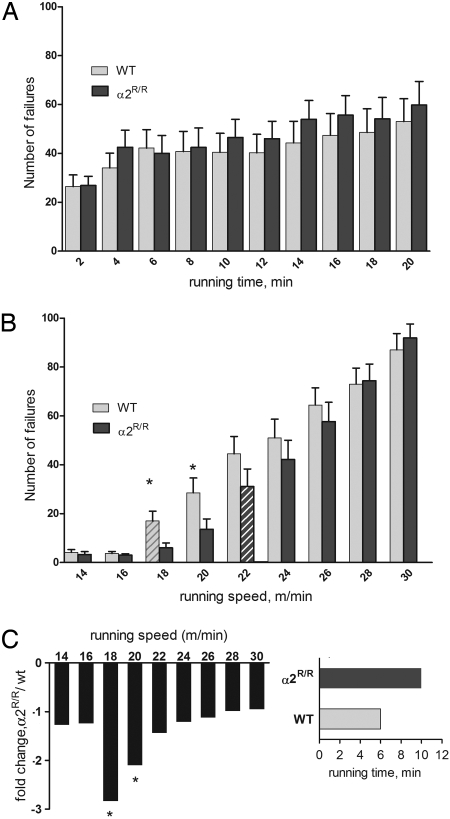
The mice were evaluated under 2 conditions—a continuous treadmill run at near maximal intensity and a graded run with stepwise increases in running speed. Fig. 5A shows that both WT and α2R/R mice perform comparably on the high-intensity, sprint-type run. Failures increased with running time, as expected, but there is no difference between genotypes. Fig. 5B shows that the α2R/R mice outperform their WT counterparts on the graded run. Their improved performance is revealed in an early window of intermediate running speeds. Both groups perform comparably at 14–16 m/min, running speeds that all mice maintain seemingly without effort. Differences become apparent at running speeds >18 m/min, a speed at which energy demands begin to rise. At this point, WT mice show a significant increase in their number of failures (striped bar; within group P < 0.05), and they continue to show more failures up to the highest running speeds. In contrast, the α2R/R mice run with significantly fewer failures at 18 and 22 m/min, and are able to run for 10 min into this test before their failure rate increases significantly (dark striped bar). The performance of α2R/R and WT mice converges at the highest running speeds, when their failures are comparable with that in the high-intensity test. Fig. 5C shows the time and intensity window in which the performance advantage of the α2R/R genotype is revealed. The α2R/R mice show 2.8-fold fewer failures at 18 m/min (n = 20, P = 0.020) and 2.1-fold fewer failures at 20 m/min (n = 21; P = 0.048). Additionally, the α2R/R mice first show a significant increase in failure rate after 10 min of running and having reached a speed of 22 m/min (P = 0.043, within group); whereas, WT mice reach this point after 6 min of running and after reaching 18 m/min (P = 0.028, within group). These findings indicate that the cardiac glycoside-binding affinity of the α2 Na,K-ATPase participates in the dynamic regulation of exercise performance. In the running animal, its contribution is revealed at early times and intermediate exercise intensities.
Fig. 5.
Exercise performance of WT and α2R/R mice. (A) Failures during each 2-min interval of treadmill running at a constant speed of 24 m/min. n = 18 WT and 20 α2R/R mice. Failures are defined as an interruption in running, measured in 1-sec intervals on the shock bar. None of the differences was significant. (B) Failures during each 2-min interval of graded running. The asterisk indicates significant difference between WT and α2R/R at 18 m/min (P = 0.016) and at 20 m/min (P = 0.048). n = 21 WT and 20 α2R/R mice. Striped bars indicate the interval when failures increased significantly above the previous interval within each genotype. (C) Enhanced performance window of α2R/R compared with WT mice, plotted as fold decrease in number of failures, and time for rate of failures to increase significantly.
Discussion
Using the α2R/R mouse, we have shown that a 1,000-fold reduction of cardiac glycoside affinity on the Na,K-ATPase α2 isoform produces phenotypic changes in Na,K-ATPase transport rate in resting and contracting skeletal muscle and improves exercise performance in the animal.
Compared with WT, mice lacking cardiac glycoside affinity on the α2 Na,K-ATPase isoform show lower resting Na,K-ATPase transport and a greater Na,K-ATPase transport rate immediately after contraction. Rest and contraction present up to a 20-fold dynamic range of demand for Na,K transport. These results demonstrate that the cardiac glycoside affinity of the Na,K-ATPase α2 isoform participates in the dynamic regulation of its transport activity in skeletal muscle. These effects cannot be explained by altered Na,K-ATPase isoform expression in the skeletal muscles, or an altered ability of the skeletal muscle Na,K-ATPase to perform active transport, in agreement with previous studies showing that the knockin of these 2 amino acids does not alter essential enzyme function or expression in multiple tissues (11).
The effects of reduced cardiac glycoside affinity on Na,K-ATPase transport in the skeletal muscles of α2R/R mice can be recapitulated in WT mice by an acute infusion of Digibind, an antibody known to sequester diverse cardiac glycosides. Compared with untreated WT mice, the Digibind-treated mice show lower resting Rb uptake and greater stimulation of Na,K-ATPase transport by muscle contraction. Thus, either the genetic removal of cardiac glycoside affinity, or the acute sequestration of endogenous ligand(s) in vivo before tissue removal, produces parallel changes in the regulation of both resting and contraction-induced Na,K-ATPase transport. This coincidence in the results obtained with 2 independent experimental models argues strongly that the regulation uncovered in this study involves the binding and/or unbinding of an endogenous ligand of the Na,K-ATPase.
In addition to these changes in the skeletal muscles, the α2R/R mice show an improved ability to perform physical exercise, with 2.1- to 2.8-fold fewer failures at early times after the onset of moderate exercise. This finding demonstrates that the cardiac glycoside-binding site can also play a physiological role in the dynamic regulation of exercise performance. The enhanced exercise performance of α2R/R mice is consistent with the greater contraction-related Na,K-ATPase transport in their skeletal muscles. Increased Na,K-ATPase activity correlates closely with increased force output, the ability to maintain excitation and contraction, and improved exercise performance (22). Alternatively, it is possible that the cardiac glycoside site of the α2 Na,K-ATPase may contribute to exercise performance via mechanisms or tissues other than, or in addition to, its actions on the skeletal muscles. The α2 isozyme is present in the heart, brain, vasculature, and other tissues, all of which participate in the adaptations to exercise in the animal.
The molecular details of this regulation and nature of the putative ligand(s) remain to be identified. The finding that transport is regulated in opposite directions in resting and contracting muscle excludes the simplest form of a 2-state, ON/OFF binding model. It suggests a more complex mechanism, perhaps by using multiple pathways or ligands, or dynamic changes in cardiac glycoside affinity. Although unlikely, the possibility that this regulation may not include a ligand binding/unbinding step cannot be completely excluded. For example, the same 2 amino acids that confer glycoside affinity may cause secondary changes in protein function, such as a change in substrate affinity for Na+ or K+. These amino acids are at the outer end of transmembrane helices TM1 and TM2 that undergo large conformational changes as the enzyme cycles from the E1/Na+ binding conformation to the E2/K+ favored conformation. However, the finding that the regulation is observed after acute infusion of Digibind in WT mice with unaltered cation affinities excludes any mechanism based on long-term effects. It remains possible that the regulatory mechanism may include a dynamic change in cation affinity in addition to a ligand binding step.
The time frame of this regulation is informative. The contraction-related change in transport comes into play within seconds to minutes of the onset of muscle contraction. This time frame is too rapid to be explained by chronic conditions or signaling through the nucleus. Previous studies have identified a hemodynamic role of the cardiac glycoside-binding site in chronic blood pressure regulation (14), and a long-term signaling role in cell growth and proliferation (35). This study reveals a direct role of cardiac glycoside affinity in the dynamic regulation of Na,K-ATPase transport rate, which can operate acutely under the control of muscle activity.
The contribution of the cardiac glycoside binding site to exercise performance likewise comes into play rapidly. It is revealed within a specific exercise time and intensity window, below running speeds of 20 m/min. The significance of this window is not known. In humans, a beneficial effect of increased Na,K-ATPase transport capacity is seen within the first 1–2 min of the onset of moderate exercise but not during high-intensity exercise (34). Our finding that both genotypes are able to handle the contraction-related bulk interstitial K+ load in vivo when stimulated at near maximal physiological rates is consistent with this observation. The speed of 20 m/min marks the point at which running mice reach the lactate threshold (36), indicating a transition from a more aerobic to a more anaerobic energy utilization. Above this transition, many factors that stimulate the Na,K-ATPase are elevated, including lactate, [H+], heat, and insulin. High-intensity exercise may provide a maximal stimulus to multiple regulatory processes, making it difficult to resolve their separate contributions.
The physiological consequences of this regulatory mechanism are not completely known. A mechanism using the binding or unbinding of an endogenous ligand under control of muscle activity could contribute to the wide dynamic range in transport capacity required of skeletal muscle during alternate periods of rest and contraction. It could also provide a rapid trigger for the systemic adaptations required during exercise, because skeletal muscle activity is the physiological drive for a broad program of cardiovascular adaptations that increase O2 delivery to the working muscles.
In summary, this study reports 2 previously unreported findings: (i) the transport activity of the Na,K-ATPase in skeletal muscle is regulated dynamically, under control of muscle activity, by a mechanism that utilizes the cardiac glycoside binding site and an endogenous ligand(s); and (ii) the cardiac glycoside binding site of the α2 Na,K-ATPase isozyme plays a physiological role in the dynamic regulation of exercise performance. These findings underscore the importance of skeletal muscle in the physiological role(s) of endogenous cardiotonic steroids and the need for further studies in this tissue.
Methods
Animals.
The mice had a WT or cardiac glycoside resistant genotype (α2R/R) and were developed and maintained as described (11). All procedures were approved by the University of Cincinnati Institutional Animal Care and Use Committee. The mice were anesthetized (2.5% Avertin, 17 mL/kg) during all surgical procedures and were euthanized after the experiment.
Western Blot Analysis.
Na,K-ATPase α-isoform expression was measured by Western blot analysis using α1- and α2-specific antibodies, as described previously (20, 36). The α3 isoform is not detected in this preparation (20). See supporting information (SI) for additional details of the protocols and statistical analysis.
Ouabain Binding.
Equilibrium ouabain binding to a partially purified preparation of skeletal muscle membranes (identical to that used for immunoblot) was measured in a reaction mix containing binding buffer [250 mM sucrose, 3 mM MgSO4,1 mM NaVO4, .02% saponin (pH 7.5)], 20 nM 3H-ouabain (15 Ci/mL), and various concentrations of unlabeled ouabain, in a volume of 150 μL. Binding was initiated by the addition of protein (15 μg) and carried out for 40 min at 37 °C. The reaction was stopped by filtration onto cellulose acetate filters (0.22 μm). Filters were washed with cold buffer and counted. Nonspecific binding was measured by using a 100-fold excess of unlabeled ouabain. The data were fitted to a standard 1-site saturation binding model for total plus nonspecific binding (Graphpad Software).
Interstitial Potassium Concentration.
The potassium concentration of the muscle interstitial space, [KISF], was measured by microdialysis. A probe (CMA/20, 0.5 × 4 mm; CMA Microdialysis) was inserted into the gastrocnemius lateralis muscle through a small slit in the proximal tendon and positioned parallel to the muscle fibers between fiber bundles. It was dialyzed with physiological saline [147 mM Na, 4 mM K, 2.3 mM Ca, 156 mM Cl (pH 7.4)] and allowed to equilibrate for 1 h before dialysate was collected. Resting [KISF] was measured by collecting the dialysate for 24 min at a flow rate of 0.5 μL/min. [KISF] in contracting muscles was measured by collecting the dialysate over successive 6-min intervals at 2 μL/min while the muscle was stimulated to contract, and for 6 min after contraction. The latter reflects a quasi steady-state average of K+ concentrations along the length of the muscle over the 6-min collection period. Contractions were evoked by extracellular stimulation of the intact sciatic nerve in the proximal area of the knee. Isotonic contractions were chosen because they are close to the in vivo contractile mode and produce large changes in Na,K-ATPase transport (18). The potassium concentration of the dialysate was determined by flame photometry and calibrated to standard curves of dialysate potassium vs. [K] standards prepared before each experiment, with an accuracy of ± 0.2 mM.
86Rb Uptake.
86Rb uptake was measured in isolated EDL muscles at rest and during evoked contraction (29). The mouse EDL is a fast-twitch muscle and has a fiber type composition (49% FG, 51% FOG) that is representative of the hind limb muscles used in running (38). The muscles were extracted from WT, α2R/R, or Digibind- (digoxin immune Fab; GlaxoSmithKline) infused mice. For the latter, 150–200 nmol/g of body weight Digibind was infused via a femoral catheter 40 min before tissue extraction. Muscles were attached vertically to a holder and allowed to shorten freely. Stimulation was delivered via transverse wire electrodes positioned at the center of the muscle. The Na,K-ATPase can be either stimulated or inhibited by contraction. For comparing the contraction-related Rb uptake in different genotypes, both WT and α2R/R mice were stimulated under identical conditions. We chose high-intensity stimulation (90 Hz, continuous, for 30 sec), a condition that elicits decreased NKA transport in the WT EDL. Paired muscles from the left and right leg were processed in parallel. The muscles were maintained in a Hepes-buffered physiological saline at room temperature during mounting and preparation (10–30 min). The muscles were then placed in a CO2 incubator maintained at 30 C, 5% CO2, and ambient oxygen concentration and transferred sequentially through the following protocol: (i) equilibration for 15 min in a Krebs buffer containing 130 mM NaCl, 30 mM NaHCO3, 4 mM KCl, 3 mM NaH2PO4, 1 mM MgCl2, 2.5 mM CaCl2, 5 mM glucose (pH 7.4–7.5); (ii) either no stimulation or continuous stimulation of the contralateral muscle for 30 sec at 90 Hz (0.1-ms pulses of amplitude 2.5-fold above threshold potential; (iii) transfer to Krebs buffer containing 0.1–0.2 μCi/mL 86Rb for 60 sec; (iv) 3 sequential 15-min washes in isotope-free bicarbonate Krebs Ringer at 0 °C with shaking; (v) transfer to plastic scintillation vials containing 4 mL of 0.3 M trichloroacetic acid for 15 min with shaking; and (vi) counting of the activity retained in the muscles by Cerenkov radiation (LS6500 Scintillation Counter; Beckman). The activity of the uptake medium was measured for each experiment and calculated as cpm/nmol of K+, by using the concentrations of 86Rb and K+ in the uptake buffer. Data were expressed as nanomoles of Rb taken up per gram of weight per minute (nmol/g·min). The contraction-related Rb uptake, Δ transport, was computed as difference between resting and stimulated uptake in paired muscles, after verifying that nonspecific uptake was not significantly different in resting and stimulated muscles. Nonspecific uptake was measured by including 1 mM ouabain in every step. Mean nonspecific 86Rb uptake was 103.3 ± 8.9 (5) and 111.5 ± 7.5 (5) nmol/g·min in resting and stimulated muscles, respectively; the mean difference in nonspecific uptake between paired stimulated and resting muscles was 10.1 nmol/g·min.
Treadmill Running.
Untrained male and female mice (42 WT weighing 25.2 ± 0.7 g, and 47 α2R/R weighing 26.7 ± 0.6 g) were exercised on a computerized treadmill (Accuscan Instruments), which logged interruptions in running time in 1-sec intervals. Exercise performance was assessed as the number of interruptions (failure to run) accumulated in each 2-min interval, during either a high-intensity or a graded treadmill run. In the high-intensity test, the mice ran at a fixed running speed of 24 m/min for 20 min at a 6° incline. The graded exercise test subjected the mice to stepwise 2 m/min increases in speed every 2 min, from 14 m/min up to 30 m/min, at a 6° incline. See SI for additional details of the treadmill protocols and statistical analysis.
Chemicals and Isotopes.
All chemicals were of analytical grade. Ouabain was from Sigma–Aldrich. 3H-ouabain (specific activity 15.7 Ci/mmol) was from PerkinElmer.
Data Analysis.
Data were expressed as the Mean ± SEM (n). Significant differences between genotypes was determined by using a 2-tailed t test for paired or unpaired observations, as appropriate, or ANOVA with Tukey's post hoc test, as indicated, with significance set at P < 0.05.
Supplementary Material
Acknowledgments.
The authors acknowledge the Physiology Research Fund, the National Institutes of Health (HL28573, HL66062), and Mr. Nicholas Heiny for their support of this research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804150106/DCSupplemental.
References
- 1.Manunta P, Ferrandi M, Messaggio E, Ferrari P. A new antihypertensive agent that antagonizes the prohypertensive effect of endogenous ouabain and adducin. Cardiovasc Hematol Agents Med Chem. 2006;4:61–66. doi: 10.2174/187152506775268811. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari P, Ferrandi M, Valentini G, Bianchi G. Rostafuroxin: An ouabain antagonist that corrects renal and vascular Na+-K+-ATPase alterations in ouabain and adducin-dependent hypertension. Am J Physiol. 2006;290:R529–R535. doi: 10.1152/ajpregu.00518.2005. [DOI] [PubMed] [Google Scholar]
- 3.Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides and their mechanisms of action. Am J Cardiovasc Drugs. 2007;7:173–189. doi: 10.2165/00129784-200707030-00004. [DOI] [PubMed] [Google Scholar]
- 4.Hamlyn JM, et al. A circulating inhibitor of (Na+-K+) ATPase associated with essential hypertension. Nature. 1982;300:650–652. doi: 10.1038/300650a0. [DOI] [PubMed] [Google Scholar]
- 5.Moreth K, Kuske R, Renner D, Schoner W. Blood pressure in essential hypertension correlates with the concentration of a circulating inhibitor of the sodium pump. Klin Wochenschr. 1986;64:239–244. doi: 10.1007/BF01711656. [DOI] [PubMed] [Google Scholar]
- 6.Bauer N, et al. Ouabain-like compound changes rapidly on physical exercise in humans and dogs: Effects of beta-blockade and angiotensin-converting enzyme inhibition. Hypertension. 2005;45:1024–1028. doi: 10.1161/01.HYP.0000165024.47728.f7. [DOI] [PubMed] [Google Scholar]
- 7.Goto A, Yamada K. Ouabain-like factor. Curr Opin Nephrol Hypertens. 1998;7:189–196. doi: 10.1097/00041552-199803000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Valdes R, Jr, Graves SW, Knight AB, Craig HR. Endogenous digoxin immunoactivity is elevated in hypertensive pregnancy. Prog Clin Biol Res. 1985;192:229–232. [PubMed] [Google Scholar]
- 9.Kaplan JH. Biochemistry of Na,K-ATPase. Annu Rev Biochem. 2002;71:511–535. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- 10.Dostanic-Larson I, et al. Physiological role of the alpha1- and alpha2-isoforms of the Na+-K+-ATPase and biological significance of their cardiac glycoside binding site. Am J Physiol. 2006;290:R524–R528. doi: 10.1152/ajpregu.00838.2005. [DOI] [PubMed] [Google Scholar]
- 11.Dostanic I, et al. The alpha2 isoform of Na,K-ATPase mediates ouabain-induced cardiac inotropy in mice. J Biol Chem. 2003;278:53026–53034. doi: 10.1074/jbc.M308547200. [DOI] [PubMed] [Google Scholar]
- 12.Price EM, Lingrel JB. Structure–function relationships in the Na,K-ATPase alpha subunit: Site-directed mutagenesis of glutamine-111 to arginine and asparagine-122 to aspartic acid generates a ouabain-resistant enzyme. Biochemistry. 1988;27:8400–8408. doi: 10.1021/bi00422a016. [DOI] [PubMed] [Google Scholar]
- 13.Qiu LY, et al. Reconstruction of the complete ouabain-binding pocket of Na,K-ATPase in gastric H,K-ATPase by substitution of only seven amino acids. J Biol Chem. 2005;280:32349–32355. doi: 10.1074/jbc.M505168200. [DOI] [PubMed] [Google Scholar]
- 14.Dostanic-Larson I, Van Huysse JW, Lorenz JN, Lingrel JB. The highly conserved cardiac glycoside binding site of Na,K-ATPase plays a role in blood pressure regulation. Proc Natl Acad Sci USA. 2005;102:15845–15850. doi: 10.1073/pnas.0507358102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He S, et al. The alpha(1)- and alpha(2)-isoforms of Na-K-ATPase play different roles in skeletal muscle contractility. Am J Physiol. 2001;281:R917–R925. doi: 10.1152/ajpregu.2001.281.3.R917. [DOI] [PubMed] [Google Scholar]
- 16.Clausen T. The Na+,K+ pump in skeletal muscle: Quantification, regulation and functional significance. Acta Physiol Scand. 1996;156:227–235. doi: 10.1046/j.1365-201X.1996.209000.x. [DOI] [PubMed] [Google Scholar]
- 17.Clausen T, Everts ME, Kjeldsen K. Quantification of the maximum capacity for active sodium-potassium transport in rat skeletal muscle. J Physiol. 1987;388:163–181. doi: 10.1113/jphysiol.1987.sp016608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nielsen OB, Clausen T. Regulation of Na+-K+ pump activity in contracting rat muscle. J Physiol. 1997;503(Pt 3):571–581. doi: 10.1111/j.1469-7793.1997.571bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clausen T. Na+-K+ pump regulation and skeletal muscle contractility. Physiol Rev. 2003;83:1269–1324. doi: 10.1152/physrev.00011.2003. [DOI] [PubMed] [Google Scholar]
- 20.Radzyukevich TL, et al. The Na(+)-K(+)-ATPase alpha2-subunit isoform modulates contractility in the perinatal mouse diaphragm. Am J Physiol. 2004;287:C1300–C1310. doi: 10.1152/ajpcell.00231.2004. [DOI] [PubMed] [Google Scholar]
- 21.Lau YH, Caswell AH, Garcia M, Letellier L. Ouabain binding and coupled sodium, potassium, and chloride transport in isolated transverse tubules of skeletal muscle. J Gen Physiol. 1979;74:335–349. doi: 10.1085/jgp.74.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clausen T. The sodium pump keeps us going. Ann NY Acad Sci. 2003;986:595–602. doi: 10.1111/j.1749-6632.2003.tb07258.x. [DOI] [PubMed] [Google Scholar]
- 23.Morris RG, Hoorn EJ, Knepper MA. Hypokalemia in a mouse model of Gitelman's syndrome. Am J Physiol. 2006;290:F1416–F1420. doi: 10.1152/ajprenal.00421.2005. [DOI] [PubMed] [Google Scholar]
- 24.Arrighi I, et al. Altered potassium balance and aldosterone secretion in a mouse model of human congenital long QT syndrome. Proc Natl Acad Sci USA. 2001;98:8792–8797. doi: 10.1073/pnas.141233398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hnik P, et al. Work-induced potassium changes in skeletal muscle and effluent venous blood assessed by liquid ion-exchanger microelectrodes. Pflugers Arch. 1976;362:85–94. doi: 10.1007/BF00588685. [DOI] [PubMed] [Google Scholar]
- 26.Hirche H, Schumacher E, Hagemann H. Extracellular K+ concentration and K+ balance of the gastrocnemius muscle of the dog during exercise. Pflugers Arch. 1980;387:231–237. doi: 10.1007/BF00580975. [DOI] [PubMed] [Google Scholar]
- 27.Juel C. Potassium and sodium shifts during in vitro isometric muscle contraction, and the time course of the ion-gradient recovery. Pflugers Arch. 1986;406:458–463. doi: 10.1007/BF00583367. [DOI] [PubMed] [Google Scholar]
- 28.Williams MW, et al. Na,K-ATPase in skeletal muscle: Two populations of beta-spectrin control localization in the sarcolemma but not partitioning between the sarcolemma and the transverse tubules. J Cell Sci. 2001;114:751–762. doi: 10.1242/jcs.114.4.751. [DOI] [PubMed] [Google Scholar]
- 29.Dorup I, Clausen T. 86Rb is not a reliable tracer for potassium in skeletal muscle. Biochem J. 1994;302(Pt 3):745–751. doi: 10.1042/bj3020745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Everts ME, Clausen T. Excitation-induced activation of the Na+-K+ pump in rat skeletal muscle. Am J Physiol. 1994;266:C925–C934. doi: 10.1152/ajpcell.1994.266.4.C925. [DOI] [PubMed] [Google Scholar]
- 31.Pullen MA, Harpel MR, Danoff TM, Brooks DP. Comparison of non-digitalis binding properties of digoxin-specific Fabs using direct binding methods. J Immunol Methods. 2008;336(2):235–241. doi: 10.1016/j.jim.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Clausen T, Hansen O, Kjeldsen K, Norgaard A. Effect of age, potassium depletion and denervation on specific displaceable [3H] ouabain binding in rat skeletal muscle in vivo. J Physiol. 1982;333:367–381. doi: 10.1113/jphysiol.1982.sp014458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hicks A, McComas AJ. Increased sodium pump activity following repetitive stimulation of rat soleus muscles. J Physiol. 1989;414:337–349. doi: 10.1113/jphysiol.1989.sp017691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nordsborg N, et al. Effect of dexamethasone on skeletal muscle Na+,K+ pump subunit specific expression and K+ homeostasis during exercise in humans. J Physiol. 2008;586:1447–1459. doi: 10.1113/jphysiol.2007.143073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides: Their roles in hypertension, salt metabolism, and cell growth. Am J Physiol. 2007;293:509–536. doi: 10.1152/ajpcell.00098.2007. [DOI] [PubMed] [Google Scholar]
- 36.Billat VL, Mouisel E, Roblot N, Melki J. Inter- and intrastrain variation in mouse critical running speed. J Appl Physiol. 2005;98:1258–1263. doi: 10.1152/japplphysiol.00991.2004. [DOI] [PubMed] [Google Scholar]
- 37.Cougnon MH, Moseley AE, Radzyukevich TL, Lingrel JB, Heiny JA. Na,K-ATPase alpha- and beta-isoform expression in developing skeletal muscles: Alpha(2) correlates with t-tubule formation. Pflugers Arch. 2002;445:123–131. doi: 10.1007/s00424-002-0898-6. [DOI] [PubMed] [Google Scholar]
- 38.Burkholder TJ, Fingado B, Baron S, Lieber RL. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J Morphol. 1994;221:177–190. doi: 10.1002/jmor.1052210207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.