Abstract
Smoothened (Smo), a 7-pass transmembrane protein, is essential for transduction of a Hedgehog (Hh) signal across the cell membrane. Smo is also the principle therapeutic target for several candidate drugs in the treatment of Hh-related diseases. Mammalian Smo translocates to the primary cilium in response to Sonic hedgehog (Shh) ligand-mediated signaling. A mechanistic understanding of Smo translocation and its interactions with drug candidates is pivotal to our understanding of Hh signaling and the design, development and application of successful drugs. We established a system in which Smo was dual-labeled with GFP and a 12-aa tag whose recognition by an enzymatic process enables the posttranslational labeling of Smo in the cell membrane within the living cell. These tools enable the simultaneous visualization of all cellular Smo, and more specifically, the cell membrane restricted subpopulation. Using this system, we demonstrate that cyclopamine, a widely used Hh antagonist, induces a cilial translocation of Smo similar to that reported for Shh ligand and several Hh agonists. In contrast, other antagonists abrogate the Shh-induced, cilial translocation of Smo. We present evidence that the majority of cilial-localized Smo originates from an intracellular source and may traffic to the primary cilium through an intraflagellar transport (IFT) pathway.
Keywords: intraflagellar transport, PPTase labeling, drug development
Hedgehog (Hh) signaling is involved in multiple developmental pathways and several adult homeostatic processes. As a consequence, a tight regulation of Hh signaling is essential in building and maintaining functional systems in the body (for review, see ref. 1). In the mammalian signaling pathway, the binding of a Hh ligand to its receptor Patched 1(Ptch1) relieves Ptch1-mediated inhibition of a second transmembrane protein Smoothened (Smo). Activation of Smo triggers an intracellular signal transduction cascade that culminates in Gli-dependent, transcriptional activities. Because genetic ablation of Smo leads to a complete loss of Hh responsiveness in target cells, Smo is clearly essential for Hh signal transduction (2). In contrast, activating mutations in Smo result in a hyperstimulation of Hh signaling (3).
Hh signaling is linked to a number of human diseases, notably several neurodegenerative diseases and a variety of cancers, including basal cell carcinoma (BCC), medulloblastoma (MB), rhabdomyosarcoma, pancreatic caner, prostate cancer, and lung cancer (reviewed in ref. 4). The identification of mutations in Hh pathway components has stimulated the identification of over a dozen Hh agonists and antagonists that fall into chemically distinct classes (4–12). Remarkably, Smo is the target protein for most of these compounds. A mechanistic understanding of Smo and how it interacts with these compounds is essential to the development of effective drugs and therapies for Hh-related diseases. Here, the mechanisms of Hh antagonist activities are of particular interest given their potential use in cancer therapy (4, 13, 14). A widely studied Hh antagonist is cyclopamine, a natural product derived from corn lily (15). Further, a number of cyclopamine derivatives have been described that offer improved pharmacological and inhibitory properties (16).
The primary cilium has emerged as a central organelle essential for Hh signal transduction in mammalian systems (reviewed in ref. 17). Multiple components of the Hh pathway localize to the primary cilium or its basal body, including Sonic Hedgehog (Shh) (10, 18), Ptch1 (10), Smo (10, 19–24), suppressor of Fused (suFU) (a key negative regulator of Gli activity), and Gli transcription factors (21, 24, 25). In the absence of Shh, Ptch1 is enriched in the primary cilium of cultured mammalian cells; its cilial localization is lost after engagement with Shh ligand (10). Conversely, Smo becomes enriched on the primary cilium upon treatment with Shh or several pathway stimulators including the small molecule agonist, Ag1.3 (also known as SAG) (6, 8, 10) and 20α-hydroxycholesterol (20-OHC), a candidate small molecule for communication between Ptch1 and Smo (7, 10). Interestingly, a tryptophan to leucine mutation in the 7th transmembrane helix bundle of Smo generates a dominant-active form (SmoA1), that constitutively localizes to the primary cilium (19, 20). Downstream of Smo, suFU and Gli proteins concentrate in or about the primary cilium (21, 24, 25). Further, their normal function depends on the intact primary cilium (23, 25–28). How all these Hh pathway components traffic in and out of the primary cilium and how the cilial localization modulates their activities is not clear.
Intraflagellar transport (IFT) is required for the assembly and maintenance of the primary cilium. Membrane proteins are thought to be transported within the primary cilium via IFT particles. These particles consist of 16 known IFT proteins that form 2 multiprotein IFT complexes, A and B. The movement of IFT particles from the base to the tip of cilium is powered by anterograde kinesin-II motor complexes, whereas retrograde transport is driven by dynein motor complexes (29). A correlation between Hh signaling and the primary cilium was first made when mouse mutations removing IFT function were shown to lead to a failure of cilial formation resulting in a spectrum of Hh-related phenotypes (23, 25–28, 30). These phenotypes may result as an indirect consequence of ciliary abnormalities or more directly if IFT trafficking is itself a component of Hh signaling.
To dynamically visualize protein distribution within the cell, genetic fusion to fluorescent protein (FP) has been a powerful strategy. However, the large mass of the FP component and their restricted spectra limit their application. Site specific posttranslational labeling is an emerging complementary technology (reviewed in ref. 31). One such approach is based on the enzymology of phosphopantetheinyl transferases (PPTases), either holo-(acyl carrier protein) synthase (AcpS) or Sfp. AcpS transfers the phosphopantetheinyl (Ppant) moiety from CoA to a conserved serine residue in the acyl carrier protein (ACP). This enzyme tolerates a wide range of modifications at the terminal thiol of CoA, allowing the transfer of a broad spectrum of fluorophores to an ACP on a tagged protein. Recent progress allows for the replacement of an ACP with 12-aa tags or even 8-aa tags (32, 33). PPTase labeling enables membrane proteins to be tracked with “built-in” high temporal and spatial resolution, with a minimal shift in molecular mass and a growing library of bright, photostable fluorophores that act across the visible spectrum. Because the time of labeling can be precisely controlled in the living cell, and the labeling reaction is rapid—typically completed within 15–30 min—multicolor pulse–chase experiments are facilitated by this approach. Further, PPTase specifically labels the extracellular component of cell membrane-bound proteins; consequently, surface and intracellular pools of a given membrane protein can be distinguished in the living cell.
In this study, we have used a Smo::FP fusion, in conjunction with AcpS labeling, to examine Smo distribution in response to a number of small molecule modulators of Hh pathway activity. Several Hh agonists and one antagonist, cyclopamine, stimulate the accumulation of Smo in the primary cilium of cultured cells. In contrast, other antagonists inhibit Shh-mediated Smo translocation to the primary cilium. Using AcpS labeling with a 12-aa tag, we provide evidence that most cilial localized Smo originates from intracellular protein pools and not from the cell membrane. The colocalization of IFT88, an anterograde IFT protein, with intracellular Smo suggests that Smo may traffic through an IFT mediated pathway.
Results
An A1::Smo::GFP Construct Is Functionally Equivalent to Endogenous Smo.
To investigate Smo localization and its trafficking mechanism, we established a system that combines conventional labeling with fluorescent proteins and a PPTase labeling technique (Fig. 1). GFP was fused to the intracellular C terminus of human Smo and a 12-aa A1 tag was added at the extracellular N terminus following the signal peptide sequence (Fig. 1A). The A1 peptide tag is specifically recognized by the AcpS enzyme. Enzymatic activity transfers a Ppant group in the CoA substrate to a serine residue in the A1 tag (Fig. 1B), thereby enabling fluorescent labeling of A1::Smo::GFP, using CoA substrates conjugated with small fluorophores such as Texas Red (TxRed).
Fig. 1.
Generation of a dual labeled A1::Smo::GFP reporter construct. (A) A 12-aa A1 tag was added to the extracellular N terminus of human Smo, whereas GFP was fused to the intracellular C terminus. (B) AcpS enzyme transfers a fluorophore to the serine in the A1 tag (marked as red in A) through a Ppant group in the CoA-fluorophore substrate, thereby labeling A1::Smo::GFP with a specific fluorophore.
A1::Smo::GFP or a control GFP construct were introduced into Shh-responsive NIH/3T3 cells by retrovirus infection. Multiple clonal lines of A1::Smo::GFP expressing cells were selected based on the expression level and translocation behavior of A1::Smo::GFP. At least two clonal lines were used to validate the results throughout this study. In the absence of Shh ligand, endogenous Smo has a diffuse distribution in cells (Fig. 2A). After treatment with ShhN (the N-terminal signaling fragment of Shh), Smo translocates to the primary cilium above the γ-tubulin-positive, basal body (Fig. 2B). A1::Smo::GFP fusion proteins showed a similar ShhN dependent distribution to endogenous mouse Smo, when they were detected with both GFP and Smo antibody (Fig. 2 C and D) (10, 19–24). Cilial accumulation of A1::Smo::GFP in response to ShhN treatment was confirmed by costaining with two additional primary cilium markers—anti-Arl13b (Fig. 2 E and F) and Inversin::Cherry (Fig. S1 A and B). Arl13b is a GTPase required for proper cilia formation (28), whereas Inversin is linked to the Wnt signaling pathway (34).
Fig. 2.
A1::Smo::GFP is functionally equivalent to endogenous Smo. (A–F) NIH/3T3 cells expressing GFP (A and B) or A1::Smo::GFP (C and D) were either untreated (A, C, and E) or treated with ShhN ligand (B, D, and F). GFP (green in A and B) and A1::Smo::GFP (green in C–F) were visualized by direct detection of GFP. Endogenous Smo (red in A–D) and A1::Smo::GFP (red in C and D) were detected with anti-Smo antibody. Cilial basal bodies were visualized with γ-tubulin antibody (blue in A–F). Arl13b was also identified with immunofluorescence (red in E and F). (Scale bars: 5 μm.) (G) Hh signaling was assessed in NIH/3T3 cells by activation of a Ptch1-promoter driven luciferase reporter in response to ShhN ligand. Cells either expressed GFP (blue bars) or A1::Smo::GFP (red bars) and in some samples they were transfected with short hairpin RNA constructs to knock down GFP (shGFP) and/or endogenous mouse Smo (shmSmo-1 and shmSmo-2; recognizing nonoverlapping regions specific to mouse and not human Smo). The result shown is the mean of 4 replicates. Error bars indicate SD.
The functionality of the A1::Smo::GFP construct in Hh signaling was addressed in Gli-luciferase assays, using Gli dependent transcription activity as a readout (Fig. 2G). An equal amount of a Ptch1-promoter driven luciferase reporter construct was introduced into either A1::Smo::GFP cells or a control cell line producing GFP only. Cotransfection of short hairpin (sh) RNA constructs against GFP and mouse Smo was used to knock down A1::Smo::GFP and endogenous Smo, respectively, in transfected cells (Fig. S2) (35, 36). Cells were serum starved to promote cilial assembly, then treated with ShhN ligand or mock medium. A1::Smo::GFP cells were equally responsive to ShhN as the control cell line, whereas knockdown of GFP did not significantly attenuate ShhN responsiveness. As expected, knockdown of mouse Smo dramatically inhibited ShhN responsiveness of the control GFP cell line but not the A1::Smo::GFP cell line (Fig. 2G). However, knockdown of both GFP and endogenous Smo dramatically inhibited ShhN responsiveness in A1::Smo::GFP cells (Fig. 2G). Together, these data demonstrate that A1::Smo::GFP is equivalent to endogenous Smo in terms of both its translocation behavior in response to ShhN ligand and Hh signaling activity in ShhN treated cells.
Different Classes of Agonists and Antagonists Differentially Influence Smo Localization.
Having established A1::Smo::GFP as a useful tool for visualizing Smo in Hh responsive cells, we compared the localization of Smo to that of the general, primary cilium marker, Arl13b, when cells were treated with a range of compounds that modulate Hh signaling (summarized in Table S1). Consistent with a previous report (10), Smo translocated to the primary cilium when NIH/3T3 cells were treated with SAG, 20-OHC (Fig. 3 A and B and Fig. S3 A and C) or a third pathway agonist, purmorphamine (Fig. S3 A and B). Surprisingly, cyclopamine, a widely used Hh antagonist, also drove Smo to the primary cilium, in contrast to a previous report examining MDCK cells (19). Conversely, other antagonists including SANT-1, SANT-2 and GANT61 abrogate the ShhN-dependent translocation of Smo to the primary cilium (Fig. 3 A and C–G and Fig. S4), whereas neither of them induces obvious localization change of Smo when applied alone (data not shown). Similar results were obtained in an A1::Smo::GFP/Inversin::Cherry cell line, where primary cilia were labeled with an Inversin::Cherry fusion protein (Fig. S1) (34). Because the cilial localization of Inversin::Cherry was unaltered by any of the above treatments (Fig. S1), the observed effects of Hh agonists and antagonists on A1::Smo::GFP cilial translocation were specific and not a more general effect of altered cilial trafficking. Importantly, Gli-luciferase reporter assays demonstrated that all small molecule treatments produced the expected effects on Hh signaling activity when compared with their published properties (Fig. S5) (4–12).
Fig. 3.
Effects on Smo localization and Hh activity by various Hh agonists and antagonists. (A–G) Cells expressing A1::Smo::GFP were treated with DMSO (A); SAG (B), cyclopamine (CYC) (C); ShhN (D); or a combination of ShhN and either SANT-1 (E), SANT-2 (F), or GANT61 (G). A1::Smo::GFP (green in A–G) was visualized by direct detection of GFP. Primary cilia were marked with Arl13b antibody (red in A–G). (Scale bars: 5 μm.) (H) Hh signaling was activated by either ShhN ligand or RNAi knock down of suFU and assessed in Gli-luciferase assays. NIH/3T3 cells were either untreated or treated with one of the Hh antagonists, either CYC, SANT-1, SANT-2, or GANT61. The result shown is the mean of 4 replicates. Error bars depict SD.
To determine how these different antagonists function in the Hh pathway, we performed an epistasis analysis in NIH/3T3 cells. All four compounds inhibited ShhN stimulated Hh activity; however, only GANT61 inhibited Gli activity when the endogenous Gli antagonist suFU was knocked down (Fig. 3H) (37, 38). These data are in good agreement with published reports (6, 9). SANT-1 and SANT-2 function upstream of suFU, most likely at the level of Smo because they both compete for cyclopamine binding of Smo (6). In contrast, GANT61 function downstream of suFU places GANT61 at the level of Gli transcriptional regulators (9).
Cilial Accumulation of Smo Originates From an Intracellular Source.
We next addressed the cellular source of Smo that accumulates on the primary cilium, using PPTase labeling technology. AcpS enzyme and CoA substrates were added to the culture medium to label A1::Smo::GFP in living cells. Because the high, negative charge of the CoA substrates prevents their penetration through the cell membrane, only A1::Smo::GFP on the cell surface can be labeled by this approach (32, 33). Thus, A1::Smo::GFP can be distinguished in cell membrane and intracellular pools.
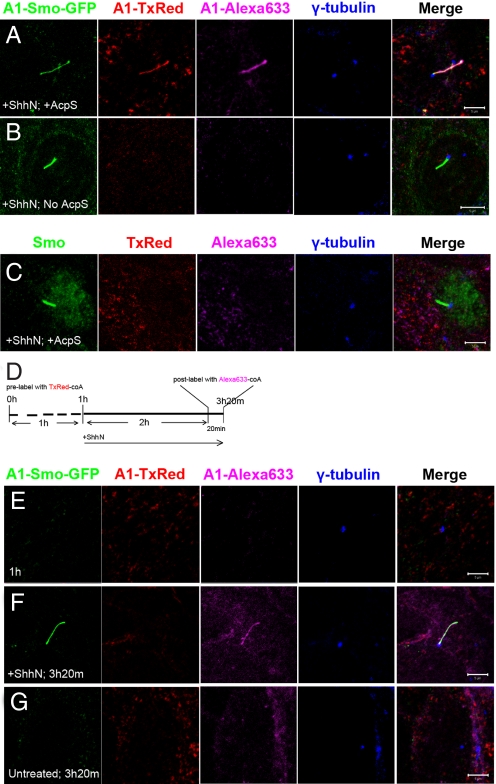
AcpS labeling efficiency was examined with two different fluorophores—TxRed and Alexa633. After ShhN stimulated accumulation of A1::Smo::GFP, a 20-min labeling reaction was performed with two CoA substrates conjugated with either TxRed or Alexa633. A1::Smo::GFP was effectively labeled with both fluorophores (Fig. 4A) in a reaction dependent on both AcpS (Fig. 4B) and A1 tag (Fig. 4C). Next, A1::Smo::GFP cells were grown in conditions that stimulate de novo assembly of the primary cilium (see Materials and Methods). Unstimulated cells were incubated for 1 h with AcpS enzyme and TxRed-CoA to saturate labeling of A1::Smo::GFP on the membrane surface (Fig. 4 D and E). After this, cells were treated with ShhN ligand for 2 h and labeled with Alexa633-CoA (Fig. 4D). A1::Smo::GFP on the primary cilium was only labeled by Alexa633 (Fig. 4 F and G). To confirm that cell surface labeling was indeed saturated after 1 h of incubation, we incubated ShhN-treated cells consecutively with two different fluorescent substrates, for 20 min each (Fig. S6A). Labeling was only observed with the first fluorophore, consistent with a rapid saturation of available A1 tag sites in the first reaction (Fig. S6B). The data suggest that the A1::Smo::GFP that translocates to the primary cilium on ShhN stimulation originates primarily from an intracellular pool and not from the cell membrane.
Fig. 4.
AcpS labeling of A1::Smo::GFP. (A and B) After ShhN treatment, A1::Smo::GFP cells were incubated with both Texas Red (TxRed)-CoA and Alexa633-CoA either with (A) or without (B) AcpS for 20 min. (C) Wildtype NIH/3T3 cells were also treated with ShhN and incubated in a reaction with AcpS. (D) Experimental procedure for a “pulse–chase” labeling with 2 colors. (E and F) The images depict labeling at 1 h (E), before ShhN treatment, and at 3 h and 20 min (F), after the second labeling with Alexa633. (G) The background level of labeling was obtained following the same process but without ShhN treatment. A1::Smo::GFP was directly visualized by detecting GFP (green in A, B, and E–G). Endogenous Smo in wildtype NIH/3T3 cells was detected by Smo antibody (green in C). Labeling signal with TxRed and Alexa633 is shown in red and purple, respectively. Cilium basal bodies are marked with γ-tubulin antibody (blue in A–C and E–G). (Scale bars: 5 μm.)
One caveat to this interpretation is the kinetics of turn-over of A1::Smo::GFP. A1::Smo::GFP could rapidly translocate from the cell membrane to the primary cilium but then turn over at this site within the 2-h assay period. To directly address A1::Smo::GFP turn-over in the primary cilium, cells were treated with ShhN ligand for 2 h, then labeled with TxRed for 20 min, to directly observe cilial protein at different periods postlabeling. No obvious decrease in signal intensity was observed 2 h after labeling. Further, label was still detected on the primary cilium 8 h after labeling (Fig. S7). Together, these data support the conclusion that intracellular trafficking from a cytoplasmic pool is primarily responsible for the elevation of Smo levels at the primary cilium on ShhN stimulation. Because SAG and cyclopamine treatments gave similar results (data not shown), this pathway is also the likely route for small-molecule driven Smo relocalization.
Smo Likely Trafficks Through an Intraflagellar Transport (IFT) Pathway.
To examine potential pathways of Smo trafficking to the primary cilium, we assayed cytoplasmic colocalization of Smo with vesicle trafficking pathways on ShhN stimulation. Interestingly, we observed noncilial associated, cytoplasmic accumulations of A1::Smo::GFP in response to ShhN ligand (Fig. 5). Further, some A1::Smo::GFP puncta colocalize with endogenous IFT88 (Polaris), an anterograde IFT protein (25), raising the possibility that Smo might traffick through an IFT mechanism. These puncta, unlike cilial A1::Smo::GFP, were not labeled by AcpS indicating that the noncilial IFT88 associated accumulations did not originate from the cell surface (Fig. 5B). Further, the bulk of A1::Smo::GFP did not colocalize with Transferin receptors (TfR), a general marker for endocytosis (Fig. S8A) (39), EEA-1, an early endosome marker (Fig. S8B) (39), or mannose 6 phosphate receptor (M6PR), a maker for late endosomes and lysosomes (Fig. S8C) (40). Together, these data argues against an endosomal pathway of membrane translocation. A BBSome complex has recently been linked to membrane vesicle trafficking to the base of the primary cilium (41). However, A1::Smo::GFP did not colocalize with PCM-1, a key component of BBSome complex. Thus, Smo is also unlikely to traffic through the BBSome pathway (41, 42).
Fig. 5.
A1::Smo::GFP colocalizes with IFT88. Cells expressing A1::Smo::GFP (green) were untreated (A) or treated with ShhN (B) and labeled at the cell surface with Alexa633 (purple), followed by staining with IFT88 (red) and γ-tubulin (blue). Arrows indicate localization to the basal bodies of primary cilia; arrowheads denote noncilial localization to intracellular puncta. (Scale bars: 5 μm.)
Discussion
Pharmacological Implications for The Different Inhibitory Mechanisms of Cyclopamine and Other Antagonists.
Understanding the normal cellular functions and properties of drug targets and how these are influenced by a drug is critical to both the development and the successful clinical application of a drug. “Mechanism-based” drug discovery, where drug development efforts are directed by identifying and better understanding the mechanisms by which drugs and their targets function, promises to increase the efficiency of drug development and to better address safety issues (43). Hh signaling is one critical pathway of interest based on its roles in both development and diseases. From many screens, Smo has emerged as the target for a majority of known compounds that modulate Hh signaling. Consequently, Smo has assumed an importance in pharmacological development of Hh agonists and antagonists. Here, we have presented evidence indicating that cyclopamine, SANT-1/2, and GANT61 have different effects on Smo translocation to the primary cilium (Fig. 3 and Figs. S1 and S4). SANT-1/2 have been suggested to act at the same level as cyclopamine given that they compete with cyclopamine for binding to Smo (6). However, SANT-1/2 inhibit a dominant active form of Smo (SmoA1) at a comparable dose to that required to inhibit wild-type Smo (6), whereas cyclopamine has to be applied at a much higher dose to block SmoA1 activity (15). Given that SANT-1/2 block Smo accumulation at the primary cilium (Fig. 3 D–F and Figs. S1 B, G, and H and Fig. S4), whereas cyclopamine triggers translocation (Fig. 3C and Figs. S1F and S4), and SANT-1/2 are chemically distinct compounds from cyclopamine (Table S1), we speculate that their binding to Smo occurs at different sites, and further, their binding induces different conformational changes in Smo protein and/or differentially disrupts Smo interaction with other cellular factors.
GANT61 functions downstream of Smo and suFU in the inhibition of Shh signaling (Fig. 3H). Because GANT61 inhibits Gli1 binding to DNA (9), GANT61 may function at the level of Gli factors modulating their transcriptional activities. Three models may explain the unexpected inhibitory effect we observe of GANT61 on cilial accumulation of Smo. First, Smo accumulation to the primary cilium may depended on active Hh signaling. Second, normal feedback mechanisms may influence Smo cilial translocation. Transcription of Ptch1, Hip1, Cdo, Boc, and Gas1 are all either positively or negatively regulated by Shh signaling and altered levels of these components may indirectly effect Smo cilial translocation. Finally, GANT61 may target cilial trafficking more generally. Because Gli processing and Smo accumulation are both dependent on the primary cilium, both processes could be influenced by a cilial trafficking defect. However, Lauth et al. report that GANT61 can act on nuclear accumulated Gli arguing against this model (9).
Cyclopamine is one of the most widely used Hh antagonists in Hh-cancer research. Prolonged systemic treatment with cyclopamine is reported to significantly diminish tumor formation in UV-irradiated Ptch1+/− mice (44). Cyclopamine can also decrease the rate of growth of mouse medulloblastoma cells in culture and in mouse allograft models (45, 46). Our finding that cyclopamine promotes Smo accumulation at the primary cilium suggests a possible issue with cyclopamine action where the Smo that has accumulated in the primary cilium may lead to a strong prolonged stimulation after drug removal. Supporting this view, we demonstrate that Smo once in the primary cilium turns over relatively slowly (Fig. S7). These results may have important ramifications in deciding on which drugs to promote for clinical development and in ensuring the maximal “target space” for Hh pathway inhibition.
Smo Trafficking and the IFT Pathway.
Our data suggest that the primary mechanism for Smo accumulation at the primary cilium is via transport from an intracellular store. The finding that Smo and IFT88 colocalize in cytoplasmic accumulations and in the primary cilium provides some evidence for a trafficking process that could move Smo to the primary cilium. In this light, mouse mutants in IFT proteins may have complicated roles in Hh signaling where both cilial structure and Smo transport may be affected. More specific mutants may be required to examine the possibility of distinct, mechanistic activities. Further study exploring the dynamic interactions between Smoothened, IFT88, and other components of the IFT pathway may shed light on their cellular relationships in conjunction with cilial trafficking. Moreover, studying the prestimulation Smo complex may give insights into the mechanism that activate Smo movement from the cytoplasm to the primary cilium. Recently, Ocbina and Anderson (47) showed that suFU also depends on IFT. Given the cilial localization of several major components in the Hh pathway, examining the broad relationships between IFT proteins and Hh pathway components may be of interest.
Short Tag PPTase Labeling, a Versatile Tool to Investigate Membrane Proteins.
Our study demonstrates the efficacy and efficiency of the PPTase labeling technology in examining cell membrane protein accumulation and movement in a living cell. PPTase labeling enabled effective multicolor labeling of A1::Smo::GFP, providing the first insights into the dynamics of Smo turnover in the primary cilium. Further, the “built-in” spatial resolution of the system, a property of the cell exclusion of CoA substrates, enabled us to study the mechanisms of Smo trafficking distinguishing cell membrane populations from intracellular pools. Future developments such as orthogonal labeling with two different PPTases, Sfp, and AcpS, will enable the investigation of multiple proteins simultaneously (32) and the development of cell permeable substrates will expand this important methodology enabling biomedical researchers to extend molecular imaging studies.
Materials and Methods
Assays for Smo translocation.
All 3T3 cell lines were plated at a density of 1 × 104 cells per well in fibronectin coated Lab-Tek chamber slides (Nunc; catalog no. 178599). The cells were grown to confluence, then switched to DMSO containing 0.5% BCS with indicated treatments for 24 h or as stated in the text. Immuno-staining was performed after fixation in 4% Paraformaldehyde (PFA) according to standard protocols. AcpS labeling reactions were performed as described in ref. 32. Fluorescent images were collected after fixing samples, using a Zeiss LSM510 META on an inverted microscope, with a 63× (1.4 NA) oil-immersion objective lens, using 4 laser lines (405, 488, 543, 633 nm).
Luciferase Assays.
Cells were plated at a density of 5 × 104 per well in 24 well plates 18 h before transfection. DNA transfection was performed using Lipofectamine2000 (Invitrogen; catalog no. 11668); the DNA introduced in each well included 100 ng of CMV driven renilla luciferase construct, 300 ng of Ptch1 promoter driven firefly luciferase construct, and 400 ng of each shRNA construct or a PBS plasmid as a control. Cells were grown for 2 days; then the medium was changed to DMEM containing 0.5% BCS and proteins/compounds indicated in the text. Cells were cultured for an additional 48 h, tryspinized, lysed and assayed using a Promega dual luciferase reporter essay system kit (E1910). Renilla luciferase signal was used to normalize the firefly luciferase signal. The RNAi efficiency was measured as previous described (35). In all luciferase assays, every condition was repeated in quadruplicate wells and experiments were repeated at least 3 times.
Further information is available in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank T. Caspary (Emory University, Atlanta, GA), X. Liu (Harvard Medical School, Boston, MA), A. Merdes (Centre National de la Recherche Scientifique-Pierre Fabre, France), L. Rubin (Harvard University, Cambridge, MA), M. Scott (Stanford University, Palo Alto, CA), J. Taipale (National Public Health Institute, Helsinki, Finland), B. Yoder (University of Alabama, Birmingham, AL) for reagents. We thank B. Allen, L. Rubin, J. Lee for critical review of our manuscript and helpful advice on the experiments. This work was supported by National Institutes of Health Grants R37 NS033642 (to A.P.M.) and GM20011 (to C.T.W.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812110106/DCSupplemental.
References
- 1.McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol. 2003;53:1–114. doi: 10.1016/s0070-2153(03)53002-2. [DOI] [PubMed] [Google Scholar]
- 2.Zhang XM, Ramalho-Santos M, McMahon AP. Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R asymmetry by the mouse node. Cell. 2001;105:781–792. [PubMed] [Google Scholar]
- 3.Xie J, et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–92. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- 4.Rubin LL, de Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov. 2006;5:1026–1033. doi: 10.1038/nrd2086. [DOI] [PubMed] [Google Scholar]
- 5.Bijlsma MF, et al. Repression of smoothened by patched-dependent (pro-) vitamin D3 secretion. PLoS Biol. 2006;4:e232. doi: 10.1371/journal.pbio.0040232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen JK, Taipale J, Young KE, Maiti T, Beachy PA. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci USA. 2002;99:14071–14076. doi: 10.1073/pnas.182542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corcoran RB, Scott MP. Oxysterols stimulate Sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proc Natl Acad Sci USA. 2006;103:8408–8413. doi: 10.1073/pnas.0602852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank-Kamenetsky M, et al. Small-molecule modulators of Hedgehog signaling: Identification and characterization of Smoothened agonists and antagonists. J Biol. 2002;1:10. doi: 10.1186/1475-4924-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lauth M, Bergstrom A, Shimokawa T, Toftgard R. Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc Natl Acad Sci USA. 2007;104:8455–8460. doi: 10.1073/pnas.0609699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 11.Sinha S, Chen JK. Purmorphamine activates the Hedgehog pathway by targeting Smoothened. Nat Chem Biol. 2006;2:29–30. doi: 10.1038/nchembio753. [DOI] [PubMed] [Google Scholar]
- 12.Williams JA, et al. Identification of a small molecule inhibitor of the hedgehog signaling pathway: Effects on basal cell carcinoma-like lesions. Proc Natl Acad Sci USA. 2003;100:4616–4621. doi: 10.1073/pnas.0732813100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimura H, Ng JM, Curran T. Transient inhibition of the Hedgehog pathway in young mice causes permanent defects in bone structure. Cancer Cell. 2008;13:249–260. doi: 10.1016/j.ccr.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 14.Romer JT, et al. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1+/−p53−/− mice. Cancer Cell. 2004;6:229–240. doi: 10.1016/j.ccr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Taipale J, et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 16.Tremblay MR, et al. Semisynthetic cyclopamine analogues as potent and orally bioavailable Hedgehog pathway antagonists. J Med Chem. 2008;51:6646–6649. doi: 10.1021/jm8008508. [DOI] [PubMed] [Google Scholar]
- 17.Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annu Rev Cell Dev Biol. 2007;23:345–373. doi: 10.1146/annurev.cellbio.23.090506.123249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chamberlain CE, Jeong J, Guo C, Allen BL, McMahon AP. Notochord-derived Shh concentrates in close association with the apically positioned basal body in neural target cells and forms a dynamic gradient during neural patterning. Development. 2008;135:1097–1106. doi: 10.1242/dev.013086. [DOI] [PubMed] [Google Scholar]
- 19.Corbit KC, et al. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 20.Han YG, et al. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11:277–284. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- 21.Kiprilov EN, et al. Human embryonic stem cells in culture possess primary cilia with hedgehog signaling machinery. J Cell Biol. 2008;180:897–904. doi: 10.1083/jcb.200706028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovacs JJ, et al. Beta-arrestin-mediated localization of smoothened to the primary cilium. Science. 2008;320:1777–1781. doi: 10.1126/science.1157983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.May SR, et al. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev Biol. 2005;287:378–389. doi: 10.1016/j.ydbio.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 24.Tran PV, et al. THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia. Nat Genet. 2008;40:403–410. doi: 10.1038/ng.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haycraft CJ, et al. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci USA. 2005;102:11325–11330. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu A, Wang B, Niswander LA. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development. 2005;132:3103–3111. doi: 10.1242/dev.01894. [DOI] [PubMed] [Google Scholar]
- 28.Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell. 2007;12:767–778. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 30.Huangfu D, et al. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 31.O'Hare HM, Johnsson K, Gautier A. Chemical probes shed light on protein function. Curr Opin Struct Biol. 2007;17:488–494. doi: 10.1016/j.sbi.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Z, et al. Genetically encoded short peptide tags for orthogonal protein labeling by Sfp and AcpS phosphopantetheinyl transferases. ACS Chem Biol. 2007;2:337–346. doi: 10.1021/cb700054k. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Z, Koglin A, Wang Y, McMahon AP, Walsh CT. An 8 residue fragment of an acyl carrier protein suffices for post-translational introduction of fluorescent pantetheinyl arms in protein modification in vitro and in vivo. J Am Chem Soc. 2008;130:9925–9930. doi: 10.1021/ja802657n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe D, et al. The left-right determinant Inversin is a component of node monocilia and other 9+0 cilia. Development. 2003;130:1725–1734. doi: 10.1242/dev.00407. [DOI] [PubMed] [Google Scholar]
- 35.Varjosalo M, Li SP, Taipale J. Divergence of hedgehog signal transduction mechanism between Drosophila and mammals. Dev Cell. 2006;10:177–186. doi: 10.1016/j.devcel.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 36.Yu J, McMahon AP. Reproducible and inducible knockdown of gene expression in mice. Genesis. 2006;44:252–261. doi: 10.1002/dvg.20213. [DOI] [PubMed] [Google Scholar]
- 37.Cooper AF, et al. Cardiac and CNS defects in a mouse with targeted disruption of suppressor of fused. Development. 2005;132:4407–4417. doi: 10.1242/dev.02021. [DOI] [PubMed] [Google Scholar]
- 38.Svard J, et al. Genetic elimination of Suppressor of fused reveals an essential repressor function in the mammalian Hedgehog signaling pathway. Dev Cell. 2006;10:187–197. doi: 10.1016/j.devcel.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 39.van Dam EM, Stoorvogel W. Dynamin-dependent transferrin receptor recycling by endosome-derived clathrin-CoAted vesicles. Mol Biol Cell. 2002;13:169–182. doi: 10.1091/mbc.01-07-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perez-Victoria FJ, Mardones GA, Bonifacino JS. Requirement of the human GARP Complex for mannose 6-phosphate-receptor-dependent sorting of cathepsin D to lysosomes. Mol Biol Cell. 2008;19:2350–2362. doi: 10.1091/mbc.E07-11-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nachury MV, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 42.Dammermann A, Merdes A. Assembly of centrosomal proteins and microtubule organization depends on PCM-1. J Cell Biol. 2002;159:255–266. doi: 10.1083/jcb.200204023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gibbs JB. Mechanism-based target identification and drug discovery in cancer research. Science. 2000;287:1969–1973. doi: 10.1126/science.287.5460.1969. [DOI] [PubMed] [Google Scholar]
- 44.Athar M, et al. Inhibition of smoothened signaling prevents ultraviolet B-induced basal cell carcinomas through regulation of Fas expression and apoptosis. Cancer Res. 2004;64:7545–7552. doi: 10.1158/0008-5472.CAN-04-1393. [DOI] [PubMed] [Google Scholar]
- 45.Dahmane N, et al. The Sonic Hedgehog-Gli pathway regulates dorsal brain growth and tumorigenesis. Development. 2001;128:5201–5212. doi: 10.1242/dev.128.24.5201. [DOI] [PubMed] [Google Scholar]
- 46.Berman DM, et al. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science. 2002;297:1559–1561. doi: 10.1126/science.1073733. [DOI] [PubMed] [Google Scholar]
- 47.Ocbina PJ, Anderson KV. Intraflagellar transport, cilia, and mammalian Hedgehog signaling: Analysis in mouse embryonic fibroblasts. Dev Dyn. 2008;237:2030–2038. doi: 10.1002/dvdy.21551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.