Abstract
Functional inactivation of p53 and constitutive activation of the NF-κB pathway has been associated with several human cancers. In this study, we show that IκB kinase 2 (IKK2/IKKβ), which is critical for NF-κB activation, also phosphorylates p53. Phosphorylation of p53 at serines 362 and 366 by IKK2 leads to its recruitment to and ubiquitination by β-TrCP1. Degradation of ubiquitinated p53 is independent of Mdm2, because it occurs in both wild-type and Mdm2−/− cells. SiRNA-mediated reduction in the levels of β-TrCP1 and other members of the SCFβ−TrCP1E3 ubiquitin ligase complex or overexpression of a dominant negative form of β-TrCP1 enhances p53 stability. Substitutions at Ser-362 and 366 of p53 by alanines (p53 AA) result in reduced phosphorylation of p53 by IKK2, decreased association with β-TrCP1, and thus increased stability of p53 and expression of p53 target genes such as p21, altering the G1 phase of the cell cycle. Our results identify IKK2 and β-TrCP1 as novel regulators of the p53 pathway and suggest that blocking of IKK2 and β-TrCP1 could be a means of regulating p53 stability and thereby modulating its biological activity.
Keywords: β-TrCP1, DNA damage, IKK2, p53 stability
NF-κB is a family of transcription factors crucial for several biological processes involved in innate and adaptive immunity, inflammation, cell survival, and cancer (1). More than 150 different types of stimuli, including proinflammatory cytokines, bacterial/viral infection, and stress, activate NF-κB–dependent transcription of target genes. Activation of the IκB kinase (IKK) complex is a prerequisite for NF-κB activation. Once activated, IKK phosphorylates serines 32 and 36 of IκB proteins (2), followed by ubiquitination by β-TrCP1, an E3 ubiquitin ligase, which targets them for proteosomal degradation (3). IKK2 has been shown to phosphorylate other cellular and viral proteins, such as β-catenin, IκBβ, IκBε, p65, HIV-VPU, and SRC-3; this phosphorylation regulates the steady-state levels and transcriptional activity of these substrates (4–7). Approximately 30% of cellular proteins contain covalently bound phosphate, even though only 500–600 protein kinases likely are encoded by the human genome (8). Therefore, it is conceivable that, like many other kinases, IKK2 also has additional substrates, and that each protein phosphorylated by IKK2 in turn can be a substrate of other kinases.
The p53 tumor suppressor plays a crucial role in the development of many cancers and is frequently mutated or deleted in human cancers (9, 10). In the absence of stress, endogenous p53 levels are very low, because of the constant recruitment of p53 to Mdm2, an E3 ubiquitin ligase that inhibits its transcriptional activity and targets it for proteosomal degradation (11, 12). In response to DNA damage and other cellular stresses, the p53 protein levels are up-regulated and its activities are induced. The inhibition of Mdm2 binding to p53 and increases in p53 transcriptional activity involve the phosphorylation and stabilization of p53.
Human p53 has been reported to contain at least 20 phosphoacceptor sites. Most of these sites are modified in response to damage or stress, but some are phosphorylated under normal growth conditions (13). Most of these specific phosphorylation sites stabilize p53 and prevent Mdm2-mediated ubiquitination; however, phosphorylation of p53 at threonine 155 by the COP9 signalsome, at threonine 55 by TAF1, and at serine 15 by Aurora kinase A targets p53 for Mdm2-induced degradation (14–16).
Given that the p53 pathway is a critical gatekeeper of cellular transformation and that its mutational inactivation is reported in more than 50% of cancers, it would seem that other cellular mechanisms are involved in inactivation of p53. Perhaps the best way to modulate p53's activity is to regulate its stability. Here we report 2 new players, IKK2 and β-TrCP1, that can regulate the stability of p53. We show that IKK2 (but not IKK1) phosphorylates p53 at serine 366, and possibly also at serine 362, after which p53 is recruited to the SCFβ−TrCP E3 ligase for ubiquitination and degradation. These findings have important clinical relevance, because activation of IKK and the NF-κB pathway also has been reported in several human cancers.
Results
IKK2 Phosphorylates p53.
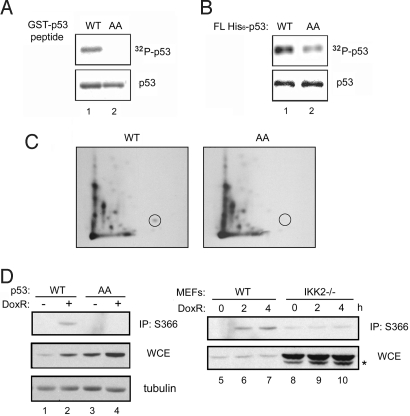
We used the consensus sequence generated from mutational analysis (supporting information (SI) Text and Fig. S1) to search for potential IKK2 substrate in the Swiss-prot database (17). One of the potential targets was p53 (Fig. S2). The ability of IKK2 to phosphorylate both GST-p53 peptide and recombinant full-length human p53 in vitro was tested. Fig. 1A shows that IKK2 phosphorylated the GST-p53 peptide. Substituting residues 362 and 366 in the GST-p53 peptide with alanine (p53 AA) abolished phosphorylation by IKK2. In addition, full-length His6-p53 could be robustly phosphorylated by IKK2 (Fig. 1B); however, phosphorylation of full-length p53 AA protein by IKK2 was significantly diminished. To further confirm that the phosphorylation indeed occurred at the two proposed serine residues, we analyzed the His6-p53 bands from the kinase assay by phosphopeptide mapping. We detected the spot corresponding to serine 366 phosphorylation only in wild-type (WT) p53 samples, not in p53 AA samples (Fig. 1C). The identity of this spot was confirmed by comigration with a synthetic phosphorylated peptide (AH*SSHLK). These results strongly establish that p53 can be phosphorylated at serines 362 and 366 in vitro.
Fig. 1.
IKK2 phosphorylates p53 at serines 362 and 366. (A and B) GST-p53 peptides (A) and full-length His6-p53 (B) were subjected to kinase assay with purified IKK2 (Top; Coomassie blue staining, Bottom). (C) The p53 bands from panel B were subjected to phosphopeptide mapping analysis. The spot in the circle was confirmed to be the peptide containing phosphorylated serine 366. (D) WT MEF cells infected with p53 WT or AA mutant lentivirus were treated with 1 μg mL−1 of doxorubicin for 4 h and lysed for immunoprecipitation with antibody to phos-Ser-366 p53 (Left), or, alternatively, WT and IKK2−/− MEF cells were infected with p53 WT lentivirus and analyzed (Right). The lower bands (denoted by *) are endogenous p53.
To further investigate the phosphorylation of serines 362 and 366 by IKK2 in vivo, we treated mouse embryonic fibroblast (MEF) cells expressing WT or AA mutant p53 with doxorubicin, and then immunoprecipitated the phosphorylated p53 proteins with antibody to phos-Ser-366 p53 (18) and immunoblotted them with p53 antibody. MEF cells infected with the lentiviral vector making p53 WT induced phosphorylation on doxorubicin treatment (Fig. 1D, lane 2). In contrast, MEF cells transduced with p53 AA lentivector exhibited no phosphorylation (lane 4). DNA damage–mediated phosphorylation of p53 was mediated by IKK2, since doxorubicin treatment increased serine 366 phosphorylation of p53 in WT MEF cells, but not in IKK2-deficient cells (Fig. 1D, lanes 5–7 vs. lanes 8–10). Based on these results, we conclude that IKK2 phosphorylates p53 in response to DNA-damaging agents like doxorubicin in vivo, and that IKK2-mediated phosphorylation of p53 likely occurs at least at serine 366. Given that Chk2 has been implicated as a major kinase for phosphorylation of serine 366 (18), we investigated whether loss of IKK2 could indirectly lead to loss of serine 366 phosphorylation due to loss of Chk2 activation; however, activation of Chk2 was completely normal in cells lacking IKK2 (Fig. S3). Because IKK2 loss leads to significant loss of serine 366 phosphorylation without affecting Chk2 activation, these results identify IKK2 as a putative p53 kinase (for serine 366) in vivo.
Phosphorylation by IKK2 Destabilizes p53.
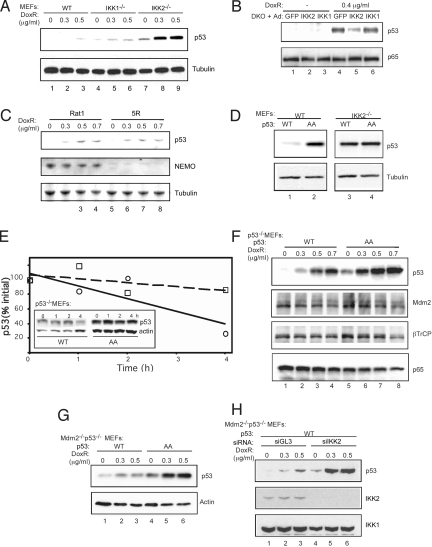
We next evaluated the physiological consequences of IKK2-mediated phosphorylation of p53. p53 stabilization occurred at a much smaller dose of doxorubicin in primary IKK2−/− MEF cells compared with primary WT or IKK1−/− MEF cells (Fig. 2A; compare lanes 2 and 5 with lane 8). Because mutations might stabilize p53 protein, we sequenced the full-length cDNA, but we found no mutations in any of these cells. The cells exhibited no differences in p53 mRNA level, confirming the notion that p53 is regulated mainly at the protein level (Fig. S4A). In addition, reconstitution of IKK2, but not IKK1, in primary IKK1/2−/− cells led to a decline in p53 levels (Fig. 2B). We also found that removing IKK2 mediated by siRNAs also could lead to an increase in p53 (Fig. S5), confirming that IKK2 indeed is a negative regulator of p53 stability.
Fig. 2.
Phosphorylation by IKK2 destabilizes p53. Primary WT, IKK1−/−, and IKK2−/− MEF cells (A); IKK1/2−/− DKO MEF cells infected with recombinant adenovirus expressing GFP, IKK2, and IKK1 (B); Rat1 and 5R rat fibroblast cells (C); Primary WT and IKK2−/− MEF cells stably infected with Flag-p53 WT or AA lentivirus (D); p53−/− MEF cells stably infected with Flag-p53 WT or AA lentivirus (F); Mdm2−/−/p53−/− MEF cells infected with Flag-p53 WT or AA lentivirus (G); Mdm2−/−/p53−/− MEF cells expressing p53 WT infected with siGL3 or siIKK2 lentivirus (H) were treated with 0.4 μg mL−1 or increasing amounts of doxorubicin for 18 h. Cell lysates were immunoblotted with indicated antibodies. (E) Doxorubicin-treated p53−/− MEF cells stably expressing recombinant Flag-p53 WT or AA protein were collected at the indicated time points after addition of cyclohexamide (CHX) for 0, 1, 2, and 4 h. The data shown in the inset were quantified by densitometry. The dashed line represents p53 AA, and the solid line represents p53 WT.
NEMO is an essential component of the IκB kinase complex. Interestingly, NEMO deficiency in rat fibroblast 5R cells did not lead to p53 stabilization compared with WT Rat1 cells (Fig. 2C), suggesting that activation of NF-κB target genes is not required for this effect. We assayed the effect of IKK2-mediated phosphorylation on the stability of p53 by expressing WT or AA variants of p53 in primary WT or IKK2−/− MEF cells. After doxorubicin treatment, p53 WT was less stabilized than p53 AA in WT MEF cells (Fig. 2D; compare lanes 1 and 2); however, both p53 WT and AA mutant were stabilized to a similar degree in primary IKK2−/− MEF cells (compare lanes 3 and 4). These results suggest that IKK2-mediated phosphorylation of serines 362 and 366 regulates the stability of p53, and that phosphorylation of p53 at these residues most likely facilitates its degradation. Incubating with cyclohexamide for 4 h after an 18-h doxorubicin treatment resulted in a more significant decrease in p53 WT compared with p53 AA mutant (Fig. 2E), suggesting higher p53 AA levels at steady state as well.
To evaluate whether phosphorylation of p53 at serines 362 and 366 also regulates its stability in response to DNA damage, we estimated the relative amount of p53 in p53−/− MEF cells infected with p53 WT or p53 AA lentivirus after treatment with increasing amounts of doxorubicin for 18 h. Quantitative PCR found equivalent mRNA levels of p53 WT and AA (Fig. S4B); however, p53 AA mutant protein levels were consistently higher than p53 WT levels (Fig. 2F). There was no significant change in Mdm2 and β-TrCP levels in p53−/− MEF cells infected with p53 WT or AA (Fig. 2F). There was a significant difference in p53 WT and AA levels in the absence of doxorubicin, likely due to the presence of basal, constitutively active IKK2 in these cells. Low-level constitutive activation of IKK2 in MEF cells has been reported, which was abrogated by genetic ablation of IKK2 (19). In fact, IKK was originally identified as a basally active kinase (20). To assess the apparent differential stability of the p53 WT and p53 AA proteins in the absence of Mdm2, Mdm2−/−/p53−/− MEF cells were infected with either p53 WT or AA mutant lentivirus (Fig. 2G) and treated with varying concentrations of doxorubicin for 18 h. Surprisingly, p53 AA was more stable than p53 WT even in the absence of Mdm2 (Fig. 2G; compare lanes 1–3 and 4–6). Furthermore, p53 WT also could be stabilized by IKK2 knockdown in Mdm2−/−/p53−/− MEF cells (Fig. 2H; compare lanes 1–3 and 4–6). Together, these results indicate that phosphorylation of serines 362 and 366 of p53 by IKK2 facilitates degradation of p53 in an Mdm2-independent manner.
β-TrCP Is Involved in IKK2-Induced Degradation of p53.
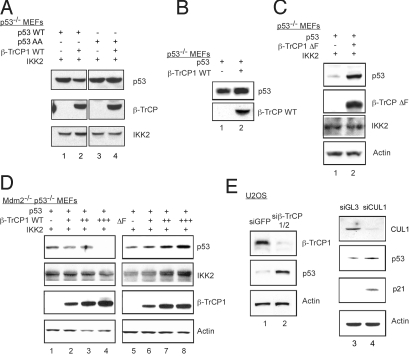
Having established that phosphorylation by IKK2 of p53 at residues 362 and 366 regulates p53's stability in response to DNA damage, we next investigated the molecular mechanism governing this phenomenon. The β-TrCP–Skp1-Cullin-F-box protein (SCF) complex is the only recognized E3 ligase for IKK2 substrates identified thus far. β-TrCP ubiquitinates IKK2 phosphorylated substrates like IκBα (21), tagging them for degradation by the 26S proteosome. We transfected p53 −/− MEF cells with a human p53 expression plasmid and a β-TrCP1 WT expression plasmid in the presence of an IKK2 expression plasmid, and measured p53 levels by immunoblot analysis 48 h later. As shown in Fig. 3A, cotransfection with β-TrCP1 WT caused a substantial decrease in the amount of p53 WT (lane 2) compared with cells cotransfected with only p53 and IKK2 expression plasmids (lane 1); however, the amount of p53 AA was not decreased by β-TrCP1 WT (lanes 3 and 4). Furthermore, β-TrCP1 WT did not cause a decrease in p53 level in the absence of exogenously transfected IKK2 (Fig. 3B).
Fig. 3.
Effect of β-TrCP1 expression on IKK2-induced degradation of p53. p53−/− MEF cells were cotransfected with Flag-p53 WT or AA, β-TrCP1 WT, and IKK2 (A); Flag-p53 WT and β-TrCP1 WT without IKK2 (B); or Flag-p53 WT, β-TrCP1 ΔF, and IKK2 (C) and treated with doxorubicin for 18 h. (D) Mdm2−/−/p53−/− MEF cells were cotransfected with Flag-p53 WT, IKK2, and increasing amounts of β-TrCP1 WT or ΔF were incubated with doxorubicin for 18 h before harvest. (E) U2OS cells were stably infected with siβ-TrCP1/2 or siGFP lentivirus, or were transfected with synthetic siRNA to Cullin1 or luciferase and treated with doxorubicin for 18 h. Immunoblot analyses were performed with the indicated antibodies.
To evaluate the role of β-TrCP1 in regulating the stability of p53, we analyzed the β-TrCP1 ΔF mutant, which lacks the F-box and hence, although it binds its substrates, cannot associate with other subunits of the SCF complex and thus functions as a dominant negative (22). Transfection of p53−/− MEF cells with a human p53 expression plasmid and a β-TrCP1 ΔF mutant expression plasmid led to the accumulation of p53 levels even in the presence of IKK2 (Fig. 3C). Under resting conditions, p53 is rapidly degraded by Mdm2, an E3 ligase for p53. To determine whether β-TrCP1–mediated regulation of p53 levels occurs independently of Mdm2, embryonic fibroblasts derived from Mdm2−/−/p53−/− double knockout were transfected with human p53 expression plasmid, along with increasing amounts of β-TrCP1 WT or ΔF expression plasmids. Increasing concentration of β-TrCP1 WT led to the degradation of p53 even in the absence of Mdm2 (Fig. 3D, lanes 1–4). In contrast, cells transfected with increasing amounts of β-TrCP1 ΔF mutant, showed accumulation of p53 (lanes 5–8). Given that β-TrCP1 ΔF mutant would function as a dominant negative of the endogenous β-TrCP1 WT protein, these results suggest that β-TrCP1 is indeed a novel E3 ligase that regulates the stability of p53 independent of Mdm2.
Previous studies have reported an overlap in the functions of the β-TrCP 1 and 2 in destabilizing IκBα and β-catenin, both of which are substrates of IKK2 (23). To confirm that β-TrCP is involved in regulating steady-state levels of p53, siRNA against both β-TrCP1 and β-TrCP2 was expressed using a lentiviral vector (24). Immunoblot analysis of lysates from U2OS cells stably infected with siβ-TrCP1/2 lentivirus showed reduced protein levels of β-TrCP1 compared with control cells stably infected with siGFP lentivirus (Fig. 3E, lane 2). Cells with the β-TrCP1/2 siRNA had higher p53 levels. siRNA to Cullin1, another key component of SCF complex, showed a similar result observed with β-TrCP1 knockdown. The levels of p53 and its target gene p21 were reproducibly increased in Cullin 1 knockdown cells (Fig. 3E, lane 4). Several studies have reported the constitutive activation of IKK2 in pathogenic states and cancer cells (25, 26). The ability of β-TrCP RNAi to stabilize p53 in U2OS, an osteosarcoma-derived cell line, is likely due to the presence of basal constitutively active IKK2 in these cells. These results suggest that β-TrCP1, like Mdm2, can regulate the steady-state levels of p53 protein, albeit in an IKK2-dependent manner.
β-TrCP1 Interacts With p53 and Promotes p53 Ubiquitination.
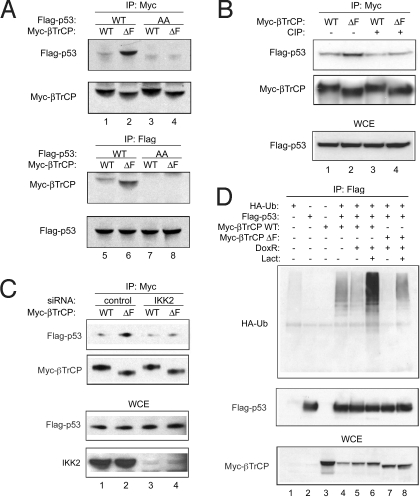
Our immunoprecipitation experiments demonstrate that p53 WT, but not AA, can bind to β-TrCP1 (Fig. 4A; compare lanes 1 and 3). The data indicate that binding of p53 to β-TrCP1 is dependent on phosphorylation of p53. Consistent with it acting as a dominant negative, the β-TrCP1 ΔF mutant was able to bring down more p53 (lane 2). Further supporting this, the interaction was significantly reduced after phosphatase treatment (Fig. 4B, lanes 3 and 4). As expected, this binding also was significantly impaired in IKK2 knockdown cells (Fig. 4C, lanes 3 and 4). To determine whether β-TrCP1, like Mdm2, can mediate ubiquitination in vivo, we cotransfected p53 plasmid in 293T cells with β-TrCP1 WT or ΔF mutant and HA-tagged ubiquitin in combination with doxorubicin and lactacystin, a proteosome inhibitor. In cells expressing p53, substantially more ubiquitination was observed in the presence of β-TrCP1 than in the presence of β-TrCP1 ΔF mutant after doxorubicin treatment (Fig. 4D, lanes 6 and 8). After doxorubicin treatment, ubiquitination of p53 was lower in cells expressing β-TrCP1 WT compared with basal or untreated cells, likely as a result of activation of p53 degradation in the absence of proteosome inhibitor (Fig. 4D, lane 5). Taken together, these results indicate that IKK2-mediated phosphorylation of p53 promotes the recruitment to and ubiquitination of p53 by β-TrCP1.
Fig. 4.
β-TrCP1 interaction with and ubiquitination of p53. (A) 293T cells were cotransfected with Flag-p53 WT or AA and Myc β-TrCP1 WT or ΔF. Cell lysates were immunoprecipitated with antibodies to Myc or Flag. (B) Cell lysates were treated with phosphatase (CIP) for 30 min at room temperature before immunoprecipitation. (C) Similar immunoprecipitation was done in siGL3 or siIKK2 stably infected 293T cells. (D) 293T cells were cotransfected with Flag-p53, HA-ubiquitin, and Myc β-TrCP1 WT or ΔF. Then, 24 h after transfection, cells were treated with doxorubicin and lactacystin for 18 h. p53 proteins were immunoprecipitated and ubiquitination was checked by HA antibody.
Phosphorylation of p53 at Serines 362 and 366 Affects the Cell Cycle.
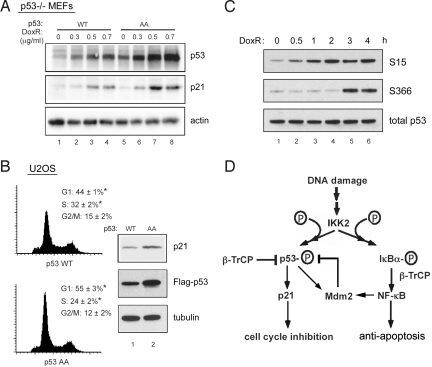
To investigate the biological role of serines 362 and 366 phophorylation of p53, we compared the effects of p53 WT and p53 AA on p53 transcriptional targets and, consequently, on p53-regulated pathways. The level of p21, a p53-inducible gene, was determined in p53-deficient MEF cells stably expressing either p53 WT or p53 AA. Treatment with increasing amounts of doxorubicin for 18 h resulted in consistently higher amounts of cyclin-dependent kinase inhibitor p21 in p53 AA mutant compared with p53 WT–expressing cells (Fig. 5A). In addition, U2OS cells that overexpressed p53 AA had a larger G1 population compared with U2OS cells with p53 WT (55% vs. 44%; Fig. 5B). Cells expressing p53 AA also exhibited greater p21 expression than cells expressing p53 WT. The greater growth arrest in the G1 phase with p53 AA could be due to stabilization of p53 and enhanced transcription of cell cycle inhibitor p21.
Fig. 5.
The mechanism and in vivo effects of IKK2 phosphorylation on p53 function. (A) p53−/− MEF cells were stably infected with p53 WT or AA lentivirus, then treated with increasing amounts of doxorubicin for 18 h. Cell lysates were immunoblotted with the indicated antibodies. (B) U2OS cells were transfected with p53 WT or AA, and cell cycle analysis was done by FACS (n = 5; *P ≤ .05). Endogenous p21 levels were checked by immunoblot analyses. (C) 293T cells were treated with 1 μg mL−1 of doxorubicin for specified period, and p53 serine 15 and 366 phosphorylation was measured. (D) Proposed model for IKK2 regulated p53 degradation. IKK2 is activated in response to DNA damage, resulting in phosphorylation of p53 at serines 362 and 366 and subsequent degradation of p53 in an Mdm2-independent manner. β-TrCP1 acts as an E3 ubiquitin ligase and targets p53 for degradation. Simultaneously, DNA damage activates the transcription factor NF-κB through IKK2. NF-κB up-regulates the expression of Mdm2, which in turn also regulates p53 turnover.
Given the fact that p53 AA leads to prolonged p53 activity, we explored whether phosphorylation at serines 362 and 366 is involved in resolving the DNA damage response. Interestingly, the kinetics of serine 366 phosphorylation differ greatly from those of serine 15 phosphorylation, the site of ATM kinase, which is very rapid (Fig. 5C); however, in agreement with the activation kinetics of IKK2 by DNA-damaging agents like doxorubicin (27), serine 366 is phosphorylated by IKK2 over 3–4 h. This suggests that IKK2 may be a bona fide kinase-phosphorylating p53 at serine 366 in vivo, and may contribute toward regulating p53 stability and activity in the physiological setting of DNA damage response.
Discussion
We have found that IKK2 phosphorylates p53 at serines 362 and 366, facilitating degradation of p53, independent of Mdm2 and NF-κB. Although we only detected p53 serine 366 phosphorylation in vivo, due to the limited availability of serine 366 phosphorylation–specific antibody, we cannot exclude the possibility of serine 362 phosphorylation. Interestingly, phosphopeptide mapping (Fig. 1C) produced much stronger signal for serine 366 phosphorylation than for serine 362 phosphorylation. Thus, phosphorylation on serine 366 by IKK2 likely plays a more important role in the cellular context.
We have previously shown that IKK2 can promote p53 destabilization through NF-κB– mediated Mdm2 expression (27). Our present findings indicate yet another mechanism through which IKK2 directly regulates p53 degradation (Fig. 5D). This alternative mechanism of p53 phosphorylation by IKK2 could provide an Mdm2-independent mechanism of attenuating p53 response. A study of the kinetics of phosphorylation of p53 immediately after DNA damage showed that S15 phosphorylation precedes phosphorylation of p53 at S366 (Fig. 5C). This may be an important mechanism in resolving p53 activity after DNA damage response.
It is well known that phosphorylation of IκBs by IKK2 results in ubiquitination of IκBs by β-TrCP, an E3 ubiquitin ligase and WD40 repeat–containing F-box protein (28). Our findings indicate that β-TrCP1 also can bind to p53, promote p53 ubiquitination, and regulate p53 steady-state levels only in the presence of IKK2. In addition, we found that β-TrCP1 functions independently of Mdm2. Although p53 lacks a consensus DS(p)GXXS(p) phospho-dependent binding motif for β-TrCP, binding of p53 to β-TrCP1 requires phosphorylation. β-TrCP has been identified as the E3 ligase for ubiquitination of Wee1, p100, and Smad3, all of which lack the consensus binding motif for β-TrCP (29–31). Therefore, it is reasonable to propose that IKK2 phosphorylation at serines 362 and 366 targets p53 for ubiquitination by β-TrCP1.
Mdm2 is a well-established important regulator of p53 turnover; however, a number of studies have shown that degradation of p53 can occur independent of Mdm2. HPV E6 protein, JNK, NAD(P)H quinone oxidoreductase 1 (NQO1), RING domain-containing protein Pirh2, and COP1 have been shown to promote p53 ubiquitination independent of Mdm2 (32–36). It has been demonstrated that adenovirus E1B55K and E4orf6 proteins function together to promote p53 ubiquitination and degradation through a Cullin-containing E3 ubiquitin ligase complex (37). β-TrCP is strikingly similar to the E4orf6 ubiquitin ligase complex, in that each contains a substrate recognition subunit linked to a Cullin/Rbx1 subcomplex through either Elongin BC or the Elongin C-like protein Skp1. Previous studies have suggested that the viral protein E4orf6 may have evolved functions similar to those of unidentified cellular regulators of p53 degradation; thus, β-TrCP possibly could be a cellular homolog of E4orf6. It is interesting to note that mice lacking Cullin1, a subunit of the β-TrCP–SCF complex, have higher levels of p53 during embryonic development (38). Similarly, p53 is more stabilized in cells in which Cullin1 has been knocked down (Fig. 3E).
There is growing evidence that inflammatory genes induced by NF-κB are critical components of tumor progression (39, 40). Although it has been shown that cytokines (such as IL-6, an NF-κB target gene) may disturb the p53 tumor-suppressor pathway (41–43), they do not modulate p53 stability. In line with this observation, we found no effect of TNF-α, which efficiently activates the NF-κB pathway, on the stability of p53 (Fig. S6). However, the presence of DNA damage in cancers arising at the sites of chronic inflammation (where IKK2 could be constitutively activated by the cytokines) could provide an apt clinical setting in which constitutively active IKK2 possibly could regulate p53 stability and function over a long period. Further in vivo demonstration of a physiological role for IKK2 in controlling p53 function has shown that cell type–specific deletion of IKK2 in mice leads to stabilization of p53 and plays an important role in p53-induced apoptosis in the intestine (44).
The programs governing tumorigenesis are complex and varied. Our findings may point toward a novel mechanism that can explain the role of constitutive IKK2 observed in the progression of several cancers. Further studies are needed to investigate whether elevated IKK2 activity and decreased p53 levels are correlated in human biopsy specimens. The role of IKK2 in regulating p53 steady-state levels makes IKK2 an attractive target for developing therapeutic strategies for human cancers.
Materials and Methods
In Vitro Kinase Assays and Phosphopeptide Mapping.
In vitro kinase assays were performed on purified WT or mutant GST-p53 fusion proteins. For phosphopeptide mapping, the 32P-labeled His6-tag WT or serines 362 and 366 alanine mutant of full-length p53 bands were cut from the gel after the in vitro kinase assay. The proteins were extracted and digested with trypsin. The peptides were separated in 2 dimensions on cellulose thin-layer plates by electrophoresis at pH 1.9 (horizontal direction) and chromatography (vertical direction).
p53 Degradation Assays.
Freshly confluent IKK1/2-deficient MEF cells were infected with recombinant adenovirus expressing GFP, IKK1 WT, or IKK2 WT. WT, IKK2-deficient, p53-deficient, and Mdm2−/−/p53−/− MEF cells were infected with either Flag-tagged p53 WT or serine 362 and 366 AA mutant lentivirus. Stably infected clones with same p53 (WT or AA) mRNA levels were used for all experiments. p53−/− and Mdm2−/−/p53−/− MEF cells were transfected with Flag-tagged p53 with either β-TrCP1 or ΔF β-TrCP1 with IKK2. The cells were then treated with 0.4 μg mL−1 of doxorubicin for 18 h, lysed in SDS loading buffer containing β-mercaptoethanol, and resolved by SDS/PAGE. Protein levels were detected by immunoblot analyses with the respective antibodies. To assess the stability of p53, p53−/− MEF cells stably infected with p53 WT and AA lentivirus were treated with 20 μg mL−1 of cyclohexamide for indicated times after 18 h of doxorubicin treatment (0.4 μg mL−1). Quantitative PCR was performed to determine p53 WT and AA mRNA levels in infected MEF cells.
siRNA Knockdown.
The following siRNA sequences were used: for siβ-TrCP1/2, 5′-GTGGAATTTGTGGAACATC-3′; for siIKK2, 5′-GGGCAGTCTTTGCACATCA-3′ (human) and 5′-GAAGATACTTGAACCAGTT-3′ (mouse); for siCullin1, 5′-AACGAAGAGUUCAGGUUUACC-3′. The efficiency of gene suppression was confirmed by immunoblot analyses and RT-PCR.
In Vivo Binding and Ubiquitination Assay.
293T cells were transfected with Flag-p53 together with either Myc-tagged β-TrCP1 WT or ΔF mutant. The cells were lysed 48 h after transfection and subjected to immunoprecipitation with anti-Myc or anti-Flag antibodies. To detect p53 ubiquitination, HA-tagged ubiquitin was cotransfected with plasmid combinations. Then, 24 h after transfection, the cells were treated with 0.4 mg mL−1 of doxorubicin and 5 μM lactacystin for 18 h. The cell lysates were immunoprecipitated overnight using anti-Flag polyclonal antibodies and then immunoblotted with monoclonal antibodies against Flag, HA, or Myc.
Cell Cycle Analysis.
Flag-tagged p53 WT and AA expression plasmids were transfected in U2OS osteosarcoma cells. The cells were incubated with 10 μg mL−1 of Hoechst for 30 min at 48 h after transfection. Cells were collected for FACS analysis (using a Becton Dickinson LSR analyzer) to determine p53-dependent growth arrest.
Supplementary Material
Acknowledgments.
We thank Ricardo Correa and Erin Gray for their help with preliminary experimental studies and critical discussions, Dr. Sheau-Yann Shieh for the phos-Ser-366 p53 antibody, Dr. You-Wei Zhang for the Cullin1 siRNA and antibody, and Jill Meisenhelder for assistance with phosphopeptide mapping. I.V. is an American Cancer Society Professor of Molecular Biology and is supported by grants from the National Institutes of Health, the Lustgarten Foundation, the Leducq Foundation, the Ellison Medical Foundation, and the H. N. and Frances C. Berger Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812256106/DCSupplemental.
References
- 1.Li Q, Verma IM. NF-κB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 2.Mercurio F, et al. IKK-1 and IKK-2: Cytokine-activated IκB kinases essential for NF-κB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 3.Spencer E, Jiang J, Chen ZJ. Signal-induced ubiquitination of IκBα by the F-box protein Slimb/β-TrCP. Genes Dev. 1999;13:284–294. doi: 10.1101/gad.13.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heilker R, Freuler F, Pulfer R, Di Padova F, Eder J. All three IκB isoforms and most Rel family members are stably associated with the IκB kinase 1/2 complex. Eur J Biochem. 1999;259:253–261. doi: 10.1046/j.1432-1327.1999.00028.x. [DOI] [PubMed] [Google Scholar]
- 5.Yang F, Tang E, Guan K, Wang CY. IKKβ plays an essential role in the phosphorylation of RelA/p65 on serine 536 induced by lipopolysaccharide. J Immunol. 2003;170:5630–5635. doi: 10.4049/jimmunol.170.11.5630. [DOI] [PubMed] [Google Scholar]
- 6.Wu RC, et al. Regulation of SRC-3 (pCIP/ACTR/AIB-1/RAC-3/TRAM-1) coactivator activity by I kappa B kinase. Mol Cell Biol. 2002;22:3549–3561. doi: 10.1128/MCB.22.10.3549-3561.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamberti C, et al. Regulation of beta-catenin function by IκB kinases. J Biol Chem. 2001;276:42276–42286. doi: 10.1074/jbc.M104227200. [DOI] [PubMed] [Google Scholar]
- 8.Cohen P. The regulation of protein function by multisite phosphorylation: A 25-year update. Trends Biochem Sci. 2000;25:596–601. doi: 10.1016/s0968-0004(00)01712-6. [DOI] [PubMed] [Google Scholar]
- 9.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 10.Vousden KH, Lu X. Live or let die: The cell's response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 11.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 12.Fang S, Jensen JP, Ludwig RL, Vousden KH, Weissman AM. Mdm2 is a RING finger-dependent ubiquitin protein ligase for itself and p53. J Biol Chem. 2000;275:8945–8951. doi: 10.1074/jbc.275.12.8945. [DOI] [PubMed] [Google Scholar]
- 13.Toledo F, Wahl GM. Regulating the p53 pathway: In vitro hypotheses, in vivo veritas. Nat Rev Cancer. 2006;6:909–923. doi: 10.1038/nrc2012. [DOI] [PubMed] [Google Scholar]
- 14.Bech-Otschir D, et al. COP9 signalosome-specific phosphorylation targets p53 to degradation by the ubiquitin system. EMBO J. 2001;20:1630–1639. doi: 10.1093/emboj/20.7.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katayama H, et al. Phosphorylation by aurora kinase A induces Mdm2-mediated destabilization and inhibition of p53. Nat Genet. 2004;36:55–62. doi: 10.1038/ng1279. [DOI] [PubMed] [Google Scholar]
- 16.Li HH, Li AG, Sheppard HM, Liu X. Phosphorylation on Thr-55 by TAF1 mediates degradation of p53: A role for TAF1 in cell G1 progression. Mol Cell. 2004;13:867–878. doi: 10.1016/s1097-2765(04)00123-6. [DOI] [PubMed] [Google Scholar]
- 17.Boeckmann B, et al. The SWISS-PROT protein knowledgebase and its supplement TrEMBL in 2003. Nucleic Acids Res. 2003;31:365–370. doi: 10.1093/nar/gkg095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ou YH, Chung PH, Sun TP, Shieh SY. p53 C-terminal phosphorylation by CHK1 and CHK2 participates in the regulation of DNA damage–induced C-terminal acetylation. Mol Biol Cell. 2005;16:1684–1695. doi: 10.1091/mbc.E04-08-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka M, et al. Embryonic lethality, liver degeneration, and impaired NF-κB activation in IKK-β–deficient mice. Immunity. 1999;10:421–429. doi: 10.1016/s1074-7613(00)80042-4. [DOI] [PubMed] [Google Scholar]
- 20.Chen ZJ, Parent L, Maniatis T. Site-specific phosphorylation of IκBα by a novel ubiquitination-dependent protein kinase activity. Cell. 1996;84:853–862. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- 21.Winston JT, et al. The SCFβ–TRCP–ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IκBα and beta-catenin and stimulates IκBα ubiquitination in vitro. Genes Dev. 1999;13:270–283. doi: 10.1101/gad.13.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang TT, Feinberg SL, Suryanarayanan S, Miyamoto S. The zinc finger domain of NEMO is selectively required for NF-κB activation by UV radiation and topoisomerase inhibitors. Mol Cell Biol. 2002;22:5813–5825. doi: 10.1128/MCB.22.16.5813-5825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakayama K, et al. Impaired degradation of inhibitory subunit of NF-κB (IκB) and beta-catenin as a result of targeted disruption of the beta-TrCP1 gene. Proc Natl Acad Sci USA. 2003;100:8752–8757. doi: 10.1073/pnas.1133216100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Busino L, et al. Degradation of Cdc25A by beta-TrCP during S phase and in response to DNA damage. Nature. 2003;426:87–91. doi: 10.1038/nature02082. [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Richmond A. Constitutive IκB kinase activity correlates with nuclear factor-κB activation in human melanoma cells. Cancer Res. 2001;61:4901–4909. [PubMed] [Google Scholar]
- 26.Krappmann D, et al. Molecular mechanisms of constitutive NF-κB/Rel activation in Hodgkin/Reed–Sternberg cells. Oncogene. 1999;18:943–953. doi: 10.1038/sj.onc.1202351. [DOI] [PubMed] [Google Scholar]
- 27.Tergaonkar V, Pando M, Vafa O, Wahl G, Verma I. p53 stabilization is decreased upon NFκB activation: A role for NFκB in acquisition of resistance to chemotherapy. Cancer Cell. 2002;1:493–503. doi: 10.1016/s1535-6108(02)00068-5. [DOI] [PubMed] [Google Scholar]
- 28.Deshaies RJ. SCF and Cullin/Ring H2-based ubiquitin ligases. Annu Rev Cell Dev Biol. 1999;15:435–467. doi: 10.1146/annurev.cellbio.15.1.435. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe N, et al. M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCF{beta}-TrCP. Proc Natl Acad Sci USA. 2004;101:4419–4424. doi: 10.1073/pnas.0307700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fong A, Sun SC. Genetic evidence for the essential role of beta-transducin repeat–containing protein in the inducible processing of NF-κB2/p100. J Biol Chem. 2002;277:22111–22114. doi: 10.1074/jbc.C200151200. [DOI] [PubMed] [Google Scholar]
- 31.Fukuchi M, et al. Ligand-dependent degradation of Smad3 by a ubiquitin ligase complex of ROC1 and associated proteins. Mol Biol Cell. 2001;12:1431–1443. doi: 10.1091/mbc.12.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin–protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- 33.Fuchs SY, et al. JNK targets p53 ubiquitination and degradation in nonstressed cells. Genes Dev. 1998;12:2658–2663. doi: 10.1101/gad.12.17.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asher G, Lotem J, Sachs L, Kahana C, Shaul Y. Mdm2- and ubiquitin-independent p53 proteasomal degradation regulated by NQO1. Proc Natl Acad Sci USA. 2002;99:13125–13130. doi: 10.1073/pnas.202480499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leng RP, et al. Pirh2, a p53-induced ubiquitin–protein ligase, promotes p53 degradation. Cell. 2003;112:779–791. doi: 10.1016/s0092-8674(03)00193-4. [DOI] [PubMed] [Google Scholar]
- 36.Dornan D, et al. The ubiquitin ligase COP1 is a critical negative regulator of p53. Nature. 2004;429:86–92. doi: 10.1038/nature02514. [DOI] [PubMed] [Google Scholar]
- 37.Querido E, et al. Degradation of p53 by adenovirus E4orf6 and E1B55K proteins occurs via a novel mechanism involving a Cullin-containing complex. Genes Dev. 2001;15:3104–3117. doi: 10.1101/gad.926401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dealy MJ, et al. Loss of Cul1 results in early embryonic lethality and dysregulation of cyclin E. Nat Genet. 1999;23:245–248. doi: 10.1038/13886. [DOI] [PubMed] [Google Scholar]
- 39.Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-κB and IκB proteins: Implications in cancer and inflammation. Trends Biochem Sci. 2005;30:43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Clevers H. At the crossroads of inflammation and cancer. Cell. 2004;118:671–674. doi: 10.1016/j.cell.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Cordon-Cardo C, Prives C. At the crossroads of inflammation and tumorigenesis. J Exp Med. 1999;190:1367–1370. doi: 10.1084/jem.190.10.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hudson JD, et al. A proinflammatory cytokine inhibits p53 tumor-suppressor activity. J Exp Med. 1999;190:1375–1382. doi: 10.1084/jem.190.10.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yonish-Rouach E, et al. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991;352:345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]
- 44.Egan LJ, et al. IκB-kinaseβ–dependent NF-κB activation provides radioprotection to the intestinal epithelium. Proc Natl Acad Sci USA. 2004;101:2452–2457. doi: 10.1073/pnas.0306734101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.