Abstract
The histone H3 demethylase Not dead yet-1 (Ndy1/KDM2B) is a physiological inhibitor of senescence. Here, we show that Ndy1 is down-regulated during senescence in mouse embryonic fibroblasts (MEFs) and that it represses the Ink4a/Arf locus. Ndy1 counteracts the senescence-associated down-regulation of Ezh2, a component of polycomb-repressive complex (PRC) 2, via a JmjC domain-dependent process leading to the global and Ink4a/Arf locus-specific up-regulation of histone H3K27 trimethylation. The latter promotes the Ink4a/Arf locus-specific binding of Bmi1, a component of PRC1. Ndy1, which interacts with Ezh2, also binds the Ink4a/Arf locus and demethylates the locus-associated histone H3K36me2 and histone H3K4me3. The combination of histone modifications driven by Ndy1 interferes with the binding of RNA Polymerase II, resulting in the transcriptional silencing of the Ink4a/Arf locus and contributing to the Ndy1 immortalization phenotype. Other studies show that, in addition to inhibiting replicative senescence, Ndy1 inhibits Ras oncogene-induced senescence via a similar molecular mechanism.
Keywords: cancer, chromatin, epigenetics, Fbxl10, JHDM1B
Cellular senescence is an irreversible growth arrest that is either developmentally programmed in dividing cells or is triggered by several types of stress, such as DNA damage, telomere shortening, and oncogene activation (1, 2). At the molecular level, senescence is characterized by the activation of the Ink4a/Arf locus, which encodes 2 proteins, p16Ink4a and p19Arf (p14Arf in humans) (1–5) that regulate the Rb and p53 pathways, respectively (1, 2). p16Ink4a inhibits the cyclin-dependent kinases Cdk4 and Cdk6 that phosphorylate and inactivate Rb (1, 2). p19Arf interacts with the ubiquitin ligase MDM2 and inhibits MDM2-mediated p53 degradation (1, 2).
Overexpression of the Polycomb group (PcG) proteins Bmi1 (6), Ezh2 (7), CBX7 (8), and CBX8 (9) delays the onset of replicative senescence in mouse and human embryonic fibroblasts by repressing the Ink4a/Arf locus. The PcG proteins are involved in the maintenance of cell identity and stem cell renewal and contribute to cell cycle regulation and oncogenesis (10, 11). PcG proteins exist in 2 distinct complexes that cooperate to maintain long-term gene silencing through chromatin modifications. Polycomb-repressive complex (PRC) 2 contains Ezh2, Eed, and Suz12 (10, 11) and methylates histone H3 at K27 via Ezh2 (12, 13). Trimethylated H3K27 facilitates the recruitment of PRC1, which contains Cbx, Ring, Bmi1, and Mel-18 and promotes gene silencing by ubiquitinating H2A at K119, a histone modification that interferes with the binding of RNA Polymerase II (RNA Pol II) (10, 11, 13–15). RNA Pol II and PRC2 are indeed known to occupy gene promoters in a mutually exclusive manner (16, 17). Replicative senescence in mouse embryonic fibroblasts (MEFs) is characterized by down-regulation of Ezh2, elimination of the H3K27me3 mark at the Ink4a/Arf locus, displacement of Bmi1, and transcriptional activation of Ink4a and Arf (7, 18, 19).
Our previous studies have shown that the histone demethylases Ndy1/KDM2B and Ndy2/KMD2A inhibit replicative senescence and immortalize MEFs (20). The inhibition of senescence may be caused, at least in part, by the ability of Ndy1 to regulate redox homeostasis and to protect cells from oxidative stress (21). Here, we show that Ndy1 protects MEFs from replicative senescence, as well as Ras oncogene-induced senescence, by also repressing the Ink4a/Arf locus. Ndy1 mRNA is down-regulated in MEFs undergoing senescence. Moreover, overexpression of Ndy1 represses p16Ink4a, and to a lesser extent p19Arf, whereas its knock down has the opposite effect. Mechanistically, Ndy1 counteracts the senescence-associated down-regulation of Ezh2 via a JmjC domain-dependent process and promotes histone H3K27 trimethylation. The trimethylation of histone H3 in the Ink4a/Arf locus at K27 facilitates the binding of Bmi1. Bmi1 and Ezh2 synergize with Ndy1 to repress the Ink4a/Arf locus, suggesting that Ndy1 represses the Ink4a/Arf locus not only by regulating the expression of Ezh2 and the binding of Bmi1 within the locus but by additional mechanisms. Data presented in this article indeed show that Ndy1 binds the Ink4a/Arf locus and promotes H3K36me2 and H3K4me3 demethylation. These histone modifications, when combined, interfere with the binding of RNA Pol II and contribute to the silencing of the Ink4a/Arf locus. The effects of Ndy1 on the modification of histones and on the silencing of the Ink4a/Arf locus are passage dependent, suggesting that Ndy1-induced histone modifications may be amplified by further facilitating the binding of polycomb complexes to this locus.
Results
Replicative Senescence in MEFs Is Associated with the Down-Regulation of Ndy1.
Ndy1 functions as a physiological inhibitor of senescence in MEFs (20), suggesting that it may be down-regulated in cells undergoing replicative senescence. The results in Fig. 1A show that, indeed, Ndy1 and, to a lesser degree, Ndy2 are down-regulated in passaged MEFs and that their down-regulation is passage dependent. Ezh2, which is known to be down-regulated, and Bmi1, Suz12, and Eed1, which are known not to be down-regulated during senescence, were used as controls. The expression of other histone demethylases was also not down-regulated [supporting information (SI) Fig. S1], suggesting an important role of Ndy1 and Ndy2 in the physiological regulation of replicative senescence.
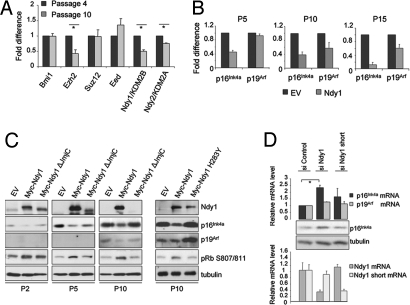
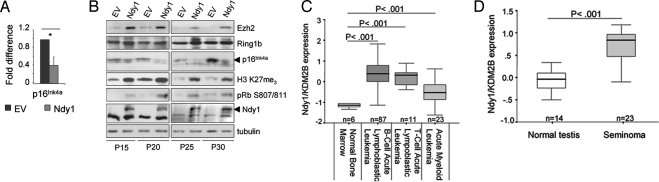
Fig. 1.
Ndy1 represses the senescence-associated up-regulation of p16Ink4a and p19Arf. (A) Total mRNA was isolated from MEFs at the indicated passage and analyzed by real-time PCR for the relative mRNA levels of Bmi1, Ezh2, Suz12, Eed, Ndy1, and Ndy2 (n = 2). *P < 0.05. (B) MEFs overexpressing Ndy1 were plated at a concentration of 105 cells per 6-cm dish and serially passaged. In passage 5 (P5), P10, and P15, total mRNA was isolated and analyzed by real-time PCR for the relative mRNA levels of p16Ink4a and p19Arf. The graphs show the fold decrease of p16Ink4a and p19Arf mRNAs in MEFs that overexpress Ndy1 normalized to control empty-vector (EV)–transduced cells from 3 independent infections. (C) As in B, MEFs were serially passaged, and whole-cell lysates from the indicated passages were analyzed by Western blotting with the indicated antibodies. p19Arf was undetectable at P2 and P5. (D) Passage 3 MEFs were transfected with siRNA, which specifically targets either the long or short form of Ndy1. Four days after transfection, cells were analyzed by Western blotting and total mRNA was collected and analyzed by real-time PCR with primers specific for p16Ink4a and p19Arf and primers specific for the long and short forms of Ndy1 (n = 3). *P < 0.05
Ndy1 Represses the Expression of p16Ink4a and p19Arf in MEFs.
Replicative senescence is characterized by the dramatic up-regulation of p16Ink4a (>100-fold) (Fig. S2) and p19Arf (>10-fold) (1, 18). Because p16Ink4a inhibits the cyclin D/CDK4–6 complex that mediates the phosphorylation of Rb at Ser-807/811 and Ndy1 promotes the phosphorylation of Rb at this site (20), we asked whether the phosphorylation of Rb in Ndy1-transduced MEFs can be linked to a defect in the induction of the Ink4a/Arf locus. To address this question, we examined the relative levels of p16Ink4a and p19Arf mRNA in passaged MEFs stably expressing Ndy1. Fig. 1 B and C and Fig. S2 show that overexpression of Ndy1 attenuated the induction of p16Ink4a in passages 5, 10, and 15 by ≈55%, 62%, and 90%, respectively, relative to empty-vector–transduced MEFs. The overexpression of Ndy1 also attenuated the induction of p19Arf in later passages (≈43% and 34% reduction at passages 10 and 15, respectively). The expression of p16Ink4a inversely correlates with the phosphorylation of Rb at Ser-807/811 (Fig. 1C), suggesting a causative relation between the attenuated induction of p16Ink4a and the enhanced phosphorylation of Rb in cells overexpressing Ndy1. Knockdown of endogenous Ndy1 in passage 3 MEFs increased the expression of p16Ink4a by ≈2.3 times (Fig. 1D). We conclude that Ndy1 is indeed a physiological repressor of p16Ink4a. The JmjC domain deletion mutant and the H283Y point mutant of Ndy1 failed to immortalize MEFs, and they were counterselected during passage (Fig. 1C) (20). Interestingly, the same mutants failed to attenuate the induction of p16Ink4a and p19Arf (Fig. 1C), suggesting that the demethylase activity of Ndy1, which is required for the immortalization of the Ink4a/Arf locus, is also required for the repression of the Ink4a/Arf locus. We have previously pointed out that Ndy1 encodes multiple protein isoforms in both humans and mice (20). One of these, the short form of Ndy1 (NM_013910), lacks the JmjC domain (20), and its overexpression promotes senescence (20). Knocking down the short form did not significantly up-regulate either p16Ink4a or p19Arf (Fig. 1D), further supporting the importance of the JmjC domain in the regulation of the Ink4a/Arf locus.
Ndy1 Up-Regulates Ezh2 and H3K27me3.
Cells undergoing replicative senescence down-regulate Ezh2, the catalytic subunit of PRC2 (7, 18). Because PRC2 is required for the PRC1-mediated repression of the Ink4a/Arf locus, its down-regulation promotes the transcriptional activation of the locus (7, 18). Ndy1 is also down-regulated in MEFs during passage (Fig. 1A). We therefore examined whether Ndy1 regulates the expression of Ezh2. Fig. 2A shows that overexpression of Ndy1 in MEFs promotes the marked induction of Ezh2 and the global increase of histone H3K27me3 as early as the second passage after infection. Consistent with these observations, knocking down the endogenous Ndy1 in MEFs decreases the expression of Ezh2 (Fig. 2B). The regulation of Ezh2 by Ndy1 was confirmed in MEFs immortalized with a floxed retroviral construct of Ndy1 (Fig. S3). MigR1-LoxP-Ndy1–immortalized MEFs were infected with a retrovirus expressing the Cre recombinase. Cre-mediated excision of Ndy1 down-regulates the Ndy1 protein (Fig. 2C) and mRNA (Fig. 2D) levels, suggesting that exogenous Ndy1 was efficiently ablated. Ndy1 ablation resulted in a dramatic up-regulation of p16Ink4a and in a lesser up-regulation of p19Arf and confirmed that Ndy1 represses the Ink4a/Arf locus. Finally, whereas excision of Ndy1 down-regulated the mRNA and protein levels of Ezh2 (Fig. 2 C and D), its overexpression up-regulated the mRNA levels of Ezh2 in MEFs and IMR90 cells (Figs. S4 and S5). We therefore conclude that Ndy1 up-regulates Ezh2. H3K27me3 has been linked to transcriptional repression (13, 18, 19). It may therefore be responsible for the repression of the Ink4a/Arf locus. Chromatin immunoprecipitation (ChIP) experiments indeed revealed that Ndy1 overexpression up-regulates H3K27 trimethylation throughout the Ink4a/Arf locus (Fig. 2E). The up-regulation was particularly prominent in the promoter and coding regions of Exon 1α (Fig. 2F, probes 7–11) and, to a lesser extent, Exons 1β, 2, and 3 (Fig. 2F, probes 1–2 and 12–13). The increase in H3K27me3 at the Ink4a/Arf locus was passage dependent and correlated with the Ndy1-driven up-regulation of Ezh2 and the global H3K27 trimethylation (Fig. 2A).
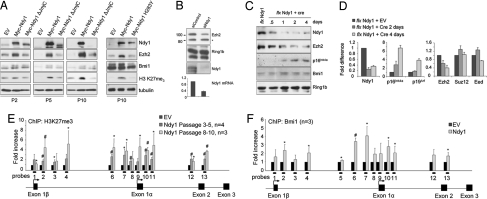
Fig. 2.
Ndy1 counteracts the senescence-associated down-regulation of Ezh2 and induces global H3K27 trimethylation. (A) The same samples described in Fig. 1C were further analyzed by Western blotting with the indicated antibodies. (B) Passage 3 MEFs were transfected with siRNA against Ndy1. Four days after transfection, cells were collected and analyzed by Western blotting. The graph at the bottom of the figure shows the efficiency of Ndy1 knockdown. *Nonspecific band. (C) Western blotting analysis of whole-cell extracts from MEFs immortalized with the MigR1-Ndy1 LoxP retroviral construct before and after infection with a retroviral construct that expresses the Cre recombinase. (D) The graph shows the fold difference of different mRNAs in MigR1-Ndy1 LoxP-immortalized MEFs before and after infection with the Cre recombinase (n = 2). (E) ChIP analysis of H3K27me3 at the Ink4a/Arf locus in early- and late-passage MEFs that overexpress Ndy1. The graph shows the fold increase of H3K27me3 methylation at the Ink4/Arf locus in MEFs that overexpress Ndy1 normalized to control empty-vector (EV)–transduced cells. (F) ChIP analysis of Bmi1 binding at the Ink4a/Arf locus in passage 3–5 MEFs that overexpress Ndy1. The graph shows the fold increase of Bmi1 binding at the Ink4a/Arf locus in MEFs that overexpress Ndy1 normalized to control EV-transduced cells. *P < 0.05; #P < 0.01.
Ndy1-Driven Histone Modifications in the Ink4a/Arf Locus Promote the Binding of Bmi1.
H3K27me3 serves as a docking site for the binding of the chromodomain protein Polycomb, a component of PRC1 that represses the Ink4a/Arf locus in a Bmi1-dependent manner (18, 19, 22). Ezh2 down-regulation in cells undergoing senescence decreases H3K27me3 at the Ink4a/Arf locus and leads to the displacement of PRC1-Bmi1 complex, with subsequent activation of transcription. Fig. 2F presents the results of ChIP analysis showing that overexpression of Ndy1 increased the binding of endogenous Bmi1 to the promoter region and Exon 1α of the Ink4a/Arf locus (Fig. 2F, probes 6, 7, 10, and 11). Of note, the binding of Bmi1 to this locus exhibited a similar pattern to that of H3K27me3 (Fig. 2E), supporting the notion that the Ndy1-induced Ezh2-dependent H3K27 trimethylation functions as a priming event for the binding of Bmi1 and the repression of p16Ink4a.
Ndy1 Cooperates with Ezh2 and Bmi1 to Repress p16Ink4a.
Ezh2 overexpression, H3K27 trimethylation, and Bmi1 binding to the Ink4a/Arf locus immortalize MEFs by repressing p16Ink4a. This raised the question as to whether Ezh2 up-regulation and Bmi1 binding to the Ink4a/Arf locus are sufficient for the Ndy1 immortalization phenotype. To address this question, we transiently knocked down Ndy1, Bmi1, or Ezh2 alone and in all possible combinations in passage 2 MEFs. Fig. 3A shows that whereas the transient knockdown of Ndy1, Bmi1, or Ezh2 individually caused a relatively slight up-regulation of p16Ink4a, the simultaneous knockdown of Ndy1 with either Ezh2 or Bmi1 or the simultaneous knockdown of Ezh2 and Bmi1 caused a significant up-regulation of p16Ink4a. This finding suggested that Ndy1 may have additional effects that are independent of Ezh2 and Bmi1. Knocking down Bmi1 in MEFs that had been immortalized by Ndy1 caused only a partial reversion of the Ndy1-mediated repression of p16Ink4a, providing additional support to the proposed hypothesis (Fig. 3B).
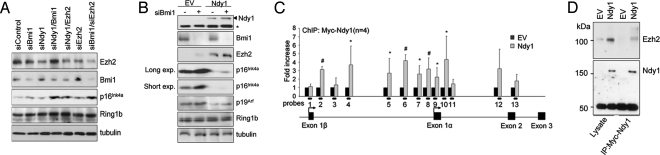
Fig. 3.
Ndy1 cooperates with Ezh2 and Bmi1 to repress the Ink4/Arf locus. (A) Passage 2 MEFs were transfected with the indicated siRNA for 4 days. Whole-cell lysates were analyzed by Western blotting with the indicated antibodies. (B) Ndy1-transduced MEFs were transfected with siRNA against Bmi1. Whole-cell lysates were analyzed by Western blotting with the indicated antibodies. (C) ChIP analysis of Ndy1 binding at the Ink4a/Arf locus in MEFs. The graph shows the fold enrichment of Myc-tagged Ndy1 at the Ink4a/Arf locus normalized to control empty-vector (EV)–transduced cells. *P < 0.05; #P < 0.01. (D) HEK293T cells were transfected with the EV and Myc-tagged Ndy1. Whole-cell lysates were immunoprecipitated with the Myc antibody and probed with an antibody against Ezh2 (Upper) and a mix of antibodies against Myc and tubulin (Lower).
Ndy1 Binds to and Promotes the Demethylation of Histones H3K36me2 and H3K4me3 in the Ink4a/Arf Locus.
We hypothesized that Ndy1 may regulate p16Ink4a not only by up-regulating Ezh2 but by promoting histone H3 demethylation at the Ink4a/Arf locus. A prerequisite for the Ndy1-dependent regional demethylation of histone H3 is the binding of Ndy1 to the Ink4a/Arf locus. ChIP experiments indeed revealed that Myc-tagged Ndy1 binds Exon1α and the transcribed regions of both the Ink4a and Arf genes (Fig. 3C). Previous studies had shown that Ezh2, which is up-regulated by Ndy1, also binds the Ink4a/Arf locus (18) and that Ndy1 interacts with Bmi1 and Ring1b (23, 24). To address the question as to whether Ezh2 facilitates the binding of Ndy1, we examined whether Ndy1 and Ezh2 interact. Fig. 3D shows that the 2 proteins indeed interact and suggests that by up-regulating Ezh2, Ndy1 facilitates its own binding to the Ink4a/Arf locus.
The specificity of the Ndy1 histone demethylase has been a matter of controversy (25, 26). Data in this article show that both mouse and human Ndy1 immunoprecipitated from MEFs and IMR90 cells possesses strong H3K36me2 and weak H3K4me3 demethylase activities (Fig. 4A and Fig. S6). Consistently, using a fluorescence-coupled demethylation assay, a bacterially expressed mouse Ndy1 fragment containing the JmjC, CXXC, and PHD domains (amino acids 171–707) demethylated both H3K36me2 and H3K4me3 peptides, with high and low efficiencies, respectively (Fig. 4B and Fig. S7). Of note, the 171–707 Ndy1 fragment containing the Y221A mutation, which abolishes the demethylase activity of the protein (20), failed to demethylate either peptide (Fig. 4B and Fig. S7). To confirm the activity and specificity of the Ndy1 histone demethylase in vivo, we used flow cytometry to assess quantitatively the global levels of histone H3K36me2 and H3K4me3 in HEK293 cells transiently transfected with Ndy1. The data confirmed that Ndy1 exhibits strong histone H3K36me2 and weak histone H3K4me3 demethylase activities in vivo (Fig. S8).
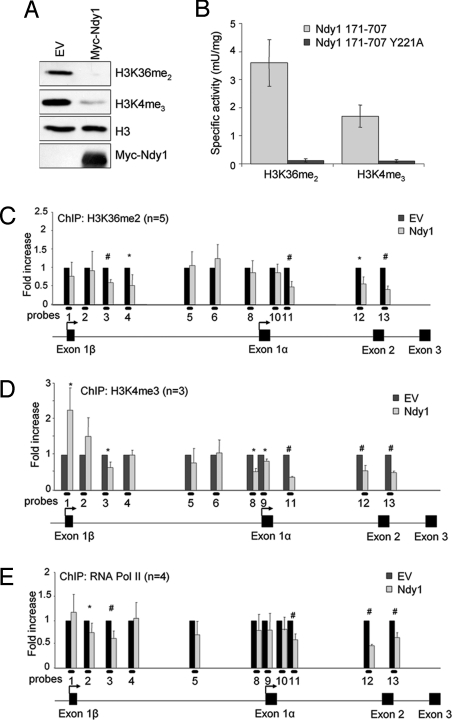
Fig. 4.
Ndy1 functions in vitro and in vivo as H3K36me2 and H3K4me3 demethylase. (A) Representative Western blot analysis of an in vitro demethylation assay with native murine Ndy1 protein isolated from MEFs overexpressing Ndy1. (B) Fluorescence-coupled demethylation assay of bacterial purified fragments of Ndy1 using the indicated peptide substrates. (C) ChIP analysis of the H3K36me2 methylation status at the Ink4a/Arf locus in MEFs that overexpress Ndy1. The graph shows the fold decrease of H3K36me2 methylation at the Ink4a/Arf locus in MEFs that overexpress Ndy1 normalized to control empty-vector (EV)–transduced cells. (D) ChIP analysis of the H3K4me3 methylation status at the Ink4a/Arf locus in MEFs that overexpress Ndy1. The graph shows the fold difference of H3K4me3 methylation at the Ink4a/Arf locus in MEFs that overexpress Ndy1 normalized to control empty-vector–transduced cells. (E) ChIP analysis of RNA Pol II binding at the Ink4a/Arf locus in MEFs that overexpress Ndy1. The graph shows the fold difference of RNA Pol II binding at the Ink4a/Arf locus in MEFs that overexpress Ndy1 normalized to control EV-transduced cells. *P < 0.05; #P < 0.01.
ChIP assays designed to address whether the Ink4a/Arf locus-bound Ndy1 functions as a regional histone demethylase showed that Ndy1 overexpression down-regulates H3K36me2 (Fig. 4C, probes 3–4 and 11–13) and H3K4me3 (Fig. 4D, probes 3 and 11–13) in the transcribed regions of both the Ink4a and Arf genes. Ndy1 also reduced H3K4me3 in the promoter region of p16Ink4a near the transcriptional start site of Exon1α (Fig. 4D, probes 8–9) but not in the promoter of p19Arf. The inability of Ndy1 to demethylate H3K4me3 in the promoter of p19Arf may explain why Ndy1 represses primarily the expression of p16Ink4a.
Ndy1-Driven Histone Modifications at the Ink4/Arf Locus Inhibit the Binding of RNA Pol II.
Whereas histone H3K36me2 and H3K4me3 correlate with active/permissive chromatin, histone H3K27me3 is a feature of inactive/repressive chromatin (13, 16, 17, 27–33). Because Ndy1 demethylates H3K36me2 and H3K4me3 and enhances both H3K27me3 and the binding of Bmi1/PRC1 to the Ink4a/Arf locus, we hypothesized that it may repress this locus by inhibiting the recruitment of RNA Pol II. ChIP analyses indeed revealed that Ndy1 overexpression significantly down-regulates the binding of RNA Pol II to the transcribed region of the Ink4a/Arf locus (Fig. 4E, probes 2–3 and 11–13). The suggested interdependence of histone H3K36me2 and H3K4me3 demethylation and RNA Pol II binding is supported by the finding that the pattern of histone demethylation and the pattern of RNA Pol II binding are similar.
Ndy1 Protects MEFs from Ras-Induced Senescence.
Overexpression of an oncogene, such as Ras, in primary fibroblasts induces premature senescence (1, 3, 4, 34, 35). To address whether Ndy1 protects cells from oncogene-induced senescence, we expressed RasV12 in MEFs either alone or in combination with Ndy1 or Bmi1, a known inhibitor of oncogene-induced senescence (6, 36). As shown in Fig. S9A, overexpression of RasV12 alone induced senescence, whereas coexpression of Ndy1 or Bmi1 inhibited senescence. Furthermore, whereas MEFs expressing RasV12, together with Ndy1 or Bmi1, formed colonies when plated at very low density, MEFs expressing only RasV12 did not (Fig. S9B). Overall, these data suggest that overexpression of Ndy1 bypasses oncogene-induced senescence and cooperates with Ras to transform MEFs. The mechanism by which Ndy1 inhibits Ras-induced senescence involves up-regulation of Ezh2 and inhibition of the induction of p16Ink4a (Fig. S9 C and D).
Ndy1 Up-Regulates Ezh2 and Represses p16Ink4a in IMR90 Cells.
To address if Ndy1 represses the Ink4a/Arf locus in human cells, we overexpressed Ndy1 in IMR90 human fibroblasts and found that it attenuated the induction of p16Ink4a during passage and enhanced the phosphorylation of Rb at Ser-807/811 (Fig. 5 A and B). In addition, we found that Ndy1 overexpression up-regulated Ezh2 and global H3K27me3, recapitulating our findings in MEFs (Fig. 5B and Fig. S5). Lastly, we searched the Oncomine online database for differences in the expression of Ndy1 between normal and tumor tissues. We found that the expression of Ndy1 was significantly increased in B- and T-cell acute lymphoblastic leukemias (37), in acute myeloid leukemias (37), and in seminomas (38) (Fig. 5 C and D, respectively). Taken together, these results suggest that Ndy1 may contribute to the development of those cancers.
Fig. 5.
Ndy1 up-regulates Ezh2 and represses p16Ink4a in IMR90 cells. (A) Total mRNA was isolated from Ndy1- or empty-vector (EV)–transduced IMR90 cells at passage 20 (P20) and analyzed by real-time PCR for the relative mRNA levels of p16Ink4a (n = 2). *P < 0.05. (B) Ndy1- or EV-transduced IMR90 cells were serially passaged and analyzed by Western blotting. (C) Data from ref. 37 reanalyzed to show expression levels of Ndy1 in normal bone marrow, B- and T-cell acute lymphoblastic leukemias, and acute myeloid leukemia. (D) Data from ref. 38 reanalyzed to show expression levels of Ndy1 in normal testis and seminomas.
Discussion
Senescence is induced by several distinct molecular mechanisms: (i) activation of the Ink4a/Arf locus, (ii) progressive shortening of telomeres, and (iii) DNA damage (1, 2, 4). Ink4a/Arf activation and telomere shortening may be developmentally programmed in dividing cells, or they may be induced in response to DNA damage. Cellular senescence contributes to both organismal aging and tumor suppression. Thus, whereas aging tissues up-regulate p16Ink4a and p19Arf, different types of human cancer harbor inactivating mutations in the Ink4a/Arf locus (1, 2).
Ndy1 protects cells from both replicative and oncogene-induced senescence (ref. 20 and this report). Our earlier studies have shown that Ndy1 may inhibit senescence by regulating redox homeostasis and by protecting cells from oxidative stress (21). Here, we show that Ndy1 represses the expression of the Ink4a/Arf locus, whose silencing also contributes to immortalization. Our data also show that Ndy1 is down-regulated during senescence, along with Ezh2. The concordant down-regulation of Ezh2 and Ndy1 suggested that Ndy1 may regulate Ezh2. Data in this report confirmed this hypothesis. In addition, they showed that by up-regulating Ezh2, Ndy1 up-regulates histone H3K27me3 both globally and within the Ink4a/Arf locus. As a result, Ndy1 promotes the binding of Bmi1, which is known to repress the Ink4a/Arf locus. However, H3K27 trimethylation and Bmi1 binding were not sufficient to explain the Ndy1 phenotype fully. Data presented here indeed show that Ndy1 binds the Ink4a/Arf locus and demethylates the locus-associated H3K36me2 and H3K4me3. These combined histone modifications inhibit the binding of RNA Pol II and contribute to the silencing of the locus. Interestingly, Ndy1 also binds Ezh2. By up-regulating Ezh2, Ndy1 may promote its own binding to the locus. The complex role of Ndy1 in the regulation of the Ink4a/Arf locus is outlined in the model in Fig. 6. According to this model, the pathways by which Ndy1 and Bmi1 repress the Ink4a/Arf locus overlap only partially with Ndy1, promoting both the binding of Bmi1 and the demethylation of H3K36me2 and H3K4me3. The placement of Bmi1 downstream of Ndy1 is suggested by the findings that Ndy1 promotes the binding of Bmi1 to the Ink4a/Arf locus and that Bmi1 alone has no effect on H3K27me3.
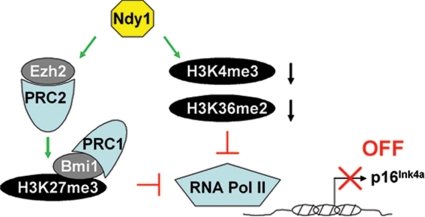
Fig. 6.
The histone demethylase Ndy1 represses the Ink4a/Arf locus. Ndy1 represses the Ink4a/Arf locus by 2 distinct mechanisms: it up-regulates Ezh2 and promotes histone H3K27 trimethylation and Bmi1 binding within the Ink4a/Arf locus, and it binds to and demethylates H3K36me2 and H3K4me3 within the Ink4a/Arf locus. These histone modifications, when combined, interfere with the binding of RNA Pol II and contribute to the silencing of the Ink4a/Arf locus.
One of the earliest molecular events triggered by Ndy1 in both mouse and human fibroblasts is the JmjC domain-dependent up-regulation of Ezh2, which results in the up-regulation of H3K27me3. Ndy1 knockdown had the opposite effects. Moreover, Ndy1 and Ezh2 were down-regulated in concert during senescence, which is characterized by Ezh2 depletion, elimination of H3K27me3, displacement of Bmi1, and transcriptional activation of the Ink4A/Arf locus (7, 19). These data suggest that the up-regulation of Ezh2 by Ndy1 plays an important role in the Ndy1 immortalization phenotype. The mechanism by which Ndy1 regulates the expression of Ezh2 remains to be determined. To date, our data show that Ndy1 up-regulates the mRNA levels of Ezh2. However, the up-regulation is modest, suggesting that Ndy1 may up-regulate Ezh2 primarily via posttranslational mechanisms. The physical interaction between Ndy1 and Ezh2 suggests that Ndy1 may up-regulate Ezh2 by stabilizing polycomb complexes (23, 24).
Overexpression of Ezh2 and histone H3K27 trimethylation promote the binding of Bmi1, which results in transcriptional repression (18). Data presented in this article show that the up-regulation of Ezh2 by Ndy1 increases H3K27me3 and Bmi1 binding at the Ink4a/Arf locus, suggesting that Ndy1 represses the Ink4a/Arf locus and inhibits senescence, at least in part, by regulating Bmi1 recruitment to the locus. However, the knockdown of Ndy1, Ezh2, and Bmi1, singly or in combination in MEFs, and the knockdown of Bmi1 in Ndy1-immortalized MEFs suggested that Ndy1 cooperates with both Ezh2 and Bmi1. These data also suggested that Ndy1 may elicit additional events that contribute to the immortalization phenotype. Studies presented in this article indeed showed that Ndy1 binds the Ink4a/Arf locus and demethylates histone H3K36me2 and H3K4me3. Both H3K36me2 and H3K4me3 are associated with active/permissive chromatin (39–41) in the transcribed region and in the promoter of a gene, respectively (16, 17, 27–31). Mechanistically, H3K36me2 recruits histone deacetylase complexes, which contribute to the restoration of normal chromatin structure in the wake of elongating RNA Pol II in the body of transcribed genes (28, 40–43), and H3K4me3 provides a docking site for transcription factor IID, which seeds the formation of the preinitiation complex (30). Therefore, by eliminating those methylation marks within the Ink4a/Arf locus, Ndy1 inhibits the recruitment of RNA Pol II and functions as a transcriptional repressor.
To fine-tune the transcription of different genes, Ndy1 may function as an integral component of polycomb complexes regulating H3K4me3 and H3K36me2 demethylation in concert with Ezh2-mediated H3K27 trimethylation. The suggested interdependence of H3K27 trimethylation and H3K36me2 and H3K4me3 demethylation is strongly supported by the passage dependence of the outcome of these activities in cells engineered to overexpress Ndy1. Ezh2 up-regulation and H3K27 trimethylation, the earliest known consequences of Ndy1 overexpression, may promote the binding of Bmi1- and Ndy1-containing complexes. Histone modifications induced by these complexes, including trimethylation of H3K27 and demethylation of H3K36me2 and H3K4me3, may further stimulate the binding of PRC complexes. This feed-forward mechanism is expected to enhance complex binding and histone modifications with each passage, as observed.
We have previously shown that Ndy1 enhanced the proliferation of but failed to immortalize human IMR90 cells in culture (20). Given that the induction of senescence in IMR90 cells, but not in MEFs, is attributable primarily to telomere shortening, these data suggested that Ndy1 protects primary cells from developmentally regulated or DNA damage-induced growth inhibitory activities, but it does not protect them from telomere shortening. Data presented in this article confirm this hypothesis by showing that Ndy1 up-regulates Ezh2, represses the expression of p16Ink4a, and up-regulates the phosphorylation of Rb at Ser-807/811 in both mouse and human fibroblasts. This suggests that despite the inability of Ndy1 to immortalize IMR90 cells in culture, the role for Ndy1 in the regulation of PRC2 and the repression of the Ink4a/Arf locus is conserved between humans and mice. This finding, combined with the observation that Ndy1 is up-regulated in several types of human tumors, suggests that Ndy1 functions as an oncogene not only in animal cancer but in human cancer.
Materials and Methods
In Vitro Histone Demethylation Assay.
Cells were harvested, and the cell pellet was resuspended in 5 packed cell volumes of buffer A [10 mM Hepes (pH 7.9), 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, and 0.2 mM PMSF] and spun at 300 × g for 5 min. Buffer A was added up to a final of 3 original packed cell volumes, and the suspension was incubated on ice for 10 min. Cells were transferred to a Wheaton A Dounce homogenizer and lysed with 10 strokes. Nuclei were pelleted by spinning at maximum speed for 5 min in a microcentrifuge. Nuclei were resuspended in buffer B [20 mM Hepes (pH 7.9), 100 mM KCl, 0.5 mM DTT, and 0.2 mM PMSF] and disrupted by sonication. After preclearing with 20 μL of protein A-agarose beads, the supernatant was incubated with either myc- or hemagglutinin-specific monoclonal antibody (5 μg/sample) and 20 μL of protein A-agarose beads overnight at 4 °C. The following day, the sample was washed 3 times with 1 mL of buffer B, and the purified immune complex was incubated overnight at 4 °C with myc or HA peptide to release Ndy1 from the beads. Demethylation reactions were carried out in 10 mM Hepes (pH 7.9), 50 mM NaCl, 1 mM α-ketoglutarate, 2 mM ascorbate, 30–70 μM Fe(NH4)2(SO4)2, and 0.25 mg/mL BSA and contained 6 μg of bulk histone per 90-μL reaction. Reactions were incubated at 37 °C for 1–3 h and were analyzed by 18% (vol/vol) SDS/PAGE and Western blotting with histone-specific antibodies. Fluorescent assays were carried out as previously described (44) but under the reaction conditions mentioned previously. One unit of enzyme activity is determined as the amount of Ndy1 that would produce 1 μmol of formaldehyde in 1 min when incubated with 340 μM peptide substrate at 37 °C under these conditions. The sequences of the H3K4(me3) and H3K36(me2) peptides used in the biochemical studies were ART-K(me3)-QTARKST and ATGGV-K(me2)-KPHRY, respectively, which were synthesized at Tufts University Core facility. All other procedures are described in SI Experimental Procedures.
Supplementary Material
Acknowledgments.
This work was supported by National Institutes of Health Grant R01CA109747.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813139106/DCSupplemental.
References
- 1.Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Gil J, Peters G. Regulation of the INK4b-ARF-INK4a tumour suppressor locus: All for one or one for all. Nat Rev Mol Cell Biol. 2006;7:667–677. doi: 10.1038/nrm1987. [DOI] [PubMed] [Google Scholar]
- 3.Sharpless NE, et al. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature. 2001;413:86–91. doi: 10.1038/35092592. [DOI] [PubMed] [Google Scholar]
- 4.Serrano M, et al. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996;85:27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- 5.Kamijo T, et al. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell. 1997;91:649–659. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs JJ, Kieboom K, Marino S, DePinho RA, van Lohuizen M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999;397:164–168. doi: 10.1038/16476. [DOI] [PubMed] [Google Scholar]
- 7.Kamminga LM, et al. The Polycomb group gene Ezh2 prevents hematopoietic stem cell exhaustion. Blood. 2006;107:2170–2179. doi: 10.1182/blood-2005-09-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gil J, Bernard D, Martinez D, Beach D. Polycomb CBX7 has a unifying role in cellular lifespan. Nat Cell Biol. 2004;6:67–72. doi: 10.1038/ncb1077. [DOI] [PubMed] [Google Scholar]
- 9.Dietrich N, et al. Bypass of senescence by the polycomb group protein CBX8 through direct binding to the INK4A-ARF locus. EMBO J. 2007;26:1637–1648. doi: 10.1038/sj.emboj.7601632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz YB, Pirrotta V. Polycomb silencing mechanisms and the management of genomic programmes. Nat Rev Genet. 2007;8:9–22. doi: 10.1038/nrg1981. [DOI] [PubMed] [Google Scholar]
- 11.Sparmann A, van Lohuizen M. Polycomb silencers control cell fate, development and cancer. Nat Rev Cancer. 2006;6:846–856. doi: 10.1038/nrc1991. [DOI] [PubMed] [Google Scholar]
- 12.Cao R, Zhang Y. The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr Opin Genet Dev. 2004;14:155–164. doi: 10.1016/j.gde.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Cao R, et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–1043. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 14.Kirmizis A, et al. Silencing of human polycomb target genes is associated with methylation of histone H3 Lys 27. Genes Dev. 2004;18:1592–1605. doi: 10.1101/gad.1200204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou W, et al. Histone H2A monoubiquitination represses transcription by inhibiting RNA polymerase II transcriptional elongation. Mol Cell. 2008;29:69–80. doi: 10.1016/j.molcel.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barski A, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Kim TH, et al. A high-resolution map of active promoters in the human genome. Nature. 2005;436:876–880. doi: 10.1038/nature03877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bracken AP, et al. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev. 2007;21:525–530. doi: 10.1101/gad.415507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bracken AP, Dietrich N, Pasini D, Hansen KH, Helin K. Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes Dev. 2006;20:1123–1136. doi: 10.1101/gad.381706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfau R, et al. Members of a family of JmjC domain-containing oncoproteins immortalize embryonic fibroblasts via a JmjC domain-dependent process. Proc Natl Acad Sci USA. 2008;105:1907–1912. doi: 10.1073/pnas.0711865105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polytarchou C, Pfau R, Hatziapostolou M, Tsichlis PN. The JmjC domain histone demethylase Ndy1 regulates redox homeostasis and protects cells from oxidative stress. Mol Cell Biol. 2008;28:7451–7464. doi: 10.1128/MCB.00688-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernandez-Munoz I, Taghavi P, Kuijl C, Neefjes J, van Lohuizen M. Association of BMI1 with polycomb bodies is dynamic and requires PRC2/EZH2 and the maintenance DNA methyltransferase DNMT1. Mol Cell Biol. 2005;25:11047–11058. doi: 10.1128/MCB.25.24.11047-11058.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanchez C, et al. Proteomics analysis of Ring1B/Rnf2 interactors identifies a novel complex with the Fbxl10/Jhdm1B histone demethylase and the Bcl6 interacting corepressor. Mol Cell Proteomics. 2007;6:820–834. doi: 10.1074/mcp.M600275-MCP200. [DOI] [PubMed] [Google Scholar]
- 24.Gearhart MD, Corcoran CM, Wamstad JA, Bardwell VJ. Polycomb group and SCF ubiquitin ligases are found in a novel BCOR complex that is recruited to BCL6 targets. Mol Cell Biol. 2006;26:6880–6889. doi: 10.1128/MCB.00630-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsukada Y, et al. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006;439:811–816. doi: 10.1038/nature04433. [DOI] [PubMed] [Google Scholar]
- 26.Frescas D, Guardavaccaro D, Bassermann F, Koyama-Nasu R, Pagano M. JHDM1B/FBXL10 is a nucleolar protein that represses transcription of ribosomal RNA genes. Nature. 2007;450:309–313. doi: 10.1038/nature06255. [DOI] [PubMed] [Google Scholar]
- 27.Kim A, Kiefer CM, Dean A. Distinctive signatures of histone methylation in transcribed coding and noncoding human beta-globin sequences. Mol Cell Biol. 2007;27:1271–1279. doi: 10.1128/MCB.01684-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joshi AA, Struhl K. Eaf3 chromodomain interaction with methylated H3–K36 links histone deacetylation to Pol II elongation. Mol Cell. 2005;20:971–978. doi: 10.1016/j.molcel.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 29.Krogan NJ, et al. The Paf1 complex is required for histone H3 methylation by COMPASS and Dot1p: Linking transcriptional elongation to histone methylation. Mol Cell. 2003;11:721–729. doi: 10.1016/s1097-2765(03)00091-1. [DOI] [PubMed] [Google Scholar]
- 30.Vermeulen M, et al. Selective anchoring of TFIID to nucleosomes by trimethylation of histone H3 lysine 4. Cell. 2007;131:58–69. doi: 10.1016/j.cell.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Bernstein BE, et al. Genomic maps and comparative analysis of histone modifications in human and mouse. Cell. 2005;120:169–181. doi: 10.1016/j.cell.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Shilatifard A. Chromatin modifications by methylation and ubiquitination: Implications in the regulation of gene expression. Annu Rev Biochem. 2006;75:243–269. doi: 10.1146/annurev.biochem.75.103004.142422. [DOI] [PubMed] [Google Scholar]
- 33.Eissenberg JC, Shilatifard A. Leaving a mark: The many footprints of the elongating RNA polymerase II. Curr Opin Genet Dev. 2006;16:184–190. doi: 10.1016/j.gde.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Brookes S, et al. INK4a-deficient human diploid fibroblasts are resistant to RAS-induced senescence. EMBO J. 2002;21:2936–2945. doi: 10.1093/emboj/cdf289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin AW, et al. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 1998;12:3008–3019. doi: 10.1101/gad.12.19.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Datta S, et al. Bmi-1 cooperates with H-Ras to transform human mammary epithelial cells via dysregulation of multiple growth-regulatory pathways. Cancer Res. 2007;67:10286–10295. doi: 10.1158/0008-5472.CAN-07-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andersson A, et al. Microarray-based classification of a consecutive series of 121 childhood acute leukemias: Prediction of leukemic and genetic subtype as well as of minimal residual disease status. Leukemia. 2007;21:1198–1203. doi: 10.1038/sj.leu.2404688. [DOI] [PubMed] [Google Scholar]
- 38.Sperger JM, et al. Gene expression patterns in human embryonic stem cells and human pluripotent germ cell tumors. Proc Natl Acad Sci USA. 2003;100:13350–13355. doi: 10.1073/pnas.2235735100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao B, Shibata Y, Strahl BD, Lieb JD. Dimethylation of histone H3 at lysine 36 demarcates regulatory and nonregulatory chromatin genome-wide. Mol Cell Biol. 2005;25:9447–9459. doi: 10.1128/MCB.25.21.9447-9459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bell O, et al. Localized H3K36 methylation states define histone H4K16 acetylation during transcriptional elongation in Drosophila. EMBO J. 2007;26:4974–4984. doi: 10.1038/sj.emboj.7601926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li B, et al. Infrequently transcribed long genes depend on the Set2/Rpd3S pathway for accurate transcription. Genes Dev. 2007;21:1422–1430. doi: 10.1101/gad.1539307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee ER, Murdoch FE, Fritsch MK. High histone acetylation and decreased polycomb repressive complex 2 member levels regulate gene specific transcriptional changes during early embryonic stem cell differentiation induced by retinoic acid. Stem Cells. 2007;25:2191–2199. doi: 10.1634/stemcells.2007-0203. [DOI] [PubMed] [Google Scholar]
- 43.Keogh MC, et al. Cotranscriptional set2 methylation of histone H3 lysine 36 recruits a repressive Rpd3 complex. Cell. 2005;123:593–605. doi: 10.1016/j.cell.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 44.Couture JF, Collazo E, Ortiz-Tello PA, Brunzelle JS, Trievel RC. Specificity and mechanism of JMJD2A, a trimethyllysine-specific histone demethylase. Nat Struct Mol Biol. 2007;14:689–695. doi: 10.1038/nsmb1273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.