Abstract
Native cytochrome c (cyt c) has a compact tertiary structure with a hexacoordinated heme iron and functions in electron transport in mitochondria and apoptosis in the cytoplasm. However, the possibility that protein modifications confer additional functions to cyt c has not been explored. Disruption of methionine 80 (M80)-Fe ligation of cyt c under nitrative stress has been reported. To model this alteration and determine if it confers new properties to cyt c, a cyt c mutant (M80A) was constitutively expressed in cells. M80A-cyt c has increased peroxidase activity and is spontaneously released from mitochondria, translocating to the cytoplasm and nucleus in the absence of apoptosis. Moreover, M80A models endogenously nitrated cyt c because nitration of WT-cyt c is associated with its translocation to the cytoplasm and nucleus. Further, M80A cyt c may up-regulate protective responses to nitrative stress. Our findings raise the possibility that endogenous protein modifications that disrupt the M80-Fe ligation (such as tyrosine nitration) stimulate nuclear translocation and confer new functions to cyt c in nonapoptotic cells.
Keywords: mitochondria, nitration, oxidation, oxidative stress, peroxynitrite
When cytochrome c (cyt c) acts as an electron shuttle in mitochondria, all 6 coordination positions of its heme iron are occupied, thus preventing its interactions with small ligands such as H2O2 and NO (1). However, the methionine 80 (M80)-Fe ligation of a subpopulation of cyt c may be endogenously disrupted in cells. Specifically, tyrosine nitration, M80 oxidation, and interactions with cardiolipin have been reported to cause loosening of the tertiary structure of cyt c and disruption of the M80-Fe ligation. Endogenous nitration of cyt c has been detected in cultured osteoclasts (2) and in kidneys after ischemia/reperfusion injury and during chronic allograft nephropathy (3, 4). In vitro nitration of cyt c has been shown to disrupt the M80-Fe ligation, increase the peroxidase activity, and inhibit the electron transport function of cyt c (5). In addition, oxidation of M80 by a variety of oxidants, including singlet oxygen and HOCl, disrupts the M80-Fe ligation (5–8). Finally, approximately 15% of cyt c tightly binds to cardiolipin in the inner mitochondrial membrane via both electrostatic and hydrophobic interactions, resulting in a loosening of the tertiary structure of cyt c and disruption of the M80-Fe ligation (9–11). Disruption of the M80-Fe ligation may increase the peroxidase activity of cyt c by increasing the access of H2O2 to the heme iron (1). Indeed, the subpopulation of mitochondrial cyt c that is tightly bound to cardiolipin has increased peroxidase activity and may catalyze cardiolipin peroxidation that is required for cyt c release from mitochondria during apoptosis (1). This subpopulation of cyt c may have other unique biological functions due to its altered conformation that have yet to be identified.
To investigate the possibility that the function of cyt c is altered by endogenous posttranslational modifications and/or cardiolipin interactions that disrupt the M80-Fe ligation, we generated a cyt c mutant with a constitutively disrupted M80-Fe ligation due to a mutation of M80 to alanine (M80A). We then investigated the effects of the M80A mutation on the subcellular localization and function of cyt c.
Results
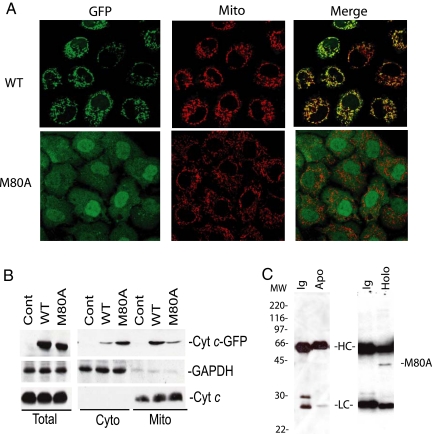
To determine if disruption of the M80-Fe ligation alters the intracellular distribution of cyt c, the subcellular localization of GFP-tagged M80A or WT cyt c expressed in HeLa cells was analyzed by confocal microscopy. The M80A mutation in iso-yeast cyt c does not cause major alterations to the tertiary structure of cyt c despite disrupting the heme ligation (12). Although WT cyt c was localized primarily in mitochondria, M80A cyt c had a nonmitochondrial nuclear and cytoplasmic distribution (Fig. 1A). Similar results were obtained when GFP-tagged M80A or WT cyt c was expressed in MCF-7 cells (data not shown). Subcellular fractionation studies confirmed that M80A cyt c had a predominantly nonmitochondrial localization (Fig. 1B). The distribution of M80A cyt c was not dependent on the GFP tag because tetracysteine-tagged M80A cyt c also had a diffuse cytoplasmic and nuclear distribution by confocal analysis (data not shown). These findings suggest that the M80A cyt c mutant is either not imported into mitochondria or is imported and then rapidly released into the cytoplasm and/or nucleus.
Fig. 1.
M80A cyt c is spontaneously released from mitochondria and translocates to the nucleus. (A) The subcellular localization of GFP-tagged M80A and WT cyt c stably expressed in HeLa cells was determined by confocal microscopy. Mitochondria were labeled with MitoTracker Red (Mito). Colocalization of green GFP-cyt c staining and red mitochondrial staining is seen as yellow in the merged images. (B) Subcellular localization of WT and M80A cyt c as determined by subcellular fractionation. Levels of cyt c-GFP (Cyt c-GFP) in equal concentrations of whole cell (Total), cytoplasmic (Cyto), or mitochondrial (Mito) lysates of untransfected HeLa cells (Control) or HeLa cells expressing WT or M80A cyt c-GFP were assessed on cyt c WBs. As loading controls and to assess the purity of subcellular fractions, glyceraldehyde-3-phosphate dehydrogenase (GAPDH, cytoplasm) and endogenous cyt c (mitochondria) WBs were also performed. The data are representative of 3 separate experiments. c. M80A cyt c is a holoprotein. Lysates of HeLa cells expressing M80A cyt c were immunoprecipitated by antibodies recognizing apocyt c (Apo), holocyt c (Holo), or by equal concentrations of control antibodies (Ig). A cyt c WB of the immunoprecipitates is shown. MW indicates molecular weight standards. HC and LC indicate Ig heavy and light chains, respectively.
Cyt c is synthesized as an apoenzyme in the cytoplasm, translocates to mitochondria, and only then acquires a heme group to form a holoenzyme. Therefore, to determine if M80A is imported into mitochondria before being released into the cytoplasm, we investigated whether M80A was immunoprecipitated by an antibody specific for heme-bound holocyt c. M80A cyt c was immunoprecipitated by an antibody specific for holocyt c, but not by an antibody that recognizes apocyt c (Fig. 1C). In addition, the molecular weight of immunoprecipitated GFP-tagged M80A cyt c as determined by liquid chromatography/mass spectrometry was nearly identical to that of GFP-tagged WT cyt c, with the observed 60 Da difference attributable to the mutation from methionine to alanine in M80A. The approximately 700 Da increase in molecular weight of WT and M80A cyt c as compared to the predicted molecular weight of the apocyt c is consistent with the addition of a heme group to both proteins (supporting information (SI) Fig. S1). Moreover, it has previously been shown in yeast that mutation of the heme-coordinating methionine does not inhibit import of cyt c into mitochondria (13). These findings suggest that M80A cyt c is imported into mitochondria, acquires a heme group, and then is spontaneously released. Although mitochondrial release and nuclear translocation of cyt c has been described during apoptosis (14), our data suggest that this event also occurs in nonapoptotic cells.
During apoptosis, cyt c release from mitochondria is dependent on either the Bcl-2 family members Bax and Bak and/or on the mitochondrial permeability transition pore (MPTP) (15). To determine if the spontaneous release of M80A cyt c from mitochondria in the absence of apoptosis was Bax- or Bak-dependent, GFP-tagged M80A or WT cyt c was expressed in mouse embryonic fibroblasts (MEFs) obtained from Bax and Bak double knock-out (DKO) mice (16). M80A cyt c has a cytoplasmic and nuclear distribution in the Bax/Bak DKO MEFs, indicating that M80A cyt c release from mitochondria is Bax/Bak-independent (Fig. S2). Moreover, inhibitors of the MPTP (cyclosporine A and bongkrekic acid) did not change the nonmitochondrial distribution of M80A (data not shown), suggesting that its release is also not dependent on the MPTP. A serine protease-dependent mechanism of mitochondrial cyt c release has also been reported (17). However, the serine protease inhibitor 4-(2-aminoethyl)benzenesulfonylfluoride also did not significantly alter the nuclear and cytoplasmic distribution of M80A (data not shown). Thus cyt c with a disrupted M80-Fe ligation may be released from mitochondria in nonapoptotic cells by a mechanism that is independent of Bax/Bak, the MPTP, and/or serine proteases.
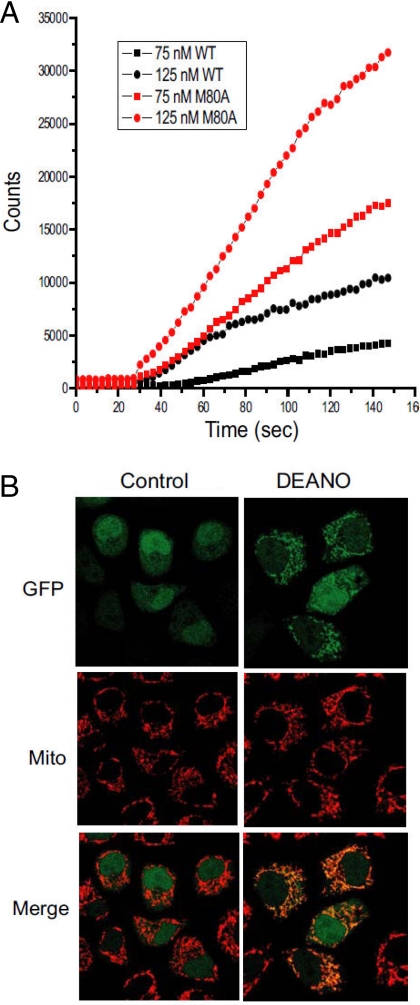
Recent data suggest that an increased peroxidase activity of cyt c during apoptosis leads to cardiolipin oxidation that is required for the release of cyt c and other pro-apoptotic factors from mitochondria (1). Therefore, M80A cyt c may be spontaneously released from mitochondria because it has increased peroxidase activity (as a result of disruption of the Fe-M80 ligation) and oxidizes cardiolipin. In support of this hypothesis, recombinant M80A cyt c has increased peroxidase activity as compared to equal concentrations of WT cyt c (Fig. 2A). In addition, the nitric oxide donor 2-(N,N-Diethylamino)diazenolate-2-oxide sodium (DEANO), an inhibitor of the peroxidase activity of cyt c (18), inhibited the release of M80A cyt c from mitochondria in a subpopulation of cells (Fig. 2B). Moreover, knock-down of M80A expression with RNAi protected cells from tert-Butyl hydroperoxide (tBuOOH)-induced death, as would be expected if M80A functioned as a peroxidase intracellularly (Fig. S3). These data raise the possibility that the increased peroxidase activity of M80A cyt c may mediate its spontaneous release from mitochondria. However, levels of cardiolipin hydroperoxide were not increased in cells expressing M80A cyt c as compared to cells expressing WT cyt c (data not shown). Thus peroxidation of a substrate other than cardiolipin may be involved in the release of M80A cyt c from mitochondria, or cardiolipin peroxidation may be rapidly reversed in cells expressing M80A.
Fig. 2.
The release of M80A from mitochondria may be peroxidase activity-dependent. (A) Recombinant M80A has higher peroxidase activity than recombinant WT cyt c. The peroxidase activity of recombinant M80A or WT cyt c was determined by luminol oxidation as described previously (32). (B) DEANO, an inhibitor of the peroxidase activity of cyt c (18), inhibits M80A cyt c release from mitochondria. HeLa cells expressing GFP-tagged M80A cyt c were treated in the presence or absence of DEANO (2 mM) for 8 h. Subcellular localization of M80A was then determined by confocal microscopy as described in Fig. 1A. The results are representative of 4 separate experiments.
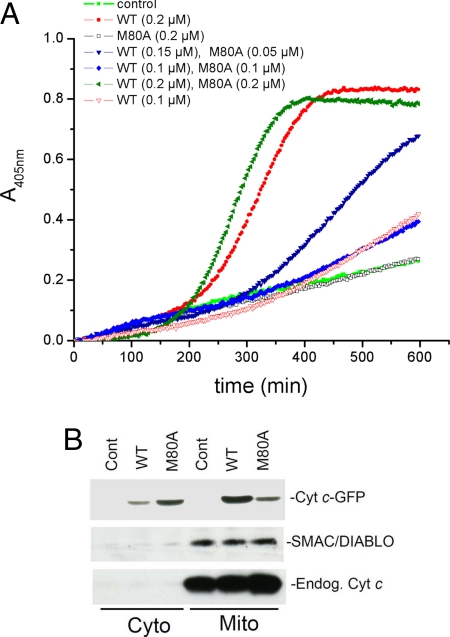
Normally, release of cyt c into the cytoplasm stimulates apoptosome formation, caspase activation, and apoptotic cell death. However, cells expressing M80A cyt c did not undergo apoptosis, suggesting that M80A cyt c may not activate caspases as efficiently as WT cyt c. In fact, recombinant M80A cyt c did not efficiently activate caspase-3 in cytoplasmic lysates and was not able to competitively inhibit the activation of caspase-3 by recombinant WT cyt c (Fig. 3A). In addition, SMAC/DIABLO, a pro-apoptotic protein that is released from mitochondria along with cyt c during apoptosis, was not released into the cytoplasm in cells expressing M80A (Fig. 3B). Of interest, endogenous cyt c was also not released from mitochondria along with M80A cyt c (Fig. 3B), indicating that M80A cyt c is selectively released. Consistent with this finding, cells expressing M80A cyt c are energy-sufficient as expected if endogenous cyt c is retained in mitochondria. These results suggest that cytoplasmic release of M80A does not trigger apoptosis both because other pro-apoptotic proteins are not released from mitochondria along with M80A and because M80A does not efficiently activate the apoptosome, perhaps due to reduced affinity for Apaf-1. Of note, similar to what we observed with M80A cyt c (Fig. 3A), nitrocytochrome c also does not efficiently activate the apoptosome as we (19) and others (20) have recently reported.
Fig. 3.
M80A cyt c does not efficiently activate caspases or induce the release of SMAC-DIABLO from mitochondria. (A) Decreased activation of caspase-3 by M80A. Equal concentrations of recombinant M80A or WT cyt c were added to cytosolic extracts of Jurkat cells in the presence of dATP and ATP. Caspase-3 activity was measured using the chromogenic substrate AcDEVDpNA. To verify the occurrence of competitive inhibition, M80A and WT cyt c were also assayed in combination, in several molar ratios. (B) M80A cyt c expression does not induce the release of SMAC/DIABLO from mitochondria. HeLa cells expressing M80A or WT cyt c or untransfected HeLa cells (Control) were separated into mitochondrial and cytoplasmic fractions and the levels of cyt c-GFP (Cyt c-GFP), SMAC/DIABLO and endogenous cyt c (Endog Cyt c) in each fraction were determined by WB analysis.
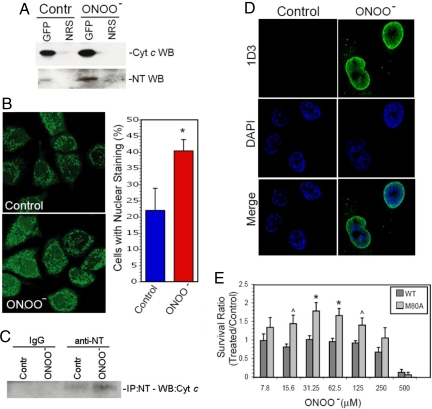
We next determined if M80A cyt c is modeling the physiologic function of endogenously nitrated cyt c because tyrosine nitration disrupts the M80-Fe ligation of cyt c (5, 6, 21), and endogenous nitration of cyt c has been detected in vivo under conditions of nitrative stress (3, 4). To determine if peroxynitrite (ONOO− an endogenously produced nitrating agent, induces nitration of WT cyt c, cells expressing WT cyt c-GFP were exposed to a low nontoxic dose of ONOO− (25 μM). Tyrosine nitration is the only detectable modification of cyt c induced by low concentrations of ONOO− (5, 6). Cyt c was then immunoprecipitated from cells and tyrosine nitration was assessed on anti-nitrotyrosine Western blots. No evidence of cyt c nitration was seen in cyt c immunoprecipitates of whole cell lysates (data not shown). However, tyrosine nitration of cyt c that increased after ONOO− treatment was detected in cyt c immunoprecipitates of nuclear-enriched cellular fractions (Fig. 4A). These findings raise the possibility that nitrated cyt c translocates to the nucleus. In support of this hypothesis, ONOO−-induced nitration of cyt c was associated with the translocation of a subpopulation of GFP-tagged WT cyt c from mitochondria to the nucleus (Fig. 4B).
Fig. 4.
Tyrosine nitration of WT cyt c is associated with its translocation to the cytoplasm and nucleus and may regulate the response of cells to further nitrative stress. (A) WT cyt c is tyrosine nitrated by nontoxic levels of ONOO−. HeLa cells expressing GFP-labeled WT cyt c were left untreated (Contr) or were treated for 30 sec with ONOO− (25 μM). The nuclear-enriched fraction of the cells was then immunoprecipitated with an anti-GFP antiserum (GFP) or with equal concentrations of normal rabbit serum (NRS). Levels of cyt c and nitrotyrosine in the immunoprecipitates were then determined on anti-cyt c (Cyt c WB) or anti-nitrotyrosine (NT WB) WBs. (B) Nontoxic levels of ONOO− induce the translocation of a subpopulation of WT cyt c from mitochondria to the cytoplasm and nucleus. HeLa cells expressing GFP-labeled WT cyt c were left untreated or were treated with ONOO− (25 μM). After 4 h, the subcellular localization of cyt c was determined by confocal microscopy. The graph shows the mean percentage of cells with nuclear cyt c-GFP staining plus SD in 4 separate experiments. * indicates P < 0.05, two-tailed t test, n = 4. (C) Endogenous cyt c with a disrupted M80-Fe ligation is detected in the nucleus of cells treated with nontoxic levels of ONOO−. Nontransfected HeLa cells were treated with 25 μM ONOO− for 30 sec, incubated overnight in regular medium and the nuclear fraction was then submitted to immunoprecipitation with an anti-nitrotyrosine (anti-NT) antibody or a control, irrelevant antibody (IgG). Shown is a cyt c Western blot analysis of the immunoprecipitates from control (Contr) and peroxynitrite-treated cells (ONOO−). (D) Cells processed as described in (C) were analyzed by confocal microscopy with the 1D3 antibody, which recognizes an epitope present when the Fe-M80 ligation is disrupted in cyt c (22). Anti-mouse FITC-conjugated antibody was used to detect 1D3 labeling. Nuclei are stained with DAPI. (E) Cells expressing M80A have higher rates of survival and/or proliferation in response to ONOO−. Equal numbers of HeLa cells expressing WT or M80A cyt c-GFP were treated with increasing doses of ONOO−. After 18 h, cell proliferation and viability was determined using the WST-1 assay. The data indicate the absorption ratio of ONOO− treated/control untreated cells (mean + SEM) in 8 separate experiments. ^ indicates P < 0.05 and * indicates P < 0.01, two-tailed t test.
To rule out the possibility that the nitration and nuclear translocation of cyt c is an artifact of GFP-cyt c overexpression, we analyzed whether mild nitrative stress is associated with a disruption of the M80-Fe ligation and the nuclear translocation of endogenous cyt c. To address this issue, nontransfected HeLa cells were treated with 25 μM ONOO−, followed by immunoprecipitation of nuclear nitrated proteins with an anti-nitrotyrosine antibody, and Western blot analysis with an anti-cyt c antibody. As shown in Fig. 4C, cyt c is detected in higher quantity in the nuclear fraction of cells treated with ONOO−. Detection of cyt c with the anti-nitrotyrosine antibody was abrogated by pretreatment of the membrane with dithionite, confirming the nitrated nature of the subpopulation of cyt c imported into the nucleus. In addition, confocal microscopic studies of untransfected HeLa cells were performed using an antibody (1D3) that does not recognize native cyt c but specifically recognizes a cyt c epitope that is exposed when the M80-Fe ligation is disrupted (22). In untreated cells, 1D3 staining was not detected (Fig. 4C). However, after cells were treated with low doses of ONOO− (25 μM), 1D3 staining was detected in the nucleus (Fig. 4D). These findings suggest that mild nitrative stress disrupts the M80-Fe ligation and leads to the nuclear translocation of the nitrated subpopulation of endogenous cyt c.
Low, nontoxic levels of ONOO− not only have been shown to nitrate cyt c (7, 12) but also have been implicated in preconditioning responses that confer increased resistance to oxidative and nitrative stress (23, 24). To determine if M80A cyt c alters the response of cells to nitrative stress, cells expressing WT or M80A cyt c were exposed to a range of concentrations of ONOO−. M80A-expressing cells had increased rates of survival when exposed to moderate to highly toxic levels of ONOO− as compared to cells expressing WT cyt c (Fig. 4E). Thus M80A may regulate the resistance of cells to nitrative stress. Our findings raise the possibility that a subpopulation of cyt c is nitrated when cells are exposed to low levels of nitrative stress, leading to disruption of the M80-Fe ligation. As a result, this subpopulation of cyt c translocates from mitochondria to the cytoplasm and nucleus and regulates the response of cells to further nitrative and/or oxidative stress.
Discussion
Our data suggest that the subcellular localization of cyt c is regulated not only by Bcl-2 family members, the MPTP, and/or serine proteases, but also by the M80-Fe ligation of cyt c. Specifically, endogenous disruption of the M80-Fe ligation by modifications such as tyrosine nitration, M80 oxidation, and/or interactions with cardiolipin may increase the peroxidase activity of cyt c and induce its translocation from mitochondria to the cytoplasm and nucleus in nonapoptotic cells. Previous data suggest that the increased peroxidase activity of cyt c during apoptosis stimulates its release from mitochondria via cardiolipin peroxidation (1). Our findings indicate that mitochondrial release of cyt c with a disrupted M80-Fe ligation in nonapoptotic cells may be peroxidase-dependent, although the peroxidation target remains to be determined. Of interest, NO and related species can both stimulate (via ONOO−-mediated tyrosine nitration) and inhibit (via heme nitrosylation) (18, 25) the peroxidase activity of cyt c. Thus the intracellular redox chemistry of NO may precisely regulate the peroxidase activity and therefore the subcellular localization of cyt c.
It is possible that the M80-Fe ligation also regulates the subcellular localization and function of cyt c during apoptosis. The 1D3 antibody, directed against an epitope exposed when the M80-Fe ligation is disrupted (22), recognizes a subpopulation of cyt c released from mitochondria during apoptosis. In addition, detection of heme nitrosylated cyt c during apoptosis also suggests that the M80-Fe ligation is disrupted in a subpopulation of cyt c (25). It remains to be determined if the translocation of cyt c to the nucleus and endoplasmic reticulum during apoptosis is regulated by protein modifications and/or alterations in the structure of cyt c (14, 26).
Several groups have shown that low levels of ONOO− are associated with the development of protective responses to further oxidative/nitrative stress in models as diverse as ischemia-reperfusion-induced heart tissue injury (27, 28), endothelial pathophysiology (29), and transplant-related kidney dysfunction (24). In addition, it has been demonstrated that mitochondria produce and are exposed to ONOO− and further that mitochondrial NOS stimulation causes cyt c release from mitochondria and that this release can be prevented by ONOO− scavengers (30). Therefore, it is possible that the ONOO−-induced disruption of M80-Fe ligation in cyt c, with subsequent release from mitochondria and nuclear translocation of cyt c, might be involved in the preconditioning response to further redox stress. Nevertheless, how the modified form of cyt c contributes to this protective effect remains to be investigated.
Our findings raise the possibility that cyt c has a spectrum of functions in cells that are dependent on the level of nitrative and/or oxidative stress. In resting cells, cyt c is predominantly hexacoordinated and functions in mitochondrial electron transport. In response to low levels of oxidative and/or nitrative stress, a subpopulation of cyt c may become nitrated and/or oxidized, translocate to the nucleus and cytoplasm, and function in a preconditioning response to further nitrative and/or oxidative stress. When cells are exposed to toxic levels of stress, pro-apoptotic bcl-2 family members may be activated, leading to increased cyt c release from mitochondria and apoptotic cell death.
In conclusion, cyt c may serve as a redox sensor that modulates the response of cells to varying levels of stress. Structural studies of M80A and nitrated cyt c may further elucidate conformational changes that underlie the functional alterations in the protein. Future studies may reveal additional roles for distinct conformations of cyt c in cell physiology and pathophysiology.
Materials and Methods
Cell Culture.
Cells were grown at 37 °C and 5% CO2 in complete medium [DMEM (Mediatech, Inc.) supplemented with 10% FBS (FBS, Nalge), 0.2 mM L-glutamine, 10 U/ml penicillin, and 10 μg/ml streptomycin (Sigma)].
Virus Production and Derivation of Cell Lines Expressing cyt c-GFP.
Transduction of cell lines with pBABE mouse cyt c-GFP virus was performed as described previously (31). Experimental details are provided in SI Methods.
Cyt c-GFP Knock-Down by siRNA.
Cells were grown in 96-well plates (WST-1 assay) or 35 mm dishes (confocal microscopy). For transfections, GFP-targeted siRNA (Dharmacon, 50 nM final concentration) was preincubated with LipofectAMINE (Gibco-Invitrogen) and subsequently added to the cell cultures in fresh DMEM containing 2% FBS for 12 h. The culture medium was then replaced with complete medium and protein knock-down was verified after 72–96 h by cyt c Western blot (WB) or confocal analysis.
Peroxidase Activity of WT and M80A cyt c.
The peroxidase activity of WT or M80A cyt c was assayed by luminol oxidation in presence of H2O2 as described previously (32).
Subcellular Fractionation.
HeLa cells expressing M80A or WT cyt c were separated into mitochondrial and cytoplasmic fractions as described previously (33). The subcellular fractions were then subjected to WB analysis as previously described (34) using antibodies to cyt c (BD Biosciences), SMAC/DIABLO (ProSciencific, Inc.) and GAPDH (Santa Cruz Biotechnology).
Immunofluorescence Detection of Endogenous cyt c.
All steps were carried out at room temperature unless otherwise stated. Following control treatment or exposure to 25 μM ONOO−, nontransfected HeLa cells grown on glass cover slips were fixed with 4% paraformaldeheyde in PBS for 10 min and incubated for an additional 10 min in 0.4% Triton X-100. Blockage was carried out with PBS containing 10% FBS, followed by overnight incubation at 4 °C with the anti-cyt c primary antibody (1D3 mouse monoclonal; ref. 22). FITC-conjugated rabbit anti-mouse (Jackson Immunoresearch) was added as the secondary antibody for 30 min in the dark. PBS washes were carried out between all steps. The cover slips were mounted on glass slides with Prolong Gold Antifade medium containing DAPI (Invitrogen). Cells were then analyzed by confocal microscopy as described in SI Methods.
Cell Viability Assay.
Cellular viability after the treatment with tBuOOH or peroxynitrite was measured using the WST-1 reagent, according to the manufacturer's instructions (Roche). The WST-1 assay measures the cleavage of tetrazolium salts to formazan by mitochondrial dehydrogenases in metabolically active cells. Experimental details are provided in the SI Methods.
Statistics.
Statistical significance was determined using a two-tailed t test for paired samples.
SI Methods.
Details concerning production of recombinant WT and M80A mutant cyt c, immunoprecipitations, detection of nitrated GFP-tagged cyt c, preparation of cell-free extracts for caspase 3 assays and confocal microscopy are provided in SI Methods.
Supplementary Material
Acknowledgments.
This work was supported by grants from National Institutes of Health (R01GM065824-05 to J.B.M.), Howard Hughes Medical Institute and International Center for Genetic Engineering (to R.R.), and Consejo Superior de Investigaciones Científicas (to L.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. G.K. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809279106/DCSupplemental.
References
- 1.Kagan VE, et al. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 2.Oursler MJ, Bradley EW, Elfering SL, Giulivi C. Native, not nitrated, cytochrome c and mitochondria-derived hydrogen peroxide drive osteoclast apoptosis. Am J Physiol Cell Physiol. 2005;288:C156–C168. doi: 10.1152/ajpcell.00092.2004. [DOI] [PubMed] [Google Scholar]
- 3.Cruthirds DL, et al. Mitochondrial targets of oxidative stress during renal ischemia/reperfusion. Arch Biochem Biophys. 2003;412:27–33. doi: 10.1016/s0003-9861(03)00039-0. [DOI] [PubMed] [Google Scholar]
- 4.MacMillan-Crow LA, Cruthirds DL, Ahki KM, Sanders PW, Thompson JA. Mitochondrial tyrosine nitration precedes chronic allograft nephropathy. Free Radic Biol Med. 2001;31:1603–1608. doi: 10.1016/s0891-5849(01)00750-x. [DOI] [PubMed] [Google Scholar]
- 5.Cassina AM, et al. Cytochrome c nitration by peroxynitrite. J Biol Chem. 2000;275:21409–21415. doi: 10.1074/jbc.M909978199. [DOI] [PubMed] [Google Scholar]
- 6.Batthyany C, et al. Time course and site(s) of cytochrome c tyrosine nitration by peroxynitrite. Biochemistry. 2005;44:8038–8046. doi: 10.1021/bi0474620. [DOI] [PubMed] [Google Scholar]
- 7.Chen YR, Deterding LJ, Sturgeon BE, Tomer KB, Mason RP. Protein oxidation of cytochrome C by reactive halogen species enhances its peroxidase activity. J Biol Chem. 2002;277:29781–29791. doi: 10.1074/jbc.M200709200. [DOI] [PubMed] [Google Scholar]
- 8.Estevam ML, et al. Changes in the spin state and reactivity of cytochrome C induced by photochemically generated singlet oxygen and free radicals. J Biol Chem. 2004;279:39214–39222. doi: 10.1074/jbc.M402093200. [DOI] [PubMed] [Google Scholar]
- 9.Belikova NA, et al. Peroxidase activity and structural transitions of cytochrome c bound to cardiolipin-containing membranes. Biochemistry. 2006;45:4998–5009. doi: 10.1021/bi0525573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernad S, et al. Interaction of horse heart and thermus thermophilus type c cytochromes with phospholipid vesicles and hydrophobic surfaces. Biophys J. 2004;86:3863–3872. doi: 10.1529/biophysj.103.025114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuominen EK, Wallace CJ, Kinnunen PK. Phospholipid-cytochrome c interaction: Evidence for the extended lipid anchorage. J Biol Chem. 2002;277:8822–8826. doi: 10.1074/jbc.M200056200. [DOI] [PubMed] [Google Scholar]
- 12.Silkstone G, Stanway G, Brzezinski P, Wilson MT. Production and characterisation of Met80X mutants of yeast iso-1-cytochrome c: Spectral, photochemical and binding studies on the ferrous derivatives. Biophys Chem. 2002;98:65–77. doi: 10.1016/s0301-4622(02)00085-6. [DOI] [PubMed] [Google Scholar]
- 13.Dumont ME, Ernst JF, Sherman F. Coupling of heme attachment to import of cytochrome c into yeast mitochondria. Studies with heme lyase-deficient mitochondria and altered apocytochromes c. J Biol Chem. 1988;263:15928–15937. [PubMed] [Google Scholar]
- 14.Nur EKA, et al. Nuclear translocation of cytochrome c during apoptosis. J Biol Chem. 2004;279:24911–24914. doi: 10.1074/jbc.C400051200. [DOI] [PubMed] [Google Scholar]
- 15.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 16.Wei MC, et al. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizuta T, Shimizu S, Matsuoka Y, Nakagawa T, Tsujimoto Y. A Bax/Bak-independent mechanism of cytochrome c release. J Biol Chem. 2007;282:16623–16630. doi: 10.1074/jbc.M611060200. [DOI] [PubMed] [Google Scholar]
- 18.Vlasova II, et al. Nitric oxide inhibits peroxidase activity of cytochrome c. cardiolipin complex and blocks cardiolipin oxidation. J Biol Chem. 2006;281:14554–14562. doi: 10.1074/jbc.M509507200. [DOI] [PubMed] [Google Scholar]
- 19.Souza JM, Castro L, Cassina AM, Batthyany C, Radi R. Nitrocytochrome c: Synthesis, purification, and functional studies. Methods Enzymol. 2008;441:197–215. doi: 10.1016/S0076-6879(08)01211-1. [DOI] [PubMed] [Google Scholar]
- 20.Rodríguez-Roldán V, García-Heredia JM, Navarro JA, Rosa MA, Hervás M. Effect of nitration on the physicochemical and kinetic features of wild-type and monotyrosine mutants of human respiratory cytochrome c. Biochemistry. 2008;47:12371–12379. doi: 10.1021/bi800910v. [DOI] [PubMed] [Google Scholar]
- 21.Abriata LA, et al. Nitration of solvent-exposed tyrosine 74 on cytochrome c triggers heme iron-methionine 80 bond disruption: Nuclear magnetic resonance and optical spectroscopy studies. J Biol Chem. 2008;284:17–26. doi: 10.1074/jbc.M807203200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jemmerson R, et al. A conformational change in cytochrome c of apoptotic and necrotic cells is detected by monoclonal antibody binding and mimicked by association of the native antigen with synthetic phospholipid vesicles. Biochemistry. 1999;38:3599–3609. doi: 10.1021/bi9809268. [DOI] [PubMed] [Google Scholar]
- 23.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 24.Rhoden EL, et al. The role of nitric oxide pathway in the renal ischemia-reperfusion injury in rats. Transpl Immunol. 2002;10:277–284. doi: 10.1016/s0966-3274(02)00079-5. [DOI] [PubMed] [Google Scholar]
- 25.Schonhoff CM, Gaston B, Mannick JB. Nitrosylation of cytochrome c during apoptosis. J Biol Chem. 2003;278:18265–18270. doi: 10.1074/jbc.M212459200. [DOI] [PubMed] [Google Scholar]
- 26.Boehning D, et al. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol. 2003;5:1051–1061. doi: 10.1038/ncb1063. [DOI] [PubMed] [Google Scholar]
- 27.Nossuli TO, Hayward R, Jensen D, Scalia R, Lefer AM. Mechanisms of cardioprotection by peroxynitrite in myocardial ischemia and reperfusion injury. Am J Physiol. 1998;275:H509–H519. doi: 10.1152/ajpheart.1998.275.2.H509. [DOI] [PubMed] [Google Scholar]
- 28.Altug S, Demiryurek AT, Kane KA, Kanzik I. Evidence for the involvement of peroxynitrite in ischaemic preconditioning in rat isolated hearts. Br J Pharmacol. 2000;130:125–131. doi: 10.1038/sj.bjp.0703280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lefer DJ, et al. Peroxynitrite inhibits leukocyte-endothelial cell interactions and protects against ischemia-reperfusion injury in rats. J Clin Invest. 1997;99:684–691. doi: 10.1172/JCI119212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghafourifar P, Schenk U, Klein SD, Richter C. Mitochondrial nitric-oxide synthase stimulation causes cytochrome c release from isolated mitochondria. Evidence for intramitochondrial peroxynitrite formation. J Biol Chem. 1999;274:31185–31188. doi: 10.1074/jbc.274.44.31185. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein JC, Waterhouse NJ, Juin P, Evan GI, Green DR. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat Cell Biol. 2000;2:156–162. doi: 10.1038/35004029. [DOI] [PubMed] [Google Scholar]
- 32.Radi R, Thomson L, Rubbo H, Prodanov E. Cytochrome c-catalyzed oxidation of organic molecules by hydrogen peroxide. Arch Biochem Biophys. 1991;288:112–117. doi: 10.1016/0003-9861(91)90171-e. [DOI] [PubMed] [Google Scholar]
- 33.Filomeni G, et al. Pro-apoptotic activity of novel Isatin-Schiff base copper(II) complexes depends on oxidative stress induction and organelle-selective damage. J Biol Chem. 2007;282:12010–12021. doi: 10.1074/jbc.M610927200. [DOI] [PubMed] [Google Scholar]
- 34.Schonhoff CM, et al. S-nitrosothiol depletion in amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2006;103:2404–2409. doi: 10.1073/pnas.0507243103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.