Abstract
Glioblastoma multiforme (GBM) is a highly lethal brain tumor for which little treatment is available. The epidermal growth factor receptor (EGFR) signaling pathway is thought to play a crucial role in GBM pathogenesis, initiating the early stages of tumor development, sustaining tumor growth, promoting infiltration, and mediating resistance to therapy. The importance of this pathway is highlighted in the fact that EGFR is mutationally activated in over 50% of GBM tumors. Consistent with this, we show here that concomitant activation of wild-type and/or mutant (vIII) EGFR and ablation of Ink4A/Arf and PTEN tumor suppressor gene function in the adult mouse central nervous system generates a fully penetrant, rapid-onset high-grade malignant glioma phenotype with prominent pathological and molecular resemblance to GBM in humans. Studies of the activation of signaling events in these GBM tumor cells revealed notable differences between wild-type and vIII EGFR-expressing cells. We show that wild-type EGF receptor signals through its canonical pathways, whereas tumors arising from expression of mutant EGFRvIII do not use these same pathways. Our findings provide critical insights into the role of mutant EGFR signaling function in GBM tumor biology and set the stage for testing of targeted therapeutic agents in the preclinical models described herein.
Keywords: glioblastoma, mouse model, receptor tyrosine kinase, mTORC1/2, STAT3
Glioblastoma multiforme is the most common and lethal primary malignant cancer of the central nervous system (CNS). Despite multimodal therapies, the median survival of GBM patients is ≈1 year. The deadly nature of GBMs resides in their explosive growth characteristic, extreme invasive behavior, and intrinsic resistance to current therapies. Despite efforts to develop novel treatments, little improvement in overall survival or progression-free survival has been achieved in the past 5 decades, reflecting an unmet need in the treatment of this cancer (1). Personalized medicine based on targeting essential molecular mechanisms for GBM survival offers an alternative therapeutic strategy.
Over the years, our knowledge of GBM biology has steadily improved. From a molecular standpoint, GBMs are a highly heterogeneous tumor with multiple signaling pathways differentially activated or silenced with converging and parallel complex interactions (2). It is these intricacies that are thought to confer GBM with its notorious plasticity in response to therapeutic interventions. Therefore, a major challenge in the clinic is to determine the appropriate events to target. The most common genetic abnormality in GBMs is the activation of receptor tyrosine kinases (RTKs), of which, aberrant expression of EGFR is the most frequent (2). Concomitant with EGFR gene amplification events is the occurrence of an intragenic in-frame deletion of exons 2−7 of the EGFR gene. This rearrangement product, known as EGFRvIII, codes for a ligand-independent receptor, which is constitutively activated and highly oncogenic (reviewed in ref. 3). During EGFR locus amplification, a portion of the amplicon rearranges to produce EGFRvIII, leading to the coexpression of WT and vIII within the same cells. Alternatively, if the rearrangement occurs early during the amplification process, most cells will express the vIII variant predominantly with very little if any WT EGFR. Thus, in individual human GBMs, expression of EGFRWT, EGFRWT/vIII, or EGFRvIII is observed (4–6). Although the prognostic value of EGFRvIII expression is still debated, recent molecular characterizations of targeted therapy resistance appear to indicate that EGFRvIII confers properties distinct from EGFR WT (7).
To understand EGFR signaling in GBM to better predict efficacy of targeted therapeutics, we developed 3 preclinical models of GBM based on overexpression of EGFR WT alone, coexpression of EGFR WT and vIII, and expression of EGFRvIII alone, thus reflecting natural occurrences in human GBMs. Using these models, we show here that ectopic expression of EGFR (both WT and vIII) in adult CNS tissues, in the context of p16Ink4a/p19ARF and PTEN inactivation, leads to the formation of GBMs de novo. We also show that EGFR-mediated tumor formation is accompanied by the activation of canonical and unexpected signaling pathways. Our findings show that these animals represent accurate model systems to study the genetic contributors to gliomagenesis and therapeutic treatment resistance in GBMs.
Results
Expression of EGFR in Adult Brain Tissues Is Not a Transforming Event.
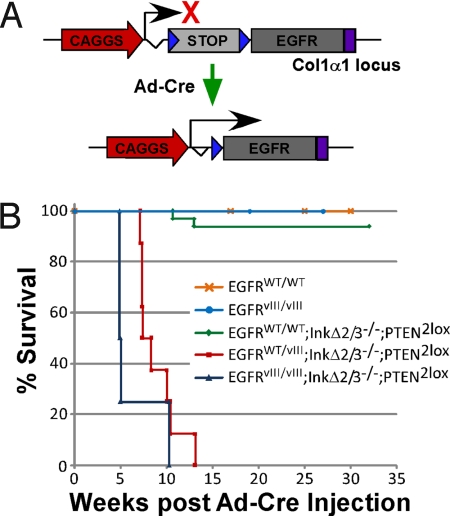
To evaluate the capacity of EGFR to induce adult-onset primary brain cancer de novo, we created Cre-Lox conditional transgenic strains of mice capable of expressing WT and/or vIII human EGF receptors. This was achieved by targeting the insertion of EGFR minigenes into the mouse collagen 1α1 gene locus. The basis of these minigenes consists of a floxed transcriptional/translational stop cassette inserted between a strong ubiquitous promoter (CAGGS) and the EGFR cDNAs (either WT or vIII) [Fig. 1A, supporting information (SI) Fig. S1, and Materials and Methods]. Two EGFR strains were produced, one expressing the wild-type receptor (referred as EGFRWT) and another expressing the oncogenic variant vIII (EGFRvIII). To obtain a strict spatiotemporal control over EGFR expression, we somatically induced the removal of the floxed stop cassette by stereotactic intracranial injections of an adenovirus transducing Cre recombinase (Ad-Cre). Recombinant adenoviruses are episomal following infection of host cells and capable of efficient expression, resulting in a transient expression of Cre with no potential for insertional mutagenesis. We injected Ad-Cre in the basal ganglia (striatum) of homozygous Col1α1-EGFRWT/WT and Col1α1-EGFRvIII/vIII mice and monitored tumor formation and survival over time. After 35 weeks postinjection, none of the injected animals developed tumors as measured by survival and histological means (Fig. 1B and data not shown).
Fig. 1.
Localized somatic expression of EGFR in adult mouse brains form tumors. (A) Schematic representation of the activation of the conditional EGFR transgenes. A strong ubiquitous promoter (CAGGS) is positioned upstream of a transcriptional stop cassette (STOP) which is flanked by 2 loxP sites (blue triangles), followed by either wild-type (WT) or mutated (vIII) human EGFR cDNAs and a polyA signal sequence (purple rectangle). The expression of EGFR is conditional to the removal of the stop cassette, which is mediated by an adenovirus expressing Cre recombinase. (B) Tumor-free survival (Kaplan-Meier) analysis of Ad-Cre-injected conditional EGFR cohorts of mice with the indicated genotypes. EGFRWT/WT, n = 6; EGFRvIII/vIII, n = 5. For EGFRWT/WT, EGFRvIII/vIII, and EGFRWT/vIII, all on an InkΔ2/3−/− and PTEN−/− background, n = 33, n = 4, and n = 8, respectively.
Loss of p16Ink4a, p19Arf, and PTEN Cooperates with EGFR in Gliomagenesis.
Recently, an extensive molecular characterization of human GBMs revealed a finite number of genetic aberrations in which RTKs are activated concomitantly with the loss of tumor suppressor gene function, such as those encoded by the Ink4a/Arf and PTEN loci (2). Given these observations, we crossed our conditional EGFR transgenic lines to strains of mice that carried dually disrupted p16Ink4a and p19Arf genes (referred to hereafter as InkΔ2/3) (8) and a conditional knockout PTEN gene (9). Cohorts of Col1α1-EGFRWT/WT, Col1α1-EGFRWT/vIII, and Col1α1-EGFRvIII/vIII animals all on an InkΔ2/3 null and conditional PTEN2lox knockout background animals were subjected to stereotactic Ad-Cre injections and monitored for tumor formation and survival (Fig. 1B). When combined with InkΔ2/3 and PTEN deficiency, expression of EGFR resulted in the formation of highly aggressive tumors. Interestingly, expression of EGFRWT was rather inefficient at creating tumors, whereas the addition of a single copy of the EGFRvIII variant allele significantly increased the penetrance and reduced the latency of tumor formation, leading to death in 100% of the animals within 13 weeks after Ad-Cre injection (Fig. 1B). Homozygous EGFRvIII/vIII-expressing animals exhibited a slightly more aggressive tumor formation phenotype with a shorter latency as compared with EGFRWT/vIII-expressing mice (Fig. 1B). Mechanistically, we found that the different potency in tumor formation between EGFR WT and vIII is not due to initial weaker expression of EGFR WT vs. vIII receptors (Fig. S2), thus showing that injection of Ad-Cre in the striatum of our EGFRWT conditional transgenic line result in expression of the receptor, and that this expression, in most cases, seems insufficient to form cancerous lesions.
Col1α1-EGFR; InkΔ2/3−/−; PTEN−/− Tumors Are Highly Infiltrative GBMs.
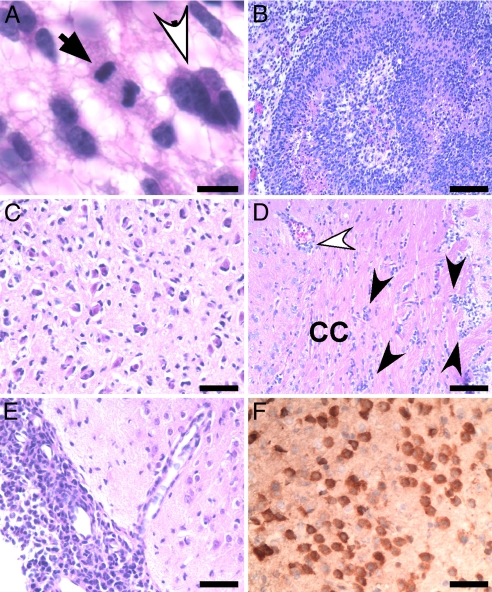
The histopathologic features of our EGFR tumors share a high degree of similarity with human GBMs. All Col1α1-EGFR; InkΔ2/3−/−; PTEN−/− tumors are highly cellular and are composed of cells containing pleomorphic nuclei (Fig. 2A, white arrow) set in a fibrillary background. Tumor cells typically have a gemistocytic appearance with eccentrically placed nucleus and abundant cytoplasm. The tumors also contain a high number of proliferating cells as detected by the presence of mitoses (Fig. 2A, black arrows). In addition, these tumors include large areas of necrosis and exhibit profound perineuronal satellitosis, both salient features of GBMs (Fig. 2 B and C). One of the hallmarks of human GBMs is the heightened infiltration capacity of the tumor cells. GBM cells typically invade the surrounding parenchyma and appear to follow distinct anatomical structures within the CNS, often egressing along white matter tracts, the basement membranes of blood vessels, or beneath the subdural sheets. In all tumors observed, a population of GBM cells infiltrated the brain by migrating along the white matter tracts (Fig. 2D) and the subarachnoid (Fig. 2E) and perivascular Virchow-Robin spaces (Fig. 2D). Using immunohistochemistry (IHC) staining for EGFR, we were able to observe single EGFR-expressing tumor cells situated away from the bulk tumor masses (Fig. 2F). The extent of this migration is widespread and can reach far distant regions of the brain (Fig. S3).
Fig. 2.
Neuropathological analysis of EGFR tumors. Representative photomicrographs of Col1α1-EGFR; InkΔ2/3−/−; PTEN−/− tumor sections stained with H&E. Expression of both WT and vIII EGFR result in similar tumor phenotypes. (A) Tumors are set on a fibrilary background and contain pleimorphic nuclei (white arrow) and mitoses (black arrow) (Scale bar: 15 μM.) (B) Tumors show marked pseudopallisading necrosis. (Scale bar: 62.5 μM.) (C) Tumor cells tend to accumulate around neurons, a common feature known as perineuronal satellitosis. The highly infiltrative nature of EGFR tumor cells is depicted. (Scale bar: 30 μM.) (D) Tumor cells (black arrowheads) are infiltrating white matter tracts such as the corpus callosum (CC) and the perivascular space (white arrowhead) and (E) migrate within the subarachnoid space. (Scale bars: 30 μM and 62.5 μM, respectively.) (F) IHC stain for human EGFR showing tumor cells infiltrating normal brain. (Scale bar: 62.5 μM.)
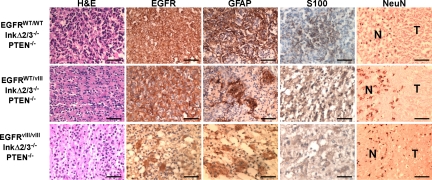
Tumors resulting from Ad-Cre-triggered expression of EGFRvIII in the striatum of mice with p16Ink4a;p19Arf and PTEN deletion typically appear as masses with variable amounts of hemorrhage and compression of adjacent brain structures (Fig. S4). At 2 weeks post-Ad-Cre injection, EGFRvIII-expressing tumors often consisting of a few clusters of neoplastic cells (Fig. S5). Over a period of an additional 2 weeks, the tumor masses increased in size and often showed perivascular infiltration (Fig. S5). At 6 and 8 weeks postadministration of Ad-Cre, tumor cells typically infiltrate the meninges, at which point tumor growth increases dramatically (Fig. S5). This explosive growth is highly reminiscent of that observed in human GBMs where tumors often remain clinically undetected until they enter a massively expansive growth rate, at which point detection typically results from neurological deficits (1). Using MRI, we measured the growth rates of EGFRvIII-expressing GBMs and show that the tumors expand swiftly and sharply (Fig. S6), ultimately reaching sizes that are incompatible with basic brain functions. EGFRvIII-expressing GBM tumors typically had irregular, thick, nodular, peripherally enhancing masses with areas of central necrosis (Fig. S6) and meningeal infiltrates appearing as hyperintense signals on T1-weighted contrast-enhanced images. IHC staining of these tumors for EGFR shows robust membrane expression, and staining for markers associated with astrocytic (GFAP and S100) and neuronal (NeuN) differentiation revealed that the neoplastic cells only express markers of astrocytic lineage (Fig. 3). Taken together, these findings suggest that expression of mutant EGFR, and to a lesser extent WT EGFR, in CNS glia cooperates with loss of the tumor suppressor loci Ink4a;Arf and PTEN gene products to form GBM tumors.
Fig. 3.
EGFR GBM tumors express markers of astrocytic differentiation. Representative photomicrographs of GBM tumors of the indicated genotypes stained with H&E, for expression of EGFR, the astrocytic markers glial fibrillary acidic protein (GFAP) and S100 and the neuronal marker NeuN by IHC. N, normal brain; T, tumor. (Scale bars: 62.5 μM.)
Signaling Pathways Initiated by EGFR in GBM Tumor Cells.
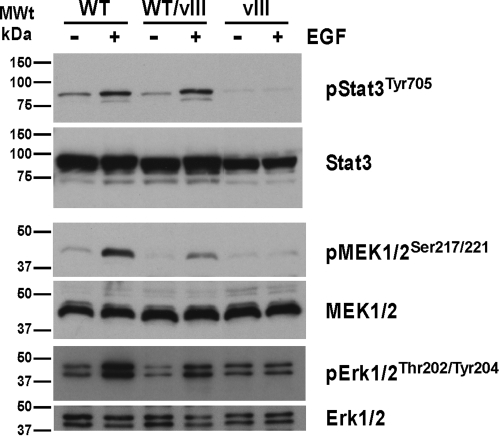
RTKs relay signals through the phosphorylation of substrate molecules and via the interaction of signaling molecules with autophosphorylation sites (reviewed in ref. 10). To better understand the mechanisms by which EGFR exerts its oncogenic potential, tumors of the genotypes EGFRWT/WT, EGFRWT/vIII, and EGFRvIII/+, all on an InkΔ2/3−/− and PTEN−/− background, were cultured ex vivo. The suitability of these cells to study signal transduction events was confirmed by comparing the levels of EGFR expression in representative samples of each genotype to human primary cultures of GBMs (11) by immunoblot analysis (Fig. S7). Using these cells, we identified EGFR autophosphorylation sites using phospho-specific anti-EGFR antibodies in immunoblot assays (Fig. S8). We detected phosphorylation on tyrosine residues 920, 992, 1045, 1068, 1148, and 1173 upon EGF stimulation of cells expressing EGFRWT. Interestingly, the only autophosphorylation we detected in EGFRvIII-expressing cells was the constitutive phosphorylation of tyrosine residue 992 (Fig. S8A). Phosphorylation on these 6 tyrosine residues has been shown to be linked to activation of the phosphatidylinositol 3-kinase (PI3K)/Akt, ras/raf/MEK/ERK, phospholipase C gamma (PLCγ), and signal transducer and activator of transcription (STAT3) signaling pathways (for a review of EGFR signaling, see ref. 12). Activation of these signaling pathways was confirmed in our ex vivo cultures by immunoblot analysis with phospho-specific antibodies against these proteins. Phosphorylation of STAT3 at tyrosine 705 is induced by stimulation of GBM tumor cells with EGF ligand in EGFRWT-expressing cells but not in constitutively activated EGFRvIII-expressing cells (Fig. 4). A similar pattern is observed for the formation of phospho-MEK1/2 (Ser-217/221) and phospho-Erk1/2 (Thr-202/Tyr-204) sites (Fig. 4). A principal consequence of PI3K activation is the activation of the protein kinase Akt, which can be monitored by detection of its phosphorylation status. We observed Akt phosphorylation on Ser 473 and Thr 308 as a result of EGF stimulation of EGFRWT-expressing GBM cells and to a lesser extent in EGFRWT/vIII-expressing cells (Fig. 5). In contrast, EGFRvIII cells did not display EGF-induced phospho-Akt but instead contained constitutively phosphorylated Akt on Ser 473 (Fig. 5). Finally, an important outcome of Akt activation is the stimulation of the mammalian target of rapamycin complex 1 (mTORC1). One of the many functions of mTORC1 is to maintain homeostatic protein synthesis through, among other proteins, the activation of ribosomal protein S6 kinases (S6Ks) (reviewed in ref. 13). To evaluate if ligand stimulated and constitutively activated Akt signals through mTORC1, we investigated the phosphorylation status of surrogate markers of mTORC1 activation, S6K and S6 ribosomal protein. Fig. 5 shows that both S6K and S6 ribosomal protein are phosphorylated upon EGF stimulation of EGFRWT-expressing cells and are not present in EGFRvIII-expressing cells.
Fig. 4.
Signaling networks usurped by EGFR in GBM tumor cells. STAT3 and MEK/ERK are activated by EGFRWT but not EGFRvIII receptors. Ex vivo cultures of cells from the indicated tumor genotypes were starved for 24 hr and stimulated with 50 ng/ml of EGF for 5 min. Immunoblots of total cell lysates were probed with the indicated antibodies.
Fig. 5.
EGFRvIII signals differently than EGFRWT. EGF ligand stimulation of EGFRWT-expressing cells stimulates the activation of mTORC1, whereas mTORC2 appears to be constitutively activated in EGFRvIII-expressing GBM tumor cells. Western blot analysis of total cell extracts from GBM tumor cells expressing the indicated receptors. Cells were serum starved and stimulated as described previously. Immunoblots were probed with antibodies as indicated.
Discussion
Here we show that somatic expression of mutant EGFRvIII in the CNS of adult mice, in the context of loss of key tumor suppressor genes, is very efficient at de novo transformation and the formation of GBM tumors in vivo. GBM's most impenetrable attribute to therapeutic intervention is its extreme invasive nature, which makes complete surgical resection virtually unachievable. Invading GBM cells tend to follow distinct anatomical structures within the CNS, often egressing along white matter tracts, the basement membranes of blood vessels, or beneath the subdural sheets. In our model, we consistently observed migration of EGFR GBM cells within all 3 spaces (Fig. 2 and Fig. S5). This reflects the ability of EGFR to activate signaling mechanisms inherent to invasive behaviors, thus making our model an accurate system to study modalities of astrocytoma cell invasion with respect to the tumor microenvironment in a de novo fashion and offer a conduit for testing anti-invasion therapeutic interventions.
Ectopic expression of oncogenes in somatic cells can lead to apoptosis or senescence. Senescence is known to be triggered by the activation of a series of molecular events that involve key cancer proteins such as p53 or p19Arf (14). The expression of EGFRvIII may induce senescence in normal cells, a hypothesis consistent with the absence of tumor formation in Col1α1-EGFRvIII mice alone (Fig. 1B). In fact, activation of EGFR is rarely seen in the absence of loss of p16INK4a/p14ARF function in GBMs (2). Therefore, deleting the integral senescence protein p19Arf in InkΔ2/3 null animals likely short circuits an oncogene-induced senescence and allows for EGFRvIII-mediated transformation to take place in these cells.
We also show that expression of WT EGF receptors under the same circumstances is rather inefficient at tumor formation. This is an unexpected result given the high rate of WT EGFR overexpression in human GBMs. This discrepancy is not due to differences in EGFR expression levels between our system and human GBM tumors (Fig. S7) or through a lack of EGFRWT expression postinduction in vivo (Fig. S2). It is possible that the EGF receptors in our system are not activated to the same level as in human tumors. It is known that human GBMs express high concentrations of EGFR ligands that form autocrine and paracrine loops with the receptors (15), events that may be absent in our system. It is likely that for those few tumors that arose in our EGFRWT animals, additional somatic genetic hits may have contributed to the formation of these tumors.
Ex vivo cultures of our GBM tumors and primary astrocytes derived from our transgenic models show that additional growth factors are required for these cells to thrive in vitro (Fig. S9 and S10). This suggests that in this context, active EGFR is inefficient to sustain growth by itself but rather acts in concert with other growth factor inputs to maintain growth of tumor cells. This reflects recent observations in human GBMs describing the importance of understanding integrative RTK signaling complexes to properly devise efficient therapeutic interventions (16, 17).
By characterizing the extent of phosphorylation events on the receptors, we were able to ascertain which signaling pathways emanate from our activated EGF receptors (Fig. S8). Indeed, many of the canonical EGFR signaling events are activated in a ligand-dependent manner in our EGFRWT model (Figs. 4 and 5). Our model also offers a unique opportunity to study the consequences of EGFRvIII expression on GBM tumor biology. For example, constitutive phosphorylation of EGFRvIII receptor on Tyr-992 would result in a persistent activation of PLCγ signaling pathways, yet the MAPK pathway remains silent in these cells. This suggests that activation of PLCγ in GBM may signal through a novel mechanism. Given EGFRvIII's potent oncogenecity, this observation underlines a role for PLCγ in GBM biology. In addition, activation of the PI3K/AKT/mTOR signaling complex pathway by EGFR is well described. Using phosphospecific antibodies, we assessed the extent of this signaling axis in EGFRWT and EGFRvIII-expressing cells and discovered that Akt phosphorylation on Thr-308 is observed only in response to EGF activation of EGFRWT, whereas expression of EGFRvIII leads to a constitutive phosphorylation of Akt on Ser-473. Phosphorylation of Akt on Thr-308 is a PDK1-mediated event, the result of PI3K activity. Thr-308 phosphorylated Akt in turn activates mTORC1 (a Rapamycin sensitive complex) through a TSC1/2/Rheb cascade (reviewed in ref. 18). Interestingly, EGFRvIII-expressing cells have constitutively high levels of pSer473 Akt proteins, which has been reported to result from mTORC2 kinase activity (18). Similarly, phosphorylation on S6 kinase protein, an mTORC1 event, is seen only in EGFRWT-activated cells, and the same is true for phosphorylated S6 ribosomal protein. These observations together allow us to propose a model whereby the expression of EGFRvIII promotes a switch in the usage of mTOR complexes from mTORC1 (rapamycin sensitive) to mTORC2 (rapamycin insensitive). Our findings have significant clinical implications as they suggest that a gain of EGFRvIII expression would render GBMs insensitive to treatment with rapamycin and its analogues. Our system offers a unique opportunity to study the clinical potential of modulating mTORC1/2 components expression by RNA interference in the context of mutant EGFR expression.
Understanding cellular transformation by WT and vIII EGFR and the signaling systems necessary for this event may have broad implications for therapeutic interventions given their frequent expression in human GBMs. The findings presented here show that mutant and WT EGF receptors contribute to gliomagenesis via the activation of different signaling events, thus offering new opportunities for therapeutic exploitations.
Materials and Methods
EGFR Conditional Transgenic Mice.
Cre/Lox-mediated conditional expression of the human EGF receptors (WT and vIII) was achieved by targeted knockin of CAGGS-floxed stop cassette EGFR cDNA minigenes into the mouse collagen 1α1 gene locus as described in SI Materials and Methods. Germline-transmitted EGFRWT and EGFRvIII founder males were mated to InkΔ2/3 (8) and conditional PTEN knockout strains (9). The combinations of strain indicated in the text were produced by crossbreeding. Activation of EGFR expression in the brain was accomplished by stereotactic intracranial injections of an adenovirus expressing Cre recombinase under the CMV promoter (Gene Transfer Vector Core, University of Iowa, Iowa City, IA). A detailed procedure is described in SI Materials and Methods.
Immunoblotting.
For immunoblots, protein extract samples were separated by SDS-PAGE and then transferred to Immobilon-P membranes (Millipore). Specific proteins were detected with antibodies listed in SI Materials and Methods.
Histology and Immunohistochemistry.
Tumor-bearing animals were transcardially perfused with cold PBS; their brains excised and rinsed in PBS; and serial coronal sections cut using a brain mold. Half of the sections were used to isolate primary cultures of tumor cells as described in SI Materials and Methods, and the other half were postfixed in 4% paraformaldehyde, embedded in paraffin, sectioned (5–10 μM), and stained with hematoxylin and eosin (H&E) (Sigma). For IHC, sections were deparaffinized and rehydrated followed by antigen target retrieval and processing as described in SI Materials and Methods. All antibodies (listed in SI Materials and Methods) were diluted in blocking solution, and immunobinding of primary antibodies was detected by biotin-conjugated secondary antibodies and Vectastain ABC Kit (Vector Labs, Inc.) using DAB (Vector Labs, Inc.) as a substrate for peroxidase activity and counterstained with haematoxylin as described in the manufacturer's protocol.
Supplementary Material
Acknowledgments.
We thank Drs. Marius Werning and Rudolf Jaenisch (Whitehead Institute, Cambridge, MA) for the CAGGS-Col1α1 plasmid and E2 ES cells, and Drs. Philip Hinds and Julia Alberta for critical review of the manuscript. We also thank Jenny Chan, Anne Yu, and Greg Wojtkiewicz for experimental assistance. This work was supported in part by a CNS/MGI Pharma Fellowship in Tumor Research (to A.B.); National Institutes of Health Grants K08HL081170 (to J.W.C.) and U54 CA119349 (to D.H., R.W., and A.C.); and The Cam Neely Foundation (A.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813314106/DCSupplemental.
References
- 1.Kleihues P, Cavenee WK. Pathology and Genetics of Tumours of the Nervous System. Lyon, France: International Agency for Research on Cancer; 2000. [Google Scholar]
- 2.Consortium TCGA. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedersen MW, Meltorn M, Damstrup L, Poulsen HS. The type III epidermal growth factor receptor mutation. Biological significance and potential target for anti-cancer therapy. Ann Oncol. 2001;12:745–760. doi: 10.1023/a:1011177318162. [DOI] [PubMed] [Google Scholar]
- 4.Ekstrand AJ, et al. Genes for epidermal growth factor receptor, transforming growth factor alpha, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res. 1991;51:2164–2172. [PubMed] [Google Scholar]
- 5.Wong AJ, et al. Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc Natl Acad Sci USA. 1992;89:2965–2969. doi: 10.1073/pnas.89.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biernat W, et al. Predominant expression of mutant EGFR (EGFRvIII) is rare in primary glioblastomas. Brain Pathol. 2004;14:131–136. doi: 10.1111/j.1750-3639.2004.tb00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mellinghoff IK, Cloughesy TF, Mischel PS. PTEN-mediated resistance to epidermal growth factor receptor kinase inhibitors. Clin Cancer Res. 2007;13:378–381. doi: 10.1158/1078-0432.CCR-06-1992. [DOI] [PubMed] [Google Scholar]
- 8.Serrano M, et al. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996;85:27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- 9.Lesche R, et al. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis. 2002;32:148–149. doi: 10.1002/gene.10036. [DOI] [PubMed] [Google Scholar]
- 10.Pawson T. Dynamic control of signaling by modular adaptor proteins. Curr Opin Cell Biol. 2007;19:112–116. doi: 10.1016/j.ceb.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Sarkaria JN, et al. Use of an orthotopic xenograft model for assessing the effect of epidermal growth factor receptor amplification on glioblastoma radiation response. Clin Cancer Res. 2006;12:2264–2271. doi: 10.1158/1078-0432.CCR-05-2510. [DOI] [PubMed] [Google Scholar]
- 12.Sebastian S, et al. The complexity of targeting EGFR signalling in cancer: From expression to turnover. Biochim Biophys Acta. 2006;1766:120–139. doi: 10.1016/j.bbcan.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 14.Sharpless NE, DePinho RA. Cancer: Crime and punishment. Nature. 2005;436:636–637. doi: 10.1038/436636a. [DOI] [PubMed] [Google Scholar]
- 15.Ramnarain DB, et al. Differential gene expression analysis reveals generation of an autocrine loop by a mutant epidermal growth factor receptor in glioma cells. Cancer Res. 2006;66:867–874. doi: 10.1158/0008-5472.CAN-05-2753. [DOI] [PubMed] [Google Scholar]
- 16.Stommel JM, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287–290. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- 17.Huang PH, et al. Quantitative analysis of EGFRvIII cellular signaling networks reveals a combinatorial therapeutic strategy for glioblastoma. Proc Natl Acad Sci USA. 2007;104:12867–12872. doi: 10.1073/pnas.0705158104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.