Abstract
Cytotoxic lymphocyte antigen-4 (CTLA-4) blockade is an active immunotherapeutic strategy that is currently in clinical trials in cancer. There are several ongoing trials of anti-CTLA-4 in the metastatic setting of prostate cancer patients with reported clinical responses consisting of decreases in the prostate specific antigen (PSA) tumor marker for some patients. Immunologic markers that correlate with these clinical responses are necessary to guide further development of anti-CTLA-4 therapy in the treatment of cancer patients. We recently reported that CD4+ inducible co-stimulator (ICOS)hi T cells that produce interferon-γ (IFN-γ) are increased in the peripheral blood and tumor tissues of bladder cancer patients treated with anti-CTLA-4 antibody. Here we present data from the same clinical trial in bladder cancer patients demonstrating a higher frequency of CD4+ICOShi T cells and IFN-γ mRNA levels in nonmalignant prostate tissues and incidental prostate tumor tissues removed at the time of radical cystoprostatectomy. Our data suggest immunologic markers that can be used to monitor prostate cancer patients who receive anti-CTLA-4 therapy and indicate that the immunologic impact of anti-CTLA-4 antibody can occur in both tumor and nonmalignant tissues. These data should be taken into consideration for evaluation of efficacy as well as immune-related adverse events associated with anti-CTLA-4 therapy.
Keywords: clinical trial, CTLA-4, T cells
Cytotoxic lymphocyte antigen-4 (CTLA-4), which is expressed on T cells only after activation, plays a crucial role in restricting T cell immune responses (1–3). Blockade of the inhibitory signals mediated by CTLA-4 has been shown to enhance T cell responses and induce tumor rejection in a number of animal models (4, 5). A monoclonal antibody to human CTLA-4 has been found to elicit objective responses in clinical trials (6–10) and is a promising immunotherapeutic agent for the treatment of cancer patients. Clinical trials with anti-CTLA-4 antibody are ongoing in cancer patients with various tumor types, including melanoma, renal cell, and prostate cancers. Prostate cancer patients have been reported to have decreases in the prostate-specific antigen (PSA) tumor marker after treatment with anti-CTLA-4 antibody (10). Clinical responses in patients with various malignancies have also included partial and complete responses with regression of all detectable tumor lesions in some patients. Disease responses after treatment with anti-CTLA-4 therapy have been limited to a subset (≈10%) of patients. Immune-related adverse events (irAEs) and toxicities have also been reported for a subset of patients. Reported irAEs include dermatitis, colitis, hepatitis, pancreatitis, and uveitis. Published studies suggest that irAEs and clinical benefit occur in the same patients (11). These data imply that the immunologic impact of anti-CTLA-4 antibody occurs in both tumor tissues and nonmalignant tissues, thereby leading to clinical benefit and irAEs, respectively, in the same patient. Immune monitoring of treated patients for correlation with clinical outcomes is underway as investigators aim to further improve the anti-tumor effects and limit the toxicities of anti-CTLA-4 therapy.
We recently reported increased expression of the inducible co-stimulator (ICOS) T cell molecule on CD4 T cells after treatment of bladder cancer patients with anti-CTLA-4 therapy (12). ICOS is a T cell-specific surface molecule that is structurally related to CD28 and CTLA-4 and is expressed only after activation (13–16). Our data from bladder cancer patients revealed an expansion of CD4+ICOShi T cells in both the peripheral blood and bladder tumor tissues of patients treated with anti-CTLA-4 antibody. This CD4+ICOShi T cell population included cells that produced the Th1 cytokine interferon-γ (IFN-γ) when stimulated by the tumor antigen NY-ESO-1 (12).
Here we report an analysis of prostate tissues obtained from the radical cystoprostatectomy surgeries of 7 treated bladder cancer patients. Three patients had incidental findings of prostate adenocarcinoma (Gleason score 6, 3 + 3) and four patients were found to have nonmalignant prostate tissues. Regardless of tumor status, all 7 prostate tissue samples revealed similar immunologic effects of anti-CTLA-4 treatment: higher frequency of CD4+ICOShi T cells and higher ratio of Th1 (IFN-γ) to Th2 (IL-10) cytokine profile. These are the first data demonstrating immunologic changes in prostate tissues after treatment of patients with anti-CTLA-4 antibody and suggest a broad based systemic impact of anti-CTLA-4 in both malignant and nonmalignant tissues. These findings have implications regarding the immunologic events that may contribute to both clinical efficacy and immune-related adverse events associated with anti-CTLA-4 therapy.
Results
Frequency of CD4+ICOShi and CD4+FOXP3+ T Cells in Peripheral Blood of Prostate Cancer Patients.
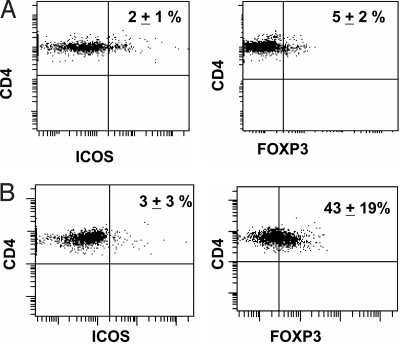
The frequency of CD4+ICOShi T cells in the peripheral blood of healthy donors (≈2%) was not different from that of untreated prostate cancer patients (Fig. 1A and B). However, the frequency of CD4+FOXP3+ regulatory T cells (17), was considerably higher in the peripheral blood of prostate cancer patients than in healthy donors (43% vs. 5%, P < 0.05, Fig. 1 A and B). We recently reported variable changes in CD4+FOXP3+ T cells after treatment of bladder cancer patients with anti-CTLA-4 therapy. In these bladder cancer patients the frequency of CD4+ICOShi T cells was as low as shown in Fig. 1 for the prostate cancer patients in pretherapy blood samples; however, there was a significant increase in the frequency of CD4+ICOShi T cells in the blood following treatment (12). Similar results would be expected in patients with different tumor types because anti-CTLA-4 antibody targets T cells and is not tumor specific.
Fig. 1.
Expression of ICOS and FOXP3 by CD4 T cells in peripheral blood of prostate cancer patients. (A) In healthy donors (n = 10), ≈2% of CD4 T cells express ICOS (Left) and 5% of CD4 T cells express FOXP3 (Right). (B) In untreated prostate cancer patients (n = 10), ≈3% of CD4 T cells express ICOS (Left) and 43% of CD4 T cells express FOXP3 (Right). A representative figure from one healthy donor (A) and one untreated prostate cancer patient (B) is shown, with numbers indicating mean percentages (± standard deviations) calculated from samples from 10 healthy donors and 10 patients with untreated prostate cancer.
Frequency of CD4+ICOShi and CD4+FOXP3+ T Cells in Prostate Cancer Tissues.
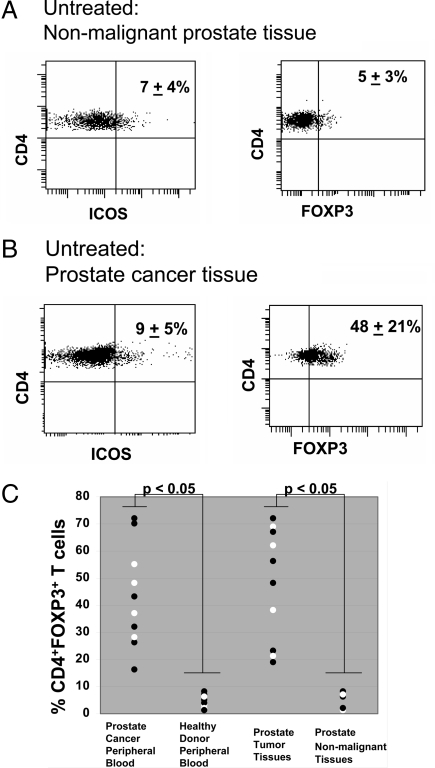
Prostate adenocarcinoma tumor tissues (Gleason scores 6 and 7) from patients undergoing radical prostatectomy as treatment for their disease were obtained and investigated for phenotypic characteristics of tumor infiltrating CD4 T cells. Similar data were obtained from nonmalignant prostate tissues procured from radical cystoprostatectomy surgeries of bladder cancer patients who had removal of both bladder and prostate organs as treatment for their disease. As shown in Fig. 2, nonmalignant prostate tissues had an average of ≈7% CD4+ICOShi T cells and ≈5% CD4+FOXP3+ T cells (Fig. 2A) while prostate cancer tissues from untreated patients had similar frequencies of CD4+ICOShi T cells (≈9%) but much higher frequencies of CD4+FOXP3+ T cells (≈48%) (Fig. 2B). CD4+ICOShi T cells were on average 3-fold higher in tumor tissues (9 ± 5%, Fig. 2B) as compared to peripheral blood (3 ± 3%, Fig. 1B) of prostate cancer patients, which suggests expansion of these cells against specific antigens present within the tumor microenvironment. CD4+FOXP3+ regulatory T cells had a wide range of expression in both peripheral blood (43 ± 19%, Fig. 1B) and tumor tissues (48 ± 21% Fig. 2B) of prostate cancer patients but, was significantly higher (P < 0.05) than observed in healthy donor blood (5 ± 2%, Fig. 1A) and nonmalignant prostate tissues (5 ± 3%, Fig. 2A). The frequency of CD4+FOXP3+ T cells measured in peripheral blood of each prostate cancer patient and healthy donor, and in each prostate tumor tissue and nonmalignant prostate tissue is provided in a graphical format (Fig. 2C). Tumor-infiltrating regulatory T cells have been reported to be similarly high in prostate cancer and other tumor types (18–20). Tumor-infiltrating regulatory T cells were previously reported as a potential mechanism for suppressing effector anti-tumor immune responses and as a correlative marker of poor clinical outcome (21–23). Conversely, increased intratumoral effector T cells and higher ratios of effector to regulatory T cells in tumor tissues have been associated with improved clinical outcome (24, 25).
Fig. 2.
Expression of ICOS and FOXP3 by CD4 T cells in normal and malignant prostate tissues of untreated patients. (A) In nonmalignant prostate tissues (n = 5), ≈7% of CD4 T cells express ICOS (Left) and 5% of CD4 T cells express FOXP3 (Right). (B) In prostate cancer tissues (n = 10), ≈9% of CD4 T cells express ICOS (Left) and 48% of CD4 T cells express FOXP3 (Right). A representative figure from one nonmalignant tissue sample (A) and from prostate cancer tissue sample from one untreated patient (B) is shown with numbers indicating mean percentages (± standard deviations) calculated from samples from 5 nonmalignant prostate tissue samples and 10 prostate cancer tissue samples. (C) Graphical representation of significantly (P < 0.05) higher frequency of CD4+FOXP3+ T cells in peripheral blood of prostate cancer patients (n = 10) as compared to peripheral blood of healthy donors, and in prostate tumor tissues (n = 10) as compared to nonmalignant prostate tissues (n = 5).
Anti-CTLA-4 Therapy Results in a Higher Frequency of CD4+ICOShi T Cells in Both Prostate Cancer and Nonmalignant Prostate Tissues.
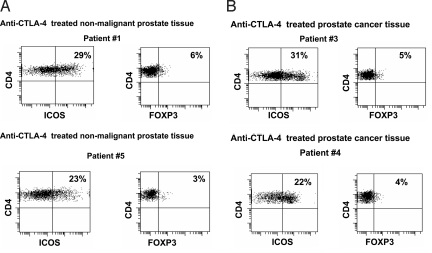
Eight consecutive patients with bladder cancer were treated on a presurgical clinical trial with 2 doses of the anti-CTLA-4 antibody ipilimumab with each dose given 3 weeks apart and radical cystoprostatectomy surgery performed 4 weeks after administration of the last dose of antibody. Seven patients had evaluable prostate tissues consisting of 3 incidental cases of prostate adenocarcinoma (Patients 3, 4, and 8) and 4 cases of nonmalignant prostate tissues (Patients 1, 5, 6 and 7) (Table S1). Patient 2 is not reported here because this patient had extension of the bladder cancer into the prostate. As shown in Fig. 3, both nonmalignant prostate tissues (Fig. 3A) and prostate cancer tissues (Fig. 3B) had increased frequencies of CD4+ICOShi T cells after treatment with anti-CTLA-4 antibody, as compared with untreated tissues (Fig. 2). Patients 1 and 5, who were found to have nonmalignant prostate tissues, had 29% and 23% CD4+ICOShi T cells, respectively, which were about 2–3 times higher as compared to untreated nonmalignant prostate tissues (7 ± 4%, Fig. 2A). Similar data regarding ICOS expression was obtained from the nonmalignant prostate tissues of patients 6 and 7 (Table S1). Patients 3 and 4, who were found to have prostate cancer, had 31% and 22% CD4+ICOShi T cells, respectively, which were also 2–3 times higher in frequency as compared to untreated prostate cancer tissues (9 ± 5%, Fig. 2B). Similar data regarding ICOS expression was observed in prostate cancer tissues from patient 8 (Table S1). CD4+FOXP3+ T cells were lower in prostate cancer tissues (Fig. 3B) after treatment with anti-CTLA-4 antibody as compared to untreated prostate cancer tissues (Fig. 2B). Patients 3 and 4 had 5% and 4% CD4+FOXP3+ T cells, respectively, as compared to untreated prostate cancer tissues (48 ± 21%, Fig. 2B). Patient 8 also had a lower frequency of CD4+FOXP3+ T cells (8%, Table S1) as compared to untreated prostate cancer.
Fig. 3.
Expression of ICOS and FOXP3 by CD4 T cells in normal and malignant prostate tissues from patients treated with anti–CTLA-4. (A) CD4 T cells had a higher frequency of ICOS expression at 29% (patient 1, Left Upper) and 23% (patient 5, Left Lower) in nonmalignant prostate tissues after patients received treatment with anti-CTLA-4 antibody while FOXP3 levels remained similarly low in both patients (Right) as compared to untreated nonmalignant tissues shown in Fig. 2A. (B) CD4 T cells had a higher frequency of ICOS expression at 31% (patient 3, Left Upper) and 22% (patient 4, Left Lower) in prostate cancer tissues after patients received treatment with anti-CTLA-4 antibody, while frequency of FOXP3 expression was lower at 5% (patient 3, Right Upper) and 4% (patient 4, Right Lower) as compared to untreated prostate cancer tissues shown in Fig. 2B.
Anti-CTLA-4 Therapy Results in Higher Levels of IFN-γ and T-bet mRNA in Both Prostate Cancer and Nonmalignant Tissues.
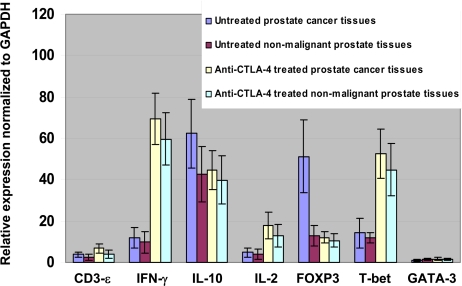
Prostate tissues were analyzed for differences in mRNA levels for several genes related to T cell function (Fig. 4). FOXP3 mRNA levels were found to be lower in prostate cancer tissues from patients treated with anti-CTLA-4 antibody as compared to untreated prostate cancer tissues, which was consistent with data presented in Figs. 2 and 3. Both prostate cancer and nonmalignant tissues had higher mRNA levels for IFN-γ, a Th1 cytokine (26), and T-bet, a transcription factor responsible for regulating IFN-γ production (27), as compared to untreated tissues. IL-2 mRNA levels were also slightly increased in treated tissues as compared to untreated tissues but this increase was not as pronounced as the increase seen for IFN-γ. However, mRNA levels for IL-10, a Th2 cytokine (28), did not differ significantly between groups. Similarly, mRNA levels for GATA-3, a transcription factor associated with IL-10 production (29), did not differ between groups. The average ratio of Th1/Th2 cytokine inferred from the ratio of IFN-γ/IL-10 was notably increased from ≈0.2/L in untreated tissues to ≈1.5/L in treated tissues, regardless of tumor status. Therefore, CTLA-4 blockade alters the Th1 to Th2 ratio in both prostate cancer and nonmalignant tissues.
Fig. 4.
Changes in expression of specific gene transcripts in prostate tissues following treatment with anti-CTLA-4. Fold induction of CD3-ε, IFN-γ, IL-10, IL-2, FOXP3, T-bet, and GATA-3 mRNA levels in untreated prostate cancer tissues, untreated nonmalignant prostate tissues, anti-CTLA-4 antibody treated prostate cancer tissues, and anti-CTLA-4 antibody treated nonmalignant prostate tissues as compared to GAPDH mRNA levels as assessed by real-time PCR. Anti-CTLA-4 treatment induced higher IFN-γ and T-bet mRNA levels in both cancer and nonmalignant tissues as well as lower FOXP3 mRNA levels in cancer tissues as compared to untreated cancer tissues.
Prostate Tumor-infiltrating CD4 T Cells from an Anti-CTLA-4 Treated Patient Recognize the NY-ESO-1 Tumor Antigen.
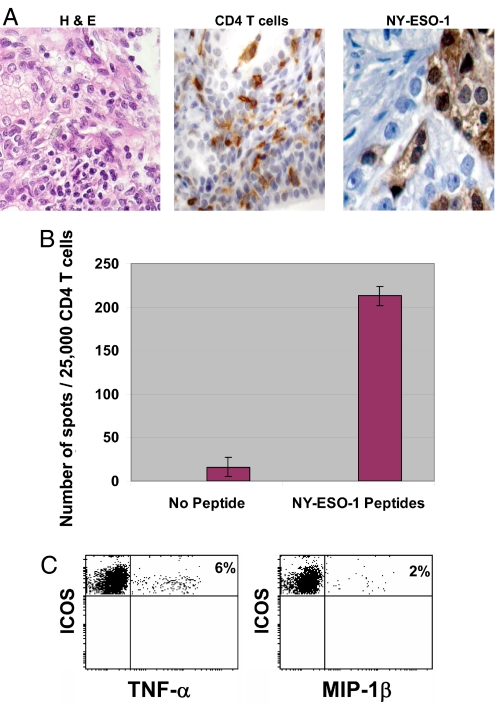
Prostate tissues from anti-CTLA-4 treated patients were infiltrated with CD4 T cells (Fig. 5A, representative sample, patient 4). We obtained tumor infiltrating CD4 T cells in sufficient quantity for functional analysis from only one patient's prostate sample (patient 4). The prostate tumor from patient 4 expressed the NY-ESO-1 tumor antigen (Fig. 5B). CD4 tumor infiltrating lymphocytes from this patient's prostate sample recognized the NY-ESO-1 tumor antigen with subsequent production of IFN-γ (Fig. 5B). As shown in Fig. 5C, these contained a population of ICOShi cells that produced additional cytokines (TNF-α) and chemokines (MIP-1β).
Fig. 5.
NY-ESO-1 is recognized by CD4 tumor-infiltrating T cells from an anti-CTLA-4 treated patient sample. (A) H & E staining of patient 4 prostate tissues (Right), demonstrating CD4 T cell infiltration (Middle) and NY-ESO-1 expression on prostate tumor cells (Left). (B) An ELISPOT assay demonstrating CD4 T cell recognition of antigen-presenting cells (APCs) pulsed with NY-ESO-1 overlapping peptides as compared to unpulsed APCs (no peptides). The assay was done in triplicate and data were plotted as mean ± standard deviation. (C) CD4+ICOShi T cells produce TNF-α (Right) and MIP-1β (Left) in the presence of APCs pulsed with NY-ESO-1 overlapping peptides.
Discussion
Anti-tumor responses and toxicities that occur as a result of anti-CTLA-4 therapy are both likely to be mediated by immunologic events. Here we present data showing that both nonmalignant prostate tissues and prostate cancer tissues are similarly affected following treatment of patients with anti-CTLA-4 antibody. Both nonmalignant prostate tissues and prostate cancer tissues from treated patients had a higher frequency of CD4+ICOShi T cells. CD4+ICOShi T cells contain a population of effector T cells leading to a higher ratio of effector to regulatory T cells in tumor tissues after treatment. Similarly, anti-CTLA-4 therapy also leads to a higher ratio of Th1 to Th2 cytokines in tumor tissues. Although these changes are expected to be beneficial in the setting of tumor tissues, and have been correlated with tumor rejection in animal models (30), it remains questionable as to whether they are detrimental in nonmalignant tissues. It is likely that many factors contribute to the development of irAEs as a result of anti-CTLA-4 therapy. We previously reported that decreased concentrations of plasma IL-10 appear to be associated with irAEs (31). In our small cohort of patients, we did not observe irAEs in all patients who demonstrated a higher frequency of CD4+ICOShi T cells or a higher ratio of Th1 to Th2 cytokines in nonmalignant tissues.
It is possible that the immunologic changes that we observed within prostate tissues are due to the anatomical proximity of the prostate and bladder and are simply an extension of the immunologic changes that are ongoing within the bladder tumor tissues of our patients. Additional tissue samples from anti-CTLA-4–treated patients would need to be studied to determine whether the immunologic changes that we observed are ongoing in other nonmalignant tissues. However, in the only other reported study to analyze tissues after anti-CTLA-4 therapy, the authors demonstrated by immunohistochemical methods that T cell infiltration in nonmalignant and malignant tissues was comparable after patients were treated, thus making it less likely that our data are related to anatomical proximity and supporting the concept of a systemic response induced by therapy. In a previous report it was shown that anti-CTLA-4 therapy led to the development of skin rash in a subset of melanoma patients and biopsies from these nonmalignant areas revealed CD4 and CD8 T cell infiltrates (32). The authors also demonstrated that biopsies from enlarged lymph nodes of treated patients revealed CD3 T cell infiltration without evidence of HMB-45 and MART-1 expressing melanoma tumor cells. Similarly, in 2 ovarian carcinoma patients who developed diarrhea after treatment with anti-CTLA-4 antibody, biopsies from the gastrointestinal tract revealed CD4 and CD8 T cell infiltration (32). These findings of dense T cell infiltration in nonmalignant tissues were also seen in biopsies of metastatic melanoma lesions after patients were treated with anti-CTLA-4 antibody (32). Therefore, it is feasible that anti-CTLA-4 therapy leads to a broad-based systemic activation of T cell responses, which can then lead to irAEs or anti-tumor responses depending on the T cell repertoire and/or additional immunological events that are ongoing in select patients. Interestingly, we could not expand CD4 T cells from tumor tissues that did not express the NY-ESO-1 antigen with our current in vitro culture techniques using the NY-ESO-1 overlapping peptides, which suggests that appropriate antigenic stimuli are likely to be necessary to expand tumor-infiltrating T cells that are specific for other tumor antigens. Similarly, CD4 T cells from nonmalignant prostate tissues did not survive and could not be expanded in vitro as was possible for CD4 T cells from the patient whose prostate cancer expressed the NY-ESO-1 tumor antigen (Fig. 5). This finding suggests that T cells within nonmalignant tissues may require additional signals before subsequent immunological responses could occur as compared to T cells from tumor tissues.
Previous studies have also documented induction of NY-ESO-1-specific antibody and T- cell responses (12, 32, 33) in patients treated with anti-CTLA-4 therapy. These antigen-specific T cells suggest that only particular subsets of T cells will be able to expand to perpetuate a tissue-destructive immune response, thereby preventing similar immune responses from occurring in nonmalignant tissues. We previously reported that NY-ESO-1–specific T cell responses can be induced in the systemic circulation of prostate cancer patients vaccinated with the NY-ESO-1 DNA vaccine but these responses were suppressed in a subset of patients as a result of regulatory T cell function (34). Here we show that NY-ESO-1-specific T cells also exist within incidental prostate adenocarcinoma samples obtained from patients treated with anti-CTLA-4 antibody. CD4+FOXP3+ T cells were less prevalent in tumor tissues after anti-CTLA-4 therapy, which possibly permitted an expansion of effector antigen-specific CD4 T cells. CD4 T cells from an anti-CTLA-4 treated prostate tumor sample had high expression of ICOS and a subset of cells produced Th1-associated factors including IFN-γ, TNF-α, and MIP-1β in response to in vitro stimulation with the NY-ESO-1 antigen. Our data imply a broad based systemic effect of anti-CTLA-4 therapy across tumor types and tissue pathology. The identification of increased ICOS expression on CD4 T cells in both tumor tissues and blood provides a relevant blood-based marker that can be used to monitor patients, including prostate cancer patients, who receive anti-CTLA-4 therapy. Additional studies are warranted to investigate the role of CD4+ICOShi T cells and cytokine levels in anti-tumor responses and irAEs.
Materials and Methods
Patients.
Tissues were collected from surgical samples including prostate samples of all male patients who were treated by radical cystoprostatectomy for bladder cancer or radical prostatectomy for prostate cancer. Surgical samples from patients who were treated with anti-CTLA-4 therapy were obtained from an ongoing clinical trial wherein bladder cancer patients receive 2 doses of ipilimumab anti-CTLA-4 antibody before surgery, which is performed 4 weeks after the last dose of antibody. Ipilimumab is a fully human monoclonal Ig (IgG1) specific for human CTLA-4 (CD152). The antibody is given at a dose of 3 mg/kg or 10 mg/kg each time, with a 3-week interval between doses. All patients were monitored for safety. This is an ongoing clinical trial. The results reported in prostate tissues reflect data obtained from 7 treated patients (4 patients with nonmalignant prostate tissues and 3 patients with prostate adenocarcinoma). Patient 2 had urothelial carcinoma of the bladder extending into the prostate and his prostate sample was not included in this reported data set. Nonmalignant prostate tissues (n = 5) were obtained from prostate samples of male bladder cancer patients who were treated by radical cystoprostatectomy and who did not participate in the anti–CTLA-4 antibody trial. These patients were consented onto a separate IRB-approved sample acquisition laboratory protocol. Nonmalignant prostate tissues were found to have no evidence of cancer within the tissues by pathology review. Untreated prostate cancer tissues (n = 10) were obtained from prostatectomy samples of patients undergoing surgery as treatment for localized prostate cancer. These patients were also consented onto a separate IRB-approved sample acquisition laboratory protocol. Blood samples from untreated prostate cancer patients with localized disease (n = 10) were acquired from patients undergoing radical prostatectomy. Healthy donor blood was obtained from volunteers (n = 10).
Blood and Tissue Processing.
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood by density gradient centrifugation using Lymphocyte Separation Medium (Mediatech) and Leucosep tubes (Greiner Bio-one ). Cells recovered from the gradient interface were washed twice in RPMI medium 1640 (Mediatech), counted, and immediately used for cytokine analysis, staining, and flow cytometry analysis. Fine needle aspirations (FNAs) of prostate tissues were washed twice with cold PBS supplemented with 2% BSA and 2 mM EDTA before performing cell surface and intracellular staining for ex-vivo flow cytometric analysis, as previously reported (12).
Flow Cytometry.
Antibodies used for flow cytometry consisted of: CD3-FITC and CD4-PerCP-Cy5.5 (BD PharMingen), FOXP3-PE (eBiosciences, clone PHC101), ICOS-biotilynated (eBiosciences) conjugated with streptavidin-APC-Cy7 (BD Biosciences). Intracellular staining for FOXP3, TNF-α, and MIP-1β were conducted as per manufacturer's guidelines. Samples were analyzed using the FACSCanto II (Becton Dickinson). Data were analyzed using BD FACSDiva software. Gates were set according to appropriate isotype controls.
Real-time PCR.
Total RNA samples from tissues were isolated with the RNeasy kit (Qiagen). Reverse transcription was performed using the SuperScript III Reverse Transcriptase kit (Invitrogen). Real-time quantitative PCR was performed with the 7500 Fast Real-time PCR system (Applied Biosystems) according to the manufacturer's instructions. Samples were used as templates in reactions to obtain the threshold cycle (Ct) that were normalized with the Ct of GAPDH from the same sample (ΔCt). To compare the relative levels of gene expression in different tissues, ΔΔCt values were calculated with the ΔCt values associated with the lowest expression levels as 1. Fold induction was calculated using 2ΔΔCt. CD3-ε, IFN-γ, T-bet, and FOXP3 probes were synthesized with the Taqman Gene Expression Assay (Applied Biosystems). Other primers used were synthesized by Integrated DNA Technologies (IDT) and were used with the SYBR Green PCR Master Mix System (Applied Biosystems). Primers used for PCR included GAPDH sense: TGCACCACCAACTGCTTAGC; anti-sense: GGCAT GGACTGTGGTCATGAG; IL-2 sense: AAGTTTTTACATACCCAAGAAGG; antisense: AAGTGAAAGTTTTTGCTTTGAGC; IL-10 sense: TGGGGGAGAACC TGAAGAC; antisense: ACAGGGAAGAAATCGATGACA; GATA-3 sense:GCTTCGGATGCAAGTCCA; antisense: GCCCCACAGTTCACACACT.
ELISPOT Assay to Detect T cell Responses to NY-ESO-1 Tumor Antigen.
CD4 T cells were sorted from FNA material obtained from tissue samples as previously described (12). To serve as antigen-presenting cells (APCs), PBMCs were depleted of CD8+ and CD4+ T cells and pulsed with a 10 μM peptide pool consisting of synthesized (Multiple Peptide Systems) 20-mer overlapping peptides (amino acids 1–20, 11–30, 21–40, 31–50, 41–60, 51–70, 61–80, 71–90, 81–100, 91–110, 101–120, 111–130, 119–143, 131–150, 139–160, 151–170, 161–180) encompassing the full-length NY-ESO-1 antigen. Pulsed APCs were added to the plates containing CD4 T cells obtained from tumor tissues and supplemented RPMI media. Sensitized CD4 T cells were then tested with target APCs without peptide or pulsed with peptide in ELISPOT assays as previously described (30). Nitrocellulose plates (MultiScreen -HA; Millipore) were coated with IFN-γ mAb (4 μg/mL, 1-D1K; Mabtech) and incubated overnight at 4 °C. The presensitized CD4 T cells and target APCs were added to each well and incubated for 20 h. Plates were then washed and IFN-γ mAb (0.2 μg/mL, 7-B6–1-biotin; Mabtech) was added, incubated for 2 h at 37 °C, washed again, and developed with streptavidin-alkaline phosphatase (1 μg/mL; Roche). Substrate (5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium; Sigma) was added and plate membranes displaying dark-violet spots representing IFNγ-secreting T cells were counted with the CTL Immunospot analyzer and software (Cellular Technologies). ICOS-expressing CD4 T cells were also obtained after in vitro presensitization and T cell responses were visualized by intracellular cytokine (TNF-α and MIP-1β) staining and compared to responses that were obtained in the absence of NY-ESO-1 peptides on APCs.
Immunohistochemistry.
Immunohistochemistry was done on formalin-fixed paraffin-embedded tissues. Immunohistochemical staining was done for NY-ESO-1 and CD4 using monoclonal antibodies E978 as previously described (34) and 1F6 (Labvision), respectively. Five-micrometer sections were applied to charged slides (Superfrost Plus, Menzel), heated overnight, and then deparaffinized and rehydrated in xylene and a series of graded alcohols. Antigen retrieval was used for all stainings by immersing slides in a buffer solution preheated for 15–30 min in a steamer at 97 °C. DAKO HipH solution was used for E978 as well as 1F6. Primary antibody incubation was done overnight at 5 °C and the Powervision kit (Vision Biosystems) was used as a secondary reagent. Diaminobenzidine served as a chromogen, and hematoxylin was used for counterstaining. Hematoxylin-eosin (H & E) staining was used to evaluate all tissue specimen. Images were obtained with 20× objective magnification.
Statistical Analyses.
Data regarding CD4+FOXP3+ regulatory T cells were compared between prostate cancer patients and healthy donors using the Wilcoxon rank-sum test to assess the differences in continuous variables between the two groups and P-values that were calculated to be less than 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments.
We thank the entire Genitourinary Medical Oncology and Urology Departments at M. D. Anderson Cancer Center for helpful suggestions in the implementation of the neoadjuvant anti-CTLA-4 antibody clinical trial; research nurses Brenda Moomey, Donnah Jones, and Dallas Williams for their assistance in conducting the clinical trial; Marla Johnson, Jason Love, Cherie Perez, Alana Bethea, and Monica Quillian for their assistance in data management and protocol amendments; Erin Horne and Loretta Patterson for coordinating patient visits and blood draws; Karen Phillips for editorial assistance; Cindy Soto and Ina Prokhovora for technical assistance in obtaining surgical tissues; Drs. Rachel Humphrey, Axel Hoos, and Ramy Ibrahim at Bristol-Myers Squibb for their enthusiastic support and guidance in designing and conducting the ipilimumab neoadjuvant clinical trial; and J.P.A. for critical reading of this manuscript. This work was supported in part by a Physician-Scientist Program Award and an Institutional Research Grant from The University of Texas M. D. Anderson Cancer Center (UTMDACC), a Prostate Cancer Foundation Award, and a Clinical Investigator Award from the Cancer Research Institute (all to P.S.). Bristol-Myers Squibb sponsored and funded the clinical trial of neoadjuvant ipilimumab for bladder cancer patients.
Footnotes
Conflict of interest statement: P.S. has served as a consultant on advisory boards for Bristol-Myers Squibb.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813175106/DCSupplemental.
References
- 1.Walunas TL, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. [PubMed] [Google Scholar]
- 2.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing affects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 5.van Elas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodi FS, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci USA. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phan GQ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korman A, Yellin M, Keler T. Tumor immunotherapy: Preclinical and clinical activity of anti-CTLA4 antibodies. Curr Opin Investig Drugs. 2005;6:582–591. [PubMed] [Google Scholar]
- 9.Saenger YM, Wolchuk JD. The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: Patient cases. Cancer Immun. 2008;8:1–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Small EJ, et al. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:1810–1815. doi: 10.1158/1078-0432.CCR-06-2318. [DOI] [PubMed] [Google Scholar]
- 11.Attia P, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liakou CI, et al. CTLA-4 blockade increases IFNγ-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci USA. 2008;105:14987–14992. doi: 10.1073/pnas.0806075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutloff A, et al. ICOS is an inducible T cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 14.Yoshinaga SK, et al. T cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402:827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- 15.Coyle AJ, et al. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity. 2000;13:95–105. doi: 10.1016/s1074-7613(00)00011-x. [DOI] [PubMed] [Google Scholar]
- 16.Dong C, et al. ICOS co-stimulatory receptor is essential for T cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 17.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 18.Miller AM, et al. CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol. 2006;177:7398–7405. doi: 10.4049/jimmunol.177.10.7398. [DOI] [PubMed] [Google Scholar]
- 19.Ahmadzadeh M, et al. FOXP3 expression accurately defines the population of intratumoral regulatory T cells that selectively accumulate in metastatic melanoma lesions. Blood. 2008;112:4953–4960. doi: 10.1182/blood-2008-06-163048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark RA, et al. Human squamous cell carcinomas evade the immune response by down-regulation of vascular E-selectin and recruitment of regulatory T cells. J Exp Med. 2008;205:2221–2234. doi: 10.1084/jem.20071190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woo EY, et al. Cutting edge: Regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168:4272–4276. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- 22.Ichihara F, et al. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res. 2003;9:4404–4408. [PubMed] [Google Scholar]
- 23.Curiel TJ, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 24.Sato E, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma P, et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci USA. 2007;104:3967–3972. doi: 10.1073/pnas.0611618104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity, and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 27.Szabo SJ, et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 28.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 29.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 30.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun J, et al. Concurrent decrease in IL-10 with development of immune-related adverse events in a patient treated with anti-CTLA-4 therapy. Cancer Immun. 2008;8:9–15. [PMC free article] [PubMed] [Google Scholar]
- 32.Hodi FS, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci USA. 2008;105:3005–3010. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan J, et al. Cytotoxic T lymphocyte-associated antigen 4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with tumor regression. Proc Natl Acad Sci USA. 2008;105:20410–20415. doi: 10.1073/pnas.0810114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gnjatic S, et al. NY-ESO-1 DNA vaccine induces T cell responses that are suppressed by regulatory T cells. Clinical Cancer Res. 2009 doi: 10.1158/1078-0432.CCR-08-2632. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jungbluth AA, et al. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int J Cancer. 2001;92:856–860. doi: 10.1002/ijc.1282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.