Abstract
Mutation in leucine-rich repeat kinase-2 (LRRK2) is the most common cause of late-onset Parkinson's disease (PD). Although most cases of PD are sporadic, some are inherited, including those caused by LRRK2 mutations. Because these mutations may be associated with a toxic gain of function, controlling the expression of LRRK2 may decrease its cytotoxicity. Here we show that the carboxyl terminus of HSP70-interacting protein (CHIP) binds, ubiquitinates, and promotes the ubiquitin proteasomal degradation of LRRK2. Overexpression of CHIP protects against and knockdown of CHIP exacerbates toxicity mediated by mutant LRRK2. Moreover, HSP90 forms a complex with LRRK2, and inhibition of HSP90 chaperone activity by 17AAG leads to proteasomal degradation of LRRK2, resulting in increased cell viability. Thus, increasing CHIP E3 ligase activity and blocking HSP90 chaperone activity can prevent the deleterious effects of LRRK2. These findings point to potential treatment options for LRRK2-associated PD.
Keywords: LRRK2, Parkinson's disease, proteasome, ubiquitin
Parkinson's disease (PD) is a progressive neurodegenerative disorder pathologically characterized by loss of dopaminergic neurons from the substantia nigra and the presence of Lewy bodies (1–3). The etiology of PD is incompletely understood but appears to involve both genetic and environmental factors. To date, 5 genes (α-synuclein, parkin, DJ-1, PINK-1, and LRRK2) are associated with genetic forms of PD that closely resemble idiopathic PD (4–10). Mutation in LRRK2 is the most frequent genetic cause of PD (11). Patients with LRRK2 mutations exhibit clinical and neurochemical phenotypes that are indistinguishable from sporadic PD (9, 10). These patients suffer neuronal loss and gliosis in the substantia nigra and development of Lewy bodies, and also exhibit pleomorphic neuropathology, including α-synuclein and tau pathology (9, 12, 13). Thus, LRRK2 is important for the pathogenesis of several major neurodegenerative disorders associated with parkinsonism.
LRRK2, a member of the ROCO protein family, contains a guanosine triphosphatase (GTPase), a C-terminal of Ras domain with a kinase effector domain (14), repeat sequences beginning at the N terminus, and a leucine-rich repeat structure near its GTPase domain (15). LRRK2 is localized to membranous structures, where it may be in involved in neuronal polarity (16–18). Mutations in LRRK2 are frequent in autosomal-dominant PD as well as sporadic PD (19–23). PD-associated LRRK2 mutants seem to enhance kinase activity, and mutant LRRK2-mediated neuronal toxicity requires GTP-binding and kinase activity (17, 24–26).
The ubiquitin proteosomal system (UPS) appears to regulate LRRK2 level, with little influence from the autophagic and lysosomal degradation pathways (17). LRRK2 also dimerizes and interacts with HSP90 (18, 27, 28), which is somehow involved in controlling LRRK2 levels. The identity of the E3 ligase and the mechanisms that regulate the stability of LRRK2 via HSP90 are not known. Whether the levels of LRRK2 are linked to toxicity also is unclear.
The carboxyl terminus of HSP70-interacting protein (CHIP) plays a critical role in quality control of cellular proteins and stress recovery systems in most cell types (29, 30). CHIP contains multiple domains, including a tetratricopeptide repeat (TPR) domain that allows it to interact with molecular chaperones, such as HSP70 and HSP90, and a U-box domain that confers its E3 ubiquitin ligase function (31, 32). Thus, CHIP functions as both a co-chaperone and an E3 ubiquitin ligase and serves as a molecular link between cellular protein folding and degradation. CHIP mediates ubiquitin attachment to the chaperone substrate and stimulates the degradation of chaperone substrates by the UPS (33, 34) CHIP has been linked to several neurodegenerative diseases characterized by protein misfolding and aggregation. Because HSP90 interacts with LRRK2 and CHIP, we explored the potential for CHIP to regulate LRRK2 levels (33). We found that CHIP interacts with and ubiqutinates LRRK2, leading to the latter's proteasomal degradation through a HSP90 chaperone–containing complex. In addition, we found that CHIP and HSP90 levels are critical determinants of LRRK2 toxicity; thus, regulating the levels and activity of CHIP and HSP90 may be potentially valid candidates for treating LRRK2-related PD.
Results
LRRK2 Interacts With CHIP.
LRRK2 dimerizes and interacts with HSP90 (18, 27, 28). Because CHIP is an ubiquitin ligase that interacts with HSP90, we explored the possibility that CHIP interacts with and ubiquitinates LRRK2 in SH-SY5Y neuroblastoma cells (Fig. 1). To investigate a possible interaction between CHIP and LRRK2, we conducted coimmunoprecipitation experiments with Myc-tagged LRRK2 and HA-tagged CHIP, and found that LRRK2 pulled down CHIP (Fig. 1A) and Myc-tagged CHIP pulled down FLAG-tagged LRRK2 (Fig. 1B). In addition, immunoprecipitation of FLAG-tagged wild-type (WT), R1441C, or G2019S LRRK2 pulled down HA-tagged CHIP, demonstrating that CHIP also interacts with mutant LRRK2 (Fig. 1C). We found no substantial differences between WT and mutant LRRK2 in terms of binding to CHIP. We observed similar results in HeLa cells (data not shown). To explore whether LRRK2 interacts with CHIP, we conducted in vivo coimmunoprecipitation experiments with brains from WT mice and LRRK2 knockout (KO) mice (Fig. 1D). Immunoprecipitation of endogenous CHIP pulled down LRRK2 from the WT mouse brain but not from the LRRK2 KO mouse brain (Fig. 1D). The failure to immunoprecipitate LRRK2 from the LRRK2 KO mouse brain indicates that the coimmunoprecipitation of CHIP and LRRK2 from WT mouse brain was specific.
Fig. 1.
LRRK2 interacts with CHIP. (A) Lysates from SH-SY5Y cells transfected with HA-tagged CHIP and Myc-tagged LRRK2 were subjected to IP with anti-Myc, followed by anti-HA immunoblotting (Middle) or with anti-Myc antibody (Bottom) to show an equivalent amount of immunoprecipitated LRRK2. (B). Lysates from SH-SY5Y cells transfected with Myc-tagged CHIP and FLAG-tagged LRRK2 subjected to IP with anti-Myc, followed by anti-FLAG immunoblotting (Middle) or anti-Myc antibody (Bottom). (C) Lysates from SH-SY5Y cells transfected with HA-tagged CHIP and FLAG-tagged WT, R1441C, or G2019S LRRK2 constructs subjected to IP with anti-FLAG, followed by anti-HA immunoblotting (Middle) or anti-FLAG antibody (Bottom). (D) In vivo interaction in mouse brain from lysates prepared from mouse brain subjected to immunoprecipitation with anti-CHIP, anti-IgG with immunoblotting using anti-CHIP or anti-LRRK2, respectively. (E) Primary cortical neurons were fixed and stained with primary antibodies against LRRK2 and CHIP, followed by detection with secondary antibodies conjugated to Cy2 (LRRK2; green) or Cy3 (CHIP; red). Superimposing 2 colors (merged) resulted in a yellow signal, indicating colocalization of the 2 proteins.
Next, we used double-labeling immunofluorescence confocal microscopic analysis to determine the cellular localizations of endogenous CHIP and LRRK2 in primary cortical neurons. We found significant colocalization between endogenous LRRK2 and CHIP in the soma and neurites of cortical neurons (Fig. 1E). These findings support a physiological interaction between CHIP and LRRK2.
LRRK2 Interacts With CHIP Through Its TPR Domain, and CHIP Associates With LRRK2 Through Its Ras of Complex Proteins (ROC) Domain.
CHIP contains 2 major structural motifs, a TPR motif and a U-box domain. The TPR domain is required for interaction with HSC70 and HSP90, whereas the U-box domain has ubiquitin ligase activity (Fig. 2A). To determine the domain of CHIP that interacts with LRRK2, we transfected Myc-tagged WT CHIP, CHIP lacking the U-box domain (CHIPΔUbox), and CHIP lacking the TPR domain (CHIPΔTPR) into SH-SY5Y cells with FLAG-tagged LRRK2. We found that LRRK2 interacted with the TPR domain of CHIP, as demonstrated by a marked reduction in the coimmunoprecipitation of LRRK2 with the CHIPΔTPR mutant compared with the interaction of LRRK2 with WT CHIP (Fig. 2A). To determine the domain of LRRK2 that interacts with the CHIP, we performed coimmunoprecipitation experiments in SH-SY5Y cells transfected with a series of deletion mutants of FLAG-tagged LRRK2 constructs and HA-tagged CHIP, and found that CHIP interacted with the ROC domain of LRRK2 (Fig. 2B).
Fig. 2.
LRRK2 interacts with the TPR domain of CHIP, and CHIP associates with the ROC domain of LRRK2 in SH-SY5Y cells. (A) Lysates from SH-SY5Y cells transfected with FLAG-LRRK2 and Myc-tagged CHIP domain constructs were subjected to IP with anti-Myc, followed by anti-FLAG immunoblotting (Middle) or anti-Myc (Bottom). The deletion domains of CHIP used are shown at the bottom of the panel. (B) Lysates from SH-SY5Y cells transfected with HA-tagged CHIP and FLAG-tagged fragments of LRRK2 subjected to IP with anti-HA antibodies, followed by anti-FLAG immunoblotting (Middle) or anti-HA (Bottom). A schematic representation of the different LRRK2 fragments used is shown.
LRRK2 Is a Substrate for the CHIP E3 Ligase.
To ascertain whether CHIP ubiquitinates LRRK2, we performed in vivo ubiquitination experiments. We cotransfected SH-SY5Y cells with FLAG-tagged LRRK2, Myc-tagged WT CHIP, CHIPΔU-box, CHIPΔTPR, and HA-tagged ubiquitin. FLAG-tagged LRRK2 was immunoprecipitated with an anti-FLAG antibody from the total cell extract. LRRK2 was ubiquitinated by CHIP, as shown by the substantial anti-HA immunoreactivity in SH-SY5Y cells (Fig. 3A). CHIP lacking the U-box still ubiquintinated LRRK2, whereas CHIP lacking the TPR domain failed to ubiquitinate LRRK2 (Fig. 3A). Moreover, proteasome inhibition with the proteosomal inhibitor clasto-lactacystin β-lactone augmented CHIP-mediated LRRK2 ubiquitination in HEK293 cells (Fig. 3C). We also found that CHIP ubiquitinated mutant R1441C and G2019S LRRK2 in a manner similar to WT LRRK2 (Fig. 3B). We noted substantially reduced ubiquitination of WT and mutant LRRK2 in the presence of CHIPΔTPR (Fig. 3B).
Fig. 3.
CHIP regulates the steady-state level of LRRK2 via UPS degradation. (A) Lysates from SH-SY5Y cells transfected with Myc-tagged WT CHIP, Myc-tagged ΔU-box or Myc-tagged ΔTPR, HA-tagged ubiquitin, and FLAG-tagged LRRK2 subjected to immunoprecipitation with anti-FLAG, followed by immunoblotting with anti-HA (Third panel) and anti-FLAG (Fourth panel) for in vivo ubiquitination assays. Lysates also were probed with an anti-Myc antibody (Second panel) and anti-HA (First panel) to demonstrate ubiquitin and CHIP expression. Brackets indicate ubiquitinated LRRK2. (B) Lysates from SH-SY5Y cells transfected with Myc-tagged WT CHIP and Myc-tagged ΔTPR, HA-tagged ubiquitin and FLAG-tagged WT and pathogenic forms (R1441C and G2019S) of LRRK2 were subjected to immunoprecipitation with anti-FLAG, followed by immunoblotting with anti-HA (Third panel) and anti-FLAG (Fourth panel). Lysates also were probed with an anti-HA (First panel) and anti- Myc antibody (Second panel) to demonstrate ubiquitin and CHIP expression. Brackets indicate ubiquitinated LRRK2. (C) HEK 293T cells were transfected with FLAG-tagged WT LRRK2 and HA-tagged ubiquitin along with either Myc-CHIP or empty vector. After 24 h, cells were treated with 10 μM β-lactone for 24 h. FLAG coimmunoprecipitation demonstrated that LRRK2-specific ubiquitination was greatly increased in the presence of CHIP and/or β-lactone. (D) SH-SY5Y cells stably expressing FLAG-tagged LRRK2 and pathogenic forms (R1441C and G2019S) of LRRK2 were transiently transfected with Myc-WT CHIP or Myc-CHIPΔTPR, and 24 h later treated with 5 μM β-lactone for 18 h. Total lysates were immunoblotted with anti-FLAG antibody to show the steady-state LRRK2 level (Upper), with anti-Myc to show relative levels of CHIP (Middle), and with anti-actin to confirm equivalent loading (Bottom). (E) HeLa cells were first transfected in triplicate with a nonsilencing control (scramble) or CHIP siRNA and then transfected with FLAG-tagged WT LRRK2 and Myc-CHIP or empty vector for 72 h, after which they were harvested for Western blot analysis of CHIP and LRRK2. Cells exposed to the CHIP siRNA exhibited a 20%–25% increase in LRRK2 level, whereas cells exposed to the WT CHIP had a 20%–25% decrease in LRRK2 level on quantitative densitometric analysis. (F) Primary cortical neurons were infected with lentiviruses delivering shRNAs for 2 days and then treated with 10 μM MG132 for 18 h. Proteins levels were monitored by Western blot analysis, with actin as a loading control. (G) Mouse brains from WT and CHIP KO mice were analyzed by Western blot analysis (41). In panels D–G, values represent optical density ± SD, normalized to actin. *P < .05; **P < .005.
To explore whether CHIP degrades LRRK2, we generated SH-SY5Y stable cell lines expressing FLAG-tagged WT, G2019S, and R1441C LRRK2 and monitored the steady-state levels of WT, G2019S, and R1441C LRRK2 in the presence of WT CHIP, CHIPΔTPR, and WT CHIP plus β-lactone (Fig. 3D). WT CHIP led to a significant and substantial reduction in the steady- state levels of WT, G2019S, and R1441C LRRK2, whereas CHIPΔTPR had no substantial effect, and β-lactone prevented the degradation of WT, G2019S, and R1441C LRRK2 by CHIP (Fig. 3D). To investigate whether reducing the CHIP level leads to an increase in LRRK2 level, we measured the LRRK2 level in HeLa cells in the presence of CHIP siRNA compared with scrambled siRNA (Fig. 3E). Knocking down the expression of endogenous CHIP led to a substantial increase in the levels of FLAG-tagged LRRK2 in HeLa cells (Fig. 3E). To examine whether in vitro knockdown of endogenous CHIP leads to the accumulation of LRRK2, we measured LRRK2 levels in primary neurons after shRNA knockdown of CHIP compared with scrambled shRNA control. We found that shRNA-mediated knockdown of CHIP increased the steady-state level of endogenous LRRK2 protein compared with control to the same degree as proteasomal inhibition with MG132 (Fig. 3F). Together, proteasomal inhibition and shRNA knockdown of CHIP modestly increases the level of endogenous LRRK2 protein. To ascertain whether CHIP regulates the levels of LRRK2 in vivo, we compared LRRK2 levels in 1-year-old WT mice and CHIP KO mice. We found a significant (4-fold) increase in LRRK2 level in the CHIP KO mice compared with the WT mice in the soluble fraction (Fig. 3G). Western blot analysis and quantification of α-synuclein and DJ-1 levels failed to exhibit any significant change in soluble extracts relative to control brain extracts (Fig. S1). Taken together, these findings indicate that LRRK2 is a substrate for the CHIP E3 ligase and that CHIP regulates the level of LRRK2 through proteasomal degradation.
CHIP Rescues LRRK2 Toxicity.
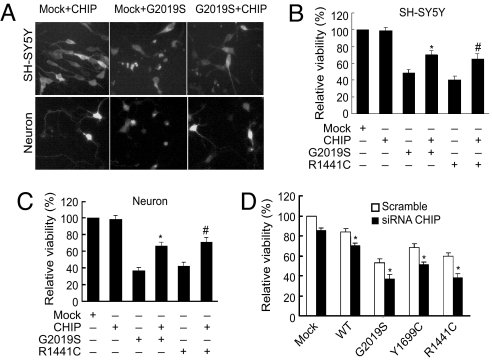
To determine whether CHIP can alter cell toxicity induced by LRRK2 (24, 25, 35), we investigated the effects of overexpression and knockdown of CHIP on LRRK2-induced cell death (Fig. 4). We cotransfected SH-SY5Y cells with GFP, CHIP, and G2019S or R1441C LRRK2 by Lipofectamine Plus (Invitrogen). Viable cells were defined as those having at least one smooth neuronal process that was twice the length of the cell body. Consistent with other observations (24, 25, 35), there was mild and decreased viability with WT LRRK2. In contrast to WT LRRK2, the G2019S and R1441C mutants caused significant cell toxicity compared with control transfected cells (Fig. 4B and C). Importantly, CHIP overexpression reduced G2019S and R1441C toxicity by ≈50% in SH-SY5Y cells (Fig. 4A, Top and B). In a similar manner, CHIP reduced G2019S and R1441C toxicity in primary cortical neurons by ≈50% (Fig. 4A, Bottom and C). Knocking down CHIP levels with siRNA significantly enhanced WT, G2019S, R1441C, and Y1699C LRRK2 toxicity compared with scrambled siRNA in SH-SY5Y cells (Fig. 4D). These results indicate that CHIP influences LRRK2 toxicity by regulating LRRK2 protein levels.
Fig. 4.
CHIP protects against and knockdown of CHIP exacerbates mutant LRRK2-induced toxicity. (A, Top) SH-SY5Y cells cotransfected with pcDNA3.1-GFP and vector pcDNA3.1, pcDNA3.1-FLAG-LRRK2-G2019S, or LRRK2-R1441C with or without Myc-CHIP at a 1:15 ratio. GFP-positive cells with 2-fold continuous extensions were counted as live cells. Representative photomicrographs for each experimental group are shown. The transfection efficiency of GFP was about 10%. (B) Quantitation of the data shown in panel A (Top), representing the cell viability of each experimental group normalized to that of cells cotransfected with empty vector and GFP. Data are mean ± SE for 3 separate experiments performed in duplicate. *P < .05 vs. cells cotransfected with LRRK2-G2019S, vector, and GFP; #P < .05 vs. cells cotransfected with LRRK2-R1441C, vector, and GFP by ANOVA. (A, Bottom) Mouse primary cortical neurons cotransfected with pcDNA3.1-GFP and indicated constructs. GFP-positive viable neurons with 2-fold continuous neurites were counted as live neurons. Representative photomicrographs for each experimental group are shown. (C) Quantitation of the data shown in A (Bottom), representing the cell viability of each experimental group normalized to that of cells cotransfected with empty vector and GFP. Data are mean ± SE for 3 separate experiments performed in duplicate. *P < .05 vs. cells cotransfected with LRRK2-G2019S, vector, and GFP; #P < .05 vs. cells cotransfected with LRRK2-R1441C, vector, and GFP by ANOVA. (D) SH-SY5Y cells transfected with nonsilencing control or CHIP siRNA for 24 h. The cells were cotransfected with pcDNA3.1-GFP and pcDNA3.1, FLAG-tagged WT, G2019S, Y1699C, or R1441C LRRK2. GFP-positive cells with 2-fold continuous extensions were counted as live cells. The quantitation of data represents the cell viability of each experimental group normalized to that of cells cotransfected with empty vector and GFP. Data are mean ± SE for 3 separate experiments performed in duplicate. *P < .05 vs. cells cotransfected with WT, LRRK2-G2019S, LRRK2-Y1699C, or LRRK2-R1441C vector and GFP by ANOVA.
HSP90 Regulates the Stability of LRRK2.
Because CHIP and LRRK2 interact with HSP90 (18, 28), we performed immunoprecipitation experiments to further explore their interactions (Fig. 5). We transfected HeLa cells with FLAG-tagged WT LRRK2 and Myc-tagged CHIP, Myc-CHIPΔU-box, Myc-CHIPΔTPR, or Myc-CHIP-K30A, followed by immunoprecipitation of Myc. We found that HSP90, HSP70, and LRRK2 coimmunoprecipitated with CHIP, but failed to coimmunoprecipitate with CHIP lacking the TPR domain or CHIP K30A mutant within the TPR domain (Fig. 5A). These findings suggest that the CHIP–LRRK2 interaction depends on CHIP's interaction with the HSPs. To ascertain whether HSP90 could regulate the steady-state levels of LRRK2, we monitored LRRK2 levels in the presence of increasing concentration of the HSP90 inhibitor, 17AAG. HSP90 and HSP70 levels increased after 17AAG treatment with a concomitant reduction in WT LRRK2 levels (Fig. 5B). Next, we monitored mutant LRRK2 levels in the presence of the HSP90 inhibitor, 17AAG (1 μM). 17AAG significantly reduce the levels of WT and mutant G2019S and R1441C LRRK2 (by 78.5%, 67.8%, and 75.2%, respectively) (Fig. 5C). To investigate the mechanism by which HSP90 inhibition reduces LRRK2 expression, we used 17AAG to assess heat-shock factor 1 (HSF1) and HSP90 expression in HeLa cells that overexpressed WT LRRK2. We found that 17AAG reduced LRRK2 levels by 50% in cells transfected with nonsilencing siRNA, but siRNA suppression of HSP90 expression abrogated the 17AAG-mediated LRRK2 reductions (Fig. 5D). Suppression of HSF1 expression by siRNA had no effect on the 17AAG-mediated LRRK2 reductions (Fig. 5E). To determine whether 17AAG protects against LRRK2 toxicity, we transfected SH-SY5Y cells with WT, G2019S, and R1441C LRRK2 and monitored cell viability. We found that a 24-h treatment with 17AAG significantly protected against G2019S- and R1441C-induced cell death (Fig. 5F).
Fig. 5.
17AAG decreases LRRK2 protein levels and associated toxicity in an HSP70/90-dependent manner. (A) HeLa cells transfected with FLAG-tagged WT LRRK2 and either Myc-tagged CHIP, Myc-tagged ΔU-box, Myc-tagged ΔTPR, Myc-CHIP with the K30A mutation, or empty vector. Myc coimmunoprecipitation shows that loss of the TPR domain or the K30A mutation abrogates LRRK2–CHIP binding. (B) HeLa cells transfected with FLAG-tagged WT LRRK2 were treated with the indicated concentrations of 17AAG or DMSO. Western blot analysis of cell lysates shows a 17AAG-mediated reduction of LRRK2 protein levels, with a corresponding HSP70 induction in those cells. (C) HeLa cells transfected with WT, G2019S, or R1441C LRRK2 were treated with 1 μM 17AAG or DMSO. LRRK2 WT, G2019S, and R1441C mutant LRRK2 protein levels are significantly decreased (by 78.5%, 67.8%, and 75.2%, respectively) with 17AAG treatment (black bars) compared with vehicle treatment (gray bars) after normalization to GAPDH and total LRRK2 levels (n = 4). (D and E) 17AAG-mediated degradation of LRRK2 requires a constitutive chaperone response. HeLa cells were transfected in duplicate with nonsilencing control, HSP90, and HSF1 siRNA and then transfected with FLAG-tagged WT LRRK2 and treated with 1 μM 17AAG or DMSO. Lysates were analyzed by Western blot. 17AAG causes a strong decrease in LRRK2 in nonsilencing control. (D) Knockdown of HSP90 increases LRRK2 levels in DMSO-treated cultures, but LRRK2 is increased only marginally in 17AAG treated cells. (E) HSF1 siRNA increases LRRK2 levels compared with nonsilencing control. Treatment with 17AAG decreases LRRK2 levels comparably to the nonsilencing control treated with 17AAG. (F) 17AAG protects against LRRK2 toxicity in SH-SY5Y cells treated with 10 nM 17AAG. Data are for 3 separate experiments done in duplicate and normalized to cells cotransfected with empty vector and GFP. Data are mean ± SE. *P < .05.
Discussion
CHIP is implicated in various neurodegenerative diseases characterized by protein misfolding and aggregation (31, 36, 37). Our major finding in the current study is that CHIP is an ubiquitin E3 ligase for LRRK2 that regulates LRRK2 levels. LRRK2 interacts, coimmunoprecipitates, and colocalizes with CHIP, and the 2 proteins coimmunoprecipitate from mouse brain. Familial associated mutations in LRRK2 do not alter the interaction with CHIP. The TPR domain of CHIP is required for binding to LRRK2, and the ROC domain of LRRK2 is required for the binding to CHIP. WT and mutant LRRK2 are polyubiquitinated by CHIP, and overexpression of CHIP decreases the steady-state level of WT and mutant LRRK2 in a proteasomal-dependent manner. LRRK2 is an authentic CHIP substrate, as demonstrated by our findings that siRNA knockdown of CHIP leads to up-regulation of LRRK2 in SH-SY5Y cells, shRNA knockdown of CHIP leads to up-regulation of LRRK2 in primary neurons, and knockout of CHIP in mice leads to the accumulation of LRRK2 in aging CHIP KO brain tissue compared with age-matched WT controls. Moreover, the up-regulation of LRRK2 by CHIP knockdown is equivalent to the level of up-regulation induced by proteasomal inhibition. The toxicity of LRRK2 toxicity depends on its level of expression, because overexpression of CHIP protects against mutant LRRK2 toxicity and siRNA knockdown of CHIP enhances WT and mutant LRRK2 toxicity. HSP90 and HSP70 also interact with LRRK2 through CHIP's TPR domain, and the binding of CHIP to LRRK2 is mediated through these HSPs as a single point mutation in the TPR domain of CHIP that prevents its interaction with HSPs, which abrogates CHIP's interaction with LRRK2. Increasing the steady-state level of HSPs with the glendamycin derivative, 17-AAG, reduces LRRK2 levels and toxicity. Together, these findings indicate that LRRK2 is a client of the CHIP–HSP chaperone system, and that regulation of LRRK2 levels through this system is potentially involved in LRRK2 toxicity.
HSP90 is a molecular chaperone crucial to the stability and function of many client proteins that promote cancer cell growth and survival (38). We and others have reported that HSP90 forms a complex with LRRK2 (18, 28), raising the possibility that HSP90 plays an important role in the maintenance of LRRK2 protein quality by regulating the balance between the folding and degradation of LRRK2. The site of LRRK2–CHIP interaction appears to be in the TPR domain, which is known to mediate interactions with chaperones, such as HSP90 and HSP70 (31). Thus, it appears that chaperone interaction is essential for LRRK2 ubiquitination and degradation by CHIP in vivo. In the present study, we found that treatment with the HSP90 inhibitor 17AAG reduced the steady-state levels of WT LRRK2 and LRRK2 mutants. HSP90 knockdown suppressed the 17AAG-mediated reductions in LRRK2 levels (Fig. 5D), whereas suppression of HSF1 expression by RNA interference prevented up-regulation of HSP70 (Fig. 5E), yet had no effect on 17AAG-mediated LRRK2 reductions. Together, these findings suggest that LRRK2 is processed through the constitutive HSP90 refolding system, where it is prone to proteasomal degradation, rather than being dependent on de novo transcription of HSP chaperones stimulated by HSF1.
In summary, our findings provide evidence that LRRK2 is a substrate for the CHIP E3 ligase and HSP90 chaperone system and that CHIP and the HSP90 chaperone system are essential for the proper degradation of LRRK2. Modulating CHIP levels may be a critical determinant in LRRK2 toxicity. Because CHIP is part of the HSP90 chaperone system, perturbations in CHIP and the chaperone system with aging may interfere with turnover of LRRK2, leading to aggregation and toxicity and subsequent Parkinson's disease. Strategies that enhance HSP90- or CHIP-mediated degradation could be possible therapeutic strategies to treat LRRK2-linked PD.
Materials and Methods
Plasmids, Antibodies, and Reagents.
Details about the materials used in this study are provided in SI Materials and Methods.
Cell Culture and Transfection.
Human neuroblastoma SH-SY5Y cells and HeLa cells were cultured as described previously (39, 40) and detailed in SI Materials and Methods.
Mouse Primary Cortical Neuronal Cultures and Electroporation Transfection.
Mouse primary cortical neuronal cultures were derived from CD-1 mice (Jackson Laboratory) at E15–16. Cortices were dissociated, plated on laminin- and polyD-lysine–coated plates (BD Biosciences), and cultured in Neurobasal medium with the addition of Glutamax, B-27 supplement, and penicillin/streptomycin (24). Under these culture conditions, 95% of cells were neurons. Transfection of LRRK2 constructs into mouse primary cortical neurons was carried out using Nucleofector (Amaxa Biosystems).
Immunoprecipitation and Western Blot Analysis.
Coimmunoprecipitation and Western blot analysis experiments were conducted using standard techniques. Details are provided in SI Materials and Methods.
In Vivo Ubiquitination Assay.
SH-SY5Y cells were transiently transfected with 2 μg of Myc-tagged WT CHIP or Myc-tagged CHIP mutants, pcDNA3.1-FLAG-tagged WT LRRK2, G2019S, R1441C and 2 μg of pMT123-HA-ubiquitin plasmids. 48 h later total cell lysates were harvested, the pellets were solubilized in 2% SDS with sonication. The samples were split for use as input and for immunoprecipitation. Antibodies against FLAG were used for immunoprecipitation followed by Western blot analysis with anti-HA, anti-Myc, and anti-FLAG antibodies and detection with Super Signal West Pico and Femto chemiluminescent substrates (Pierce Biotechnology).
Measurement of Cell Viability.
SH-SY5Y and neuron cell viability assay were conducted as described previously (24) and as detailed in SI Materials and Methods.
Statistical Analysis.
Quantitative data are expressed as arithmetic mean ± SE based on at least 3 separate experiments performed in duplicate or quadruplicate. The difference between 2 groups was analyzed using Student's t-test or 1-way ANOVA. Significance was defined at P < .05.
Supplementary Material
Acknowledgments.
This work was supported by the Morris K. Udall Parkinson's Disease Research Center; National Institutes of Health/National Institute of Neurological Disorders and Stroke Grants NS38377, NS54207, and NS04826, and National Institute of Aging Grant AG017216; the National Parkinson Foundation; and the American Parkinson Disease Association. T.M.D. is the Leonard and Madlyn Abramson Professor in Neurodegenerative Diseases.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810123106/DCSupplemental.
References
- 1.Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- 2.Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 3.Dauer W, Przedborski S. Parkinson's disease: Mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 4.Polymeropoulos MH, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 5.Kruger R, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 6.Kitada T, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 7.Bonifati V, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 8.Valente EM, et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 9.Zimprich A, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Paisan-Ruiz C, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 11.Savitt JM, Dawson VL, Dawson TM. Diagnosis and treatment of Parkinson disease: Molecules to medicine. J Clin Invest. 2006;116:1744–1754. doi: 10.1172/JCI29178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Funayama M, et al. An LRRK2 mutation as a cause for the parkinsonism in the original PARK8 family. Ann Neurol. 2005;57:918–921. doi: 10.1002/ana.20484. [DOI] [PubMed] [Google Scholar]
- 13.Ross OA, et al. Lrrk2 R1441 substitution and progressive supranuclear palsy. Neuropathol Appl Neurobiol. 2006;32:23–25. doi: 10.1111/j.1365-2990.2006.00693.x. [DOI] [PubMed] [Google Scholar]
- 14.Bosgraaf L, Van Haastert PJ. Roc, a Ras/GTPase domain in complex proteins. Biochim Biophys Acta. 2003;1643:5–10. doi: 10.1016/j.bbamcr.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Marin I. The Parkinson disease gene LRRK2: Evolutionary and structural insights. Mol Biol Evol. 2006;23:2423–2433. doi: 10.1093/molbev/msl114. [DOI] [PubMed] [Google Scholar]
- 16.Biskup S, et al. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann Neurol. 2006;60:557–569. doi: 10.1002/ana.21019. [DOI] [PubMed] [Google Scholar]
- 17.West AB, et al. Parkinson's disease–associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci U S A. 2005;102:16842–16847. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gloeckner CJ, et al. The Parkinson disease–causing LRRK2 mutation I2020T is associated with increased kinase activity. Hum Mol Genet. 2006;15:223–232. doi: 10.1093/hmg/ddi439. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez DG, et al. Clinical and positron emission tomography of Parkinson's disease caused by LRRK2. Ann Neurol. 2005;57:453–456. doi: 10.1002/ana.20401. [DOI] [PubMed] [Google Scholar]
- 20.Khan NL, et al. Mutations in the gene LRRK2 encoding dardarin (PARK8) cause familial Parkinson's disease: Clinical, pathological, olfactory and functional imaging and genetic data. Brain. 2005;128:2786–2796. doi: 10.1093/brain/awh667. [DOI] [PubMed] [Google Scholar]
- 21.Gasser T. Genetics of Parkinson's disease. Curr Opin Neurol. 2005;18:363–369. doi: 10.1097/01.wco.0000170951.08924.3d. [DOI] [PubMed] [Google Scholar]
- 22.Gilks WP, et al. A common LRRK2 mutation in idiopathic Parkinson's disease. Lancet. 2005;365:415–416. doi: 10.1016/S0140-6736(05)17830-1. [DOI] [PubMed] [Google Scholar]
- 23.Farrer M, et al. LRRK2 mutations in Parkinson disease. Neurology. 2005;65:738–740. doi: 10.1212/01.wnl.0000169023.51764.b0. [DOI] [PubMed] [Google Scholar]
- 24.Smith WW, et al. Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat Neurosci. 2006;9:1231–1233. doi: 10.1038/nn1776. [DOI] [PubMed] [Google Scholar]
- 25.West AB, et al. Parkinson's disease–associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum Mol Genet. 2007;16:223–232. doi: 10.1093/hmg/ddl471. [DOI] [PubMed] [Google Scholar]
- 26.Greggio E, et al. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis. 2006;23:329–341. doi: 10.1016/j.nbd.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Greggio E, et al. The Parkinson's disease–associated leucine-rich repeat kinase 2 (LRRK2) is a dimer that undergoes intra-molecular autophosphorylation. J Biol Chem. 2008;283:16906–16914. doi: 10.1074/jbc.M708718200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, et al. The chaperone activity of heat shock protein 90 is critical for maintaining the stability of leucine-rich repeat kinase 2. J Neurosci. 2008;28:3384–3391. doi: 10.1523/JNEUROSCI.0185-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dickey CA, Patterson C, Dickson D, Petrucelli L. Brain CHIP: Removing the culprits in neurodegenerative disease. Trends Mol Med. 2007;13:32–38. doi: 10.1016/j.molmed.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Qian SB, McDonough H, Boellmann F, Cyr DM, Patterson C. CHIP-mediated stress recovery by sequential ubiquitination of substrates and Hsp70. Nature. 2006;440:551–555. doi: 10.1038/nature04600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ballinger CA, et al. Identification of CHIP, a novel tetratricopeptide repeat–containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol Cell Biol. 1999;19:4535–4545. doi: 10.1128/mcb.19.6.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang J, et al. CHIP is a U-box-dependent E3 ubiquitin ligase: Identification of Hsc70 as a target for ubiquitylation. J Biol Chem. 2001;276:42938–42944. doi: 10.1074/jbc.M101968200. [DOI] [PubMed] [Google Scholar]
- 33.Connell P, et al. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat Cell Biol. 2001;3:93–96. doi: 10.1038/35050618. [DOI] [PubMed] [Google Scholar]
- 34.Meacham GC, Patterson C, Zhang W, Younger JM, Cyr DM. The Hsc70 co-chaperone CHIP targets immature CFTR for proteasomal degradation. Nat Cell Biol. 2001;3:100–105. doi: 10.1038/35050509. [DOI] [PubMed] [Google Scholar]
- 35.Smith WW, et al. Leucine-rich repeat kinase 2 (LRRK2) interacts with parkin, and mutant LRRK2 induces neuronal degeneration. Proc Natl Acad Sci U S A. 2005;102:18676–18681. doi: 10.1073/pnas.0508052102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cyr DM, Hohfeld J, Patterson C. Protein quality control: U-box–containing E3 ubiquitin ligases join the fold. Trends Biochem Sci. 2002;27:368–375. doi: 10.1016/s0968-0004(02)02125-4. [DOI] [PubMed] [Google Scholar]
- 37.Dickey CA, et al. The high-affinity HSP90–CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J Clin Invest. 2007;117:648–658. doi: 10.1172/JCI29715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neckers L. Heat shock protein 90: The cancer chaperone. J Biosci. 2007;32:517–530. doi: 10.1007/s12038-007-0051-y. [DOI] [PubMed] [Google Scholar]
- 39.Andrabi SA, et al. Poly(ADP-ribose) (PAR) polymer is a death signal. Proc Natl Acad Sci U S A. 2006;103:18308–18313. doi: 10.1073/pnas.0606526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang L, et al. Mitochondrial localization of the Parkinson's disease–related protein DJ-1: Implications for pathogenesis. Hum Mol Genet. 2005;14:2063–2073. doi: 10.1093/hmg/ddi211. [DOI] [PubMed] [Google Scholar]
- 41.Ko HS, et al. Accumulation of the authentic parkin substrate aminoacyl-tRNA synthetase cofactor, p38/JTV-1, leads to catecholaminergic cell death. J Neurosci. 2005;25:7968–7978. doi: 10.1523/JNEUROSCI.2172-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.