Abstract
Invasive fungal infections are a leading cause of mortality among immunocompromised individuals. Treatment is notoriously difficult with the limited armamentarium of antifungal drugs, whose efficacy is compromised by host toxicity, a limited activity spectrum, or the emergence of drug resistance. We previously established that the molecular chaperone Hsp90 enables the emergence and maintenance of fungal drug resistance. For the most prevalent fungal pathogen of humans, Candida albicans, Hsp90 mediates resistance to azoles, which inhibit ergosterol biosynthesis and are the most widely deployed antifungals in the clinic. For the emerging opportunistic pathogen Aspergillus terreus, Hsp90 is required for basal resistance to echinocandins, which inhibit β(1, 3)-glucan synthesis and are the only new class of antifungals to reach the clinic in decades. Here, we explore the therapeutic potential of Hsp90 inhibitors in fungal disease using a tractable host-model system, larvae of the greater wax moth Galleria mellonella, and a murine model of disseminated disease. Combination therapy with Hsp90 inhibitors that are well tolerated in humans and an azole rescued larvae from lethal C. albicans infections. Combination therapy with an Hsp90 inhibitor and an echinocandin rescued larvae from infections with the most lethal mold, Aspergillus fumigatus. In a murine model of disseminated candidiasis, genetic compromise of C. albicans HSP90 expression enhanced the therapeutic efficacy of an azole. Thus, harnessing Hsp90 provides a much-needed strategy for improving the treatment of fungal disease because it enhances the efficacy of existing antifungals, blocks the emergence of drug resistance, and exerts broad-spectrum activity against diverse fungal pathogens.
Keywords: antifungal, Aspergillus fumigatus, azole, Candida albicans, drug resistance
Invasive fungal infections are a leading cause of human mortality worldwide, especially among immunocompromised individuals. The frequency of fungal infections continues to increase in concert with the growing immunocompromised patient population, including individuals infected with HIV as well as those undergoing chemotherapy, major surgery, or transplantation of solid organs or hematopoietic stem cells (1, 2). The economic burden of invasive fungal disease is staggering, with conservative estimates of $2.6 billion annually in the United States alone (2). Chief among the opportunistic fungal pathogens are Candida albicans and Aspergillus fumigatus, the leading causal agents of mycotic death in patients with cancer and neutropenia (3). C. albicans is the fourth most common cause of hospital-acquired infectious disease and the primary cause of systemic candidiasis, with mortality rates approaching 50% (4). A. fumigatus is the primary causal agent of invasive aspergillosis and reigns as the most deadly mold, with mortality rates up to 90% (5), and remaining at 40% with state-of-the-art antifungal therapy (6).
Treatment of invasive fungal infections is notoriously difficult. There are a limited number of antifungal drugs with highly selective toxicity to fungi, in large part because of the close evolutionary relationships fungi share with their human hosts (7–9). Three of the 5 classes of antifungals in clinical use target the biosynthesis or function of ergosterol, the major sterol in fungal membranes (8–10). One class inhibits synthesis of nucleic acids, while the remaining class inhibits synthesis of β(1, 3)-glucan, a key component of the fungal cell wall (8–10). This paucity of targets is problematic, given the prevalence of cross-resistance to all drugs with a common target. The efficacy of most antifungals currently deployed is compromised by static rather than cidal activities that block fungal growth but do not eradicate the pathogen population. Therapeutic challenges are exacerbated by the frequent emergence of drug resistance in pathogen populations (8–10), demanding the development of new therapeutic strategies for fungal infectious disease.
We previously established that Hsp90, an essential molecular chaperone that regulates the form and function of many key signal transducers (11–13), enables the emergence and maintenance of drug resistance in diverse fungal species (14, 15). For the most prevalent fungal pathogen of humans, C. albicans, Hsp90 mediates resistance to the azoles, which inhibit ergosterol biosynthesis and are the most widely used class of antifungals in the clinic. Pharmacological inhibition of Hsp90 blocks the emergence of azole resistance and abrogates resistance of laboratory mutants and strains that evolved resistance in a human host (14, 15). For the emerging opportunistic pathogen Aspergillus terreus, separated from C. albicans by ≈1 billion years of evolution (16), Hsp90 inhibitors reduce resistance to echinocandins, which inhibit β(1, 3)-glucan synthesis and are the only new class of antifungal drug to reach the clinic in decades (15). Hsp90's role in drug resistance is to enable specific cellular signaling required for crucial responses to cellular stress induced by drug exposure, such as changes in the composition of cell membranes and cell walls (8). Several small-molecule Hsp90 inhibitors are currently in clinical or late-stage preclinical investigation for anticancer activity (17–19). Our in vitro studies demonstrate that at concentrations that are well tolerated in humans, Hsp90 inhibitors prevent the emergence of drug resistance and render existing antifungals more efficacious (14, 15), suggesting that Hsp90 may provide a unique therapeutic target for improving the outcome of patients with life-threatening fungal disease.
Here, we explored the therapeutic potential of harnessing Hsp90 function in the treatment of fungal disease using 2 distinct host-model systems. We used a tractable invertebrate model, the greater wax moth Galleria mellonella, to study both candidiasis and aspergillosis. G. mellonella larvae have been used as a model for pathogenicity studies of fungi, including C. albicans, A. fumigatus, and Cryptococcus neoformans (20–22). There is a strong correlation between virulence in this model and virulence in mice and, furthermore, the efficacy of antifungal therapies in the larvae correlates well with efficacy in humans. Using the G. mellonella model for C. albicans pathogenesis, we demonstrated profound therapeutic benefit of combining the most widely used azole, fluconazole (FL), with clinically relevant Hsp90 inhibitors that are structurally related to the natural product geldanamycin (GdA). At drug exposures with minimal activity on their own, the Hsp90 inhibitors 17-(allylamino)-17-demethoxygeldanamycin (17-AAG) and 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (17-DMAG) dramatically increased the efficacy of FL against C. albicans. In vitro, GdA reduced A. fumigatus resistance to the echinocandin caspofungin (CS), as was the case for Aspergillus terreus (15). In the G. mellonella model, combination therapy with GdA and CS improved survival of larvae with A. fumigatus infections that were lethal, despite monotherapy with either agent. Finally, in a murine model of C. albicans disseminated disease (23, 24), genetic compromise of fungal Hsp90 expression markedly enhanced the therapeutic efficacy of FL in a mammalian host. Our results establish Hsp90 as an attractive target for the development of powerful, broadly effective combination therapy strategies against life-threatening fungal disease.
Results
Hsp90 Inhibitors That Are Well Tolerated in Humans, 17-AAG and 17-DMAG, Have Synergistic Antifungal Activity with FL Against C. albicans.
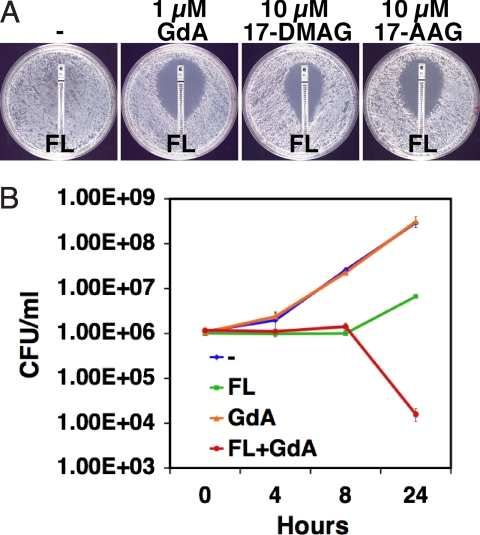
To achieve the greatest clinical relevance, we selected 2 Hsp90 inhibitors in clinical development as anticancer agents, 17-AAG and 17-DMAG. While these molecules are structurally very similar to GdA, they have different pharmacological properties, which may influence their ability to access Hsp90 in the cytosol of different fungal species. These Hsp90 inhibitors bind with high affinity to Hsp90's unusual ATP binding pocket, thereby inhibiting ATP-dependent chaperone function (18, 25). To determine if the synergy that we previously identified with GdA and azoles (14, 15) was also observed with 17-AAG and 17-DMAG, we tested their impact on FL resistance of a C. albicans clinical isolate recovered from an HIV-infected patient during treatment with FL (Fig. 1A). On a defined medium that is most relevant to host conditions, both 17-AAG and 17-DMAG reduced FL resistance without detectable effect on growth on their own (see Fig. 1A). A lower concentration of GdA was required to achieve a comparable effect. The Hsp90 inhibitors also demonstrated synergistic antifungal activity with azoles on rich medium (data not shown). Thus, Hsp90 inhibitors that are well tolerated in humans can abrogate C. albicans azole resistance in culture.
Fig. 1.
Pharmacological inhibition of Hsp90 enhances the efficacy of FL, generating a fungicidal drug combination. (A) Hsp90 inhibitors reduce FL-resistance of C. albicans clinical isolate CaCi-2 on defined RPMI medium. Antifungal test strips (Etest, AB Biodisk) produce a gradient of drug concentration with the highest at the top. Plates contained vehicle control (DMSO), GdA, 17-DMAG, or 17-AAG, as indicated. (B) Inhibition of Hsp90 is fungicidal in combination with FL. Cells were cultured in rich medium with FL (10 μg/ml) or GdA (5 μM), as indicated. Growth and viability was measured by serial dilution and plating. Plotted are means for duplicate cultures and SDs.
Inhibition of Hsp90 Function Converts the Fungistatic Activity of FL into a Fungicidal Combination.
Azoles are fungistatic against C. albicans and inhibit growth but do not eradicate fungal burden, creating conditions that favor the emergence of drug resistance. To determine whether Hsp90 inhibitors are fungicidal in combination with FL, we performed viability assays. Inhibition of Hsp90 with GdA had no impact on growth or viability of a clinical isolate relative to the untreated control (Fig. 1B). Treatment with FL resulted in an initial growth arrest followed by a modest increase in viable cell number. The combination of FL and GdA completely blocked growth and was accompanied by dramatic reduction of viability (see Fig. 1B). Thus, inhibition of Hsp90 creates a fungicidal combination with FL.
Combination Therapy with Hsp90 Inhibitors and FL Rescues G. mellonella Infected with a Lethal Dose of C. albicans.
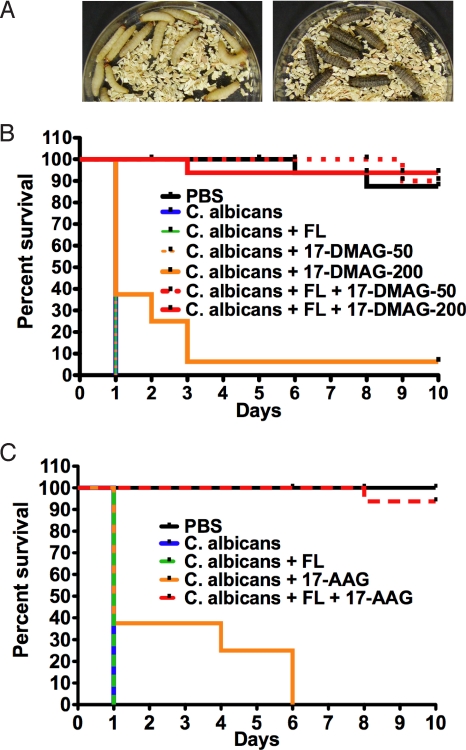
To evaluate the benefits of combination therapy, we used a stringent model involving injection of a large fungal burden that results in rapid killing of larvae. Healthy larvae are light colored and active, while larvae killed by C. albicans are dark and immobile (Fig. 2A), facilitating scoring for killing assays. Injection of control PBS had no effect on survival of larvae (Fig. 2B). Injection of 107 cells of a C. albicans clinical isolate caused 100% death within 1 day, demanding potent and rapid therapeutic effects. Treatment of infected larvae with FL alone at 14 mg/kg had no effect on survival (see Fig. 2B), as expected, because of the FL resistance of the isolate (see Fig. 1A). Treatment with 17-DMAG on its own at 50 mg/kg had no effect on survival (see Fig. 2B), while combination therapy with these doses of 17-DMAG and FL resulted in complete rescue (P < 0.0001, Log-rank test). Notably, treatment with 200 mg/kg of 17-DMAG on its own improved survival relative to the untreated larvae (P = 0.0075), but the benefit of combination therapy relative to monotherapy remained striking (P < 0.0001). Consistent with these results, we observed a modest therapeutic benefit of 17-AAG on its own at 100 mg/kg relative to treatment with FL (Fig. 2C) (P = 0.0075), and a complete rescue with the combination of 17-AAG and FL (see Fig. 2C). Thus, combination therapy with Hsp90 inhibitors that are well tolerated in humans and an azole rescues lethal C. albicans infections in an invertebrate model.
Fig. 2.
Pharmacological inhibition of Hsp90 enhances the therapeutic efficacy of FL in the Galleria mellonella host-model system. (A) G. mellonella larvae are light colored when healthy and darken when killed by fungal infection. (B) The Hsp90 inhibitor in clinical development 17-DMAG shows profound therapeutic benefits in combination with FL in the G. mellonella model of C. albicans pathogenesis. Sixteen larvae per treatment group were infected with 107 cells of CaCi-2. Larvae were treated with 17-DMAG (50 or 200 mg/kg) or with FL (14 mg/kg) once immediately after infection, as indicated, and were scored daily. (C) The Hsp90 inhibitor in clinical development 17-AAG shows profound therapeutic benefits in combination with FL in the G. mellonella model. Sixteen larvae per treatment group were infected with 107 cells of CaCi-2. Larvae were treated with 17-AAG (100 mg/kg) or with FL (14 mg/kg) once after infection, as indicated, and scored daily.
Hsp90 Inhibitors Reduce Caspofungin Resistance of A. fumigatus.
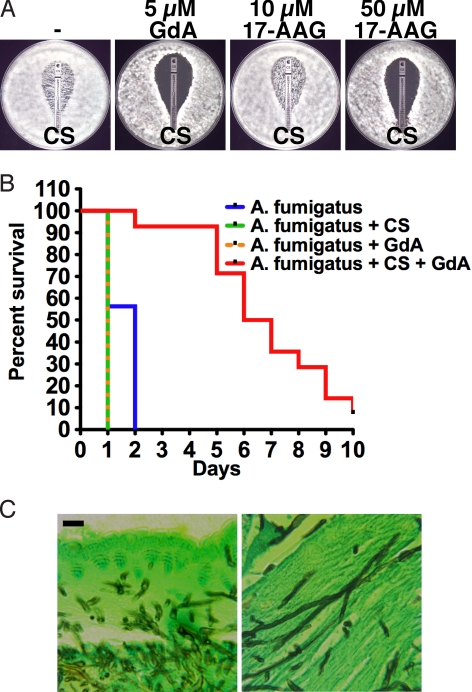
Given that A. fumigatus is among the leading fungal killers of humans and that curative treatment of aspergillosis remains a major challenge, we next sought to determine if Hsp90 inhibitors enhance the efficacy of relevant antifungals. Based on our work with A. terreus (15), we postulated that Hsp90 inhibitors would have synergistic effects with echinocandins. Echinocandins have static activity against A. fumigatus such that growth persists even at high drug concentrations (Fig. 3A). We tested the impact of Hsp90 inhibition on resistance of an A. fumigatus clinical isolate to the echinocandin CS. Inhibition of Hsp90 with GdA enhanced the efficacy of CS on both defined and rich media (see Fig. 3A and Fig. S1). Hsp90 inhibition also enhanced the efficacy of voriconazole (VO), a newer generation azole with activity against A. fumigatus, on defined medium but had little effect on rich medium (see Fig. S1). Thus, inhibition of Hsp90 enhances the activity of antifungal drugs against A. fumigatus.
Fig. 3.
Pharmacological inhibition of Hsp90 enhances the efficacy of CS both in vitro and in the Galleria mellonella host-model system. (A) Hsp90 inhibition reduces CS resistance of an A. fumigatus clinical isolate on defined RPMI medium. Antifungal test strips (Etest, AB Biodisk) produce a gradient of drug concentration with the highest at the top. Plates contained vehicle control (DMSO), GdA, or 17-AAG, as indicated. (B) The Hsp90 inhibitor GdA exerts therapeutic benefits in combination with CS in the G. mellonella model of A. fumigatus pathogenesis. Sixteen G. mellonella larvae per treatment group were infected with 107 conidia of a clinical isolate. Larvae were treated with GdA (100 mg/kg) or with CS (1.5 mg/kg) once immediately after infection, as indicated, and were scored daily. (C) A. fumigatus conidia germinate into hyphae during infection of G. mellonella. Tissue sections were taken 24 h after infection, silver stained to identify the fungus, and examined at 100× magnification using a Zeiss Axiovert microscope. (Scale bar, 10 μm.)
Combination Therapy with an Hsp90 Inhibitor and CS Rescues G. mellonella Larvae Infected with a Lethal Dose of A. fumigatus.
For the in vivo studies, we first set out to determine if clinically relevant Hsp90 inhibitors abrogate the basal echinocandin tolerance of A. fumigatus. GdA enhanced the efficacy of CS at a concentration of 5 μM, while even at 10 μM, 17-DMAG and 17-AAG had little effect (see Fig. 3A and data not shown). A higher concentration of 17-AAG (50 μM), however, was sufficient reduce CS resistance. The potent synergy between GdA and CS validated testing the benefits of combination therapy in vivo in the G. mellonella model. Injection of 107 conidia of the clinical isolate resulted in 100% death of larvae within 2 days (Fig. 3B). The slower killing compared to C. albicans may be because of the time required for conidia to germinate into growing hyphae, consistent with recent findings that inducing A. fumigatus germination before injection into Galleria increases virulence (22). Tissue sections of infected larvae 24 h after injection revealed extensive hyphal growth (Fig. 3C). Treatment with either CS or GdA alone had no therapeutic benefit (see Fig. 3B). The slightly faster killing compared to untreated controls may reflect additional stress because of multiple injections. The combination of GdA and CS markedly improved survival of larvae relative to those receiving monotherapy with either agent (P < 0.0001).
Genetic Compromise of HSP90 Expression Enhanced the Therapeutic Efficacy of FL in a Murine Model of Disseminated C. albicans Disease.
Evaluating the therapeutic potential of Hsp90 inhibitors requires careful testing in a mammalian model to address complexities of immune responses, drug metabolism, and potential toxicity issues. Initial experiments in mice indicated that inhibition of host Hsp90 is not well tolerated in the context of an acute fungal infection (data not shown). Because fungal-specific Hsp90 inhibitors have not yet been identified, we turned to genetic regulation of C. albicans HSP90 to establish proof of principle.
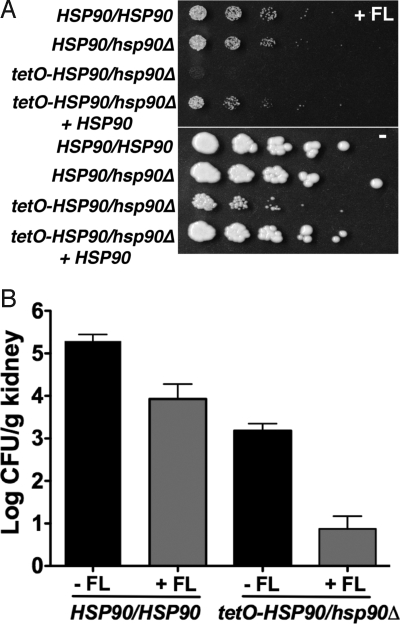
We engineered a strain in which the only HSP90 allele is under the control of a tetracycline-repressible promoter (tetO). In the absence of tetracycline, this strain has comparable Hsp90 levels to a strain in which the only HSP90 allele is under the control of the native stress-inducible promoter (data not shown). While expression of HSP90 from the native promoter is induced in response to an increase in temperature or in response to FL exposure, expression of HSP90 from the tetO promoter is not (Fig. S2). The tetO-HSP90/hsp90Δ strain is hypersensitive to FL in vitro compared to a strain with its only HSP90 allele controlled by the native promoter (Fig. 4A). Restoring a wild-type HSP90 allele to the tetO-HSP90/hsp90Δ strain complements the FL-hypersensitivity (see Fig. 4A).
Fig. 4.
Genetic compromise of C. albicans HSP90 renders FL more efficacious both in vitro and in a murine model of disseminated disease. (A) Compromising HSP90 expression in the tetO-HSP90/hsp90Δ strain results in FL-hypersensitivity, even in the absence of tetracycline. Fivefold dilutions of cells (from 1 × 106/ml) were spotted on YPD with FL (1.5 μg/ml) (Top) and without FL (Bottom). (B) Compromising C. albicans HSP90 expression enhances the therapeutic efficacy of FL in a murine model of disseminated disease. CD1 mice were infected with an inoculum of 200 μl of 1 × 106 CFU/ml of a strain expressing wild-type HSP90 levels. Inoculum for the strain with its only HSP90 allele regulated by tetO was 200 μl of 1 × 107cells/ml, corresponding to ≈4-fold higher CFU/ml compared to the wild type (see Materials and Methods). FL was administered at 2-mg/kg i.p. at 1 h after infection and then daily, as indicated.
For maximal sensitivity to detect antifungal drug efficacy, we used a well-established murine model in which fungal inoculum is delivered by tail-vein injection and progresses from the bloodstream to deep-seated infection of major organs, such as the kidney, coupled with quantitative analysis of kidney fungal burden (23). Mice infected with the tetO-HSP90/hsp90Δ strain demonstrated significantly reduced fungal burden relative to those infected with a strain expressing wild-type HSP90 levels (P < 0.001, Kruskal-Wallis) (Fig. 4B). There was no significant difference in fungal burden between mice infected with a strain expressing wild-type HSP90 levels or an HSP90 heterozygote (data not shown). Treatment of mice with FL reduced fungal burden in all cases (P < 0.001) (see Fig. 4B). FL was significantly more efficacious in mice infected with the tetO-HSP90/hsp90Δ strain compared to the wild type, with a 3.6-fold and 1.4-fold reduction in fungal burden compared to untreated counterparts, respectively (see Fig. 4B) (P < 0.01, for posthoc comparison between tetO-HSP90/hsp90Δ with and without FL, versus P > 0.05 for posthoc comparison between wild type with and without FL, Dunn's Multiple Comparison). Tetracycline-mediated transcriptional repression of HSP90 resulted in complete clearance of the infection (Shapiro RS, Uppuluri P, A.K.Z., C.C., Senn H, J.R.P., Heitman J, and L.E.C., submitted). These findings establish proof of principle in a mammalian model that Hsp90 provides an attractive therapeutic target for fungal disease.
Discussion
Ideally, a new therapeutic strategy for fungal infectious disease would enhance the efficacy of existing antifungal drugs, block the emergence of drug resistance, have fungicidal activity, and demonstrate broad efficacy against diverse fungal pathogens. We have established that harnessing fungal Hsp90 meets all of these criteria and has profound therapeutic benefits in combination with antifungal drugs in 2 well-established metazoan models of fungal disease.
Hsp90 regulates antifungal drug resistance of the 2 leading fungal pathogens of humans separated by ≈1 billion years of evolution. For C. albicans, Hsp90's role in regulating cellular signaling required to survive the membrane stress exerted by azoles is now well established (see Fig. 1A and refs. 8, 14, and 15). Compromising Hsp90 function blocks the de novo emergence of azole resistance and abrogates resistance that has already evolved. Principal limitations of the clinical efficacy of azoles include their fungistatic action against Candida species and their vulnerability to the rapid emergence of resistance in these pathogens. We demonstrate that in combination with Hsp90 inhibition, the fungistatic azoles become fungicidal (see Fig. 1B). This broadens the therapeutic implications of combination therapy with Hsp90 inhibitors to encompass benefits of both reversing antifungal drug resistance and enhancing azole efficacy against drug-sensitive isolates. Consistent with previous findings with A. terreus, the most striking synergy with Hsp90 inhibitors against A. fumigatus is with the echinocandins (see Fig. 3 and Fig. S1). The specificities of synergistic interactions of Hsp90 inhibitors with antifungal drugs likely reflect the key cellular circuitry underpinning responses to drug-induced stress. A key mediator of Hsp90-dependent azole resistance in C. albicans is the protein phosphatase calcineurin, which regulates crucial cellular responses to the membrane stress exerted by azoles (8, 9, 14, 15, 26, 27). With Aspergillus, Hsp90's role in echinocandin resistance again tracks with calcineurin's central function in mediating crucial responses to the cell wall stress exerted by echinocandins (28, 29). These findings support a broader paradigm of targeting regulators of signaling and cellular stress responses in the treatment of fungal disease.
Paradoxically, it is Hsp90's role as a key regulator of cellular signaling and stress response that positions it as an ideal therapeutic target and that complicates its utility in therapeutic interventions. Hsp90 is essential in all eukaryotes tested and highly conserved, such that the human protein can complement deletion of genes encoding Hsp90 in yeast (30). While structurally diverse Hsp90 inhibitors are in clinical development as anticancer agents and are well tolerated in humans (17–19), Hsp90 is intimately connected with cellular circuitry regulating host immune and stress responses important in the context of microbial infection. For example, drugs such as cyclosporin A and FK506, which target the Hsp90 client protein calcineurin, are potent immunosuppressants; they abrogate fungal drug resistance but render patients more susceptible to fungal infection, limiting their utility in antifungal therapy (29).
We found that the challenge of translating in vitro results into model systems in vivo was coupled with complexity of the system. As predicted by in vitro data, combination therapy with an Hsp90 inhibitor and an antifungal drug rescues lethal C. albicans and A. fumigatus infections in the invertebrate host G. mellonella (see Figs. 2 and 3). The lack of complications because of host toxicity may reflect the relative simplicity of this organism's immunological function and physiology. Preliminary experiments in a mouse model of disseminated C. albicans infection using 2 structurally unrelated Hsp90 inhibitors revealed significant toxicity associated with inhibition of host Hsp90 in the context of acute fungal infection and precluded detection of a benefit to combination therapy on survival. Consequently, we turned to a genetic system to regulate HSP90. Compromising C. albicans HSP90 expression renders FL more efficacious both in vitro and in a mouse model of disseminated disease (see Fig. 4). Hypersensitivity to FL is not a general characteristic of strains with reduced growth rates. Thus, these results establish that genetic compromise of C. albicans Hsp90 expression renders FL more efficacious. Further genetic depletion of C. albicans HSP90 results in complete clearance of the fungal infection in the mouse model of disseminated disease.
We note that a recombinant antibody against C. albicans Hsp90 demonstrated therapeutic benefits in a clinical trial in combination with the antifungal drug amphotericin B, which targets ergosterol (31). However, this must work via a mechanism distinct from pharmacological inhibition: the antibody would not be able to cross the fungal cell wall and gain access to the fungal cytosol where the Hsp90 inhibitors exert their effects. Instead, Hsp90 antibodies likely affect some aspect of host immune responses to the pathogen. Indeed, heat-shock proteins provide immunodominant antigens for recognition of many pathogens and play a central role in mediating both innate and adaptive immune responses (32, 33).
Given its function in chaperoning key regulators of the cellular circuitry governing many aspects of organismal biology, Hsp90 has center stage as a therapeutic target for diverse diseases, including cancer and neurodegeneration. In the context of cancer, the clinical development of Hsp90 inhibitors is moving rapidly. New chemotypes with improved pharmacology have been identified and their activity is being tested in clinical trials either alone or in combination with other agents against diverse malignancies (17–19). Our findings in mice that current broadly active Hsp90 inhibitors can have adverse effects in the context of acute C. albicans infection highlight the need to identify fungal-selective inhibitors and advise great caution in the treatment of patients with active fungal infection. In the process of developing anticancer Hsp90 inhibitors, extensive compound collections have been generated that could provide a rich, as yet unexploited resource for the identification of potentially fungal-selective inhibitors of the classical Hsp90 N-terminal ATPase site. Such selectivity could be achieved based on divergence between fungal and human Hsp90 orthologs in terms of sequence, ATPase activity, or equilibrium of conformational states (34). Indeed, we recently conducted a screen in Saccharomyces cerevisiae for Hsp90 pathway inhibitors and recovered compounds that block maturation of diverse Hsp90 clients in yeast but not mammalian cells. Biochemical analysis revealed a unique mechanism of action for one class of hits in that they do not compete at the conserved ATP-binding pocket targeted by classical inhibitors. Genetic analysis confirms, however, that they work by modulating function of the Hsp90 chaperone system (L.W., C. McLellan, and S.L., unpublished data).
Hsp90 inhibitors may provide an even broader therapeutic paradigm for the treatment of life-threatening infectious diseases caused by eukaryotes other than fungi. One key mediator of Hsp90-dependent drug resistance is the client protein calcineurin (14, 15). Inhibitors of Hsp90 and calcineurin both possess potent antimalarial activity, thus extending their impact to the protozoan parasite Plasmodium falciparum (35, 36). High-throughput genomic and proteomic studies reveal that Hsp90 interacts with up to 10% of the yeast proteome (37), while a systems analysis in P. falciparum indicates that Hsp90 interacts with a complex network of client proteins (36). Targeting a highly conserved protein that has an essential role in governing diverse cellular signaling pathways may minimize the probability of the emergence of resistance to Hsp90 inhibitors. Notably, Hsp90 inhibitors also have antiviral activity and evolutionary constraints on chaperone-mediated protein folding may provide a strategy refractory to the development of drug resistance (38). Clearly, the challenge ahead for successfully harnessing Hsp90 function in the treatment of infectious disease now lies in developing potent and specific inhibitors capable of discriminating between the chaperone machineries of pathogen and host.
Materials and Methods
Strains and Culture Conditions.
The C. albicans clinical isolate (CaCi-2) used in this study was recovered from an HIV-infected patient at an early time point during FL treatment (15, 39). Construction of the tetracycline-repressible HSP90 system is described in the SI Text. The A. fumigatus clinical isolate was from a patient at Children's Hospital in Boston, MA (CH 03–197 0861). Species identification was performed as described in the SI Text. C. albicans strains were grown in either rich YPD medium or a defined medium, RPMI, prepared according to standard protocols (15). A. fumigatus was grown on potato dextrose agar (PDA) at 35 °C to induce conidiation.
Antifungal Susceptibility Testing.
The effects of Hsp90 inhibitors on antifungal drug resistance were determined with E-test strips (AB Biodisk) on a rich medium (0.5X YPD) and a defined medium (RPMI) essentially as described (15). For C. albicans, cell densities of overnight cultures were determined by optical density at 595 nm and by hemacytometer counts; ≈105 cells were plated before application of an antifungal test strip. For A. fumigatus, conidia were prepared by suspension in PBS, the concentration was determined by hemacytometer count, and ≈105 conidia were plated before application of a test strip. GdA, 17-AAG, and 17-DMAG were obtained from Invivogen; drug stocks were prepared in DMSO (Sigma Aldrich Co.). Plates were photographed after 48 h at 35 °C in the dark.
Viability Assay.
Duplicate cultures for each condition were grown in liquid YPD at 30 °C with constant agitation. The starting inoculum was normalized to 1 × 106 cells/ml; samples were removed at the indicated times and CFU counts assessed by serial dilution and plating.
G. mellonella Killing Assay.
Injection of fungal pathogens and antifungal drugs was performed essentially as described (21). In brief, larvae in the final instar were obtained from Vanderhorst, Inc.. Sixteen larvae (330 ± 25 mg) were used per group. Each injection of 10 μl of fungus, drug, or control into the hemocoel was performed via a distinct proleg. Drugs were injected individually after inoculum delivery. Larvae were incubated at 37 °C and the number of dead larvae scored daily. C. albicans inoculum was prepared by growing 50-ml YPD cultures overnight at 30 °C. Cells were pelleted at 1,308 × g for 10 min followed by 3 washes in PBS. Cell densities were determined by hemacytometer count and dilutions prepared in PBS. A. fumigatus inoculum was prepared in PBS by suspending conidia from PDA. Drug stocks were prepared as indicated in the SI Text. Kill curves were plotted and estimation of differences in survival (Log-rank test) analyzed by the Kaplan-Meier method (GraphPad Prism). Histology of infected larvae is described in the SI Text.
Spotting.
Strains were grown to saturation in YPD and cell concentrations were standardized based on optical density. Fivefold dilutions (from ≈1 × 106 cells/ml) were spotted on media using a spotter (Frogger, V&P Scientific, Inc). The plates were photographed after 2 days in the dark at 30 °C.
Murine Model of C. albicans Infection.
C. albicans was cultured from frozen stocks on Sabouraud agar and incubated at 35 °C for 24 h (48 h for tetO-HSP90/hsp90Δ because of slower growth). Multiple colonies were suspended in PBS to a concentration of 1 × 106 cells/ml and 150 μl was inoculated into Sabouraud broth and incubated for 15 h at 37 °C at 250 rpm (Thermo Fischer Scientific Inc., Forma Model 490 Incubator Shaker). Cultures were centrifuged at 234 × g for 15 min, washed with PBS 3 times and diluted to the desired concentration (1 × 106 cells/ml) by hemacytometer counting. Concentration was verified by serial dilution and culture. We observed discordance between cell counts and CFU measurements for the tetO-HSP90/hsp90Δ strain, such that CFU values were ≈60% lower than expected. A 10-fold increase in inoculum concentration for tetO-HSP90/hsp90Δ (1 × 107 cells/ml) produced ≈4-fold higher CFU/ml than the inoculum for the wild type, and thus was chosen as the dose of tetO-HSP90/hsp90Δ for these experiments (see SI Text).
For murine exposure, male CD1 mice (Charles River Laboratories) aged 8 weeks (30–34 g) were infected via the tail vein with 200 μl of a 1 × 106 CFU/ml PBS suspension for the wild type (n = 13 mice for untreated and 7 for FL treated), an inoculum previously determined to produce morbidity but not mortality when using strain SC5314 at 4 days following injection. The inoculum for tetO-HSP90/hsp90Δ was 200 μl of a 1 × 107 cells/ml PBS suspension which corresponds to an ≈4-fold increase in CFU/ml relative to the wild type (n = 22 mice for untreated and 10 for FL treated). For groups receiving FL (Pfizer, Inc.), 2 mg/kg was administered i.p. at 1 h after infection and then daily. Mice were observed 3 times daily for signs of illness and weighed daily. At day 4 following injection, mice were killed by CO2 asphyxiation and the left kidney was removed aseptically, placed in PBS, homogenized via bead beating using a FastPrep 120 (QBiogene) and serial dilutions plated for determination of kidney fungal burden. CFU values in kidneys were expressed as CFU/g of tissue, log-transformed and compared using a Kruskal-Wallis test with posthoc testing of significance between groups by Dunn's Multiple Comparison Test (GraphPad Prism). All murine work was performed under a protocol approved by the Institutional Animal Use and Care Committee at Duke University Medical Center.
Supplementary Material
Acknowledgments.
We thank Eileen Gorss for A. fumigatus isolates, Sara Vargas for histopathology, and Rochelle Bagatell for caspofungin. L.E.C. was supported by Damon Runyon Cancer Research Foundation Grant DRG-1765–03 and a Genzyme Fellowship, and is now supported by a Career Award in the Biomedical Sciences from the Burroughs Wellcome Fund, by a Canada Research Chair in Microbial Genomics and Infectious Disease, and by Canadian Institutes of Health Research Grant MOP-86452. S.D.S. is supported by a University of Toronto Open Fellowship; A.K.Z. is supported by National Institutes of Health/National Institute of Allergies and Infectious Diseases Grant K08AI065837–04; and J.R.P. is supported by Public Health Service Grants AI73896 and AI28388. S.L. is supported by the G. Harold and Leila Y. Mather Charitable Foundation and by the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813394106/DCSupplemental.
References
- 1.Enoch DA, Ludlam HA, Brown NM. Invasive fungal infections: a review of epidemiology and management options. J Med Microbiol. 2006;55:809–818. doi: 10.1099/jmm.0.46548-0. [DOI] [PubMed] [Google Scholar]
- 2.Wilson LS, et al. The direct cost and incidence of systemic fungal infections. Value Health. 2002;5:26–34. doi: 10.1046/j.1524-4733.2002.51108.x. [DOI] [PubMed] [Google Scholar]
- 3.Segal BH, et al. Prevention and early treatment of invasive fungal infection in patients with cancer and neutropenia and in stem cell transplant recipients in the era of newer broad-spectrum antifungal agents and diagnostic adjuncts. Clin Infect Dis. 2007;44:402–409. doi: 10.1086/510677. [DOI] [PubMed] [Google Scholar]
- 4.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh N, Paterson DL. Aspergillus infections in transplant recipients. Clin Microbiol Rev. 2005;18:44–69. doi: 10.1128/CMR.18.1.44-69.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herbrecht R, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408–415. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 7.Baldauf SL, Roger AJ, Wenk-Siefert I, Doolittle WF. A kingdom-level phylogeny of eukaryotes based on combined protein data. Science. 2000;290:972–977. doi: 10.1126/science.290.5493.972. [DOI] [PubMed] [Google Scholar]
- 8.Cowen LE. The evolution of fungal drug resistance: modulating the trajectory from genotype to phenotype. Nat Rev Microbiol. 2008;6:187–198. doi: 10.1038/nrmicro1835. [DOI] [PubMed] [Google Scholar]
- 9.Cowen LE, Steinbach WJ. Stress, drugs, and evolution: the role of cellular signaling in fungal drug resistance. Eukaryotic cell. 2008;7:747–764. doi: 10.1128/EC.00041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson JB. Evolution of antifungal-drug resistance: mechanisms and pathogen fitness. Nat Rev Microbiol. 2005;3:547–556. doi: 10.1038/nrmicro1179. [DOI] [PubMed] [Google Scholar]
- 11.Pearl LH, Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem. 2006;75:271–294. doi: 10.1146/annurev.biochem.75.103004.142738. [DOI] [PubMed] [Google Scholar]
- 12.Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med. 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- 13.Wandinger SK, Richter K, Buchner J. The Hsp90 chaperone machinery. J Biol Chem. 2008;283:18473–18477. doi: 10.1074/jbc.R800007200. [DOI] [PubMed] [Google Scholar]
- 14.Cowen LE, Carpenter AE, Matangkasombut O, Fink GR, Lindquist S. Genetic architecture of Hsp90-dependent drug resistance. Eukaryot Cell. 2006;5:2184–2188. doi: 10.1128/EC.00274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cowen LE, Lindquist S. Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science. 2005;309:2185–2189. doi: 10.1126/science.1118370. [DOI] [PubMed] [Google Scholar]
- 16.Heckman DS, et al. Molecular evidence for the early colonization of land by fungi and plants. Science. 2001;293:1129–1133. doi: 10.1126/science.1061457. [DOI] [PubMed] [Google Scholar]
- 17.Taldone T, Gozman A, Maharaj R, Chiosis G. Targeting Hsp90: small-molecule inhibitors and their clinical development. Curr Opin Pharmacol. 2008;8:370–374. doi: 10.1016/j.coph.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 19.Workman P, Burrows F, Neckers L, Rosen N. Drugging the cancer chaperone HSP90: Combinatorial therapeutic exploitation of oncogene addiction and tumor stress. Ann N Y Acad Sci. 2007;1113:202–216. doi: 10.1196/annals.1391.012. [DOI] [PubMed] [Google Scholar]
- 20.Brennan M, Thomas DY, Whiteway M, Kavanagh K. Correlation between virulence of Candida albicans mutants in mice and Galleria mellonella larvae. FEMS Immunol Med Microbiol. 2002;34:153–157. doi: 10.1111/j.1574-695X.2002.tb00617.x. [DOI] [PubMed] [Google Scholar]
- 21.Mylonakis E, et al. Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect Immun. 2005;73:3842–3850. doi: 10.1128/IAI.73.7.3842-3850.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Renwick J, Daly P, Reeves EP, Kavanagh K. Susceptibility of larvae of Galleria mellonella to infection by Aspergillus fumigatus is dependent upon stage of conidial germination. Mycopathologia. 2006;161:377–384. doi: 10.1007/s11046-006-0021-1. [DOI] [PubMed] [Google Scholar]
- 23.Blankenship JR, et al. Calcineurin is essential for Candida albicans survival in serum and virulence. Eukaryot Cell. 2003;2:422–430. doi: 10.1128/EC.2.3.422-430.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filler SG, Kullberg BJ. In: Candida and Candidiasis. Calderone RA, editor. Washington, DC: ASM Press; 2002. pp. 341–348. [Google Scholar]
- 25.Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci USA. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blankenship JR, et al. Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J. 2002;21:546–559. doi: 10.1093/emboj/21.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanglard D, Ischer F, Marchetti O, Entenza J, Bille J. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol Microbiol. 2003;48:959–976. doi: 10.1046/j.1365-2958.2003.03495.x. [DOI] [PubMed] [Google Scholar]
- 28.Steinbach WJ, et al. Calcineurin inhibition or mutation enhances cell wall inhibitors against Aspergillus fumigatus. Antimicrob Agents Chemother. 2007;51:2979–2981. doi: 10.1128/AAC.01394-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinbach WJ, Reedy JL, Cramer RA, Jr, Perfect JR, Heitman J. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat Rev Microbiol. 2007;5:418–430. doi: 10.1038/nrmicro1680. [DOI] [PubMed] [Google Scholar]
- 30.Piper PW, et al. Yeast is selectively hypersensitised to heat shock protein 90 (Hsp90)-targeting drugs with heterologous expression of the human Hsp90beta, a property that can be exploited in screens for new Hsp90 chaperone inhibitors. Gene. 2003;302:165–170. doi: 10.1016/s0378-1119(02)01102-2. [DOI] [PubMed] [Google Scholar]
- 31.Pachl J, et al. A randomized, blinded, multicenter trial of lipid-associated amphotericin B alone versus in combination with an antibody-based inhibitor of heat shock protein 90 in patients with invasive candidiasis. Clin Infect Dis. 2006;42:1404–1413. doi: 10.1086/503428. [DOI] [PubMed] [Google Scholar]
- 32.Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002;2:185–194. doi: 10.1038/nri749. [DOI] [PubMed] [Google Scholar]
- 33.Stewart GR, Young DB. Heat-shock proteins and the host-pathogen interaction during bacterial infection. Curr Opin Immunol. 2004;16:506–510. doi: 10.1016/j.coi.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Southworth DR, Agard DA. Species-dependent ensembles of conserved conformational states define the Hsp90 chaperone ATPase cycle. Mol Cell. 2008;32:631–640. doi: 10.1016/j.molcel.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar R, Musiyenko A, Barik S. Plasmodium falciparum calcineurin and its association with heat shock protein 90: mechanisms for the antimalarial activity of cyclosporin A and synergism with geldanamycin. Mol Biochem Parasitol. 2005;141:29–37. doi: 10.1016/j.molbiopara.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 36.Pavithra SR, Kumar R, Tatu U. Systems analysis of chaperone networks in the malarial parasite Plasmodium falciparum. PLoS Comp Biol. 2007;3:1701–1715. doi: 10.1371/journal.pcbi.0030168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao R, et al. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the Hsp90 chaperone. Cell. 2005;120:715–727. doi: 10.1016/j.cell.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 38.Geller R, Vignuzzi M, Andino R, Frydman J. Evolutionary constraints on chaperone-mediated folding provide an antiviral approach refractory to development of drug resistance. Genes Dev. 2007;21:195–205. doi: 10.1101/gad.1505307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White TC. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1997;41:1482–1487. doi: 10.1128/aac.41.7.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.