Abstract
HIV-1 Vpu enhances the release of virions from infected cells. Recent work identified Bst-2/CD317/tetherin as a host factor whose inhibitory activity on viral release is counteracted by Vpu. A current working model proposes that Bst-2 inhibits virus release by tethering viral particles to the cell surface. Here, we analyzed endogenous Bst-2 with respect to its effect on virus release from HeLa cells, T cells, and macrophages. We noted significant cell type-dependent variation in Bst-2 expression. Vpu caused a reduction in Bst-2 expression in transfected HeLa cells and long-term infected macrophages. However, Vpu expression did not result in cell surface down-modulation of Bst-2 or a reduction in intracellular Bst-2 expression in CEMx174 or H9 cells, yet virus replication in these cells was Vpu-responsive. Surprisingly, Bst-2 was undetectable in cell-free virions that were recovered from the surface of HeLa cells by physical shearing, suggesting that a tethering model may not explain all of the functional properties of Bst-2. Taken together we conclude that enhancement of virus release by Vpu does not, at least in CEMx174 and H9 cells, require cell surface down-modulation or intracellular depletion of Bst-2, nor does it entail exclusion of Bst-2 from viral particles.
Keywords: CD317, host restriction, tetherin, virus assembly
Vpu is an 81-aa type 1 integral membrane protein (1, 2) that has been shown to cause proteasomal degradation of CD4 (3, 4), enhance the release of virions from infected cells (5), and prevent endocytosis of nascent viral particles (6–11). These latter biological activities of Vpu are mechanistically distinct from CD4 degradation and involve different structural domains in Vpu. Vpu does not affect assembly of viral particles but facilitates the detachment of virions from the cell surface (5, 6). Several mechanisms of Vpu-mediated virus release have been proposed. First, Vpu has the ability to assemble into a cation-specific ion channel (12–16). Randomization of the Vpu transmembrane (TM) domain did not affect membrane association or the ability to induce CD4 degradation but did inhibit Vpu ion channel activity and, at the same time, impaired its ability to regulate virus release (14, 17). This suggested a functional correlation between Vpu ion channel activity and virus release. However, it remained unclear exactly how a Vpu ion channel activity might trigger increased detachment of particles from the cell surface. It also became clear that Vpu dependence of virus release was tissue-specific, suggesting the involvement of host factors in Vpu function (18, 19). Several host factors have since been implicated in the Vpu-sensitive inhibition of virus release, including Task-1 and UBP (20, 21). More recently, two additional host factors, Bst-2/CD317/tetherin/HM1.24 (22, 23) and calcium-modulating cyclophilin ligand (CAML) (24) have been correlated with Vpu-sensitive restriction of HIV-1 virus release.
Our current work focuses on the characterization of Bst-2/CD317/tetherin/HM1.24 (for simplicity referred to as Bst-2 for the remainder of this work). Bst-2 was originally identified as a membrane protein in terminally differentiated human B cells of patients with multiple myeloma (25, 26). Bst-2 is a 30- to 36-kDa type II transmembrane protein consisting of 180 aa (27). The protein has an unusual topology in that it has both an N-terminal transmembrane domain and a C-terminal glycosylphosphatidyl inositol (GPI) anchor (28). Bst-2 associates with lipid rafts at the cell surface and was identified on internal membranes, presumably the trans-Golgi network (28). However, the precise function of Bst-2 remains unknown. Bst-2 mRNA was constitutively expressed in cell types such as HeLa, Jurkat, and at low levels in CD4+ T cells but not 293T or HT1080 cells (29) and thus correlated with cell types known to depend on Vpu for efficient virus release. Bst-2 expression was inducible by IFN treatment in 293T or HT1080 cells, consistent with the previous observation that cell lines that did not normally require Vpu for efficient virus release became Vpu-dependent upon IFN treatment (22). Although Vpu had no overt effects on ectopically expressed Bst-2 levels and cellular distribution (29), Vpu clearly down-regulated cell surface expression of Bst-2 in 293T cells (23) and caused a reduction of endogenous Bst-2 expression in HeLa cells (30). Randomization of the Vpu TM domain abolished the ability of Vpu to overcome the inhibitory effects of Bst-2 on virus release (23), consistent with our previous data on the importance of the Vpu TM domain for this effect (17).
Here, we analyzed endogenous Bst-2 with respect to its effect on virus release from HeLa cells, T cells, and macrophages. We noted significant cell type-dependent variation of total intracell ular Bst-2 expression. However, with the exception of Jurkat cells, all Vpu-dependent cell types analyzed exhibited surface expression of Bst-2. Interestingly, whereas Vpu caused a reduction in Bst-2 expression in transfected HeLa cells and long-term infected macrophages, HIV-1 replication in CEMx174 and H9 cells did not result in cell surface down-modulation of Bst-2 or a reduction of intracellular Bst-2 levels; yet virus release was Vpu-responsive. Surprisingly, Bst-2 was undetectable in virions that had been removed from the surface of cells by physical agitation, an observation that is difficult to explain by the tethering model of Bst-2. We conclude that enhancement of virus release by Vpu does not require cell surface down-modulation or intracellular depletion of Bst-2, nor does it involve exclusion of Bst-2 from viral particles.
Results
Expression of Bst-2 in Primary Cells and Cell Lines Is IFN-Inducible.
To characterize endogenous expression of Bst-2 protein, we compared 293T cells, where Bst-2 is not constitutively expressed, with HeLa cells, where Bst-2 expression is constitutive (29). It has been reported that upon treatment with IFNα, 293T cells express Bst-2 mRNA (29). To investigate the effect of IFNα on the expression of endogenous Bst-2 protein, we cultured 293T and HeLa cells in the presence or absence of IFNα for 24 h and examined expression of Bst-2 by immunoblotting. As expected, Bst-2 was expressed in HeLa cells in the absence of IFNα (Fig. 1A, lane 3) but was below the level of detection in 293T cells (Fig. 1A, lane 1). Upon treatment with IFNα, Bst-2 expression was induced in 293T cells (Fig. 1A, lane 2). Surprisingly IFNα also augmented intracellular Bst-2 levels in HeLa cells (Fig. 1A, lane 4). Of note, Bst-2 appears as multiple bands on the gel with estimated sizes of 25–35 kDa presumably because of glycosylation as reported (30). FACS analysis revealed low but detectable surface expression of Bst-2 in untreated 293T cells that was strongly up-regulated after IFNα treatment [supporting information (SI) Fig. S1A]. IFNα treatment also led to increased Bst-2 surface expression in HeLa cells, consistent with the increased intracellular protein levels (Fig. S1A).
Fig. 1.
Expression of Bst-2 in primary cells and cell lines is IFN-inducible. (A) HeLa and 293T cells were cultured in the absence (lanes 1 and 3) or presence (lanes 2 and 4) of IFNα (1 ng/mL) for 24 h. Whole-cell lysates were subjected to immunoblotting by using a Bst-2-specific polyclonal rabbit antibody. The same blot was subsequently probed with a tubulin-specific antibody (tub) as internal reference for sample loading. (B) HeLa cells, MDM, and PBMC were cultured in the absence of IFNα (lanes 1, 3, and 7) or were treated for 24 h with 0.1 ng/mL (lane 4), 1 ng/mL (lanes 2, 5, and 8), or 10 ng/mL (lane 6) IFNα. For comparison, PBMC were stimulated with CD3/CD28 antibodies as described in Materials and Methods (lane 9). Whole-cell lysates were processed for immunoblotting as described in A except that actin was used as internal reference for sample loading. (C) CEMx174, A3.01, H9, and Jurkat cells were analyzed with or without prior IFNα treatment (1 ng/mL) as in A.
We next examined the level of Bst-2 expression in monocyte-derived macrophages (MDM) and peripheral blood mononuclear cells (PBMC), where we had observed varying levels of Vpu dependence for efficient virus release (31, 32). As shown in Fig. 1B, MDM expressed only low levels of Bst-2 in the absence of IFNα treatment compared with HeLa cells (Fig. 1B, compare lanes 1 and 3). However, Bst-2 expression was induced by IFNα treatment (Fig. 1B, lanes 4–6). Low levels of IFNα (0.1 ng/mL) were sufficient to induce near-maximal expression of Bst-2 in MDM (Fig. 1B, lane 4), and increasing amounts of IFNα to 1 ng/mL (Fig. 1B, lane 5) or 10 ng/mL (Fig. 1B, lane 6) had little additional benefit for Bst-2 expression. Of note, even at the highest concentration of IFNα, Bst-2 levels in MDM remained well below those observed in unstimulated HeLa cells when normalized for actin levels (Fig. 1B, compare lanes 1 and 6). Also, the overall Bst-2 modification profile seems to differ for HeLa cells, MDM, and PBMC. Our analysis of PBMC revealed that levels of Bst-2 in unstimulated cells were below the limit of detection (Fig. 1B, lane 7). Upon treatment with IFNα (1 ng/mL), Bst-2 protein could be detected in PBMC (Fig. 1B, lane 8), although at a relatively lower level than in the other cell types examined. We conclude that Bst-2 expression is low to undetectable in primary T cells and macrophages but can be induced by IFNα in all cell types examined, including HeLa cells, which already constitutively express high levels of Bst-2. However, activation of PBMC with CD3/CD28 (Fig. 1B, lane 9) did not stimulate Bst-2 expression, suggesting that Bst-2 levels remain undetectable in these cells even after T cell activation.
Analysis of Bst-2 expression in various T cell lines revealed that CEMx174 cells express high levels without IFNα stimulation (Fig. 1C, lane 1) whereas expression in H9 cells was low but IFNα-inducible (Fig. 1C, lanes 5 and 6). Bst-2 expression in A3.01 (lanes 3 and 4) and Jurkat cells (lanes 7 and 8) was near or below the limit of detection even after IFNα stimulation. This result is surprising because both A3.01 and Jurkat T cell lines were found to require Vpu for efficient virus release (1, 10). FACS analyses confirmed the absence of cell surface Bst-2 in Jurkat cells whereas H9 cells were positive for surface Bst-2 (Fig. S1B). Despite our inability to detect Bst-2 in A3.01 cells by immunoblotting, the cells were Bst-2-positive based on FACS analysis. However, the assay does not allow direct comparison of Bst-2 surface levels across cell types, and it is possible that surface expression of Bst-2 is low in A3.01 cells despite mean fluorescence intensity similar to that of CEMx174 cells (Fig. S1B).
Vpu Reduces Bst-2 Levels.
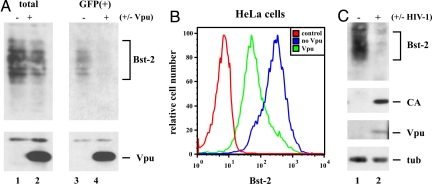
Bartee et al. (30) had noted a reduction of cellular Bst-2 levels after expression of Vpu from an adenovirus vector. To explore the possible effects of Vpu on Bst-2 expression or stability, HeLa cells were transfected with pcDNA-Vphu, a codon-optimized vector for the expression of NL4-3 Vpu (33), together with pCMV-GFP as tracer. Control cells were transfected with the GFP vector only. Whole-cell extracts were prepared 24 h after transfection from unsorted (Fig. 2A, total) or GFP-positive [Fig. 2A, GFP(+)] cells and analyzed by immunoblotting for expression of Bst-2 (Upper) or Vpu (Lower). A small reduction in Bst-2 steady-state levels was apparent in unsorted cells (Fig. 2A, lanes 1 and 2) but was more pronounced in the GFP+ population, which was enriched for Vpu-expressing cells (lanes 3 and 4). Transfected cells from Fig. 2A were also used for FACS analysis to study the effect of Vpu on cell surface expression of Bst-2 in HeLa cells (Fig. 2B). Signals were gated for GFP+ cells. Vpu induced a strong down-regulation of cell surface Bst-2 within 24 h of transfection consistent with recent data (23). Thus, when overexpressed from a codon-optimized vector, Vpu reduced both surface expression and steady-state levels of endogenous Bst-2. To analyze the effects of Vpu on Bst-2 expression under more physiological conditions, we compared Bst-2 levels of uninfected MDM with those infected for 24 days with HIV-1 Ada as described in ref. 34. Consistent with the results from HeLa cells, we noted a reduction of endogenous Bst-2 levels (Fig. 2C). Thus, Vpu has the ability to reduce steady-state expression of Bst-2 in transiently transfected HeLa cells and in HIV-infected macrophages.
Fig. 2.
Vpu reduces Bst-2 levels in transfected HeLa cells and in HIV-infected macrophages. (A) HeLa cells were transfected with 1 μg of pEGFP-N1 (Clontech) with (+) or without (−) 1 μg of pcDNA-Vphu. Total DNA was adjusted to 5 μg with empty-vector DNA. Cells were harvested 24 h after transfection and split into two fractions. One fraction was used to prepare whole-cell lysates (total); the other fraction was sorted for GFP-positive cells [GFP(+)] before cell lysis with a FACS Aria (BD Biosciences) as reported in ref. 42. Cell lysates were analyzed by immunoblotting with antibodies to Bst-2 (Upper) or Vpu (Lower). Proteins are identified on the right. (B) HeLa cells were transfected as in A, stained with Bst-2 antibodies as described in Materials and Methods, and analyzed on a FACS Calibur. Transfected cells were gated for GFP-positive cells. As a control, untransfected HeLa cells were stained with preimmune serum (control). (C) Human MDM were infected with HIV-1 Ada as described in ref. 34 (lane 2). Uninfected macrophages were cultured in parallel as a control (lane 1). Cells were harvested 24 days after infection, and whole-cell lysates were analyzed by immunoblotting for Bst-2, viral capsid protein (CA), or Vpu. The blot was subsequently reprobed with tubulin-specific antibody to control for sample loading.
Replication of HIV-1 in CEMx174 and H9 Cells Is Vpu-Responsive but Does Not Necessitate Bst-2 Down-Regulation.
To investigate the effect of Vpu on Bst-2 during acute infection of T cells, we first chose the CEMx174 cell line because these cells were highly susceptible to HIV infection and had the highest steady-state level of Bst-2 among the cell lines tested (Fig. 1C). We also wanted to examine further our previous conclusions on the importance of the Vpu TM domain, which we found to be important for enhanced virus release, and two phosphoserine residues in the cytoplasmic domain, which we had found to be critical for CD4 degradation but negligible for the virus release function of Vpu (17). CEMx174 cells were infected with wild-type NL4-3 (WT), its Vpu-deficient variant NL4-3/Udel (Udel) (5), or viruses containing a scrambled Vpu TM domain (Urd), or having serine-to-asparagine mutations of the two cytoplasmic phosphoserine residues S52 and S56 (U26) (17). Virus release was monitored by determining the viral reverse transcriptase activity in the culture supernatants over time (Fig. 3A). As observed previously, WT NL4-3 and NL4-3/U26 released similar levels of RT activity into the culture supernatant, whereas release of NL4-3/Udel and NL4-3/Urd virions was impaired. Peak virus production for all cultures was on day 4, at which time cells were removed from the culture and used for immunoblot analysis to assess Bst-2 steady-state levels (Fig. 3B) and for FACS analysis to determine surface expression of Bst-2 (Fig. 3C). As seen in Fig. 3B, all cultures produced similar amounts of viral Gag proteins (Top). Also, similar levels of WT Vpu, Vpurd, and Vpu26 were found in the day 4 cell lysates (second panel from Top, lanes 2, 4, and 5). Constant levels of tubulin (Bottom) verified equal sample loading. Interestingly, even though levels of Bst-2 exhibited slight variations, there were no changes that could be attributed to Vpu function (third from Top). Furthermore, cell surface levels of Bst-2 were virtually unchanged even though ≈90% of the cells were HIV-infected (Fig. 3C). Only the WT NL4-3-infected culture revealed a modest reduction of Bst-2 in ≈5% of the p24-positive cell population. Similar results were observed in H9 cells, which express lower levels of Bst-2 than CEMx174 (Fig. S2B). Accumulation of cell-free viruses was less Vpu-responsive in H9 cells than in CEMx174 cells (Fig. S2A), which is consistent with the lower levels of Bst-2 in these cells (see Fig. 1). FACS data are shown for days 6 and 8 (Fig. S2B). Thus, unlike transfected HeLa cells and infected macrophages, HIV-1-infected CEMx174 and H9 cells did not exhibit Vpu-induced down-regulation of Bst-2.
Fig. 3.
Vpu does not reduce Bst-2 levels and does not induce cell surface down-regulation of Bst-2 during replication in CEMx174 cells. (A) CEMx174 cells were infected with equal reverse transcriptase units of WT NL4-3, NL4-3/Udel, NL4-3/Urd, and NL4-3/U26 virus stocks produced in 293T cells. Virus replication was monitored by measuring the virus-associated reverse transcriptase activity in the culture supernatants. Error bars reflect mean error from duplicate independent infections. (B and C) On day 4 after infection, ≈40% of the cells were removed from the infected cultures and divided into two aliquots. One aliquot of each sample was used for preparation of whole-cell extracts, followed by immunoblotting (B); the other part of the cells was used for Bst-2 cell surface staining followed by staining for intracellular p24 as described in Materials and Methods (C).
Packaging of Bst-2 into HIV-1 Virions.
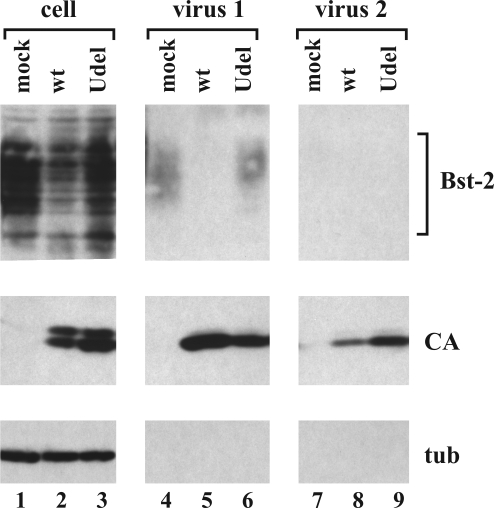
Bst-2 was proposed to function as a “tetherin,” physically linking virus to the plasma membrane and linking viral particles to each other (29). At least the latter function would require Bst-2 to be present in viral particles. To address this issue, we investigated the possible encapsidation of Bst-2 into WT and vpu-deficient NL4-3 virions produced from Bst-2-expressing HeLa cells (Fig. 4). Mock-infected HeLa cells were analyzed in parallel. Cells were detached 24 h later by scraping and pelleted. Virus-containing supernatants were removed (Fig. 4, virus 1). Cells were then suspended in an equal volume of complete culture medium and agitated for 10 s by vortexing as described in ref. 5. Cells were then pelleted, and supernatants were removed (Fig. 4, virus 2). Cells and virus-containing supernatants were then processed for immunoblotting as described in Materials and Methods. Consistent with our previous data (5), physical shearing of cells removed more vpu-defective particles than WT virions. Interestingly, however, neither WT nor vpu-deficient viruses removed by physical shearing contained detectable amounts of Bst-2 (Fig. 4, lanes 8 and 9). There were trace amounts of Bst-2 in the viral fraction 1 (Fig. 4, lane 6); however, similar levels of Bst-2 were also found in the mock sample (Fig. 4, lane 4). We conclude that Bst-2, despite its presence at the cell surface, is excluded from WT and vpu-deficient HIV-1 viral particles. The fact that Bst-2 is absent from WT and vpu-deficient particles suggests that Bst-2 is excluded from HIV-1 virions through a mechanism that is unrelated to cell surface down-modulation by Vpu.
Fig. 4.
Packaging of endogenous Bst-2 into HIV-1 virions. HeLa cells were transfected with 5 μg each of WT pNL4-3 (lanes 2, 5, and 8) or NL4-3/Udel (lanes 3, 6, and 9). A mock-transfected culture was analyzed in parallel (lanes 1, 4, and 7). Viral fractions (virus 1 and virus 2) were collected as described in the text. Cell lysates and concentrated viral supernatants were subjected to immunoblotting with antibodies to Bst-2 (Top). Viral capsid protein (CA) was identified with an HIV-positive patient serum (Middle). The blot was reprobed with an antibody to tubulin (tub) as internal reference (Bottom). Proteins are identified on the right.
Discussion
The recent identification of Bst-2 as an IFN-inducible host factor whose expression renders cells restrictive for vpu-defective viruses (29) and whose intracellular expression and surface presentation are reduced by the HIV-1 Vpu protein (23, 30) has spurred new interest in the mechanism of Vpu-mediated virus release. The Vpu TM domain plays a critical function in the process of virus release although its exact function remains a mystery. Two known functions of the Vpu TM domain are the assembly into homooligomeric complexes (35) and the formation of ion-conductive membrane pores (reviewed in ref. 36). However, it is unclear whether the interference with Bst-2 function requires Vpu oligomerization. Vpu does traffic to the cell surface (37), and it is conceivable that it regulates virus release from the cell surface although the site of Vpu function has yet to be established. The model proposed by Neil et al. (29) suggesting that Bst-2 functions as a tether to prevent the release of vpu-deficient virions from the cell surface has a lot of appeal. Our observation of extensive cell type-specific variations in intracellular Bst-2 levels versus relatively constant surface expression in most cell types tested is consistent with a model that involves cell surface Bst-2 in inhibiting virus release. One possible mechanism of Vpu would then be to induce surface down-modulation of Bst-2. Indeed, we confirmed data first reported by Van Damme et al. (23) that given enough time as is the case in long-term infected macrophages or when overexpressed in HeLa cells, Vpu down-modulates surface Bst-2. We are currently investigating the possibility that this mechanism is operative in primary CD4+ cells, which express very low levels of Bst-2. However, the observation that Bst-2 surface expression was little if at all altered in the course of a rapidly spreading infection of CEMx174 and H9 cells suggests that cell surface down-modulation of Bst-2 may not be the only function of Vpu.
Our inability to detect Bst-2 in virus particles even when Bst-2 was not down-modulated by Vpu (e.g., in vpu-deficient virions) and when particles were removed from the cell surface by physical shearing argues against a simple tethering function of Bst-2. Also, Bst-2 identified in the “virus-1” fraction in Fig. 4 did not copurify with the viral fraction in linear sucrose gradients, suggesting that this Bst-2 was nonspecifically released from the cells. The question remains of course how many Bst-2 molecules would be required to physically tether a mature virion to the cell surface. We were able to detect nonspecifically secreted Bst-2 in our experiment (Fig. 4, lanes 4 and 6). Therefore, we believe that the sensitivity and specificity of our antibody are excellent; however, we cannot rule out the presence of miniscule amounts of Bst-2 in viral particles that are below the limit of detection. Until these questions can be answered, alternative mechanisms of Bst-2 and Vpu function may need to be considered in addition to the tetherin model. It is possible that Bst-2 functions in concert with other host factor(s) (e.g., CAML). This would explain why silencing of either Bst-2 or CAML in HeLa cells resulted in Vpu-independent release of particles (24, 29). It will therefore be interesting to investigate the potential functional interplay of Bst-2 and CAML. We confirmed that Vpu has the ability to reduce intracellular Bst-2 in HeLa cells. However, it remains to be shown whether the reduction of Bst-2 levels in transfected HeLa cells and infected macrophages is caused by protein degradation or is an indirect consequence of Vpu dysregulation of NF-κB, both of which require functional phosphoserine motifs in Vpu (38). It is also important to note that reduced secretion of vpu-deficient viruses cannot be equated with reduced virus spread. In fact, replication kinetics of WT and vpu-deficient NL4-3 are generally quite similar irrespective of the cell type used (Fig. 3A and Fig. S2A), and there is strong evidence that vpu-deficient viruses are capable of spreading cell-to-cell despite reduced levels of cell-free virus. Until the true significance of Vpu-enhanced virus release is fully understood for in vivo dissemination of HIV-1, the question of whether Bst-2 should be added to the ever-growing list of antiviral host factors remains open.
Materials and Methods
Plasmids.
All plasmids have been described: pNL4-3 (39), pNL4-3/Udel (5), pNL4-3/Urd and pNL4-3/U26 (17), and pcDNA-Vphu (33).
Antisera.
Anti-Bst-2 antiserum was elicited in rabbits by using a bacterially expressed MS2-Bst-2 fusion protein composed of amino acids 1–91 of the MS2 replicase and amino acids 41–162 of Bst-2, generating a polyclonal antibody against the extracellular portion of Bst-2. Polyclonal anti-Vpu serum (rabbit), directed against the hydrophilic C-terminal cytoplasmic domain of Vpu expressed in Escherichia coli (35), was used for detection of Vpu. Serum from an HIV-positive patient was used to detect HIV-1-specific capsid (CA) and Gag precursor (Pr55gag) proteins. Tubulin and actin were identified by using monoclonal antibodies to α-tubulin and actin, respectively (both from Sigma–Aldrich).
Tissue Culture and Transfections.
Cell maintenance and transfections were performed by using standard techniques as detailed in SI Materials and Methods.
Preparation of MDM and PBMC and Stimulation with IFNα.
PBMC were isolated from the leukophoresed blood of HIV-seronegative donors after gradient separation with lymphocyte separation medium (Organon Teknika–Cappel). Cells (2 × 106 each) were cultured for 24 h in 1.5 mL of RPMI medium 1640 (10% FCS, 4 mM l-glutamine, 2 mM sodium pyruvate, 50 μg/mL gentamicin, and 50 μM 2-mercaptoethanol) either alone or in the presence of 1 ng/mL IFNα-2c or 10 ng/mL anti-CD3 plus 5 μg/mL anti-CD28 (both BD Biosciences PharMingen) for 24 h. Cells were harvested 24 h later and lysed in 300 μL of sample buffer. Boiled samples (50 μL each) were processed for immunoblotting.
Monocytes from normal human donors were prepared as described in ref. 40 to allow differentiation into MDM as reported in ref. 34. IFNα-2c was added as indicated in the text and detailed in SI Materials and Methods.
Virus Preparation.
Virus stocks for infection were prepared by transfecting 293T cells with appropriate plasmid DNAs as described in ref. 41 and SI Materials and Methods.
Immunoblotting.
For immunoblot analysis of intracellular proteins, whole-cell lysates were prepared as described in ref. 41 and detailed in the SI Materials and Methods.
FACS Analysis.
Cells were washed twice with ice-cold 20 mM EDTA-PBS, followed by two washes in ice-cold 1% BSA-PBS. Cells were blocked for 10 min with 50 μg of mouse IgG (Millipore). Cells were incubated with primary antibody (α-Bst-2) for 30 min at room temperature, washed twice with ice-cold 1% BSA-PBS followed by the addition of allophycocyanin-conjugated anti-rabbit IgG secondary antibody (Jackson ImmunoResearch Laboratories) in 1% BSA-PBS. Incubation was for 30 min at room temperature in the dark. Cells were then washed twice with ice-cold 1% BSA-PBS and fixed with 1% paraformaldehyde in PBS. Finally, cells were analyzed on a FACS Calibur (BD Biosciences Immunocytometry Systems). Data analysis was performed by using CellQuest Pro (BD Biosciences) and Flow Jo (Tree Star). For staining of intracellular p24, cells were stained with primary and secondary antibodies as described. Before the fixation step, cells were permeabilized for 10 min at room temperature in the dark by using 1× FACS permeabilization buffer (Becton Dickinson). Cells were then washed twice with ice-cold 1% BSA-PBS and stained for 30 min at room temperature in the dark with α-p24-Gag antibody (KC57 RD1; Beckman Coulter). Cells were washed twice with ice-cold 1% BSA-PBS, fixed, and analyzed as above.
Supplementary Material
Acknowledgments.
We thank Mohammad Khan, Ritu Goila-Gaur, Robert Walker, and Ronald Willey for helpful discussion and critical comments. We thank Jason Brenchley for help with cell sorting, Bernard Lafont for help with FACS, and Kathleen Clouse and Linda Tiffany (Center for Drug Evaluation and Research, Beltsville, MD) for providing monocyte-derived macrophages. IFNα-2c was a generous gift from Kathryn C. Zoon (National Institute of Allergy and Infectious Diseases, Bethesda, MD). This work was supported by a grant from the National Institutes of Health Intramural AIDS Targeted Antiviral Program (to K.S.) and by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813223106/DCSupplemental.
References
- 1.Strebel K, Klimkait T, Martin MA. A novel gene of HIV-1, vpu, and its 16-kilodalton product. Science. 1988;241:1221–1223. doi: 10.1126/science.3261888. [DOI] [PubMed] [Google Scholar]
- 2.Cohen EA, Terwilliger EF, Sodroski JG, Haseltine WA. Identification of a protein encoded by the vpu gene of HIV-1. Nature. 1988;334:532–534. doi: 10.1038/334532a0. [DOI] [PubMed] [Google Scholar]
- 3.Willey RL, Maldarelli F, Martin MA, Strebel K. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J Virol. 1992;66:7193–7200. doi: 10.1128/jvi.66.12.7193-7200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willey RL, Maldarelli F, Martin MA, Strebel K. Human immunodeficiency virus type 1 Vpu protein regulates the formation of intracellular gp160–CD4 complexes. J Virol. 1992;66:226–234. doi: 10.1128/jvi.66.1.226-234.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klimkait T, Strebel K, Hoggan MD, Martin MA, Orenstein JM. The human immunodeficiency virus type 1-specific protein Vpu is required for efficient virus maturation and release. J Virol. 1990;64:621–629. doi: 10.1128/jvi.64.2.621-629.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neil SJ, Eastman SW, Jouvenet N, Bieniasz PD. HIV-1 Vpu promotes release and prevents endocytosis of nascent retrovirus particles from the plasma membrane. PLoS Pathog. 2006;2:e39. doi: 10.1371/journal.ppat.0020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harila K, et al. Vpu and Tsg101 regulate intracellular targeting of the human immunodeficiency virus type 1 core protein precursor Pr55gag. J Virol. 2006;80:3765–3772. doi: 10.1128/JVI.80.8.3765-3772.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harila K, Salminen A, Prior I, Hinkula J, Suomalainen M. The Vpu-regulated endocytosis of HIV-1 Gag is clathrin-independent. Virology. 2007;369:299–308. doi: 10.1016/j.virol.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Strebel K, Klimkait T, Maldarelli F, Martin MA. Molecular and biochemical analyses of human immunodeficiency virus type 1 Vpu protein. J Virol. 1989;63:3784–3791. doi: 10.1128/jvi.63.9.3784-3791.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terwilliger EF, Cohen EA, Lu YC, Sodroski JG, Haseltine WA. Functional role of human immunodeficiency virus type 1 Vpu. Proc Natl Acad Sci USA. 1989;86:5163–5167. doi: 10.1073/pnas.86.13.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Damme N, Guatelli J. HIV-1 Vpu inhibits accumulation of the envelope glycoprotein within clathrin-coated, Gag-containing endosomes. Cell Microbiol. 2008;10:1040–1057. doi: 10.1111/j.1462-5822.2007.01101.x. [DOI] [PubMed] [Google Scholar]
- 12.Piller SC, Ewart GD, Premkumar A, Cox GB, Gage PW. Vpr protein of human immunodeficiency virus type 1 forms cation-selective channels in planar lipid bilayers. Proc Natl Acad Sci USA. 1996;93:111–115. doi: 10.1073/pnas.93.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ewart GD, Sutherland T, Gage PW, Cox GB. The Vpu protein of human immunodeficiency virus type 1 forms cation- selective ion channels. J Virol. 1996;70:7108–7115. doi: 10.1128/jvi.70.10.7108-7115.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schubert U, et al. Identification of an ion channel activity of the Vpu transmembrane domain and its involvement in the regulation of virus release from HIV-1-infected cells. FEBS Lett. 1996;398:12–18. doi: 10.1016/s0014-5793(96)01146-5. [DOI] [PubMed] [Google Scholar]
- 15.Marassi FM, et al. Correlation of the structural and functional domains in the membrane protein Vpu from HIV-1. Proc Natl Acad Sci USA. 1999;96:14336–14341. doi: 10.1073/pnas.96.25.14336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma C, et al. Expression, purification, and activities of full-length and truncated versions of the integral membrane protein Vpu from HIV-1. Protein Sci. 2002;11:546–557. doi: 10.1110/ps.37302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schubert U, et al. The two biological activities of human immunodeficiency virus type 1 Vpu protein involve two separable structural domains. J Virol. 1996;70:809–819. doi: 10.1128/jvi.70.2.809-819.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varthakavi V, Smith RM, Bour SP, Strebel K, Spearman P. Viral protein U counteracts a human host cell restriction that inhibits HIV-1 particle production. Proc Natl Acad Sci USA. 2003;100:15154–15159. doi: 10.1073/pnas.2433165100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakai H, Tokunaga K, Kawamura M, Adachi A. Function of human immunodeficiency virus type 1 Vpu protein in various cell types. J Gen Virol. 1995;76:2717–2722. doi: 10.1099/0022-1317-76-11-2717. [DOI] [PubMed] [Google Scholar]
- 20.Hsu K, Seharaseyon J, Dong P, Bour S, Marban E. Mutual functional destruction of HIV-1 Vpu and host TASK-1 channel. Mol Cell. 2004;14:259–267. doi: 10.1016/s1097-2765(04)00183-2. [DOI] [PubMed] [Google Scholar]
- 21.Callahan MA, et al. Functional interaction of human immunodeficiency virus type 1 Vpu and Gag with a novel member of the tetratricopeptide repeat protein family. J Virol. 1998;72:5189–5197. doi: 10.1128/jvi.72.6.5189-5197.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neil SJ, Sandrin V, Sundquist WI, Bieniasz PD. An interferon-α-induced tethering mechanism inhibits HIV-1 and Ebola virus particle release but is counteracted by the HIV-1 Vpu protein. Cell Host Microbe. 2007;2:193–203. doi: 10.1016/j.chom.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Damme N, et al. The interferon-induced protein BST-2 restricts HIV-1 release and is down-regulated from the cell surface by the viral Vpu protein. Cell Host Microbe. 2008;3:245–252. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varthakavi V, et al. Identification of calcium-modulating cyclophilin ligand as a human host restriction to HIV-1 release overcome by Vpu. Nat Med. 2008;14:641–647. doi: 10.1038/nm1778. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Goto T, et al. A novel membrane antigen selectively expressed on terminally differentiated human B cells. Blood. 1994;84:1922–1930. [PubMed] [Google Scholar]
- 26.Ohtomo T, et al. Molecular cloning and characterization of a surface antigen preferentially overexpressed on multiple myeloma cells. Biochem Biophys Res Commun. 1999;258:583–591. doi: 10.1006/bbrc.1999.0683. [DOI] [PubMed] [Google Scholar]
- 27.Ishikawa J, et al. Molecular cloning and chromosomal mapping of a bone marrow stromal cell surface gene, BST2, that may be involved in pre-B-cell growth. Genomics. 1995;26:527–534. doi: 10.1016/0888-7543(95)80171-h. [DOI] [PubMed] [Google Scholar]
- 28.Kupzig S, et al. Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic. 2003;4:694–709. doi: 10.1034/j.1600-0854.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- 29.Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 30.Bartee E, McCormack A, Fruh K. Quantitative membrane proteomics reveals new cellular targets of viral immune modulators. PLoS Pathog. 2006;2:e107. doi: 10.1371/journal.ppat.0020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schubert U, Clouse KA, Strebel K. Augmentation of virus secretion by the human immunodeficiency virus type 1 Vpu protein is cell type-independent and occurs in cultured human primary macrophages and lymphocytes. J Virol. 1995;69:7699–7711. doi: 10.1128/jvi.69.12.7699-7711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schubert U, Bour S, Willey RL, Strebel K. Regulation of virus release by the macrophage-tropic human immunodeficiency virus type 1 AD8 isolate is redundant and can be controlled by either Vpu or Env. J Virol. 1999;73:887–896. doi: 10.1128/jvi.73.2.887-896.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen KL, et al. Codon optimization of the HIV-1 vpu and vif genes stabilizes their mRNA and allows for highly efficient Rev-independent expression. Virology. 2004;319:163–175. doi: 10.1016/j.virol.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 34.Miyagi E, et al. APOBEC3G-independent reduction in virion infectivity during long-term HIV-1 replication in terminally differentiated macrophages. Virology. 2008;379:266–274. doi: 10.1016/j.virol.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maldarelli F, Chen MY, Willey RL, Strebel K. Human immunodeficiency virus type 1 Vpu protein is an oligomeric type I integral membrane protein. J Virol. 1993;67:5056–5061. doi: 10.1128/jvi.67.8.5056-5061.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strebel K. HIV accessory genes vif and vpu. Adv Pharmacol. 2007;55:199–232. doi: 10.1016/S1054-3589(07)55006-4. [DOI] [PubMed] [Google Scholar]
- 37.Bour S, Perrin C, Strebel K. Cell surface CD4 inhibits HIV-1 particle release by interfering with Vpu activity. J Biol Chem. 1999;274:33800–33806. doi: 10.1074/jbc.274.47.33800. [DOI] [PubMed] [Google Scholar]
- 38.Akari H, Bour S, Kao S, Adachi A, Strebel K. The human immunodeficiency virus type 1 accessory protein Vpu induces apoptosis by suppressing the nuclear factor κB-dependent expression of antiapoptotic factors. J Exp Med. 2001;194:1299–1311. doi: 10.1084/jem.194.9.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adachi A, et al. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gruber MF, Weih KA, Boone EJ, Smith PD, Clouse KA. Endogenous macrophage CSF production is associated with viral replication in HIV-1-infected human monocyte-derived macrophages. J Immunol. 1995;154:5528–5535. [PubMed] [Google Scholar]
- 41.Kao S, et al. The human immunodeficiency virus type 1 Vif protein reduces intracellular expression and inhibits packaging of APOBEC3G (CEM15), a cellular inhibitor of virus infectivity. J Virol. 2003;77:11398–11407. doi: 10.1128/JVI.77.21.11398-11407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goila-Gaur R, Khan MA, Miyagi E, Strebel K. Differential sensitivity of “old” versus “new” APOBEC3G to human immunodeficiency virus type 1 Vif. J Virol. 2009;83:1156–1160. doi: 10.1128/JVI.01734-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.