Abstract
Learning increases the survival of new cells that are generated in the hippocampal formation before the training experience, especially if the animal learns to associate stimuli across time [Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ (1999) Nat Neurosci 2:260–265]. All relevant studies have been conducted on male rats, despite evidence for sex differences in this type of learning. In the present study, we asked whether sex differences in learning influence the survival of neurons generated in the adult hippocampus. Male and female adult rats were injected with one dose of bromodeoxyuridine (BrdU; 200 mg/kg), to label one population of dividing cells. One week later, half of the animals were trained with a temporal learning task of trace eyeblink conditioning, while the other half were not trained. Animals were killed 1 day after training (12 days after the BrdU injection). Hippocampal tissue was stained for BrdU and a marker of immature neurons, doublecortin. Both sexes learned to emit the conditioned eyeblink response during the trace interval. As a consequence, more new neurons remained in their hippocampi than in sex-matched controls. In individual animals, the number of surviving cells correlated positively with asymptotic performance; those that expressed more learned responses retained more new neurons. However, animals that learned very well retained even more new cells if they required many trials to do so. Because females emitted more learned responses than males did, they retained nearly twice as many new cells per unit volume of tissue. This effect was most evident in the ventral region of the hippocampal formation. Thus, sex differences in learning alter the anatomical structure of the hippocampus. As a result, male and female brains continue to differentiate in adulthood.
Keywords: eyeblink conditioning, learning, neurogenesis, sex differences, stem cell
Gender differences in cognition are well documented. In general, women tend to outperform men on tasks that involve verbal communication and memory of personal experiences, whereas men perform better on tasks that require the manipulation of complex spatial information (1). Sex differences in learning also occur in laboratory rodents (2). Adult neurogenesis in the rat hippocampus is affected by learning and is perhaps involved in related processes (3–7). Specifically, it has been shown that learning a new task enhances the survival of the new cells that were generated in the dentate gyrus (DG) of the hippocampus 1 week before the training began (8–13), while it has no effect on other neurogenic regions, such as the subventricular zone (11). The vast majority of these surviving cells differentiate into neurons and many are incorporated into the circuitry of the hippocampus (7, 12, 14, 15). However, this effect of training on cell survival only occurs if animals are trained on a relatively difficult task and only if they learn the task well (8, 13, 16). Learning a trace eyeblink conditioning task is relatively difficult and successful learning ensures the survival of many, if not most, of the new cells that are available for rescue at the time of training (8, 11–13, 17). Learning a similar task, known as delay conditioning, is much easier and does not rescue new neurons from death (11, 13, 17).
The studies addressing the effects of learning on neuronal survival have been conducted exclusively in male rodents, despite evidence for sex differences in associative learning and hippocampal neurogenesis, both of which are enhanced when estrogen levels are high (18–22). In the present study, we asked whether sex differences in trace conditioning would be evident even when females were trained when estrogen levels are relatively low (diestrus). Sex differences were expressed, and as a consequence females retained proportionately more new neurons in their hippocampi than did males.
Results
Females Outperform Males During Training with Trace Eyeblink Conditioning.
Before training, males and females expressed similar levels of spontaneous blinking (P > 0.05; data not shown). They responded similarly to a white-noise stimulus before any training occurred (P > 0.05; 1–2 sensitized blinks out of 10) and did not respond to the conditioned stimulus (CS; white noise) on the first trial of training (P > 0.05; one animal in each group emitted a response during the trace interval of the first trial). Upon presentations of paired stimuli (Fig. 1B), both males and females learned to associate the conditioned stimulus with the unconditioned stimulus (US; eyelid stimulation) and emitted conditioned responses (CRs) during the trace interval in anticipation of the US (effect of trials of training: F (11, 176) = 54.6, P < 0.001).
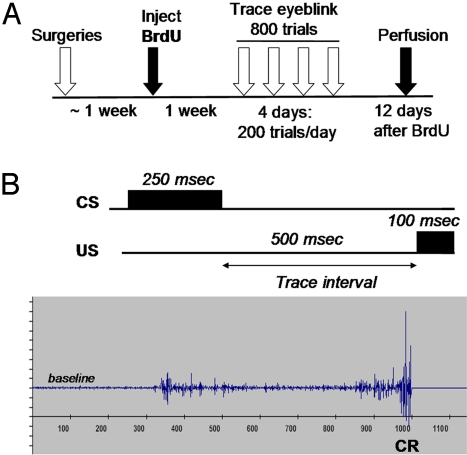
Fig. 1.
Experimental design. (A) Male and female rats were surgically implanted with electrodes to measure the electromyographic response (EMG) of the eyelid. Afterward, animals were injected with 1 dose of bromodeoxyuridine (BrdU), which labels 1 population of dividing cells. Females were cycling normally and injected during estrus. One week later, males and females were trained with trace eyeblink conditioning each day for 4 days. One day later, trained and untrained animals were killed to assess the number of new cells in the dentate gyrus of the hippocampus (12 days after the BrdU injection). (B) During trace conditioning, a CS of white noise was paired with a US (periorbital stimulation), which elicits an eyeblink response. The 2 conditioning stimuli were separated by a trace interval or temporal gap of 500 ms. As an animal learns that the 2 stimuli are associated, it blinks during the trace interval and in anticipation of the US. Eyeblinks were detected by an increase in the magnitude of the EMG. Those that occurred during the trace interval were considered CRs. The electrophysiological record shows an example of a CR that occurred just before the onset of the US.
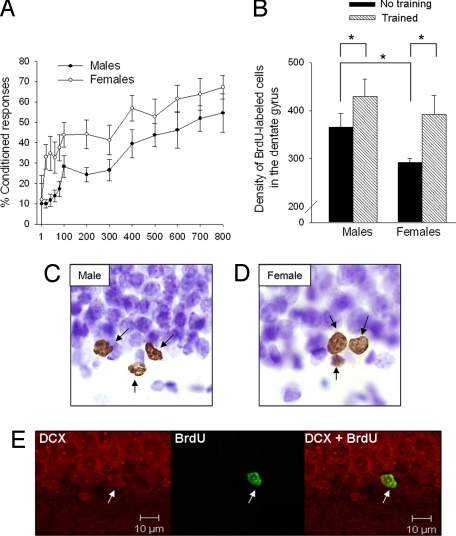
Overall, females emitted more CRs across the 800 trials of training than did males (sex difference effect: F (1, 16) = 4.7, P < 0.05) (Fig. 3A). This effect was pronounced during the first session of training (the first 200 trials). Females also emitted late CRs sooner than did males. Late CRs were those blinks that occurred within 250 ms of the US (late CRs on the first day, sex difference effect: F (1, 16) = 7.49, P = 0.01; percent late CRs in males: 8 ± 2; females: 22 ± 5). Because late blinks more accurately predict the onset of the US, they are considered adaptive (23, 24). Minimally, the present results indicate that females learned to time the CR sooner in training than did males. As a group, the percentage of eyeblinks continued to increase in males, even during the last session of training, indicating that some animals had not reached asymptote. There was no sex difference in responding at the end of training (P > 0.05). However, more females than males reached a criterion of 60% responding (60% CRs); 7 out of 8 females reached criterion, whereas 7 out of 10 male rats reached the same criterion.
Fig. 3.
Females learned better than males and rescued a greater proportion of new neurons. (A) Both sexes acquired the conditioned response (P < 0.05), but females emitted more CRs than males did across trials of training (P < 0.05). More females (7 out of 8) reached a learning criterion than did males (7 out of 10). (B) The volume of the dentate gyrus was calculated to assess the density of new cells that were generated and maintained in the male versus female hippocampus with and without training. Overall, the density of BrdU-labeled cells in the dentate gyrus (GCL/SVZ and hilus) was higher in trained than untrained animals, irrespective of sex. However, the percent increase was greater in trained females (34%) than in trained males (17%). The density of new cells in untrained females was lower than that in untrained males (significant differences are noted with an *). (C and D) The adult-generated cells were labeled with BrdU (brown) and counterstained with cresyl violet (purple). Representative images from a male and female hippocampus were magnified (1,000×) with an optical microscope. (E) The vast majority of new cells (≈80%) differentiated into neurons by the end of training (12 days after the BrdU injection). These cells expressed BrdU (green) and DCX (red), which is expressed in immature neurons. Representative images of a new cell expressing DCX alone (Left), BrdU alone (Middle), and both DCX and BrdU (Right) were obtained with a confocal scanning microscope.
Learning Increases Survival of the New Cells in the Hippocampus.
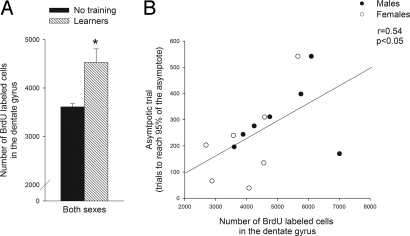
The overall effect of training on the survival of new neurons was similar in males and females. The number of cells labeled with BrdU after the training experience was increased in animals that were trained versus those that were not trained (F (1, 31) = 6.2, P < 0.05). In previous studies, the increase in cell survival after training was preferentially expressed in animals that learned, defined as those animals that expressed CRs on at least 60% of the trials in a session. The increase in survival did not occur in animals that were exposed to the same number of trials but did not learn to express the CR (8). In the present study, we replicated this finding and extend it to include females; the number of surviving cells was elevated in animals emitting CRs on at least 60% of the trials within a session (n = 7 males and n = 7 females) (F (1, 27) = 7.4, P = 0.01) (Fig. 2A). As previously reported, the number of new cells remaining in the hippocampi of animals that did not learn (n = 4) was not different from the number in those that were not trained (P > 0.05; data not shown) (8).
Fig. 2.
Learning increased the survival of new cells in the hippocampus. (A) Learning during trace conditioning increased the number of new cells that survived after training (* indicates that the effect of training on cell number was significant: P < 0.05). The number of BrdU-labeled cells in the dentate gyrus (GCL/SGZ and hilus) of male and female animals that learned (n = 14) is shown in contrast to the number of new cells remaining in animals that were not trained (n = 17). (B) The number of BrdU-labeled cells that were present in an animal after training correlated positively and significantly with the period during training when the animal learned to emit the CR during the trace interval. The asymptotic trial was used to estimate the trial when an animal's conditioned response rate was 95% or more of its asymptote. The asymptotic trial did not differ between male and female animals that learned (P > 0.05).
In the next analysis, we only considered animals that learned the CR. We considered animals to have learned if they expressed 60% CRs during training. In those animals, we then determined the trial at which each animal reached its highest level of responding (25, 26). This trial, hereafter referred to as the “asymptotic trial,” was the trial at which an animal reached 95% of its asymptotic performance. This trial correlated positively with the number of cells labeled with BrdU after training (r = 0.54, P < 0.05) (Fig. 2B). To verify this relationship, we used a more traditional measure of learning during eyeblink conditioning: the trial on which an individual animal emitted 8 out of 9 CRs on consecutive trials. This trial was also correlated with the number of BrdU-labeled cells in the hippocampus (r = 0.533; P < 0.05). Thus, both analyses suggest that animals that learn and require more trials to do so also retain more of the new neurons in their DG. It is important to reiterate that animals that learn retain more new cells after training than animals that do not learn, but within those animals that learn well, those that require more trials to do so retain the most new cells. This relationship between new cell survival and trials to criterion has also been demonstrated before in males (13).
Learning Increases the Density of New Neurons More in Females than in Males.
In the next analyses, we compared the effects of training on the absolute number versus density of surviving cells. This analysis was necessary because the female hippocampus is significantly smaller in volume than the male hippocampus (27, 28). To conduct the analysis, all animals were included, regardless of their performance on the trace memory task. The analysis revealed significant main effects of training and sex differences on the absolute number of BrdU-labeled cells that were detected in the DG (F (1, 31) = 6.2, P < 0.05; F (1, 31) = 10.1, P < 0.01, respectively), with no interaction (BrdU-labeled cells: males with no training: 4,056 ± 333; males with training: 4,908 ± 338; females with no training: 3,114 ± 89; females with training: 3,834 ± 383). Next, we assessed the effects of training on the proportion of new cells that survive in the male versus the female hippocampus. This was conducted to account for 2 sex differences in the hippocampal structure. First, 12 days after the one injection of BrdU, females that were not trained (left in home cage) possessed fewer BrdU-labeled cells (3,114 ± 89) than did males that were not trained and left in their home cage (4,056 ± 333). It is during this time period that many of the new cells die (11, 13). Thus, more cells may die in females than in males across time. The second issue is that the female hippocampus is smaller than the male hippocampus (27, 28). To account for these sex differences, we calculated the density of new cells in the DG (cells per mm3). Indeed, the volume of the DG [granular cell layer/subgranular zone (GCL/SGZ) and hilus] was smaller in females than in males (sex effect: F (1, 31) = 7.7, P = 0.01). Training with trace conditioning did not alter the volume of the DG in either sex (males with no training: 11.1 ± 0.3 mm3; males with training: 11.6 ± 0.5 mm3; females with no training: 10.7 ± 0.2 mm3; females with training: 9.9 ± 0.4 mm3). Nonetheless, training with the trace-conditioning memory task increased the density of BrdU-labeled cells in the DG of both males and females (main effect of training: F (1, 31) = 6.7, P = 0.01; with no main effect of sex: F (1, 31) = 3.0, P > 0.05). The density of new cells in the animals that were not trained was lower in females than in males (1-way ANOVA: F (1, 15)> = 0.4, P < 0.05), but was not different between males and females that were trained (P > 0.05) (Fig. 3B). As a result of training, twice as many new cells per unit volume survived in the female (34%) than in the male hippocampus (17%) (see Fig. 3B). The effect size was similar when the analysis was limited to animals that learned (reached 60% CRs). In this case, there was a 40% increase in the proportion of new cells that remained in the DG of females versus 22% in males.
Nearly 80% of the cells labeled with BrdU also expressed the immature neuronal marker doublecortin (DCX) (16). The percentage of BrdU-labeled cells that coexpressed DCX was similar in males and females, as reported previously (19). These data suggest that by the end of training, the vast majority of new cells have already begun to differentiate into neurons and the sex of the animals does not alter that process (Fig. 3E).
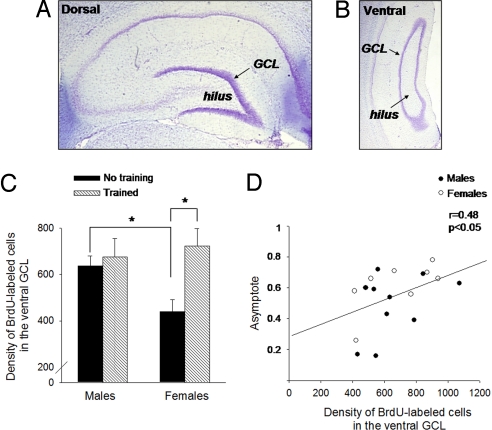
Recent data suggest that dorsal and ventral regions of the hippocampus differentially contribute to learning the trace eyeblink response (29, 30). If this is true, new cells generated in these two regions may also respond preferentially to trace conditioning (31). To examine this possibility, we estimated the density of the new cells in the dorsal versus the ventral hippocampus, based on methods described previously (32) (Fig. 4 A and B). In response to training alone, the density of new cells that survived in the GCL/SGZ of the dorsal hippocampus of male and female rats was similar. However, the effect of training on the density of surviving cells was more pronounced in the ventral part of the female hippocampus. Training increased the density of BrdU-labeled cells in the ventral hippocampus (F (1, 31) = 4.7, P < 0.05), with an interaction between training and sex differences (F (1, 31) = 3.8, P = 0.05). This effect was exacerbated by the fact that fewer new cells survived in the ventral hippocampus of females that were not trained versus males that were not trained (P < 0.05) (Fig. 4C). As before, the density of BrdU-labeled cells correlated positively with the highest level of responding that animals achieved across training (r = 0.48, P < 0.05), (Fig. 4D) (8). The correlation was stronger in females than in males (females: r = 0.63, P = 0.09; males: r = 0.39, P = 0.2), probably because more females than males learned well.
Fig. 4.
Learning increased the density of new cells that survived in the ventral hippocampus of females. (A and B) The hippocampal formation was arbitrarily divided into the dorsal (A) and the ventral (B) regions. Cell layers were stained with cresyl violet, illustrating the GCL and the hilus. Images were obtained with an optical microscope (20×). (C) Training increased the density of new cells that resided in the ventral region of the female hippocampus (P < 0.05). The density of new cells in the ventral GCL of untrained females was lower than that in untrained males. (* indicates significant posthoc tests). (D) The level of responding that an animal achieved by the end of training (“asymptote”) correlated with the density of new cells that survived in the ventral GCL, especially in females.
Discussion
Learning rescues new neurons from death in the adult hippocampus (8, 11–13, 17). Here, we report that sex differences in learning predict how many new cells will survive to become neurons after the training experience is over. Using a task that is known to enhance new cell survival, trace eyeblink conditioning, we observed that females learned to accurately time the conditioned response sooner than did males and, as a result, more of them reached a learning criterion. As a further consequence, twice as many new cells per unit volume survived in the female than in the male hippocampus, when both sexes were compared to animals that were not trained. In both sexes, the degree to which each animal expressed the learned response related in a positive way to the number and density of the new cells that survived. Consistent with previous studies, individual differences in learning predicted how many new cells would survive after training (8–10, 12, 13, 16, 33). However, and perhaps more importantly, in those animals that did learn, those that required more trials to reach the learning criterion retained more of the new neurons. Together, these data indicate that at least 2 factors are essential to rescue the new cells from death. First, the animals must learn and learn well, consistently expressing the conditioned response across hundreds of trials. Second, animals that learn well and require more trials to do so tend to retain most of the new neurons (present study and ref. 13).
How this effect of learning on neurogenesis occurs at the neuronal level is not known. It is well-established that the mature and established neurons in the hippocampal formation, especially the pyramidal cells in area CA1 of the hippocampus, become more active during acquisition of the trace eyeblink conditioning (29, 34). Also, individual differences in learning predict in a positive way the degree of the activity that occurs (35). Thus, increases in neuronal activity throughout the hippocampus during training may contribute to the preservation of these new neurons (7, 13). In animals that do learn, those that maintain an increase in cellular activity across more trials of training may retain more new cells after the learning experience has occurred.
The present study shows for the first time that sex differences in learning influence the survival of new neurons in the adult hippocampus. Females learned better than males and more of them reached the criterion of 60% CRs (good learners). As a result, females retained a higher percentage of new cells per unit volume at the end of training. Because the new cells were labeled with BrdU 1 week before training began the effects are not likely to reflect changes in the production of new cells (proliferation). Rather, because some of the new cells have already begun to die when the training begins, the effect reported here reflects an increase in new cell survival (7). Why females learn the trace memory better than males and what it is about their learning that increases cell survival is not known, but there are clues. Overall, females emitted more learned responses early in training. During this period, they also emitted CRs closer to the onset of the US. These so-called “late” CRs indicate that an animal has accurately learned to predict the onset of the US. Thus, females emitted more well-timed responses than did males, suggesting that they more readily learned to time the CR. Some studies suggest that the hippocampus is involved in the precise timing of the CR (23, 34). Thus, timing of the CR may influence the effect of learning on cell survival. Finally, it is important to note that before training, females did not emit more spontaneous blinks or sensitized responses to the CS than did males.
Sex differences during eyeblink conditioning occur in other species, including humans (36). In rats, they are most evident when females begin training in proestrus, when estrogen levels are elevated (18, 20, 22). In the present study, the differences in trace conditioning were not limited to stages of estrus when estrogen levels are elevated. Rather, females began training in diestrus, a phase of the estrous cycle characterized by lower levels of estrogen and progesterone. As far as neurogenesis is concerned, females produce more new cells in proestrus than in estrus (19). In the present study, we purposely injected all females with BrdU during a phase of the cycle (i.e., estrus) when the numbers of proliferating cells in the hippocampus are similar between sexes (19). However, at the time of their being killed (12 days later), control females that were not trained possessed fewer of the BrdU-labeled cells than did males that were not trained. Thus, without stimulation, fewer new cells seem to survive in naive females than in males. Perhaps the presence of testosterone enhances cell survival in males (37). In any event, we do not know exactly how many new cells were available to rescue at the beginning of training in these experiments. In males, virtually all cells that were available to rescue at the beginning of training were rescued from death by trace conditioning, again provided that the animal learned slowly and well (13). Thus, it is possible that in the present study more new cells were available to rescue in females than in males at the beginning of training, and that in the absence of training, more of those cells died. It is further noted that females also learn delay conditioning better than do males (21, 22), but since delay conditioning does not enhance new cell survival (11), there should be no consequence for neurogenesis per se.
In humans, some sex differences in learning are related to gender and thus influenced by social and environmental factors (38). Other sex differences in learning are mediated by the presence of sex hormones and sex differences in the structure of the brain (27, 28, 39–41). As presented here, the total volume of the dentate gyrus is greater in males than in females (27, 42). This sex difference is dependent on the organizational effect of gonadal hormones because it can be reversed if females are masculinized with testosterone at birth (27). Similarly, sex differences in trace eyeblink conditioning are organized, at least in part, by the presence of gonadal hormones (43). Females that are masculinized at birth by the presence of testosterone acquire the trace memory at a similar rate to males when trained as adults (43). Ultimately then, the neurogenic response to learning in males versus females is likely organized by the presence of gonadal hormones during early brain development. However, the present results demonstrate the influence of learning on brain anatomy; they do not address the anatomy that underlies sex differences in learning.
Recently, a great deal of focus has been placed on distinguishing the dorsal from the ventral region of the hippocampus. These two regions have differing degrees of input from afferent structures, as well as differences in efferent connections (31, 44, 45). During trace conditioning, mature neurons in both regions are activated (29), but they possibly play distinctive roles in the learning process (29, 30, 46, 47). In the present study, we found suggestive evidence that new neurons generated in the ventral hippocampus may be especially responsive to learning. In females, the effect of trace conditioning on new cell survival was preferentially expressed in the ventral hippocampus. During spatial learning, mature neurons in the dorsal hippocampus are primarily activated, whereas the immature neurons are more active in the ventral regions (48). It was suggested that the cells in the ventral hippocampus respond more to emotional aspects of learning (49). Trace eyeblink conditioning is a form of aversive and, by association, emotional learning (50). In cycling females, these emotional processes may be especially influential. Females possess a greater density of estrogen receptors in the ventral than in the dorsal hippocampus (51). Because sex differences in eyeblink conditioning are mediated, at least in part, by the presence of estrogen, estrogen receptors in this region may be involved in the enhanced new cell survival in females (18, 21). On the other hand, the preferential expression of new cells in the ventral region of females may simply reflect the fact that more females learned well than did males.
To summarize, females outperformed males while learning to associate stimuli across time; as a consequence, they retained a greater percentage of adult-generated neurons in their hippocampi than did males. The number of new cells, most of which went on to become neurons, correlated in a positive way with the expression of the trace memory. Thus, sex differences in learning are not only mediated by sex differences in brain anatomy, they can also induce sex differences in that anatomy.
Materials and Methods
Subjects.
Experiments were approved by the Rutgers University Animal Care and Facilities Committee. Adult (70–90 days old) male (300–350 g) and female (250–350 g) Sprague−Dawley rats were individually housed and maintained on a 12-hour light/dark cycle. Stages of estrus were determined in female rats with daily vaginal smears, as described (20, 52). Only adult females that demonstrated at least two 4 to 5 day cycles with all stages of estrus were included in the study.
Eyeblink Conditioning and BrdU Injections.
Male (n = 10) and female (n = 10) rats were implanted with electrodes for eyeblink conditioning. During surgery, rats were first anesthetized with pentobarbital (15 mg/kg, i.p.) and maintained on isoflurane and oxygen. Two pairs of electrodes were attached to a headstage and implanted through the upper eyelid (8). Naive male (n = 9) and female (n = 9) rats were kept undisturbed in their home cages.
Following recovery, animals were injected i.p. with one dose of BrdU (200 mg/kg), to label 1 population of dividing cells 1 week before training (8, 11, 53) (see Fig. 1A). BrdU is incorporated into the DNA of dividing cells during the S-phase of the cell cycle (53, 54). All females were injected with BrdU during the same phase (estrus) of the cycle, to control for differences in proliferation across the estrous cycle (19). Two operated females and 1 naive female were excluded from the study before training because of irregular cycles. Seven days after the one BrdU injection, rats in the training groups were given 45 min to acclimate (no stimuli presented) to the conditioning environment. Twenty-four hours after acclimation and 8 days after the BrdU injection, male and female rats were placed again in the same conditioning chambers. For 20 min, no stimuli were presented while the numbers of spontaneous blinks were recorded. Then, all rats were exposed to 10 trials with the white-noise stimulus alone (82 db, 250 ms). Blinks in response to the noise were used to determine whether either sex emitted sensitized responses to the CS before training. Immediately after, they were exposed to 800 trials of trace eyeblink conditioning (200 trials/day). A white-noise generator attached to a speaker administered a white noise as a CS and a shock generator delivered an eyelid shock (0.65 mA) as the US. Each block of conditioning consisted of 100 trials with every 10-trial sequence composed of 1 CS-alone presentation, 4 paired presentations of the CS and US, 1 US-alone presentation, and 4 paired presentations of the CS and US. The intertrial interval was 25 ± 5 s. A 250-ms CS was followed by a 500 ms trace interval which was followed by a 100-ms US (Fig. 1B). Eyeblinks that occurred during the trace interval were considered conditioned responses (CRs) and were detected by changes in eyelid electromyographic activity, as described (8, 12) [See supporting information (SI) Methods for details]. We also assessed how well the animals learned to time the CR. To do that, we counted late CRs, defined as those that occurred within 250 ms of the US.
Immunohistochemistry for BrdU.
Animals were perfused 24 h after the last training session (12 days after BrdU injection), along with their respective naive controls. For killing, rats were deeply anesthetized with sodium pentobarbital (100 mg/kg) and intracardially perfused with 4% paraformaldehyde in 0.1 M phosphate buffer. Brains were extracted, postfixed, and coronal sections (40 μm) were cut through the entire DG of one hemisphere of the brain. For BrdU peroxidase staining, one of every twelfth section were collected and stained as before (8, 13) (See SI Methods for details). The primary mouse anti-BrdU was purchased from Becton Dickinson ImmunoSys. After BrDU staining, slides were counterstained with cresyl violet.
Quantitative Analysis and Volume.
Estimates of total numbers of BrdU-labeled cells were made using a modified unbiased stereology protocol (8, 11, 55, 56). This was accomplished by counting the number of BrdU-labeled cells in the SGZ, GCL, and hilus on every twelfth unilateral section throughout the DG (21.88 to 24.52 mm anteroposterior) (57). Slides were coded and the cells were counted blind to the experimental conditions, avoiding cells in the outermost focal plane. The tissue was magnified by 1,000 with a Nikon Eclipse E400 light microscope. The number of cells was multiplied by 24 to obtain an estimate of the total number of BrdU-labeled cells in the hippocampus. To account for sex differences in the size of the hippocampus, we also assessed the total volume of the DG (GCL, SGZ, and hilus) from cross-sectional area measurements obtained with Scion Image software (19, 58). The area of the DG was calculated from digital pictures of every twelfth unilateral section throughout the rostrocaudal hippocampus. To calculate the volume of the DG, the Cavalieri's principle was used (59) (See SI Methods for details). BrdU counts were expressed as density of BrdU-labeled cells per volume of the total DG or GCL/SGZ alone (number of BrdU-labeled cells/mm3). Finally, the density of BrdU-labeled cells was examined separately in the rostral (interaural 3.70 to 6.88 mm) and caudal (interaural 2.28 to 3.70 mm) hippocampus (57), as described in detail elsewhere (32). The dorsal hippocampus is associated with the rostral area, whereas the ventral area is more caudal.
Double Labeling: BrdU with DCX.
Double labeling for BrdU and DCX was performed in separate hippocampal free-floating sections slices, as before (16). Primary antibodies were goat anti-DCX (Santa Cruz Biotechnology), and mouse anti-BrdU (Becton-Dickinson ImmunoSys). Secondary antibodies were Rhodamine Red-X anti-goat (Jackson Immunoresearch) and Fluro 488 anti-mouse (Molecular Probes). The number of cells that expressed both markers was determined using a confocal laser scanning microscope (16). Twenty cells per subject (n = 4) were counted on random sections throughout the hippocampus (See SI Methods for details). The percentage of the new cells that differentiated into neurons (≈80%) was similar to that reported in previous studies (11–13, 16, 17, 19).
Behavioral Analysis.
All results are presented as means ± one standard error. The percentage of CRs across training trials was analyzed using repeated measures ANOVA with sex as the between-subjects independent variable and trials the within-subjects independent variable. A closer look at the individual data suggested that many subjects did not display a gradual acquisition curve. Instead, vigorous conditioned responding often appeared abruptly, after a period of no appreciable responding, the duration of which varied from rat to rat. To identify when these changes in responding occurred, we used an algorithm developed by Gallistel that detects changes in the slope of the cumulative record (25, 26, 60). The slope of the cumulative record indicates a momentary rate of responding (61). Therefore, abrupt changes in performance are often indicated by similarly abrupt changes in the slope (See SI Methods for details). To characterize acquisition, we defined 2 measures of learning: (i) the estimate of the asymptote for each rat, which was defined as the mean percentage of CRs during the last 2 sessions of training (even though some animals continued to increase their responding) and (ii) the asymptotic trial, which was the trial of the change point which led to a slope of 95% or more of the asymptote. This measure provided an estimate of how many trials of training were necessary to reliably express the trace CR.
Supplementary Material
Acknowledgments.
This work was supported by the National Science Foundation Grant IOB-0444364 and the National Institutes of Health National Institutes of Mental Health Grant MH59970 (to T.J.S.). This research was supported by a Marie Curie International Fellowship (to C.D.), within the sixth European Community Framework Program.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809650106/DCSupplemental.
References
- 1.de Frias CM, Nilsson LG, Herlitz A. Sex differences in cognition are stable over a 10-year period in adulthood and old age. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2006;13:574–587. doi: 10.1080/13825580600678418. [DOI] [PubMed] [Google Scholar]
- 2.Shors TJ. Stressful experience and learning across the lifespan. Annu Rev Psychol. 2006;57:55–85. doi: 10.1146/annurev.psych.57.102904.190205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saxe MD, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shors TJ, et al. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- 5.Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- 6.Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- 7.Shors T. From stem cells to grandmother cells: how neurogenesis relates to learning and memory. Cell Stem Cell. 2008;3:253–258. doi: 10.1016/j.stem.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Dalla C, Bangasser DA, Edgecomb C, Shors TJ. Neurogenesis and learning: acquisition and asymptotic performance predict how many new cells survive in the hippocampus. Neurobiol Learn Mem. 2007;88(1):143–148. doi: 10.1016/j.nlm.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drapeau E, Montaron MF, Aguerre S, Abrous DN. Learning-induced survival of new neurons depends on the cognitive status of aged rats. J Neurosci. 2007;27:6037–6044. doi: 10.1523/JNEUROSCI.1031-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epp JR, Spritzer MD, Galea LA. Hippocampus-dependent learning promotes survival of new neurons in the dentate gyrus at a specific time during cell maturation. Neuroscience. 2007;149:273–285. doi: 10.1016/j.neuroscience.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 11.Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 12.Leuner B, et al. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J Neurosci. 2004;24:7477–7481. doi: 10.1523/JNEUROSCI.0204-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waddell J, Shors TJ. Neurogenesis, learning and associative strength. European J Neurosci. 2008;27:3020–3028. doi: 10.1111/j.1460-9568.2008.06222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- 15.Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Integration of new neurons into functional neural networks. J Neurosci. 2006;26:12237–12241. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sisti HM, Glass AL, Shors TJ. Neurogenesis and the spacing effect: learning over time enhances memory and the survival of new neurons. Learn Mem. 2007;14:368–375. doi: 10.1101/lm.488707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leuner B, Waddell J, Gould E, Shors TJ. Temporal discontiguity is neither necessary nor sufficient for learning-induced effects on adult neurogenesis. J Neurosci. 2006;26:13437–13442. doi: 10.1523/JNEUROSCI.2781-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leuner B, Mendolia-Loffredo S, Shors TJ. High levels of estrogen enhance associative memory formation in ovariectomized females. Psychoneuroendocrinology. 2004;29:883–890. doi: 10.1016/j.psyneuen.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodes GE, Shors TJ. Distinctive stress effects on learning during puberty. Horm Behav. 2005;48:163–171. doi: 10.1016/j.yhbeh.2005.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci USA. 1998;95:4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bangasser DA, Shors TJ. The hippocampus is necessary for enhancements and impairments of learning following stress. Nat Neurosci. 2007;10:1401–1403. doi: 10.1038/nn1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kishimoto Y, Nakazawa K, Tonegawa S, Kirino Y, Kano M. Hippocampal CA3 NMDA receptors are crucial for adaptive timing of trace eyeblink conditioned response. J Neurosci. 2006;26:1562–1570. doi: 10.1523/JNEUROSCI.4142-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit's classically conditioned nictitating membrane response. Behav Neurosci. 1986;100:729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- 25.Gallistel CR, Fairhurst S, Balsam P. The learning curve: implications of a quantitative analysis. Proc Natl Acad Sci USA. 2004;101(36):13124–13131. doi: 10.1073/pnas.0404965101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papachristos EB, Gallistel CR. Autoshaped head poking in the mouse: a quantitative analysis of the learning curve. J Exp Anal Behav. 2006;85:293–308. doi: 10.1901/jeab.2006.71-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roof RL, Havens MD. Testosterone improves maze performance and induces development of a male hippocampus in females. Brain Res. 1992;572:310–313. doi: 10.1016/0006-8993(92)90491-q. [DOI] [PubMed] [Google Scholar]
- 28.Zhang JM, Konkle AT, Zup SL, McCarthy MM. Impact of sex and hormones on new cells in the developing rat hippocampus: a novel source of sex dimorphism? Eur J Neurosci. 2008;27:791–800. doi: 10.1111/j.1460-9568.2008.06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weible AP, O'Reilly JA, Weiss C, Disterhoft JF. Comparisons of dorsal and ventral hippocampus cornu ammonis region 1 pyramidal neuron activity during trace eye-blink conditioning in the rabbit. Neuroscience. 2006;141:1123–1137. doi: 10.1016/j.neuroscience.2006.04.065. [DOI] [PubMed] [Google Scholar]
- 30.Yoon T, Otto T. Differential contributions of dorsal vs. ventral hippocampus to auditory trace fear conditioning. Neurobiol Learn Mem. 2007;87(4):464–475. doi: 10.1016/j.nlm.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 32.Banasr M, Soumier A, Hery M, Mocaer E, Daszuta A. Agomelatine, a new antidepressant, induces regional changes in hippocampal neurogenesis. Biol Psychiatry. 2006;59:1087–1096. doi: 10.1016/j.biopsych.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 33.Ambrogini P, et al. Spatial learning affects immature granule cell survival in adult rat dentate gyrus. Neurosci Lett. 2000;286:21–24. doi: 10.1016/s0304-3940(00)01074-0. [DOI] [PubMed] [Google Scholar]
- 34.McEchron MD, Disterhoft JF. Hippocampal encoding of non-spatial trace conditioning. Hippocampus. 1999;9:385–396. doi: 10.1002/(SICI)1098-1063(1999)9:4<385::AID-HIPO5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 35.Gilmartin MR, McEchron MD. Single neurons in the dentate gyrus and CA1 of the hippocampus exhibit inverse patterns of encoding during trace fear conditioning. Behav Neurosci. 2005;119:164–179. doi: 10.1037/0735-7044.119.1.164. [DOI] [PubMed] [Google Scholar]
- 36.Spence KW, Spence JT. Sex and anxiety differences in eyelid conditioning. Psychol Bull. 1966;65:137–142. doi: 10.1037/h0022982. [DOI] [PubMed] [Google Scholar]
- 37.Spritzer MD, Galea LA. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Dev Neurobiol. 2007;67:1321–1333. doi: 10.1002/dneu.20457. [DOI] [PubMed] [Google Scholar]
- 38.Hausmann M, Schoofs D, Rosenthal HE, Jordan K. Interactive effects of sex hormones and gender stereotypes on cognitive sex differences: A psychobiosocial approach. Psychoneuroendocrinology. 2008 doi: 10.1016/j.psyneuen.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 39.Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62:847–855. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- 41.Stark R, et al. Influence of the stress hormone cortisol on fear conditioning in humans: evidence for sex differences in the response of the prefrontal cortex. Neuroimage. 2006;32:1290–1298. doi: 10.1016/j.neuroimage.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 42.Barker JM, Galea LA. Repeated estradiol administration alters different aspects of neurogenesis and cell death in the hippocampus of female, but not male, rats. Neuroscience. 2008;152:888–902. doi: 10.1016/j.neuroscience.2007.10.071. [DOI] [PubMed] [Google Scholar]
- 43.Shors TJ, Miesegaes G. Testosterone in utero and at birth dictates how stressful experience will affect learning in adulthood. Proc Natl Acad Sci USA. 2002;99:13955–13960. doi: 10.1073/pnas.202199999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gage FH, Thompson RG. Differential distribution of norepinephrine and serotonin along the dorsal-ventral axis of the hippocampal formation. Brain Res Bull. 1980;5:771–773. doi: 10.1016/0361-9230(80)90220-8. [DOI] [PubMed] [Google Scholar]
- 45.Milner TA, Loy R, Amaral DG. An anatomical study of the development of the septo-hippocampal projection in the rat. Brain Res. 1983;284:343–371. doi: 10.1016/0165-3806(83)90017-2. [DOI] [PubMed] [Google Scholar]
- 46.Burman MA, Starr MJ, Gewirtz JC. Dissociable effects of hippocampus lesions on expression of fear and trace fear conditioning memories in rats. Hippocampus. 2006;16:103–113. doi: 10.1002/hipo.20137. [DOI] [PubMed] [Google Scholar]
- 47.Trivedi MA, Coover GD. Neurotoxic lesions of the dorsal and ventral hippocampus impair acquisition and expression of trace-conditioned fear-potentiated startle in rats. Behav Brain Res. 2006;168:289–298. doi: 10.1016/j.bbr.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 48.Snyder JS, Radik R, Wojtowicz JM, Cameron HA. Anatomical gradients of adult neurogenesis and activity: Young neurons in the ventral dentate gyrus are activated by water maze training. Hippocampus. 2008 doi: 10.1002/hipo.20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bannerman DM, et al. Regional dissociations within the hippocampus−memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 50.Lee T, Kim JJ. Differential effects of cerebellar, amygdalar, and hippocampal lesions on classical eyeblink conditioning in rats. J Neurosci. 2004;24:3242–3250. doi: 10.1523/JNEUROSCI.5382-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shughrue PJ, Merchenthaler I. Evidence for novel estrogen binding sites in the rat hippocampus. Neuroscience. 2000;99:605–612. doi: 10.1016/s0306-4522(00)00242-6. [DOI] [PubMed] [Google Scholar]
- 52.Dalla C, Edgecomb C, Whetstone AS, Shors TJ. Females do not express learned helplessness like males do. Neuropsychopharmacology. 2008;33:1559–1569. doi: 10.1038/sj.npp.1301533. [DOI] [PubMed] [Google Scholar]
- 53.Christie BR, Cameron HA. Neurogenesis in the adult hippocampus. Hippocampus. 2006;16:199–207. doi: 10.1002/hipo.20151. [DOI] [PubMed] [Google Scholar]
- 54.Nowakowski RS, Lewin SB, Miller MW. Bromodeoxyuridine immunohistochemical determination of the lengths of the cell cycle and the DNA-synthetic phase for an anatomically defined population. J Neurocytol. 1989;18:311–318. doi: 10.1007/BF01190834. [DOI] [PubMed] [Google Scholar]
- 55.Shors TJ, et al. Neurogenesis and helplessness are mediated by controllability in males but not in females. Biol Psychiatry. 2007;62:487–495. doi: 10.1016/j.biopsych.2006.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- 57.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Fourth Edition. Elsevier: New York; 2004. [Google Scholar]
- 58.Leuner B, Mirescu C, Noiman L, Gould E. Maternal experience inhibits the production of immature neurons in the hippocampus during the postpartum period through elevations in adrenal steroids. Hippocampus. 2007;17:434–442. doi: 10.1002/hipo.20278. [DOI] [PubMed] [Google Scholar]
- 59.Gundersen HJ, et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. Apmis. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- 60.Gallistel CR, Mark TA, King AP, Latham PE. The rat approximates an ideal detector of changes in rates of reward: implications for the law of effect. J Exp Psychol Anim Behav Process. 2001;27:354–372. doi: 10.1037//0097-7403.27.4.354. [DOI] [PubMed] [Google Scholar]
- 61.Skinner BF. Farewell, My LOVELY! J Exp Anal Behav. 1976;25:218. doi: 10.1901/jeab.1976.25-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.