Abstract
Benign prostatic hyperplasia (BPH) is usually described as a pathological proliferation of prostatic fibroblasts/myofibroblasts and epithelial cells. In the present study of BPH samples, we have made a morphological and immunohistochemical study of BPH prostatic sections using markers of proliferation, apoptosis, hormone receptors, and TGF-β signaling. We found no evidence of proliferation in the stroma but in the epithelium of some ducts; 0.7% of the basal and 0.4% of luminal cells were positive for Ki67 and PCNA. Androgen receptor and estrogen receptor beta (ERβ)1 and ERβcx were abundant in both stromal and epithelial compartments but cells expressing ERα were very rare. What was very common in all BPH samples was the following: (i) regions of the ductal epithelium where the epithelial cells did not express E-cadherin, had lost their polarization, and become spindle shaped (the nuclei of these cells were strongly positive for pSmad 3 and Snail); and (ii) regions where the walls of the blood vessels were extremely thick and there was loss of endothelial layer. Loss of E-cadherin, increased pSmad 3, and high expression of Snail are all characteristic of epithelial-mesenchymal transition (EMT). We conclude that BPH is not a disease of prostatic stromal proliferation but rather of accumulation of mesenchymal-like cells derived from the prostatic epithelium and the endothelium. TGF-β is thought to play a key role in EMT. Our data suggests that TGF-β/Smad should be considered as targets for treatment of BPH.
Keywords: proliferation, TGF beta signaling
In 1649, Riolan described for the first time the enlargement of the prostate and its most common clinical manifestation, the obstruction of the bladder outlet (1). Since then, much research has been focused on the etiology and the clinical management of benign prostatic hyperplasia (BPH). BPH has been defined as a progressive hyperplasia of glandular and stromal tissues around the urethra. It is characterized by a four-fold increase of the stromal component and a nearly doubling of the glandular elements of the prostate and because of these changes, BPH has been perceived as mainly a proliferative stromal disease (2).
More than two-thirds of men older than 50 years have histological evidence of BPH and, after age 70, the proportion increases to 80% (3). At present there is no completely effective treatment for BPH. The mainstay of therapies is the combination of 5α-reductase inhibitors, which regulate the levels of 5α-dihydrotestosterone (DHT), and alpha adrenergic-blockers, which decrease adrenergic tone. However, in some patients surgery, transurethral resection of the prostate (TURP), is the only effective intervention (4).
Despite the prevalence of BPH, its pathogenesis still remains largely unresolved. Several different partially overlapping theories have been proposed, all of which seem to be operative to some extent. Men castrated before puberty do not develop BPH (5) and, in men with genetic disorders that inhibit androgen production or androgen action, prostatic growth is impaired (6). Thus, androgen is thought to play a permissive role in BPH and, although levels of DHT are not elevated in BPH (7, 8), 5α-reductase inhibitors do relieve the symptoms of the disease (6).
In the mid-1970s, McNeal postulated that BPH results from a reawakening of embryonic inductive interactions between the prostatic stroma and the epithelium, which in turn induces epithelial hyperplasia. He proposed the idea that the initial lesion of BPH is not a stromal nodule but a formation of glandular budding and branching toward a central focus that occurs primarily in the transition zone, a situation reminiscent of embryonic development (9). Whether abnormal growth in BPH is due to embryonic reawakening (9), stem cell defects (10), chronic inflammation (11, 12), imbalance between androgen/estrogen signaling (13), increased TGF-β signaling (14), or to other so-far undefined factors, is an area of intense investigation.
With aging, changes in the ratio of local concentrations of androgens and estrogens occur with a decrease in DHT levels, leading to an overall increase in the relative levels of E2 (15). In addition, estrogens can be produced locally in the prostate gland via conversion of testosterone to 17β-estradiol by aromatase expressed within the stroma (16). With an increased estrogen:androgen ratio, epithelial cells secrete TGF-β, a pleiotropic factor that induces smooth muscle differentiation and increases the extracellular matrix (ECM) in the surrounding stromal cells (14, 17).
In the present study, we have analyzed samples obtained by TURP from BPH patients and report evidence of dysregulation of TGF-β signaling. Smad 3 (in its phosphorylated state), Snail, and Slug, three important downstream elements in the TGF-β signaling, were up-regulated in BPH tissue. We found high levels of ERβ1 and ERβcx in both the stroma and epithelium of BPH but ERα expression was very rare. Proliferation markers, PCNA and Ki67, revealed proliferating epithelial cells in many ducts but no stromal proliferation. We here propose that BPH is not a proliferative disease of the stroma but the result of accumulation of myofibroblasts and smooth muscle cells, as a consequence of epithelial proliferation and epithelial-mesenchymal transition (EMT). This phenomenon involves changes in gene expression that disrupt epithelial polarity and establish a mesenchymal phenotype, with concomitant alterations in cytoskeletal organization, cell adhesion, and production of extracellular matrix. The TGF-β/Smad3 is a key signaling pathway associated with the induction and maintenance of EMT (18). We suggest targeting of TGF-β/Smad signaling as a therapeutic approach in the treatment of BPH.
Results
Immunohistochemical Localization of Steroid Receptors in BPH.
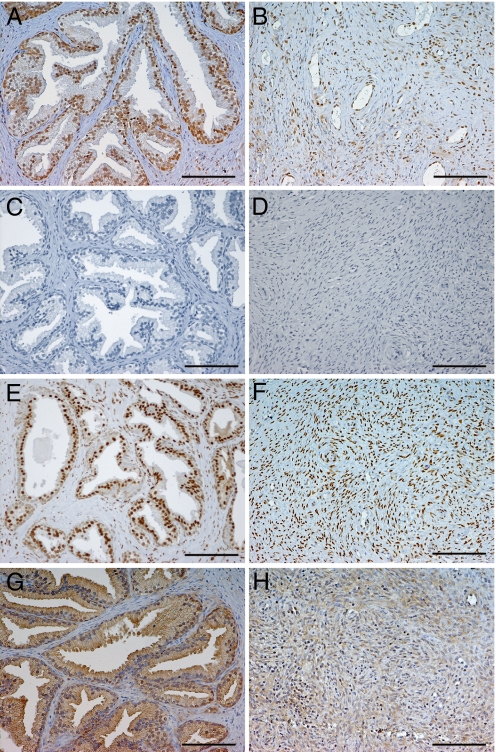
Sections from 16 patients were stained for ERα, ERβ1, ERβcx, and AR (Fig. 1). ERβ1 was expressed in the nuclei of both basal and luminal cells and in many stromal cells in all BPH specimens. In contrast, ERα was only detectable in three patients and its expression was confined to very small areas of the stroma. AR was expressed in the nuclei of luminal epithelial cells but not in the basal cells. AR was also more abundant in the stroma than was ERβ1, with the majority of nuclei of fibroblasts positive for AR. AR staining was also detected in several nuclei of endothelial cells and in smooth muscle cells and fibroblasts in blood vessel walls. The pattern of expression of ERβcx was quite similar to that of ERβ1. In most cases, ERβcx and ERβ1 seem to be co-expressed in the same cells, suggesting that ERβcx might be modulating ERβ1 function.
Fig. 1.
Expression of ERβ1, ERα, AR, and ERβcx in the representative BPH tissue samples. Immunostaining of ERβ1 (A and B). ERβ1 is expressed both in the basal and luminal cells in the glandular epithelium. It is also present in the nuclei of some stromal cells. Both epithelium and stroma are almost negative for ERα (C and D). AR is consistently found in the nuclei of luminal cells, as well as stromal cells in high proportion (E and F). ERβcx is detected mostly in the epithelium of the ducts, both in basal and luminal cells (G) and less frequently in the stroma (H). (Scale bar, 100 μm.)
Evaluation of Cell Proliferation and Apoptosis in BPH.
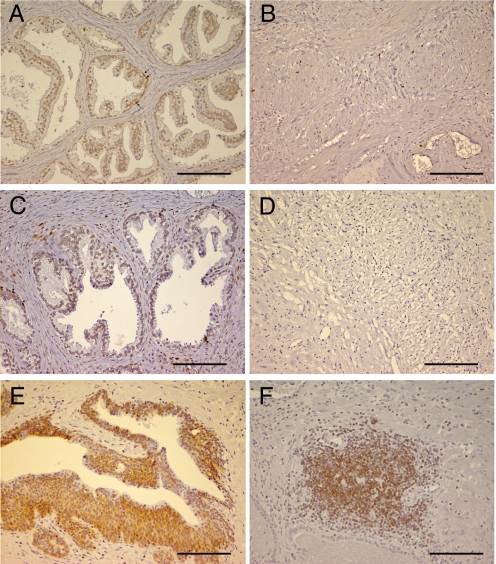
Using the proliferation markers, Ki67 and PCNA, we found no signs of proliferation in the stroma as shown in Fig. 2 B and D. In the epithelium (Fig. 2 A and C), the percentage of Ki67-positive basal and luminal cells was 0.7% and 0.4%, respectively. With PCNA antibodies, 0.8% and 0.4% of basal and luminal cells, respectively, were positive. Approximately 40% of the ducts analyzed showed some positive staining for Ki67 or PCNA.
Fig. 2.
Proliferation and apoptosis in prostate samples from BPH patients. Index of proliferation was calculated by using the markers Ki67 and PCNA. (B and D) Shown is the lack of expression of proliferation in the stromal compartment. In the epithelium of some ducts, Ki67-positive (A) or PCNA-positive (C) were detected mostly in the basal layer. The antiapoptotic protein Bcl-2 was strongly detected in epithelium (E) and in some infiltrations of immune cells in the stroma (F). (Scale bar, 100 μm.)
Moreover, the antiapoptotic factor, Bcl-2, was highly expressed in both the basal and secretory epithelia in the most hyperplastic acini, whereas in adjacent normal epithelium, Bcl-2 expression was limited (Fig. 2E). Thus lack of apoptosis probably contributes to the accumulation of prostate epithelial cells.
Disintegration of Cell-Cell Adhesions and Loss of Epithelial Polarity in BPH Ducts.
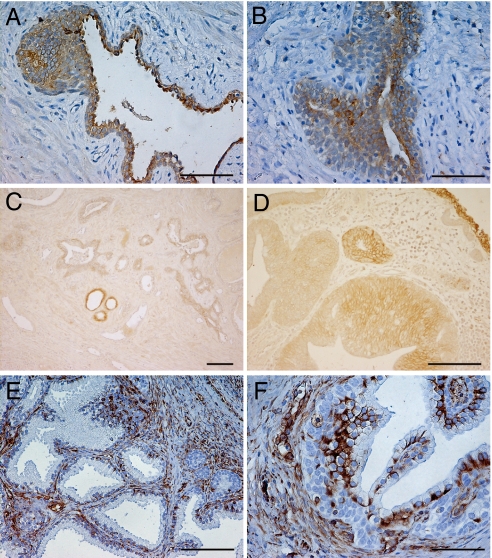
Within normal prostate epithelium, basal cells express CK5/14, luminal cells express CK8/18, and an intermediate cell population can be identified by co-expression of CK5/18. Although the pattern of expression of cytokeratin 8 (CK8) was normal in some well-conserved ducts, there was a marked decrease in the expression of this cytokeratin in regions where the epithelium was multilayered and the cells were elongated and spindle-shape (Fig. 3 A and B).
Fig. 3.
Distribution of CK8, E-cadherin, and vimentin in BPH prostatic glandular epithelium. CK8 expression was detected by using LMW antibody. A down-regulation of this cytokeratin is observed in multilayer epithelium ducts (A and B) that are losing epithelium organization. E-cadherin is almost absent in BPH ducts as is shown in C and in more detail in D. An increase in vimentin expression is detected in hyperplastic glands and surrounding stroma (E and F). (Scale bar, 100 μm.)
A substantial reduction of E-cadherin was observed in the epithelial layer in all BPH specimens (Fig. 3 C and D). In contrast, there was more abundant expression of the mesenchymal marker, vimentin, in hyperplastic glands than in adjacent normal glands. This increase was located largely in the subnuclear region of the epithelium of BPH glands (Fig. 3F) and also in the stromal cells associated with these glands (Fig. 3E). Taken together, these data indicate that there is a progressive switch from an epithelial to a mesenchymal phenotype in BPH tissues.
There were also regions in the BPH stroma where the walls of the blood vessels were extremely thick with a concomitant loss of endothelial layer. In the worst areas, there was occlusion of the vessels. These observations suggested that an endothelium to mesenchymal transition process might also be contributing to the accumulation of mesenchymal-type cells that occurs in BPH.
TGF-β Signaling Is Implicated in EMT and in the Development of BPH.
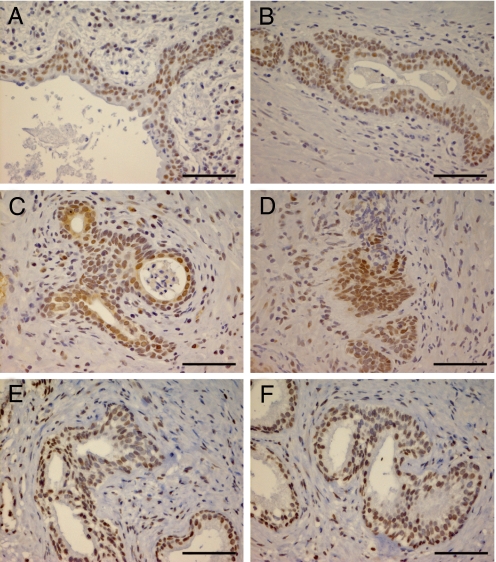
As Fig. 4 shows, we found an up-regulation of the transcription factors pSmad 3, Snail, and Slug, in specific areas where epithelial cells were losing adhesion contacts and invading through ECM. Therefore we conclude that TGF-β/Smad signaling pathway is activated in the pathogenesis of BPH and is associated with EMT.
Fig. 4.
Detection of EMT regulators in BPH tissue. The expression of the transcriptional factors pSmad 3 (A and B), Snail (C and D), and Slug (E and F) were analyzed in BPH samples. We observe a markedly nuclear staining of these factors in areas of epithelium remodeling where cells are getting an elongated appearance. (Scale bar, 50 μm.)
Discussion
Benign prostate hyperplasia (BPH) is one of the most frequent prostate abnormalities and an important cause of urinary difficulties in elderly men (19). Despite its high incidence, little is known with any certainty about the etiopathology of BPH. One factor accounting for this gap in knowledge is absence of adequate animal and in vitro models that closely reflect the biochemical and physiological features of the disease in men. In the present study we have analyzed samples from BPH patients by using markers of proliferation, apoptosis, hormone receptors, and TGF-β signaling.
Over the years, the question of whether or not proliferation plays the main role in the development of BPH has been raised. Claus et al. (20) reported that enlargement of the prostate was associated with an increase of its weight, but that there was no significant correlation between proliferation rate and prostate weight. In the present study, we observed some proliferation in the prostatic epithelium but none in the stroma in BPH tissue. We conclude that BPH is not a proliferative disease but a disease of accumulation of cells that are resistant to death. We observed that most of the replicating epithelial cells were basal cells in contrast to malignant prostatic lesions in which luminal as well as basal cells proliferate (21). Moreover, the observed increase in expression of the antiapoptotic factor, Bcl-2 in BPH may also account for accumulation of cells (22, 23). The question then shifts to the origin of the accumulated cells.
We have come to the conclusion that the BPH stroma is derived from the epithelium by a process called EMT, which means that epithelial cells lose their epithelial characteristics, particularly their orientation and attachment to the basement membrane, and acquire a mesenchymal phenotype. Normally, epithelial cells anchor to the basement membrane, establishing an aligned apical-basal polarity. This association with the basement membrane ensures that epithelial cells maintain their positioning within the epithelium and preclude their entrance into the underlying extracellular matrix (ECM). During EMT, the epithelial cells lose this stability and become more migratory, fibroblast-like cells with concomitant loss of expression of epithelial markers, such as cytokeratins, E-cadherin, desmoplakin, and vinculin (24).
In the present study, we observed that E-cadherin was down regulated in regions where the epithelial cells were assuming an elongated shape and were no longer attached to the basement membrane. Moreover there was a decrease of CK8 and an increased expression of vimentin in hyperplastic glands. Vimentin is the mesenchymal marker most commonly associated with EMT (25) and has been described to be up-regulated in BPH (26).
Several stromal and epithelial growth factors and cytokines have been reported to be overexpressed in BPH. Among all of these factors, special attention has been focused on TGF-β (27, 28). Members of TGF-β superfamily have been implicated in EMT. TGF-β stimulates transdifferentiation of prostatic fibroblasts into myofibroblasts and smooth muscle cells along with induction of ECM proteins (29, 30). In response to TGF-β binding to TGF-β receptors, there is phosphorylation of Smad 2 and Smad 3 (31). Phosphorylated Smads partner with cytoplasmic Smad 4 and translocate to the nucleus where Smad complexes control transcription of target genes. TGF-β activates both transcription factors Snail and Slug directly through Smad 3. Snail and Slug are repressors of E-cadherin.
We observed an intense expression of the transcription factors, pSmad 3, Snail, and Slug in selected areas, which suggests that TGF-β/Smad signaling may play an important role in the increased stromal accumulation and epithelial growth and that an epithelial-mesenchymal transition is related to the progression of BPH.
Estrogen receptors are present in human prostate. ERβ1 is the predominant ER subtype, expressed in the majority of the epithelial as well as the stromal cells whereas ERα is found in the stroma of peripheral zone (PZ) but not the transitional zone (TZ) (32). Some studies report an increase in estradiol within BPH tissue (13) and ERα has been suggested to mediate stromal proliferation in BPH (33). In the present study we observed expression of ERβ1, but not ERα in epithelial and stromal cells. This lack of ERα is compatible with the idea that BPH develops in the ERα-negative TZ.
TGF-β signaling is one of the most important lines of communication between stroma and epithelium (34) and estrogen influences TGF-β signaling. More recently, ERβ1 has been shown to play an important role in TGF-β signaling because it regulates the expression of the TGF-β early response gene (35). ERβ1 in the prostate may therefore be responsible for regulating TGF-β signaling. The presence of ERβcx together with ERβ1 in BPH prostate may be an indication of abnormal ERβ signaling. ERβcx is a splice variant of ERβ1, which does not bind estrogen but can repress the function of ERα and ERβ1 (36, 37). ERβcx is expressed in many cancers but is not normally expressed in the prostate (38).
AR was expressed in the luminal epithelium of BPH samples, and much more remarkably, in the stroma. Apart from the classical role of AR already described, an alternative function of AR in BPH might be to contribute in the accumulation of ECM. Versican, a chondroitin sulfate proteoglycan, is one of the main components of ECM, and it has been described to be overexpressed in BPH (39, 40). Versican transcription is induced by androgen-stimulated AR transactivation in human prostate cancer cells (41). Thus AR may participate in the remodeling and accumulation of ECM that occurs in BPH, through modulation of stromal cell secretion of macromolecules, such as versatin.
In summary, we suggest abnormalities in ERβ and TGF-β signaling in the etiology of BPH and that the therapeutic targeting of TGF-β/Smad pathway should be considered in the prevention and treatment of this disease.
Materials and Methods
Tissue.
Sixteen BPH prostate specimens obtained by transurethral resection of the prostate (TURP) were collected. TURP was performed because of lower urinary tract symptoms due to obstructive benign prostatic disease. Men with prostate cancer were excluded from the study. For TURP we used a bipolar “Olympus” resection unit, working in isotonic saline solution. The specimens were immediately frozen by −40 °C after performing TURP and stored in the Danube Hospital, Vienna, Austria. For analyses they were sent to the Karolinska Institute, Stockholm, Sweden. The mean prostate specific antigen (PSA) in our study was 3.5 ng/ml (range 1.8 to 5.9). The mean prostate volume was 36.9 ml (range 29 to 64 ml). Usage of patient material was approved by an institutional ethical committee (reg. number EK 05–094-0905).
Immunohistochemistry.
Representative blocks of paraffin-embedded tissues were cut at a 4-μm thickness, dewaxed, and rehydrated. Antigen retrieval was performed by microwaving sections in 10 mM citrate buffer (pH 6.0). Endogenous peroxidase was blocked by incubation for 30 min with a solution of 0.5% hydrogen peroxidase in 50% methanol. To block nonspecific binding, sections were incubated in 3% BSA plus 0.1% Nonidet P-40 in PBS for 1 h at room temperature. Primary antibodies were diluted in 1% BSA plus 0.1% Nonidet P-40 in PBS. Antibodies and conditions used are indicated in the Table 1. After washing, sections were incubated with the corresponding secondary antibodies for 1 h at room temperature. The Vectastain ABC kit (Vector Laboratories) was used for the avidin-biotin complex (ABC) method according the manufacturer′s instructions. Peroxidase activity was visualized with 3, 3′-diaminobenzidine (DAKO). The sections were lightly counterstained with hematoxylin, dehydrated through an ethanol series to xylene, and mounted. Appropiate positive controls were included in each immunohistochemical run to verify the specificity of the staining and negative controls were produced by substituting the primary antibody with PBS in duplicate sections. The visualizations were done using a light microscope.
Table 1.
Primary antibodies and conditions
| Ab | Source | Dilution | Retrieval | Incubation |
|---|---|---|---|---|
| ERα | DAKO | 1:35 | 20′ | Overnight 4°C |
| ERβ | Homemade | 1:300 | 20′ | Overnight 4°C |
| ERβcx | Homemade | 1:50 | 5′ | Overnight 4°C |
| AR | Santa Cruz | 1:200 | 20′ | Overnight 4°C |
| Ki67 | Novocastra | 1:1,000 | 2X5′ | Overnight 4°C |
| PCNA | Abcam | 1:6,000 | 20′ | 1 h RT |
| Bcl-2 | Novocastra | 1:50 | 2′ | Overnight 4°C |
| CK8 | DAKO | 1:50 | 20′ | Overnight 4°C |
| E-cadh | Santa Cruz | 1:150 | No retrieval | Overnight 4°C |
| Vimentin | DAKO | 1:100 | 2′ | Overnight 4°C |
| pSmad 3 | Chemicon | 1:2,000 | 20′ | Overnight 4°C |
| Snail | Abcam | 1:400 | 20′ | Overnight 4°C |
| Slug | Abcam | 1:5,000 | 20′ | 1 h RT |
RT, room temperature.
Quantification of Proliferation.
To evaluate the index of proliferation in BPH samples, the markers Ki67 and PCNA were used. Randomly selected microscopic fields (10–15) at 20× magnification per slide were used to count the Ki67 and PCNA positive cells. At least 25 ducts and 1,500 cells were counted for each section. The labeling index is the percentage of labeled cell nuclei over the total number of counted epithelial cell nuclei.
Acknowledgments.
We thank Dr Thomas Schwend for enlightening discussions. This study was supported by the Swedish Cancer Society and KaroBioAB.
Footnotes
Conflict of interest statement: J.-Å.G. is a shareholder and consultant of KaroBio AB.
Potential conflict of interest.
Jan-Åke Gustafsson is shareholder and consultant of KaroBio AB.
References
- 1.Riolan J Lutetiae Parisiorum, editor. Opera Anatomica, Vetera; Recognita and Auctiora. 1649. pp. 874–930. [Google Scholar]
- 2.Rohr HP, Bartsch G. Human benign prostatic hyperplasia: a stromal disease? New perspectives by quantitative morphology. Urology. 1980;16:625–633. doi: 10.1016/0090-4295(80)90577-4. [DOI] [PubMed] [Google Scholar]
- 3.Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132:474–479. doi: 10.1016/s0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- 4.Tiwari A, Krishna NS, Nanda K, Chugh A. Benign prostatic hyperplasia: an insight into current investigational medical therapies. Expert Opin Investig Drugs. 2005;14:1359–1372. doi: 10.1517/13543784.14.11.1359. [DOI] [PubMed] [Google Scholar]
- 5.Schroder FH. Medical treatment of benign prostatic hyperplasia: the effect of surgical or medical castration. Prog Clin Biol Res. 1994;386:191–196. [PubMed] [Google Scholar]
- 6.Bartsch G, Rittmaster RS, Klocker H. Dihydrotestosterone and the concept of 5alpha-reductase inhibition in human benign prostatic hyperplasia. World J Urol. 2002;19:413–425. doi: 10.1007/s00345-002-0248-5. [DOI] [PubMed] [Google Scholar]
- 7.Krieg M, Bartsch W, Janssen W, Voigt KD. A comparative study of binding, metabolism and endogenous levels of androgens in normal, hyperplastic and carcinomatous human prostate. J Steroid Biochem. 1979;11:615–624. doi: 10.1016/0022-4731(79)90090-6. [DOI] [PubMed] [Google Scholar]
- 8.Walsh PC, Hutchins GM, Ewing LL. Tissue content of dihydrotestosterone in human prostatic hyperplasis is not supranormal. J Clin Invest. 1983;72:1772–1777. doi: 10.1172/JCI111137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNeal JE. Origin and evolution of benign prostatic enlargement. Invest Urol. 1978;15:340–345. [PubMed] [Google Scholar]
- 10.Lin VK, et al. Prostatic stromal cells derived from benign prostatic hyperplasia specimens possess stem cell like property. Prostate. 2007;67:1265–1276. doi: 10.1002/pros.20599. [DOI] [PubMed] [Google Scholar]
- 11.Kramer G, Mitteregger D, Marberger M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur Urol. 2007;51:1202–1216. doi: 10.1016/j.eururo.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Feder-Mengus C, et al. High expression of indoleamine 2,3-dioxygenase gene in prostate cancer. Eur J Cancer. 2008;44:2266–2275. doi: 10.1016/j.ejca.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 13.Kozak I, Bartsch W, Krieg M, Voigt KD. Nuclei of stroma: site of highest estrogen concentration in human benign prostatic hyperplasia. Prostate. 1982;3:433–438. doi: 10.1002/pros.2990030503. [DOI] [PubMed] [Google Scholar]
- 14.Huang X, Lee C. Regulation of stromal proliferation, growth arrest, differentiation and apoptosis in benign prostatic hyperplasia by TGF-beta. Front Biosci. 2003;8:s740–749. doi: 10.2741/1093. [DOI] [PubMed] [Google Scholar]
- 15.Shibata Y, et al. Changes in the endocrine environment of the human prostate transition zone with aging: simultaneous quantitative analysis of prostatic sex steroids and comparison with human prostatic histological composition. Prostate. 2000;42:45–55. doi: 10.1002/(sici)1097-0045(20000101)42:1<45::aid-pros6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 16.Tsugaya M, et al. Testosterone metabolism in primary cultures of epithelial cells and stroma from benign prostatic hyperplasia. Urol Res. 1996;24:265–271. doi: 10.1007/BF00304775. [DOI] [PubMed] [Google Scholar]
- 17.Wu Q, et al. Regulation of proliferation and differentiation of prostatic stromal cells by oestradiol through prostatic epithelial cells in a paracrine manner. BJU Int. 2008;101:497–502. doi: 10.1111/j.1464-410X.2007.07340.x. [DOI] [PubMed] [Google Scholar]
- 18.Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 19.Levitt JM, Slawin KM. Prostate-specific antigen and prostate-specific antigen derivatives as predictors of benign prostatic hyperplasia progression. Curr Urol Rep. 2007;8:269–274. doi: 10.1007/s11934-007-0072-y. [DOI] [PubMed] [Google Scholar]
- 20.Claus S, Wrenger M, Senge T, Schulze H. Immunohistochemical determination of age related proliferation rates in normal and benign hyperplastic human prostates. Urol Res. 1993;21:305–308. doi: 10.1007/BF00296825. [DOI] [PubMed] [Google Scholar]
- 21.McNeal JE, Haillot O, Yemoto C. Cell proliferation in dysplasia of the prostate: analysis by PCNA immunostaining. Prostate. 1995;27:258–268. doi: 10.1002/pros.2990270505. [DOI] [PubMed] [Google Scholar]
- 22.Kyprianou N, Tu H, Jacobs SC. Apoptotic versus proliferative activities in human benign prostatic hyperplasia. Hum Pathol. 1996;27:668–675. doi: 10.1016/s0046-8177(96)90396-2. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Zhang Q, Zhang Z, Na Y, Guo Y. Apoptosis profiles in benign prostatic hyperplasia: close associations of cell kinetics with percent area density of histologic composition. Urology. 2006;68:905–910. doi: 10.1016/j.urology.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilles C, et al. Transactivation of vimentin by beta-catenin in human breast cancer cells. Cancer Res. 2003;63:2658–2664. [PubMed] [Google Scholar]
- 26.Fraga CH, True LD, Kirk D. Enhanced expression of the mesenchymal marker, vimentin, in hyperplastic versus normal human prostatic epithelium. J Urol. 1998;159:270–274. doi: 10.1016/s0022-5347(01)64080-1. [DOI] [PubMed] [Google Scholar]
- 27.Mori H, et al. Increased expression of genes for basic fibroblast growth factor and transforming growth factor type beta 2 in human benign prostatic hyperplasia. Prostate. 1990;16:71–80. doi: 10.1002/pros.2990160108. [DOI] [PubMed] [Google Scholar]
- 28.Lucia MS, Lambert JR. Growth factors in benign prostatic hyperplasia: basic science implications. Curr Urol Rep. 2008;9:272–278. doi: 10.1007/s11934-008-0048-6. [DOI] [PubMed] [Google Scholar]
- 29.Peehl DM, Sellers RG. Induction of smooth muscle cell phenotype in cultured human prostatic stromal cells. Exp Cell Res. 1997;232:208–215. doi: 10.1006/excr.1997.3525. [DOI] [PubMed] [Google Scholar]
- 30.Tuxhorn JA, et al. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res. 2002;8:2912–2923. [PubMed] [Google Scholar]
- 31.Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 32.Tsurusaki T, et al. Zone-dependent expression of estrogen receptors alpha and beta in human benign prostatic hyperplasia. J Clin Endocrinol Metab. 2003;88:1333–1340. doi: 10.1210/jc.2002-021015. [DOI] [PubMed] [Google Scholar]
- 33.Schulze H, Claus S. Histological localization of estrogen receptors in normal and diseased human prostates by immunocytochemistry. Prostate. 1990;16:331–343. doi: 10.1002/pros.2990160408. [DOI] [PubMed] [Google Scholar]
- 34.Fleisch MC, Maxwell CA, Barcellos-Hoff MH. The pleiotropic roles of transforming growth factor beta in homeostasis and carcinogenesis of endocrine organs. Endocr Relat Cancer. 2006;13:379–400. doi: 10.1677/erc.1.01112. [DOI] [PubMed] [Google Scholar]
- 35.Hawse JR, et al. Estrogen receptor beta isoform-specific induction of transforming growth factor beta-inducible early gene-1 in human osteoblast cells: an essential role for the activation function 1 domain. Mol Endocrinol. 2008;22:1579–1595. doi: 10.1210/me.2007-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore JT, et al. Cloning and characterization of human estrogen receptor beta isoforms. Biochem Biophys Res Commun. 1998;247:75–78. doi: 10.1006/bbrc.1998.8738. [DOI] [PubMed] [Google Scholar]
- 37.Ogawa S, et al. Molecular cloning and characterization of human estrogen receptor betacx: a potential inhibitor ofestrogen action in human. Nucleic Acids Res. 1998;26:3505–3512. doi: 10.1093/nar/26.15.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Esslimani-Sahla M, et al. Increased estrogen receptor betacx expression during mammary carcinogenesis. Clin Cancer Res. 2005;11:3170–3174. doi: 10.1158/1078-0432.CCR-04-2298. [DOI] [PubMed] [Google Scholar]
- 39.Sakko AJ, et al. Versican accumulation in human prostatic fibroblast cultures is enhanced by prostate cancer cell-derived transforming growth factor beta1. Cancer Res. 2001;61:926–930. [PubMed] [Google Scholar]
- 40.Cross NA, et al. The expression and regulation of ADAMTS-1, -4, -5, -9, and -15, and TIMP-3 by TGFbeta1 in prostate cells: relevance to the accumulation of versican. Prostate. 2005;63:269–275. doi: 10.1002/pros.20182. [DOI] [PubMed] [Google Scholar]
- 41.Read JT, et al. Androgen receptor regulation of the versican gene through an androgen response element in the proximal promoter. J Biol Chem. 2007;282:31954–31963. doi: 10.1074/jbc.M702099200. [DOI] [PubMed] [Google Scholar]