Abstract
Hyperbaric oxygen (HBO2) therapy is reported to cause pain relief in several conditions of chronic pain. A single 60-min session of HBO2 treatment produced a prolonged antinociceptive effect in mice that persisted for 90 min after cessation of treatment. The HBO2-induced antinociception was significantly attenuated by pretreatment prior to HBO2 exposure with the opioid antagonist naltrexone, the non-specific nitric oxide synthase (NOS)-inhibitor NG-nitro-L-arginine methyl ester (L-NAME) and the selective neuronal NOS-inhibitor S-methyl-L-thiocitrulline (SMTC) but not the selective endothelial NOS-inhibitor N5-(1-iminoethyl)-L-ornithine (L-NIO). The antinociception was also significantly reduced by central pretreatment with a rabbit antiserum against dynorphin1-13 but not by rabbit antisera against either β-endorphin or methionine-enkephalin. The prolonged antinociceptive effect at 90 min after HBO2-induced treatment was also significantly attenuated by naltrexone but not L-NAME administered 60 min following HBO2 treatment but prior to nociceptive testing. These findings indicate that the antinociception that persists for 90 min after HBO2 exposure is mediated by nitric oxide (NO) and opioid mechanisms but that the NO involvement is critical during the HBO2 treatment and not at the time of nociceptive testing. These results are consistent with the concept that HBO2 may induce an NO-dependent release of opioid peptide to cause a long-acting antinociceptive effect.
Words for Indexing: Hyperbaric Oxygen, Nitric Oxide, Opioid, Antinociception, Mice
Introduction
Hyperbaric oxygen (HBO2) therapy is the clinical application of 100% oxygen at atmospheric pressures higher than sea level for limited periods of time (60–90 min) daily to achieve therapeutic outcomes. Normally at sea level or 1 atmosphere absolute (1 ATA), the partial pressure of oxygen (PaO2) at the lungs breathing air (21% oxygen) is 102 mm Hg. By breathing 100% oxygen, the PaO2 is elevated to 673 mm Hg at 1 ATA. But if the pressure is increased to 2 ATA, it is increased to 1433 mm Hg, a 14-fold increase in PaO2 as compared to breathing air at 1 ATA, thus representing a significant increase in oxygen delivery to tissues12.
The Committee on Hyperbaric Oxygen Therapy of The Undersea & Hyperbaric Medical Society (UHMS) evaluates clinical indications for HBO2 treatment and makes recommendations in its Hyperbaric Oxygen Therapy Committee Report9. These recommendations have been approved by the U.S. Food and Drug Administration (FDA). The use of HBO2 therapy for indications other than the thirteen UHMS-approved indications is considered off-label.
Among conditions that are reportedly responsive to HBO2 are a variety of clinical conditions of pain for which exposure to HBO2 causes a long-lasting analgesic effect. These painful conditions include complex regional pain syndrome14,21,28, fibromyalgia syndrome30, migraine and cluster headache5,16,29, and pain associated with radiotherapy of cancer4,13,17.
These reports suggest that HBO2 treatment activates an endogenous pain-relieving mechanism whose continued activation does not require continued exposure to HBO2 for persistence of pain relief. The purpose of the present study was to examine the duration of antinociceptive effect induced by a single 60-min HBO2 treatment in comparison with another well-studied pharmacological gas, nitrous oxide (N2O). Another aim was to determine whether this antinociceptive effect of HBO2 is similar to that of N2O in being mediated by opioid- and nitric oxide (NO) mechanisms.
Materials and Methods
Animals
Male NIH Swiss mice, weighing 18–22 g, were purchased from Harlan Laboratories (Indianapolis, IN) and used in this study, which was approved by an institutional animal care and use committee with post-approval review and carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23, revised 1996).
Mice were housed five per cage in the AAALAC-accredited Wegner Hall Vivarium with access to food and water ad libitum. The facility was maintained on a 12-h light:dark cycle (lights on 0700–1900 h) under standard conditions (22 ± 1°C room temperature, 33% humidity). Mice were kept in the holding room for at least four days after arrival in the facility for acclimation prior to experimentation. All measures to minimize pain or discomfort were taken by the investigators.
Exposure to Hyperbaric Oxygen (HBO2)
Cages of five mice each were placed in a B-11 research hyperbaric chamber (Reimers Systems, Inc., Lorton, VA) as previously described20. The chamber was ventilated with 100% O2, U.S.P. (A-L Compressed Gases, Inc., Spokane, WA) at a flow rate of 20 L/min to minimize CO2 accumulation. The pressure within the cylindrical clear acrylic chamber (27.9 cm diameter × 55.9 cm L) was increased at a rate of 1.0 ATA/min to the desired pressure and maintained for 60 min. The mice were allowed to breathe spontaneously during HBO2 treatment. After completion of the HBO2 exposure, mice were then decompressed at a rate of 1.0 ATA/min. Control groups of mice were exposed to compressed air (A-L Compressed Gases) circulated through the chamber at 1.0 ATA and maintained for 60 min. Decompression occurred as described above.
Exposure to Nitrous Oxide (N2O)
N2O, U.S.P. and O2, U.S.P. (A-L Compressed Gases) were mixed and delivered using a dental-sedation system (Porter, Hatfield, PA) at a total flow rate of 10 L/min. Mice were individually exposed in a clear Plexiglas® exposure chamber (35 cm L × 20 cm W × 15 cm H) with gas inlet and outlet ports. The final gaseous mixture concentration of 70% N2O and 30% O2 in the box was verified using a POET II® anesthetic monitoring system (Criticare, Milwaukee, WI). The total exposure time was 60 min. Exhausted gases were routed by polyethylene tubing to a nearby fume hood.
Antinociceptive Testing
Antinociceptive responsiveness was assessed as previously described7, using the abdominal constriction test at various times following cessation of HBO2 or N2O treatment. Mice were treated i.p. with 0.1 ml per 10 g body weight of 0.6% glacial acetic acid and placed into the Plexiglas® chamber. Exactly 5 min later, the number of abdominal constrictions—lengthwise stretches of the torso with concave arching of the back—in each animal was counted for 6-min period. Multiple raters were used for some but not all experiments; at least one of the raters was blinded to the drug treatment. All experiments were consistently conducted between 1300 and 1700 h. The control reference group was exposed to room air. The degree of antinociception (inhibition of abdominal constrictions) produced in various treatment groups of mice was calculated as:
Drugs
The following drugs were used in this research: naltrexone hydrochloride (NTX) (Tocris Bioscience, Ellisville, MO); NG-nitro-L-arginine methyl ester (L-NAME) (Sigma Aldrich Research Biochemicals, Inc., St. Louis, MO); S-methyl-L-thiocitrulline (SMTC) (Sigma Aldrich); N5-(1-iminoethyl)-L-ornithine (L-NIO) (Alexis Biochemicals Corporation, San Diego, CA); and rabbit antiserum against rat dynorphin A1-13 (DYN AS), β-endorphin (β-EP AS) and methionine-enkephalin (ME AS) (Bachem/Peninsula Laboratories, LLC, San Carlos, CA).
NTX, L-NAME, SMTC and L-NIO were freshly prepared in 0.9% physiological saline solution. NTX and L-NIO were administered systemically (30-min pretreatment time) and L-NAME and SMTC were administered i.c.v. (15-min pretreatment time). In one set of experiments (#1, #2, and #3), opioid antagonists and NOS-inhibitors were administered 15–30 min prior to the 60-min HBO2 treatment (180 min prior to antinociceptive testing). In another experiment (#4), opioid antagonist and NOS-inhibitor pretreatment was administered 60 min following cessation of the 60-min HBO2 treatment (15–30 min prior to antinociceptive testing). For i.p. or s.c. pretreatments, the volume of injection was 0.1 ml/10 g body weight with control animals receiving an i.p. or s.c. injection of vehicle (sterile saline) only. For i.c.v. pretreatments, the volume of microinjection was 5.0 μl per mouse with control animals receiving an i.c.v. microinjection of vehicle (sterile saline) only.
DYN AS, β-EP AS and ME AS lyophilates were reconstituted in distilled water; the final antiserum solutions contained 0.1-M phosphate buffered saline (PBS, pH 7.4). The antisera were microinjected in an i.c.v. dose of 10 μg 30 min prior to the 60-min HBO2 treatment (180 min prior to antinociceptive testing). The volume of i.c.v. microinjection was 5.0 μl per mouse. Control animals received an i.c.v. microinjection of vehicle (sterile saline or PBS) only.
Intracerebroventricular (i.c.v.) Microinjection Procedure
Intracerebroventricular (i.c.v.) pretreatments were made using a modification of a published microinjection technique10. Briefly, mice were anesthetized with IsoFlo® (isoflurane, U.S.P., Abbott Laboratories, North Chicago, IL). A short incision was made along the midline of the scalp using a scalpel, and the skin was pulled back to expose the calvarium. The i.c.v. microinjection was made using a 10-μl microsyringe (Hamilton, Reno, NV) with a 26-gauge cemented needle. The microsyringe was held vertically by hand at a point on the calvarium 2.0 mm lateral and 1.0 mm caudal from bregma to a depth of −2.0 mm from the skull surface. Penetration was controlled by a large-bore needle through which the microsyringe needle was inserted through a large-bore hypodermic needle which served as a collar to limit penetration of the microsyringe needle to 2.0 mm. A volume of 5.0 μl of drug solution was delivered directly into the lateral cerebral ventricle over 30 sec.
Statistical Analysis of Data
A two-way analysis of variance (2 ANOVA) and a one-way ANOVA (1 ANOVA) with a post-hoc Bonferroni multiple comparison test was used to analyze the time-dependent antinociceptive effects of HBO2 and N2O. Percent changes in antinociception were arcsine-transformed prior to statistical analysis. A one-way ANOVA with a post-hoc Bonferroni multiple comparison test was used to compare HBO2-induced antinociception various pretreatment groups.
Results
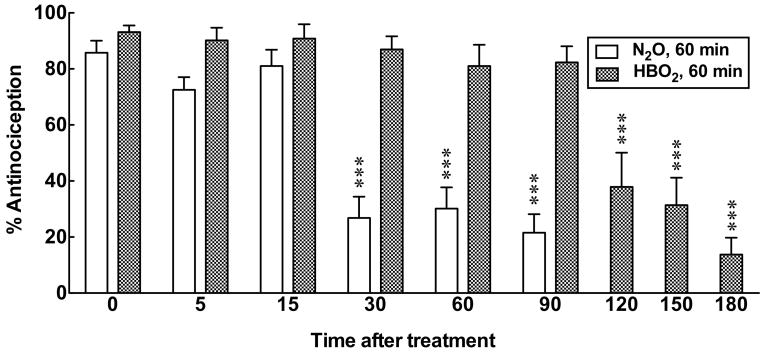
In Experiment #1, mice were subjected to 60 min of HBO2 or N2O treatment then removed to room air. Different groups of mice were injected with 0.6% glacial acetic acid and assessed for antinociception at 5, 15, 30, 60, 90, 120, 150 or 180 min following exposure. The 0-time groups were removed to room air following 60 min of HBO2 or N2O treatment and were immediately injected with glacial acetic acid for antinociceptive testing 5 min later [2 ANOVA between 0–90 min, Treatment; F1,101 = 114.4, p < 0.0001, Time; F5,101 = 18.8, p < 0.0001, Interaction; F5,101 = 10.45, p < 0.0001]. HBO2 treatment produced responses that were 80–95% of the maximal antinociceptive effect through 90 min (Fig. 1). At 120 min, the antinociceptive effect fell to 40% of maximum and further declined to under 20% by 180 min after HBO2 treatment [1 ANOVA; F = 20.25, p < 0.0001]. By comparison, N2O treatment caused mice to exhibit at least 70% antinociception at 5 and 15 min after exposure, but the response declined to approximately 20% by 30 through 90 min [1 ANOVA; F = 25.24, p < 0.0001].
Fig. 1.
A comparison of the duration of HBO2- versus N2O-induced antinociceptive effects. Each bar represents the mean percent of antinociceptive response ± S.E.M. of 8–12 mice per group. Significance of difference: ***, p < 0.001, compared to the 0 time N2O or HBO2 control group (post-hoc Bonferroni test).
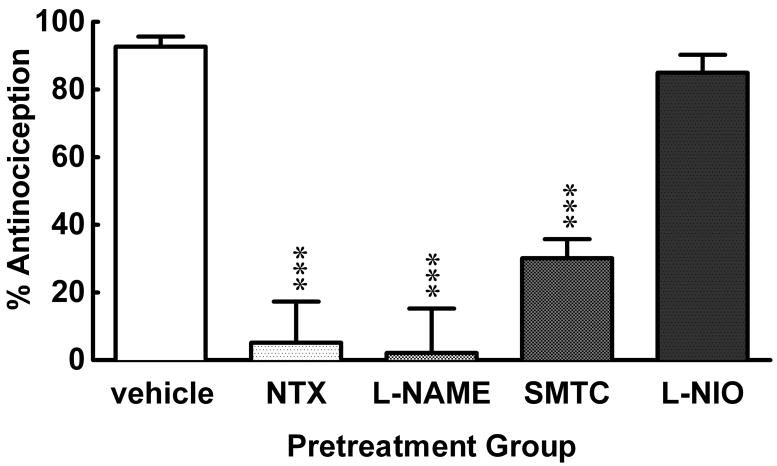
In Experiment #2, different groups of mice were pretreated with NTX (3.0 mg/kg, i.p.), L-NAME (1.0 μg/mouse, i.c.v.), SMTC (1.0 μg/mouse, i.c.v.) or L-NIO (3.0 mg/kg, s.c.) 15–30 min prior to HBO2 treatment. The antinociceptive effect assessed 90 min after HBO2 treatment was completely abolished by NTX and L-NAME, antagonized by two-thirds by SMTC and largely unaffected by L-NIO [1 ANOVA; F = 25.57, p < 0.0001] (Fig. 2). In preliminary experiments, different groups of animals were treated with NTX, L-NAME, SMTC or L-NIO in the absence of HBO2 treatment; none of these pretreatments alone (at the doses used) had any appreciable effect on the number of acetic acid-induced abdominal constrictions (data not shown).
Fig. 2.
Influence of opioid antagonist and NOS-inhibitor pretreatments administered prior to HBO2 treatment in mice. The data are expressed as the mean ± S.E.M. of 8–12 mice per group. Significance of difference: ***, p < 0.001, compared to the vehicle pretreatment group (post-hoc Bonferroni test).
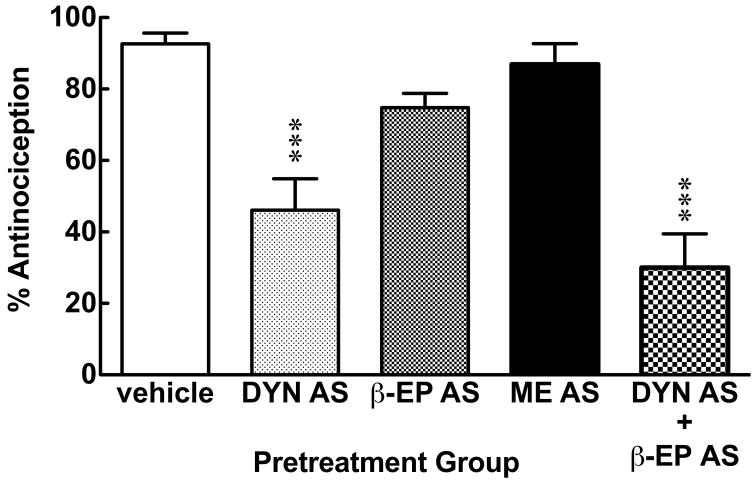
In Experiment #3, different groups of mice were pretreated with DYN AS (10 μg/mouse, i.c.v.), β-EP AS (10 μg/mouse, i.c.v.), and ME AS (10 μg/mouse, i.c.v.) or a cocktail of DYN AS (10 μg/mouse, i.c.v.) and β-EP AS (10 μg/mouse, i.c.v.) 30 min prior to HBO2 treatment [1 ANOVA; F = 27.49, p < 0.0001]. DYN AS was the most effective antagonist of the antinociception assessed 90 min following cessation of HBO2 treatment, reducing the magnitude of the response by one-half (Fig. 3). The β-EP antiserum reduced HBO2-induced antinociception by about one-fifth, and the ME antiserum did not appreciably influence the antinociceptive effect. The combination of DYN AS and β-EP AS reduced HBO2-induced antinociception by two-thirds, but this was not statistically different from the response of mice pretreated with DYN AS. In preliminary experiments, different groups of animals were treated with DYN AS, β-EP AS or ME AS in the absence of HBO2 treatment; none of these antiserum pretreatments alone (at the doses used) had any appreciable effect on the number of acetic acid-induced abdominal constrictions (data not shown).
Fig. 3.
Influence of opioid peptide antiserum pretreatment administered before HBO2 treatment in mice. The data are expressed as the mean ± S.E.M. of 6–12 mice per group. Significance of difference: ***, p < 0.001, compared to the vehicle pretreatment group (post-hoc Bonferroni test).
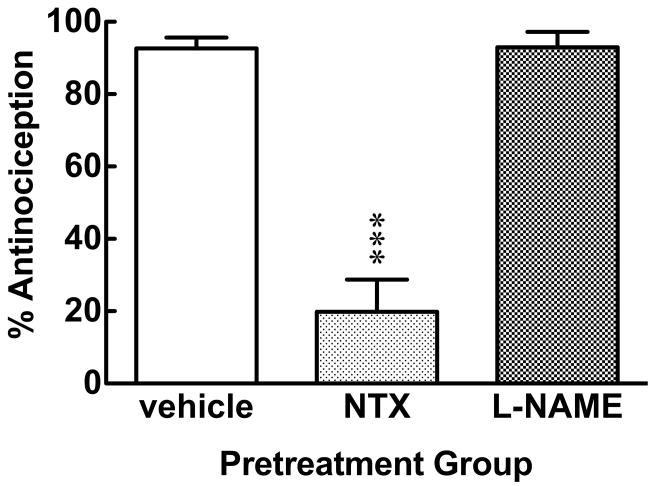
In Experiment #4, different groups of mice were pretreated with either NTX (3.0 mg/kg, i.p.) or L-NAME (1.0 μg/mouse, i.c.v.) 60 min after HBO2 treatment and 30 min prior to antinociceptive testing [F = 58.04, p < 0.0001]. NTX significantly antagonized the magnitude of the HBO2-induced antinociception assessed 90 min after HBO2 treatment, while the L-NAME pretreatment had no effect at all (Fig. 4).
Fig. 4.
Influence of opioid antagonist and NOS-inhibitor pretreatment administered after HBO2 treatment and prior to antinociceptive testing in mice. The data are expressed as the mean ± S.E.M. of 8–12 mice per group. Significance of difference: ***, p < 0.001, compared to the vehicle pretreatment group (post-hoc Bonferroni test).
Discussion
An involvement of NO in mediating the effects of HBO2 treatment is in agreement with previous studies. In in vivo brain microdialysis experiments conducted in rats, HBO2 treatment at 3 ATA for 120 min increased hippocampal and striatal levels of NO metabolites; both these increases in dialysate levels of NO metabolites were blocked by pretreatment with L-NAME6. In another study, NO-specific and O2-specific electrodes were implanted in the brains of anesthetized rats subsequently exposed to HBO2 at 2.0–2.8 ATA for ~20 min; the increase in tissue NO levels increased in proportion to increases in the tissue O2 concentration and was sensitive to antagonism by 7-nitroindazole, a neuronal-selective NOS-inhibitor27.
Our laboratory also recently demonstrated that HBO2-specific NO changes increases in different regions of the rat brain and spinal cord20. Exposure to 100% O2 alone (NBO2) generally decreased regional brain and spinal cord levels of the NO metabolites, nitrite and nitrate, while exposure to compressed air at 2.5 ATA (NBA) had little effect on tissue levels of NO metabolites. However, the combination of 100% oxygen and pressure (i.e., HBO2) generally increased tissue levels of nitrite and nitrate, which is indicative of increased NO in selected brain regions, most notably in the corpus striatum, brainstem, cerebellum and spinal cord.
HBO2 is approved by the FDA for certain clinical indications9, although there is anecdotal evidence and clinical reports of HBO2 efficacy in a broader range of conditions. Among conditions that are reportedly responsive to HBO2 are a variety of clinical conditions of pain. Patients suffering from complex regional pain syndrome (CRPS)—formerly called reflex sympathetic dystrophy (RSD)—experienced less pain following HBO2 therapy14,21,28. Significant pain reduction was also reported in patients with generalized allodynia/hyperalgesia as a consequence of fibromyalgia syndrome (FMS) 30. Patients suffering from migraine headache or cluster headache also reportedly experienced pain relief following HBO2 therapy5,16,29. Pain associated with radiotherapy of cancer has also been reported to be alleviated by HBO2 treatment4,13,17.
A number of these clinical studies indicate pain relief for varying periods of time following one or more sessions of HBO2 therapy. This suggests that HBO2 treatment activates an endogenous pain-relieving mechanism whose continued activation does not require continued exposure to HBO2 for persistence of pain relief. To determine whether HBO2 treatment (2.5 ATA for 60 min) in our animals activated pain-relieving mechanisms that outlast the period of its actual exposure sojourn, different groups of mice were tested for antinociceptive responsiveness at varying time intervals after cessation of HBO2 treatment.
The results demonstrate clearly that both HBO2 and N2O treatments can reduce the number of acetic acid-induced abdominal constrictions in mice as we have previously demonstrated31. But, the N2O-induced antinociception starts to dissipate after 15 min following termination of 60-min exposure to N2O, the HBO2-induced antinociception lasts at least 90 min following a single 60-min session of HBO2 treatment. N2O is not metabolized by the body and, due to its low blood/gas partition coefficient, is largely eliminated in the expired air25. The antinociceptive effect that lasts for 15 min following termination of exposure may reflect the residual pharmacological activity of the neuronally-released endogenous opioid peptide(s) which is limited by enzymatic degradation.
By comparison, the duration of the post-HBO2 antinociception is six times longer than that of N2O. During and following the HBO2 treatment (2.5 ATA, 100% O2), tissue O2 concentrations would be higher, resulting in significantly increased biosynthesis of NO26. The increased tissue NO levels may lead to a greater and more prolonged release of endogenous opioid peptide as compared to the N2O exposure at 1 ATA (30% O2). Another possible explanation is that HBO2 exposure activates an endogenous antinociceptive pathway that is longer acting than the pathway activated by N2O.
This HBO2-induced antinociceptive effect was nearly completely antagonized by systemic pretreatment with NTX, an opioid receptor blocker, and by an i.c.v. administration of the non-selective NOS-inhibitor, L-NAME. L-NIO, selective endothelial NOS-inhibitor, produced a slight (10%) reduction in HBO2-induced antinociception which was not statistically significant. However, following pretreatment with SMTC (i.c.v.), a selective inhibitor of neuronal NOS, the HBO2-induced antinociception was significantly antagonized. These results indicate that the antinociceptive pathway involves the O2-induced biosynthesis of NO via neuronal NOS and implicates the opiate receptors. This agrees with our earlier findings with respect to N2O-induced antinociception11,15,24. The antinociceptive response was likewise antagonized by NTX, L-NAME and SMTC. We have previously hypothesized that N2O-induced antinociception results from a stimulated NO-dependent neuronal release of endogenous opioid peptides8.
The antinociceptive effect produced by HBO2 treatment was significantly antagonized by pretreatment with DYN AS, the magnitude of the antinociceptive response being reduced by roughly one-half. Neither β-EP AS or ME AS produced a significant reduction in the HBO2-induced response. When mice were pretreated with a cocktail of DYN AS and β-EP AS, the HBO2-induced antinociceptive effect was reduced by two-thirds; however, this was not significantly different from the DYN AS-antagonized response to HBO2. It would appear that DYN—an opioid peptide with known antinociceptive properties17,22—is the primary mediator of HBO2-induced antinociception. Once again, this is similar to observations made in studies of N2O-induced antinociception. Supraspinal pretreatment of mice with DYN AS significantly antagonized the antinociceptive of N2O, while β-EP AS and ME AS were without effect1,2.
In other studies, i.c.v. administration of the NO precursor L-arginine (L-ARG) or the NO-donor 3-morpholinosydnoimine (SIN-1) evoked antinociceptive effects in the mouse abdominal constriction test3. These antinociceptive responses were sensitive to antagonism by naloxone and DYN AS, suggesting the antinociceptive response resulted from DYN activating opioid receptors in the brain. The functional link between NO and release of DYN was provided by the finding that pretreatment with the neuronal-selective NOS-inhibitor SMTC blocked the antinociceptive response to L-ARG but not SIN-1. This is reinforced by results from microdialysis experiments conducted in rats, in which N2O-induced increases in levels of both β-EP and NO metabolites in samples collected from the arcuate nucleus were antagonized by pretreatment with L-NAME19.
These findings are consistent with the hypothesis that both N2O and HBO2 may produce antinociception through stimulation of an NO-dependent neuronal release of the opioid peptide DYN8. If NO does, indeed, play a regulatory role in the neuronal release of opioid peptides, this might explain why NOS-inhibitors can antagonize HBO2-induced antinociception during the actual HBO2 treatment rather than at the time of nociceptive testing. Once endogenous opioid peptides have been released from their neuronal sources, NO is no longer required for expression of the antinociceptive response.
This research will potentially identify molecular targets that can cause prolonged activation of endogenous pain-relieving systems. It remains to be seen whether multiple sessions of HBO2 treatment might produce an antinociceptive response of even longer duration. Although HBO2 therapy is not at present indicated in the treatment of pain per se, it may represent a new weapon in the pharmacological armamentarium for clinical management of pain.
Acknowledgments
This research was supported by NIH Grant GM-77153 (R.M.Q.) and funds from the WSU College of Pharmacy, the Allen I. White Distinguished Professorship and the Chico Hyperbaric Center. None of the authors have a conflict of interest in this work.
Footnotes
Perspective
This article present evidence of a persistent antinociceptive effect of hyperbaric oxygen treatment that is mediated by opioid and nitric oxide mechanisms. Further elucidation of the underlying mechanism could potentially identify molecular targets to cause a longer-acting activation of endogenous pain-modulating systems.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Branda EM, Ramza JT, Cahill FJ, Tseng LF, Quock RM. Role of brain dynorphin in nitrous oxide antinociception in mice. Pharmacol Biochem Behav. 2000;65:217–222. doi: 10.1016/s0091-3057(99)00202-6. [DOI] [PubMed] [Google Scholar]
- 2.Cahill FJ, Ellenberger EA, Mueller JL, Tseng LF, Quock RM. Antagonism of nitrous oxide antinociception in mice by intrathecally administered opioid peptide antisera. J Biomed Sci. 2000;7:299–303. doi: 10.1007/BF02253248. [DOI] [PubMed] [Google Scholar]
- 3.Chung E, Burke B, Bieber AJ, Doss JC, Ohgami Y, Quock RM. Dynorphin-mediated antinociceptive effects of l-arginine and SIN-1 (an NO donor) in mice. Brain Res Bull. 2006;70:245–250. doi: 10.1016/j.brainresbull.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Dall’Era MA, Hampson NB, Hsi RA, Madsen B, Corman JM. Hyperbaric oxygen therapy for radiation induced proctopathy in men treated for prostate cancer. J Urol. 2006;176:87–90. doi: 10.1016/S0022-5347(06)00491-5. [DOI] [PubMed] [Google Scholar]
- 5.Di Sabato F, Fusco BM, Pelaia P, Giacovazzo M. Hyperbaric oxygen therapy in cluster headache. Pain. 1993;52:243–245. doi: 10.1016/0304-3959(93)90137-E. [DOI] [PubMed] [Google Scholar]
- 6.Elayan IM, Axley MJ, Prasad PV, Ahlers ST, Auker CR. Effect of hyperbaric oxygen treatment on nitric oxide and oxygen free radicals in rat brain. J Neurophysiol. 2000;83:2022–2029. doi: 10.1152/jn.2000.83.4.2022. [DOI] [PubMed] [Google Scholar]
- 7.Emmanouil DE, Dickens AS, Heckert RW, Ohgami Y, Chung E, Han S, Quock RM. Nitrous oxide-antinociception is mediated by opioid receptors and nitric oxide in the periaqueductal gray region of the brain. Eur Neuropsychopharmacol. 2008;18:194–199. doi: 10.1016/j.euroneuro.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emmanouil DE, Quock RM. Advances in understanding the actions of nitrous oxide. Anesth Prog. 2007;54:9–18. doi: 10.2344/0003-3006(2007)54[9:AIUTAO]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldmeier JJ, editor. The UHMS Hyperbaric Oxygen Therapy Committee Report. Durham, NC: Undersea and Hyperbaric Medical Society; 2003. Hyperbaric Oxygen 2003 – Indications and Results. [Google Scholar]
- 10.Haley TJ, McCormick WG. Pharmacological effects produced by intracerebral injections of drugs in the conscious mouse. Br J Pharmacol. 1957;12:12–15. doi: 10.1111/j.1476-5381.1957.tb01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishikawa M, Quock RM. Role of nitric oxide synthase isoforms in nitrous oxide antinociception in mice. J Pharmacol Exp Ther. 2003;306:484–489. doi: 10.1124/jpet.103.049551. [DOI] [PubMed] [Google Scholar]
- 12.Jain JJ. Physical, physiological and biochemical aspects of hyperbaric oxygenation. In: Jain KK, editor. Textbook of Hyperbaric Medicine. 3. Seattle: Hogrefe and Huber Publishers; 1999. p. 18. [Google Scholar]
- 13.Jones K, Evans AW, Bristow RG, Levin W. Treatment of radiation proctitis with hyperbaric oxygen. Radiother Oncol. 2006;78:91–94. doi: 10.1016/j.radonc.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Kiralp MZ, Yildiz S, Vural D, Keskin I, Ay H, Dursun H. Effectiveness of hyperbaric oxygen therapy in the treatment of complex regional pain syndrome. J Int Med Res. 2004;32:258–262. doi: 10.1177/147323000403200304. [DOI] [PubMed] [Google Scholar]
- 15.McDonald CE, Gagnon MJ, Ellenberger EA, Hodges BL, Ream JK, Tousman SA, Quock RM. Inhibitors of nitric oxide synthesis antagonize nitrous oxide antinociception in mice and rats. J Pharmacol Exp Ther. 1994;269:601–608. [PubMed] [Google Scholar]
- 16.Myers DE, Myers RA. A preliminary report on hyperbaric oxygen in the relief of migraine headache. Headache. 1995;35:197–199. doi: 10.1111/j.1526-4610.1995.hed3504197.x. [DOI] [PubMed] [Google Scholar]
- 17.Nakabayashi M, Beard C, Kelly SM, Carr-Locke DL, Oh WK. Treatment of a radiation-induced rectal ulcer with hyperbaric oxygen therapy in a man with prostate cancer. Urol Oncol. 2006;24:503–508. doi: 10.1016/j.urolonc.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Nakazawa T, Ikeda M, Kaneko T, Yamatsu K. Analgesic effects of dynorphin-A and morphine in mice. Peptides. 1985;6:75–78. doi: 10.1016/0196-9781(85)90079-8. [DOI] [PubMed] [Google Scholar]
- 19.Ohgami Y, Chung E, Quock RM. Nitrous oxide-induced nitric oxide-dependent release of β-endorphin from the arcuate nucleus to stimulate opioid receptors in the periaqueductal gray to cause antinociception in the rat. 2008 Exptl Biol Meeting Abst. 2008a [on CD-ROM], abstract 711.18. [Google Scholar]
- 20.Ohgami Y, Chung E, Shirachi DY, Quock RM. Influence of hyperbaric oxygen on regional brain levels and spinal cord levels of nitric oxide metabolites in rat. Brain Res Bull. 2008;75:668–673. doi: 10.1016/j.brainresbull.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Peach G. Hyperbaric oxygen and the reflex sympathetic dystrophy syndrome: a case report. Undersea Hyperb Med. 1995;22:407–408. [PubMed] [Google Scholar]
- 22.Przew ocki R, Stala L, Greczek M, Shearman GT, Przew ocka B, Herz A. Analgesic effects of mu-, delta- and kappa-opiate agonists and, in particular, dynorphin at the spinal level. Life Sci. 1983;33(Suppl 1):649–652. doi: 10.1016/0024-3205(83)90586-6. [DOI] [PubMed] [Google Scholar]
- 23.Quock RM, Kouchich FJ, Tseng LF. Does nitrous oxide induce release of brain opioid peptides? Pharmacology. 1985;30:95–99. doi: 10.1159/000138056. [DOI] [PubMed] [Google Scholar]
- 24.Quock RM, Graczak LM. Influence of narcotic antagonist drugs upon nitrous oxide analgesia in mice. Brain Res. 1988;440:35–41. doi: 10.1016/0006-8993(88)91156-0. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds JEF, editor. Martindale: The Extra Pharmacopoeia. London: The Pharmaceutical Press; 1989. pp. 1123–1124. [Google Scholar]
- 26.Thom SR, Bhopale V, Fisher D, Manevich Y, Huang PL, Buerk DG. Stimulation of nitric oxide synthase in cerebral cortex due to elevated partial pressures of oxygen: An oxidative stress response. J Neurobiol. 2002;51:85–100. doi: 10.1002/neu.10044. [DOI] [PubMed] [Google Scholar]
- 27.Thom SR, Buerk DG. Nitric oxide synthesis in brain is stimulated by oxygen. Adv Exp Med Biol. 2003;510:133–137. doi: 10.1007/978-1-4615-0205-0_22. [DOI] [PubMed] [Google Scholar]
- 28.Tuter NV, Danilov AB, Poliakova LV. The treatment of a complex regional pain syndrome. Zh Nevrol Psikhiatr Im S S Korsakova. 1997;97:33–35. [PubMed] [Google Scholar]
- 29.Wilson JR, Foresman BH, Gamber RG, Wright T. Hyperbaric oxygen in the treatment of migraine with aura. Headache. 1998;38:112–115. doi: 10.1046/j.1526-4610.1998.3802112.x. [DOI] [PubMed] [Google Scholar]
- 30.Yildiz S, Kiralp MZ, Akin A, Keskin I, Ay H, Dursun H, Cimsit M. A new treatment modality for fibromyalgia syndrome: hyperbaric oxygen therapy. J Int Med Res. 2004;32:263–267. doi: 10.1177/147323000403200305. [DOI] [PubMed] [Google Scholar]
- 31.Zylstra CC, Ohgami Y, Chung E, Shirachi DY, Quock RM. Comparison of the antinociceptive effect of two pharmacological gases, nitrous oxide (N2O) and hyperbaric oxygen (HBO2) 2008 Exptl Biol Meeting Abst. 2008 [on CD-ROM], abstract 711.16. [Google Scholar]