Abstract
The amygdala detects aversive events and coordinates with rostral anterior cingulate cortex to adapt behavior. We assessed error-related activation in these regions and its relation to task performance using functional MRI and a saccadic paradigm. Both amygdalae showed increased activation during error versus correct antisaccade trials that was correlated with error-related activation in the corresponding rostral anterior cingulate cortex. Together, activation in right amygdala and right rostral anterior cingulate cortex predicted greater accuracy. In contrast, left amygdala activation predicted a higher error rate. These findings support a role for amygdala in response monitoring. Consistent with proposed specializations of right and left amygdala in aversive conditioning, we hypothesize that right amygdala-rostral anterior cingulate cortex interactions mediate learning to avoid errors, while left error-related amygdala activation underpins detrimental negative affect.
Keywords: response monitoring, reinforcement learning, emotion, amygdala, anterior cingulate cortex, antisaccade, errors, limbic system
In humans, both the amygdala and rostral anterior cingulate cortex activate in response to aversive outcomes, and the amygdala response has been correlated with autonomic indices of conditioned fear [1]. Extensive evidence from both animal and human studies suggests that these structures mediate emotional and reward-based learning and help organisms adapt to their environment [for review see: 2, 3, 4]. Errors on a cognitive task are both salient (unexpected) and aversive (representing the non-achievement of a goal). As failures of performance, they should prompt avoidance learning. While the response of the rostral anterior cingulate cortex to errors is extensively documented [for review see: 5], despite the similarities between errors and other events to which the amygdala responds, the amygdala’s response to errors has seldom been explored. Intracranial recordings from patients with epilepsy reveal error-related responses in the amygdala [6]. We previously reported error-related activation in rostral anterior cingulate cortex and amygdala that was related to error rate in healthy participants and those with schizophrenia [7]. These findings suggest that the amygdala is a component of the response monitoring circuitry that detects errors and adapts behavior to avoid their recurrence. Here we examined the interactions of amygdala and rostral anterior cingulate cortex during error commission and their relation to task performance. We expected these structures to interact during error commission based on evidence that ventromedial prefrontal cortex, and more specifically rostral anterior cingulate cortex, modulates the amygdala response to salient events [e.g., 8, 9, 10], an interchange that is supported by extensive reciprocal connections [11, 12]. If amygdala-rostral anterior cingulate cortex interactions during error commission serve to adapt behavior, then they may correlate with task performance.
We used a saccadic paradigm and event-related functional magnetic resonance imaging (fMRI) to test our hypothesis of interactions between amygdala and rostral anterior cingulate cortex during error commission that mediate task performance. Participants performed a pseudorandom sequence of prosaccade and antisaccade trials. Prosaccades require a gaze towards a suddenly appearing stimulus, while antisaccades require suppression of the prepotent prosaccade, and a gaze in the opposite direction. Analyses were restricted to antisaccades because only they produced sufficient errors. Given evidence of hemispheric specialization in amygdala responses [e.g., 1, 13] we considered activation in each hemisphere separately.
Methods
[For greater methodological detail see, 14].
Subjects
Twenty-one healthy right-handed participants were recruited from the community. Data from two were excluded, one due to near perfect antisaccade performance (0.1% error rate) and one due to eye tracker malfunction. Analyses were conducted on the remaining 19 (12 male; mean age: 33±11 years). In addition to a base pay rate of pay, participants received a 5-cent bonus for each correct response. All participants gave written informed consent and the study was approved by the Partners Human Research Committee.
Apparatus
Images were acquired with a 3.0 Tesla Siemens Trio whole body high-speed imaging device equipped for echo planar imaging (Siemens Medical Systems, Erlangen, Germany). Task stimuli were generated by a Macintosh G4 Powermac using programs written in C++ on the Vision Shell programming platform (MicroML, St. Hyacinthe, Quebec). Eye position was sampled at a rate of 60 Hz by ISCAN’s RK-726PCI high resolution Pupil/Corneal reflection tracker.
Saccadic paradigm
Each task run consisted of a pseudorandom sequence of prosaccade and antisaccade trials, balanced for right and left movements, and fixation intervals of 2, 4 or 6 s. Participants performed six runs of 5 minutes 22 s each, generating a total of 211 prosaccade, 211 antisaccade, and 80 fixation trials. Saccadic trials lasted 4000 ms and began with an instructional cue, indicating a pro- or antisaccade, at screen center. After 300 ms, the cue was replaced by a white fixation ring. At 2000 ms, the ring shifted to the right or left. This was the stimulus to which the participant responded. The white ring remained in the peripheral location for 1000 ms, and then returned to the center for 1000 ms.
Image acquisition
Two high-resolution structural images were acquired using a three-dimensional magnetization prepared rapid gradient echo (MPRAGE) sequence (repetition time/echo time/flip angle = 7.25/3/7°; voxel size:1.3 × 1.3 × 1 mm). Functional magnetic resonance images were collected using a gradient echo T2* weighted sequence (repetition time/echo time/flip angle = 2000ms/30ms/90°). Twenty contiguous horizontal slices parallel to the intercommissural plane (voxel size: 3.13 × 3.13 × 5 mm) were acquired interleaved. The functional sequences included prospective acquisition correction (PACE) for head motion [15].
Analysis of imaging data
All analyses were conducted using FreeSurfer [16] and FreeSurfer Functional Analysis Stream (FS-FAST) software [17]. Functional scans were corrected retrospectively for motion, intensity normalized, smoothed using a three-dimensional 8 mm full-width, half-max Gaussian kernel, and aligned to the averaged MPRAGE scans. The averaged MPRAGE scans were also used to construct inflated (two-dimensional) models of individual cortical surfaces that were also spatially normalized for averaged group analyses. Finite impulse response estimates of the event-related hemodynamic responses for each of four trial types (correct prosaccades, error prosaccades, correct antisaccades, and error antisaccades) were calculated for each participant at 12 time points with an interval of 2 s (corresponding to the repetition time) ranging from 4 s prior to the start of a trial to 18 s after the start. Analyses focused on the 6 s time point at which error-related activation is maximal [14].
Regions of interest (ROI) definition
Left and right amygdalae were defined using an automated subcortical parcellation program [18]. Left and right rostral anterior cingulate cortex were defined using an automated surface-based parcellation system that provided an anterior cingulate cortex label [19], which was then divided into dorsal and rostral regions in each participant by placing a line at the anterior boundary of the genu of the corpus callosum that was perpendicular to the intercommisural plane [12]. Each of the ROI labels was visually inspected for accuracy either in the volume (amygdalae) or on the inflated cortical surface (rostral anterior cingulate cortex) for each participant. ROI activation in each of the contrasts of interest (error versus correct, correct versus fixation, and error versus fixation) was examined for outliers based on individual participants’ z-scores (≥3), their effect on normality (i.e., skewness (>|1.5|) and kurtosis (>|3|)), and on leverage (>4/n). The one participant who exceeded all three cut-offs for non-normality was excluded from the analyses.
Quantification of error-related amygdala activation
Activation for error and correct antisaccades was computed by averaging across all voxels in the anatomically-defined ROIs and compared across participants using paired t-tests. We also examined activation in each condition relative to the fixation baseline using one-sample t-tests.
Relation of error-related amygdala and rostral anterior cingulate cortex activation
We regressed error-related amygdala activation onto estimates of raw blood oxygenation level dependent (BOLD) signal in the error versus correct contrast at 6 s at each vertex of the cortical surface in each hemisphere separately. We quantified error-related activation in the amygdala as percent signal change averaged across voxels showing a positive sign in the error versus correct contrast at 6 s. To correct for multiple comparisons we ran 10,000 Monte Carlo simulations of synthesized white Gaussian noise using a probability value of < 0.01 and the smoothing, resampling, and averaging parameters of the functional analyses. We restricted the simulations to our a priori rostral anterior cingulate cortex ROIs. This determines the likelihood that a cluster of a certain size in rostral anterior cingulate cortex would be found by chance (cluster-wise probability-value: CWP). To facilitate comparison with other studies, approximate Talairach coordinates were derived by mapping the surface-based maxima back to the original structural volume for each participant, registering the volumes to the Montreal Neurological Institute (MNI305) atlas [20] and averaging the coordinates that corresponded to the surface maxima across participants. The resulting coordinates were transformed to standard Talairach space using an algorithm developed by Matthew Brett (http://imaging.mrccbu.cam.ac.uk/imaging/MniTalairach).
Results
Error-related amygdala activation
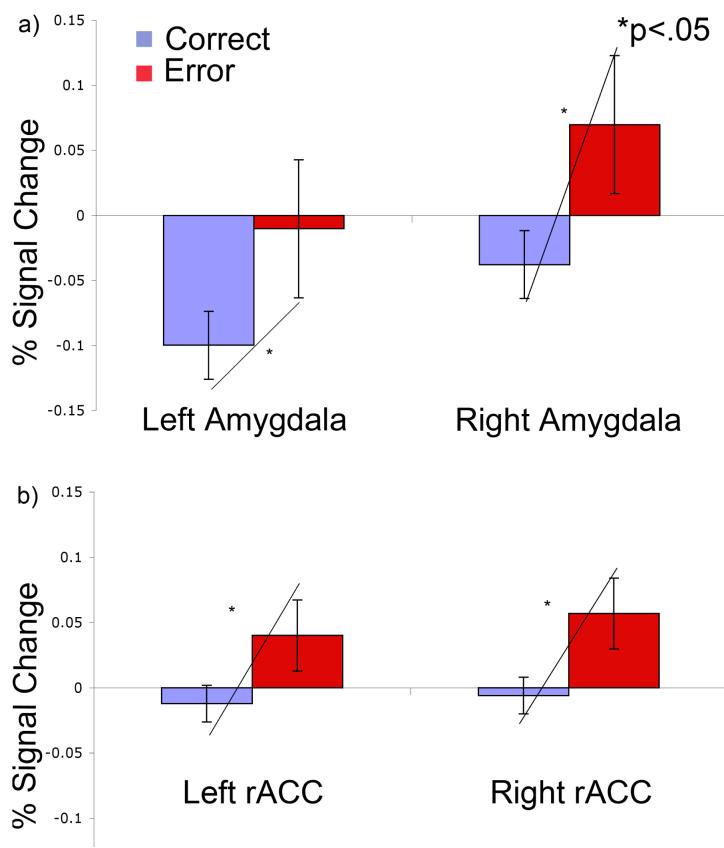
Antisaccade error rate was 9 ±6%. Error versus correct antisaccades were associated with increased amygdala (left-hemisphere: t(18)=2.29, p=.04; right-hemisphere: t(18)=2.87, p=.01) and rostral anterior cingulate cortex (left-hemisphere: t(17)=2.26, p=.05; right-hemisphere: t(17)=2.72, p=.01) activation bilaterally (Figure 1). The source of increased error-related activation differed by hemisphere. In the left amygdala, it was due to deactivation during correct trials (t(17)=−3.77, p=.002), rather than an increase during error trials (t(17)=.59, p=.85). In contrast, in the right amygdala the increase was primarily due increased activation during error trials (trend: t(17)=1.93, p=.07) rather than deactivation during correct trials (t(17)=1.38, p=.18). The rostral anterior cingulate cortex showed significant error-related activation in both hemispheres that was primarily due to greater activation for errors (left-hemisphere: t(17)=1.51, p=.15; right-hemisphere: t(17)=2.38, p=.03) rather than to deactivation during correct trials (left-hemisphere: t(17)=−.88, p=.40; right-hemisphere: t(17)=−.62, p=.54).
Figure 1.
Percent signal change with standard error bars for error and correct antisaccades in the left and right a) amygdalae and b) rostral anterior cingulate cortex (rACC) regions of interest (ROIs).
Relation of error-related amygdala and rostral anterior cingulate cortex activation
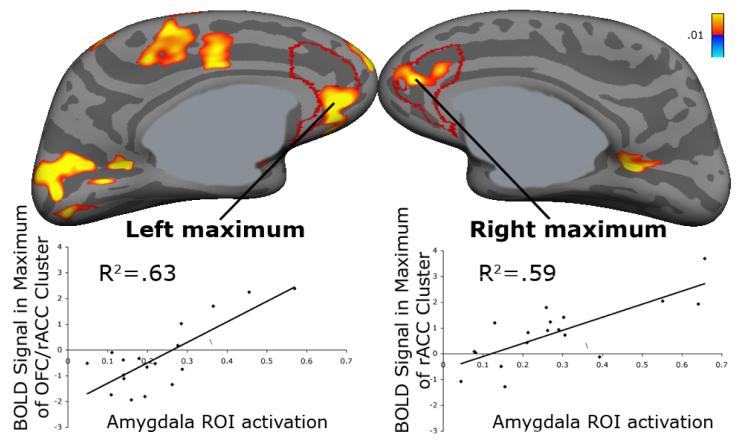
Error-related activation in both amygdalae was positively correlated with activation in the corresponding rostral anterior cingulate cortex (Figure 2). For right amygdala, as expected, the maximum fell in rostral anterior cingulate cortex (13, 47, 12, R2=.59, p<.001; CWP<.001). For left amygdala the maximum fell in orbitofrontal cortex and extended into subgenual rostral anterior cingulate cortex (−7, 44, −6). This region met cluster-wise correction for the entire cortical surface (R2=.63, p<.001; CWP=.01).
Figure 2.
Statistical maps of regressions of errorrelated amygdala activation onto error-related activation displayed on the inflated medial cortical surfaces. Scatter plots show percent signal change in the amygdala plotted against raw BOLD signal on the surface. Gray masks cover non-cortical regions in which any activation is displaced. rACC = rostral anterior cingulate cortex; OFC = orbitofrontal cortex; ROI = region of interest.
Relation of error-related amygdala and rostral anterior cingulate cortex activation to performance
To examine whether error-related amygdala and rostral anterior cingulate cortex activation and their interactions predicted antisaccade performance, we performed a standard linear regression, which tests the unique variance contributed by each component after removing the shared variance, using error rate as the dependent variable. The model had eight terms (Table 1) and accounted for 51% of the variance in error rate after adjusting for the number of parameters (p=.05). While greater error-related activation of the left amygdala significantly predicted a higher error rate, the right amygdala showed a trend to improve accuracy that was augmented by right rostral anterior cingulate cortex, and counteracted by left rostral anterior cingulate cortex as indicated by the significant interactions. To summarize, together, right rostral anterior cingulate cortex and right amygdala error-related activation predicted better performance, while error-related activation in the left amygdala was associated with worse performance.
Table 1.
t and p-values of parameter estimates in regression of error-related amygdala and rostral anterior cingulate cortex (rACC) activation on error-rate. LH = left-hemisphere; RH = right-hemisphere.
| t | p-value | |
|---|---|---|
| LH amgydala | 2.58 | .03* |
| RH amygdala | -1.96 | .08 |
| LH rACC | -1.19 | .26 |
| RH rACC | -.052 | .61 |
| LH amgydala × LH rACC | -1.42 | .19 |
| RH amygdala × RH rACC | -2.31 | .05* |
| LH amgydala × RH rACC | 1.93 | .09 |
| RH amygdala × LH rACC | 2.34 | .04* |
Discussion
Our findings support the hypothesis that amygdala interacts with rostral anterior cingulate cortex to participate in response monitoring. Both amygdalae showed greater activation during error than correct trials that correlated with error-related activation in the corresponding rostral anterior cingulate cortex, a region that is functionally and structurally connected with amygdala [11, 12, 21] and that modulates the amygdala’s response to salient events [e.g., 8, 9, 10]. Interestingly, the source of error-related amygdala activation and its interactions with rostral anterior cingulate cortex in predicting error rate differed by hemisphere. In the right-hemisphere, error-related activation of the amygdala was primarily due to increased activation during error trials and it correlated with error-related activation in a right perigenual rostral anterior cingulate region that also showed significantly increased error-related activation [reported elsewhere, 14]. Together with right rostral anterior cingulate cortex, right amygdala predicted fewer errors. Given the hypothetical role of rostral anterior cingulate cortex in appraising the affective or motivational significance of errors [22, 23], these findings suggest that right amygdala and right rostral anterior cingulate cortex work together to modulate affective responses to errors and to learn to prevent their occurrence. They are consistent with animal and human studies that suggest a right amygdala dominance for aversive conditioning [e.g., 1, 13]. Here, learning is presumably in the service of avoiding errors.
In contrast, error related activation in the left amygdala was not due to greater activation on error trials, instead it primarily reflected deactivation from baseline during correct responses. It was correlated with error-related activation in orbitofrontal cortex, extending into subgenual rostral anterior cingulate cortex. Both of these regions are reciprocally interconnected with amygdala [11, 24], and are involved in autonomic control and emotional expression [3, 25]. Although error-related activation in left orbitofrontal cortex and subgenual rostral anterior cingulate cortex was correlated with that in left amygdala, neither of these regions showed significant error-related activation [reported elsewhere, 14]. Finally, in contrast to the right amygdala/rostral anterior cingulate cortex interaction, error-related activation in left amygdala predicted more, not fewer, errors. In humans, cerebral blood flow and metabolism in the amygdala, subgenual rostral anterior cingulate cortex, and orbitofrontal cortex of the left-hemisphere are abnormally elevated in major depression, and left amygdala activity positively correlates with depression severity and normalizes with effective treatment [for review, 26]. Thus, a speculative interpretation of the differential response to error versus correct responses in left amygdala, and its association with activation in orbitofrontal cortex and subgenual rostral anterior cingulate cortex, is that it reflects negative affect that interferes with performance. Thus, only the right amygdala appeared to function in a way that was consistent with our prediction that the amygdala would detect errors as aversive events and act with rostral anterior cingulate cortex to adapt behavior. The apparently different contributions of the right and left amygdalae to task performance are consistent with their proposed specializations: right amygdala may be dominant for fear conditioning [e.g., 1, 13], while left amygdala may mediate negative affect [26].
Conclusion
During response monitoring, right amygdala and rostral anterior cingulate cortex seem to mediate aversive conditioning to errors, while left amygdala may underpin detrimental negative affect concerning performance. Optimal activity in these structures may help you learn from your mistakes, and not get too upset about them.
Acknowledgements
With gratitude to Matt Cain, Katy Thakkar, Bruce Fischl and Doug Greve.
Support: NIMH F31 MH72120 (FEP); R01 MH67720 (DSM); NARSAD (DSM); K08 NS01920 (JJSB); MIND Institute (DOE DE-FG02 99ER62764; NCRR 5 P41 RR14075 A 05.
References
- 1.LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20(5):937–45. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- 2.Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev. 2003;41(1):88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- 3.Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48(8):813–29. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- 4.Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3(7):563–73. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- 5.Taylor SF, Stern ER, Gehring WJ. Neural systems for error monitoring: recent findings and theoretical perspectives. Neuroscientist. 2007;13(2):160–72. doi: 10.1177/1073858406298184. [DOI] [PubMed] [Google Scholar]
- 6.Brazdil M, Roman R, Falkenstein M, Daniel P, Jurak P, Rektor I. Error processing--evidence from intracerebral ERP recordings. Exp Brain Res. 2002;146(4):460–6. doi: 10.1007/s00221-002-1201-y. [DOI] [PubMed] [Google Scholar]
- 7.Polli FE, Barton JJ, Thakkar KN, Greve DN, Goff DC, Rauch SL, Manoach DS. Reduced error-related activation in two anterior cingulate circuits is related to impaired performance in schizophrenia. Brain. 2008;131(Pt 4):971–86. doi: 10.1093/brain/awm307. [DOI] [PubMed] [Google Scholar]
- 8.Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62(5):446–54. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, Weinberger DR. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62(2):146–52. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- 10.Hariri AR, Drabant EM, Weinberger DR. Imaging genetics: perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biol Psychiatry. 2006;59(10):888–97. doi: 10.1016/j.biopsych.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Barbas H, De Olmos J. Projections from the amygdala to basoventral and mediodorsal prefrontal regions in the rhesus monkey. J Comp Neurol. 1990;300(4):549–71. doi: 10.1002/cne.903000409. [DOI] [PubMed] [Google Scholar]
- 12.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 13.Coleman-Mesches K, McGaugh JL. Muscimol injected into the right or left amygdaloid complex differentially affects retention performance following aversively motivated training. Brain Res. 1995;676(1):183–8. doi: 10.1016/0006-8993(95)00108-3. [DOI] [PubMed] [Google Scholar]
- 14.Polli FE, Barton JJ, Cain MS, Thakkar KN, Rauch SL, Manoach DS. Rostral and dorsal anterior cingulate cortex make dissociable contributions during antisaccade error commission. Proc Natl Acad Sci U S A. 2005;102(43):15700–15705. doi: 10.1073/pnas.0503657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn Reson Med. 2000;44(3):457–65. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 16.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 17.Burock MA, Dale AM. Estimation and detection of event-related fMRI signals with temporally correlated noise: a statistically efficient and unbiased approach. Hum Brain Mapp. 2000;11(4):249–60. doi: 10.1002/1097-0193(200012)11:4<249::AID-HBM20>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 19.Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 20.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18(2):192–205. [PubMed] [Google Scholar]
- 21.Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8(6):828–34. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 22.Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychol Sci. 2003;14(1):47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- 23.Taylor SF, Martis B, Fitzgerald KD, Welsh RC, Abelson JL, Liberzon I, Himle JA, Gehring WJ. Medial frontal cortex activity and loss-related responses to errors. J Neurosci. 2006;26(15):4063–70. doi: 10.1523/JNEUROSCI.4709-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115(4):1261–79. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- 25.Barbas H, Saha S, Rempel-Clower N, Ghashghaei T. Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neurosci. 2003;4:25. doi: 10.1186/1471-2202-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci. 1999;877:614–37. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]