Abstract
The hallmark of HIV/SIV infections is the progressive depletion of CD4+ T cells that ultimately renders the host incapable of defending against AIDS defining opportunistic infections and malignancies. Although many potential mechanisms have been proposed to explain CD4+ T cell loss, we review here the growing evidence that fibrotic ‘scarring’ and consequent damage to the lymphatic tissue niche contributes to CD4+ T cell decline and limits CD4+ T cell re-population with retroviral therapy.
INTRODUCTION
As a consequence of CD4+ T cell depletion, individuals infected with the Human Immunodeficiency Virus (HIV), the causative agent of the Acquired Immune Deficiency Syndrome (AIDS) eventually succumb to opportunistic infections and malignancies if they do not receive antiretroviral therapy (ART). The World Health Organization estimates 25 million people have already died from AIDS since it was first recognized 25 years ago and that more than 32 million people are currently living with HIV-1 infection (1).
Inhibiting viral replication with ART and reconstituting immunity, measured by increases in peripheral blood CD4+ T cells has had great impact on this terrible morbidity and mortality of HIV-1 infection. Patients are living longer-healthier lives and mortality in the treated population of HIV+ patients has significantly declined. However up to 20% of treated individuals receive no clinical benefit because, despite suppression of replicating virus in plasma, immune reconstitution is limited or absent (2, 3). Further, even among patients with significant increases in peripheral blood CD4+ T cells, few reconstitute to normal levels. While the data are clear that significant increases may be sufficient to avert opportunistic infections, there is increasing recognition that these individuals may still be at risk for complications of a subtler kind of immune suppression. Recent data indeed suggest that rates of malignancy appear to be increasing in the ARV treated HIV+ population, even among those with significant reconstitution(4–8).
It is not clear why immune reconstitution is robust with ART in some individuals and not in others. One potential explanation for the variable immune reconstitution with ART we review here is inflammation induced structural damage to the lymphatic tissue niche that normally maintains CD4+ T cell populations. We propose that this damaged niche is an important mechanism both in limiting reconstitution and in CD4+ T cell loss.
DEPLETION OF CD4+ T CELLS IN LYMPHATIC TISSUES AND THE DAMAGED NICHE HYPOTHESIS
CD4+ T cell depletion in peripheral blood and secondary lymphatic tissues of LN and GALT where most (98%) of CD4+ T cells reside is the hallmark of HIV infection. Severe depletion occurs within 14 days of HIV acquisition (i.e. during the period of seroconversion) in the lamina propria of GALT (the effector site) and by the time the individual progresses to the chronic stage of disease > 50% of CD4+ T cells in LN are lost (9–15).
Multiple mechanisms responsible for this depletion have been described, including virus induced cytopathicity, antigen specific CTL-mediated lysis, reduced expansion of memory cells, and increased cell turnover (16–18). Recently an additional mechanism of inflammatory damage to the specialized structures in LN that support homestatic mechanisms used to maintain normal population of CD4+ T cells has been described (19, 20). This has been called the damaged niche hypothesis and attributes CD4+ T cell depletion and limited reconstitution to the deposition of collagen and consequent disruption and access to cytokines and growth factors required for CD4+ T cell survival and proliferation.
Structure and function of secondary lymph nodes
The primary function of secondary lymphatic tissues is to bring together foreign antigens, antigen presenting cells (APCs) and antigen-specific B and T cells. The anatomic structures of these organs are uniquely suited to support this function. In LNs the APCs and lymphocytes in lymphatic fluid enter through the afferent lymphatic vessels that drain into a subscapular sinus and then into the medullary sinus (Figure 1). From there they enter the paracortical T cell zone (TZ) that stretches from the base of B cell follicles to the medullary cords where APCs are positioned to encounter and activate the rare antigen-specific T lymphocytes. The superficial cortex consisting mostly of B lymphocytes in primary B cell follicles lies beneath the subcapsular sinus and above and adjacent to the deeper cortical TZ. B cell follicles support the formation of germinal centers and the development of effective memory B cell and high affinity antibody responses following immunization with T cell–dependent antigens (21–27).
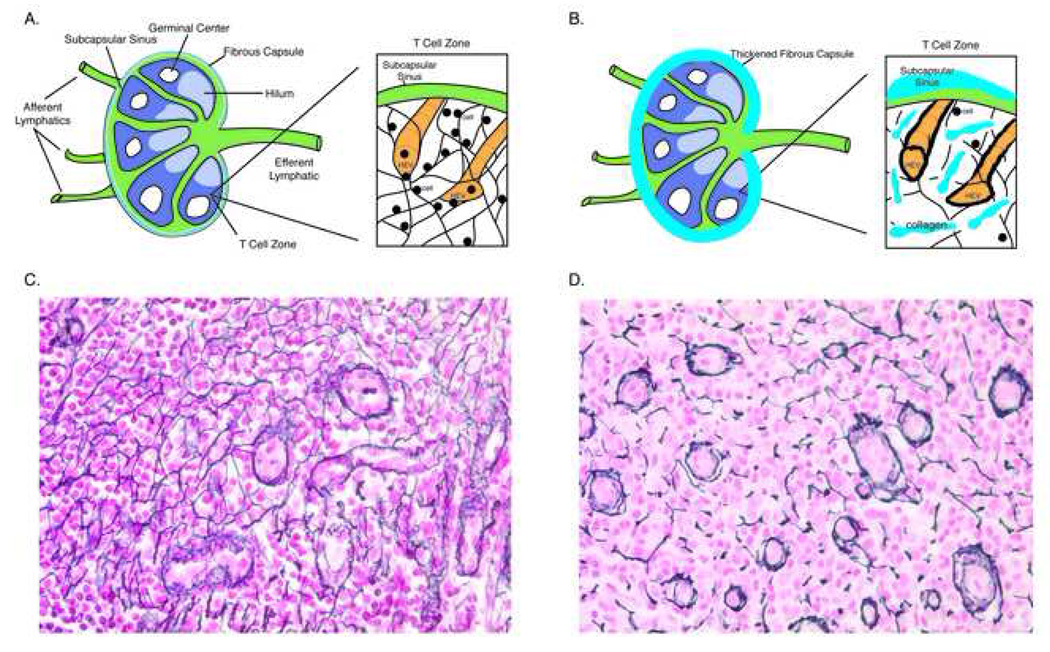
Figure 1. Lymph Node Architecture and the T Cell Zone Niche.
Normal anatomy of a secondary lymph node is presented in Panel A with an enlargement of the T cell zone niche showing the High Endothelial Venules (HEV) and the reticulin network of hollow fibers anchoring the HEV and the subcapsular sinus. The small black circles represent lymphocytes rolling along the reticulin fibers using homing signals embedded in the fibers that promote eventual interaction between antigen presenting cells and naïve CD4 T cells. Panel B shows the fibrotic damage to the lymph node as a result of HIV replication within the organ. The capsule becomes thickened with collagen and collagen is deposited in the T cell zone significantly disrupting the reticulin network. Associated with this change are significant reductions in the size of the population of naïve CD4 T cells (see Figure 3 below), likely the result of impaired homeostatic mechanisms used to maintain this population. In Panel C a section of lymph node from an HIV negative individual is stained with silver stain to identify reticulin fibers (stained black). The network of reticulin fibers is complex and the HEV are normal in appearance. In contrast, Panel D shows a similarly stained section from an individual with HIV disease. The reticulin network is severely disrupted and the HEV’s are thickened.
APCs and lymphocytes gain access to the TZ by two routes. APCs and memory lymphocytes can enter through the afferent lymphatic vessels and then migrate across the floor of the subcapsular sinus to enter the TZ. In contrast, intravascular lymphocytes, in particular naïve lymphocytes, enter the TZ via high endothelial venules (HEV), which are vessels specialized to facilitate binding and transmigration of circulating lymphocytes (28). Once lymphocytes enter the parenchyma of the LN via HEVs, they migrate along the fibers of fibroblast reticular cells (FRCs), which collectively provide the three-dimensional network where T cells can interact with DCs colocalized to the same network (29–33). This spatial organization facilitates ameboid cellular crawling along preformed paths of least resistance, while the basement membrane-like extracellular membrane of FRCs delimits a conduit system for fluid transport for delivery of low molecular weight molecules to HEVs (32–34). Lymphocytes can spend 10 to 100 minutes in this FRC reticular network, receiving inflammatory mediators, cytokines and chemokines from the conduit system, interacting with hundreds of dendritic cells (30, 35).
B cells on the other hand move through the cords to the follicles where they interact with antigens on the follicular dendritic cell network (FDCs) (30, 31, 36). Thus, the reticular network and conduit system of LNs distributes chemokines, cytokines, and inflammatory mediators within lymphoid organs that facilitate lymphocyte transmigration across HEVs and cellular migration, localization and compartmentalization of lymphocyte populations after entering the LN parenchyma that is critical for the efficient development of productive adaptive immune responses.
Naïve CD4+ T cells, depend on this complex structure of the TZ for signaling necessary for survival, locomotion, stimulation and proliferation (37–43). Naïve CD4+ T cells are the critical reservoir for central memory CD4+ T cells, which in turn, provide immunologic memory for previously encountered antigens. The overall size of this population has recently been shown to have prognostic significance in a non-human primate model of SIV infection with larger populations associated with longer survival (44).
Several investigators have shown that one important factor in maintaining the size of the population of naïve CD4+ T cells is the integrity of the TZ. Dai and colleagues found in a thymectomized adult mouse model that the maintenance of the naïve CD4+ T cell populations is dependent on the presence of secondary lymphoid tissues and trafficking of naïve CD4+ T cells through secondary lymphoid organs (45). In another study Link et al showed a critical function for lymph node access in naïve CD4+ and CD8+ T cell homeostasis and identified T cell zone FRCs as the main source of the critical homeostatic signals (i.e. IL-7 and CCL19) (46). Thus, the presence and integrity of secondary lymphatic tissues is critically important in providing the microenvironment needed for the maintenance of CD4+ T cell populations.
HIV REPLICATION AND INFLAMMATORY DAMAGE TO LYMPH NODE STRUCTURE
Transmission and establishment of the lymphatic tissue reservoir
HIV-1 is primarily transmitted across mucosal surfaces, globally now most commonly by intravaginal exposure (1). More than 90 percent of the first productively infected cells are a recently activated but ostensibly immunophenotypicaly ‘resting’ CD4+ T cells (47, 48), and expansion of infection from small founder populations of these infected cells ‘broadcasts’ virus and infected cells, first to the draining lymph nodes and then systemically, in sufficient numbers to establish and maintain virus production in the lymphoid tissues throughout the host (47, 48).
These secondary lymphatic tissues represent a reservoir of virus production and site of vast viral storage (49–55). While ART can rapidly reduce the numbers of productively infected cells in LN within weeks (56), recent work suggests that suppression is not complete, because infected cells can still be found (albeit at a very low frequency) in LN and GALT of virtually all HIV infected persons on ART, highlighting the importance of these sites as reservoirs of persistent viral replication (57, 58).
Pathological changes in the lymphatic tissue reservoir
HIV infection is thus primarily a disease of lymphatic tissues where ongoing replication directly and indirectly causes pathologic damage to lymphatic structures. One of the first descriptions of the lymphatic tissue pathology in HIV disease was of severe lymphadenopathy with pathologic features of hyperplasia leading to follicular lysis and eventually involution (59, 60). These changes were correlated with the clinical stage of HIV infection, with hyperplasia most often seen in the early and presymptomatic stage of disease and lysis and involution seen as the patient progresses to AIDS (53). By contrast, LNs from individuals referred to as long-term non-progressors, because of their relatively good control of viral replication and slower progression to disease, were shown to have less hyperplasia, preserved integrity of the architecture and stromal environment of LNs, and little activation and minimal germinal center formation when compared to LNs from HIV progressors (61, 62). Thus, persistent virus replication and deposition of virus onto the FDC structures in follicles correlated with the destruction of the lymphatic tissue and the loss of the ability to respond to HIV and other pathogens.
As early as 1985 it was recognized there were important pathological changes related to inflammation and fibrotic processes in LN, particularly in the TZ, but the functional consequences of these changes have only recently been appreciated (63). In HIV-1 and SIV infections, it has been shown there is a gradual and progressive deposition of collagen into the TZ of secondary LN and follicular aggregates and Peyer’s patches in GALT (20, 64–67). Staining LN and GALT specimens for collagen from HIV+ persons at all stages of infection for comparison to HIV- individuals reveals this change (20, 64) (Figure 2). The delicate structures of the TZ niche are replaced by collagen, and quantitative image analysis of this fibrotic process in LN shows that the amount of collagen in the TZ at any given time point is correlated with stage of disease (20, 66). By contrast, this relationship does not hold in GALT because of the rapidity of collagen accumulation during acute infection (64).
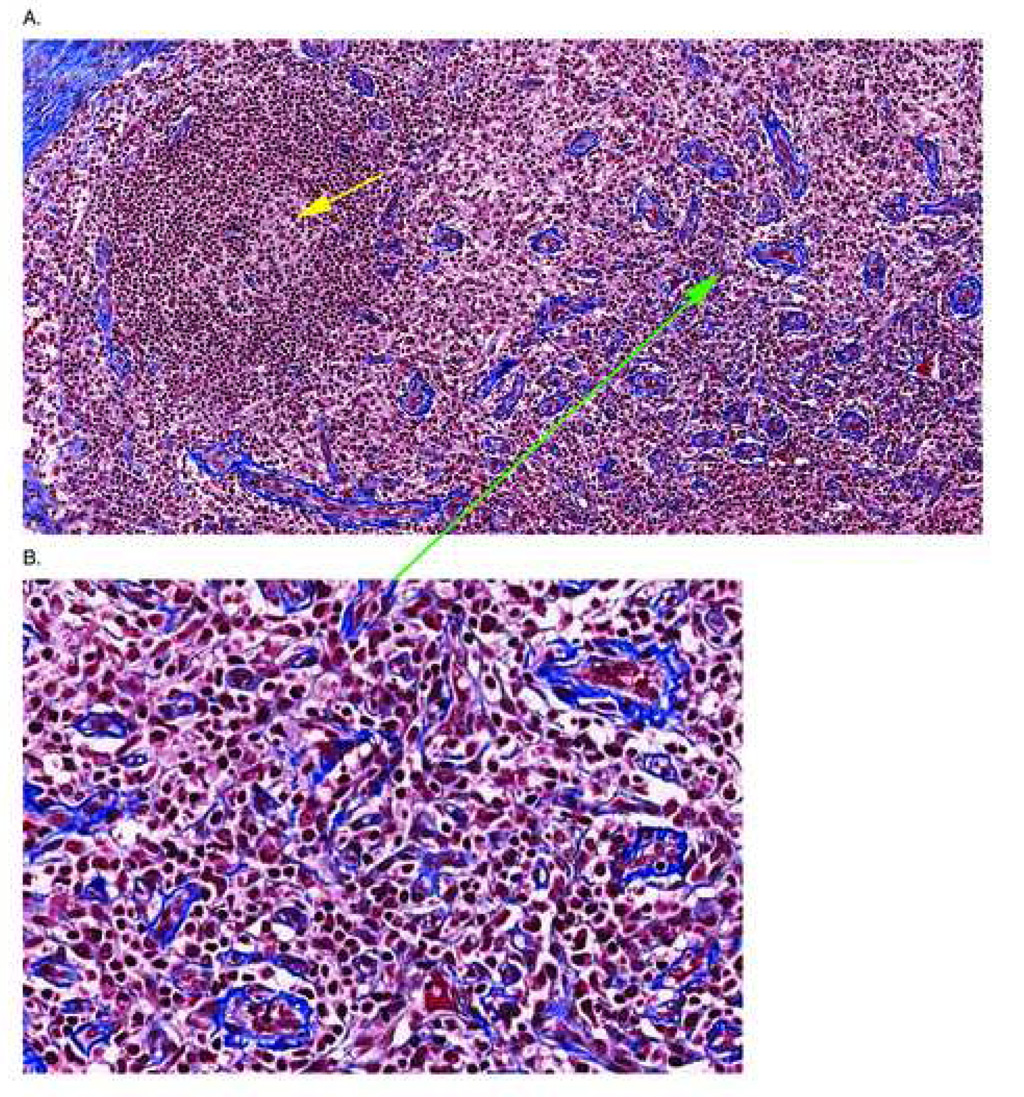
Figure 2. Representative Image of a T Cell Zone Stained With Trichrome.
A section of lymph node from a patient with AIDS is stained with trichrome to reveal collagen deposition in the tissues. In Panel A the T cell zone is evident by the numerous HEV’s (green arrow) showing thickened walls and there is significant deposition of collagen in the tissues of the T cell zone surrounding the HEV’s (more evident in the enlargement of the area around the green arrow shown in Panel B). The yellow arrow shows an involuted, burned out follicle that is characteristic in lymphatic tissues of individuals with advanced disease
Relationship between collagen deposition and size of CD4 T cell populations before and with ART
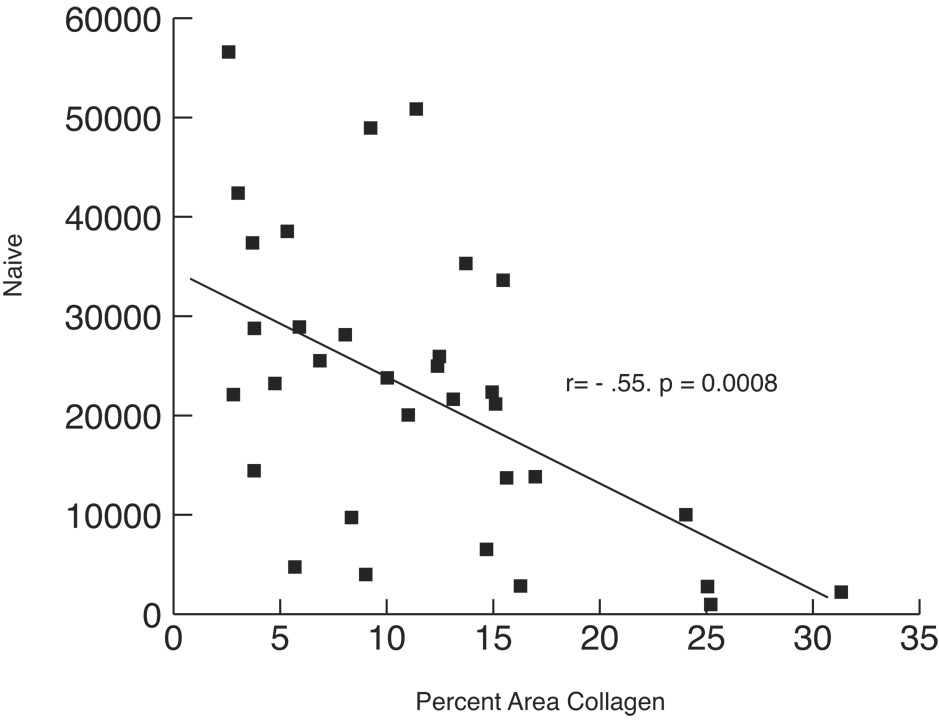
There is a relationship between the size of the CD4+ T cell population and the amount of fibrosis occupying that space (20, 66). The greater the amount of collagen measured in the TZ, the fewer numbers of total CD4+ T cells, and, most significantly, the smaller the naïve CD4+ T cell population (Figure 3). In addition, the amount of collagen in the TZ of secondary LN predicts the degree of reconstitution of the total CD4+ T cell population in peripheral blood after 6 months of ART (67).
Figure 3. T Cell Zone Fibrosis is Correlated With the Size of the LT Naive Population.
This figure (reprinted from reference 66) shows the relationship between collagen deposition in the T cell zone and the size of the naïve CD4 population. The greater the accumulation of collagen in the T cell zone, the smaller will be the size of the naïve CD4 T cell population available to act as a reservoir for the critical central memory population of CD4 + T cells.
Collagen deposition, the damaged niche hypothesis and CD4 T cell depletion and limited reconstiitution
These observations offer an explanation for why reconstitution of naïve and memory CD4+ T cell populations in lymphatic tissues is slow when ART was so effective in suppressing viral replication in these tissues (56, 68). The deposition of collagen disrupts migration and access of CD4 T cells to cytokines such as IL-7 that are critical for their survival and proliferation, and in this way contribute to CD4 T cell loss in proportion to the degree that different subsets depend on IL-7, with greatest impact on naïve CD4+ T cells, but also depletion of central memory and effector memory CD4+ T cell populations generated from the naïve population. Unpublished data from our laboratories suggest that the fibrotic damage to the TZ does not reverse with 6 months of ART, and thus the damage niche also limits repletion of these populations.
Speculations on treatment strategies to limit or reverse fibrosis/early ART for GALT reconstitution
Larger scale studies of longer duration are needed to determine if ART alone can reverse fibrosis and improve reconstitution of particularly naïve CD4+ T cell populations. We also think there may be a role for adjunctive therapies to inhibit and reverse fibrosis, such as those currently under study for idiopathic pulmonary fibrosis and other conditions associated with pathologic fibrosis.
Earlier initiation of ART may also be beneficial, as there are data to suggest that ART can limit progression of fibrosis and preserve naïve and central memory populations of CD4+ T cells in LT in blood, and to a lesser extent in LNs (64). However, ART had to be initiated in the acute/early stage of infection (within 2–3 months of HIV acquisition) for significant increase in the central memory population in the Peyer’s Patch (64). This fact along with the observation of early and more complete fibrosis of the inductive sites of GALT offer a potential explanation for the rapid depletion of CD4+ T cells in GALT and lack of reconstitution with ART.
Role of TGFβ1 in LT collagen deposition and CD4+ T cell depletion in early infection
Several animal models of fibrosis point to induction of TGFβ as an important mediator of pathologic fibrosis (reviewed in depth elsewhere (69–72)). Demonstration of TGFβ as a mechanism of collagen deposition in human HIV infection would be difficult because of the rapidity with which the process occurs early after acquistion and the difficulty in obtaining serial tissues as the patient progresses to AIDS. However, in a non-human primate model of SIV infection, TGFβ has been linked to collagen deposition in the TZ of LT (65), and in a complex way to CD4 T cell depletion. In this model, fibrotic damage was already apparent by 7 days post-infection, with >8-fold increases in collagen levels compared with the pre-infection levels. The deposition of collagen was both rapid and progressive, so that by 28 days after infection (comparable to the beginning of the presymptomatic phase of human infection) the mean fold increase from the preinfection level was 20.5 (65). Collagen deposition continued unabated throughout all stages of disease in the absence of intervention until AIDS defining illness and death ensued, and the extent of collagen deposition was significantly related to decreased numbers of CD4 T cells.
As in HIV infections, immune activation and inflammation appeared to be the driving stimulus to collagen deposition. The extent and timing of the deposition of collagen in the T cell zone paralleled increases in immune activation (Ki67+ cells) and TGFβ1+ cells, previously shown to include Treg cells (65, 73). TGFβ1+ cells were spatially localized in the T cell zone within regions of high collagen deposition during acute infection and correlated with the magnitude of increases in collagen. Expression of TGFβ by Tregs was directly linked to collagen deposition in an in vitro model using TGFβ-expressing induced Tregs co-cultured with primary autologous or allogeneic fibroblasts derived from human secondary lymphatic tissue, in which TGFβ1+ Treg cells, but not conventional activated T cells, stimulated collagen type I protein expression in primary fibroblasts (65). The importance of immune activation driving the induction of TGFβ+ Tregs and collagen deposition in vivo was highlighted by the lack of these processes in apathogenic infections of sooty mangabeys. Thus the TGFβ1+ Treg response appears to induce distinct deleterious outcomes by i) dampening the antiviral immune response (73) and ii) causing harmful effects on CD4+ T cell homeostasis by inducing collagen deposition in lymphatic tissues (65).
HOW FIBROSIS IN THE TZ NICHE MIGHT LIMIT CD4+ T CELL POPULATIONS
There are at least four potential ways that collagen deposition within secondary lymphatic tissues might impact CD4+ T cell population sizes before and during ART (Figure 4). First, collagen deposition and lymphatic tissue scarring could physically limit the space or ‘niche’ that T cells could occupy. Progressive collagen deposition in both HIV and SIV infections can account for up to one-third of the area of the T cell zone (20, 65–67), thus placing a physical limitation on the space in which CD4+ T cells normally reside and migrate. Second, fibrotic ‘scarring’ of the T cell zone of secondary lymphatic tissue could limit/hinder CD4+ T cell migration across high endothelial venules (HEVs). These structures are severely thickened early in the infection (65) and thickening of HEVs might limit ingress of naïve CD4+ T cells into the TZ where cellular growth factors and cytokine signals needed for survival and maintenance through homeostatic proliferation are located. This would explain (at least in part) the finding that the most substantial decrease in CD4+ T cells was within the naïve CD4+ T cell population(66), which was in turn strongly correlated with the extent of LT collagen deposition (66).
The third and fourth possibilities are also migration and access-mechanisms related to the critical roles FRCs play in LTs. It has recently been shown that FRCs constitute the main stromal population in secondary lymphatic tissues and form the internal framework, the reticular network (30, 74–76), whose functions are required for lymphocyte cellular survival and homeostatic maintenance (46). T cells migrate in constant contact with and along the reticular fibers of FRCs, gaining access to such important survival and growth factors as IL-7 of which FRCs are major producers. One can therefore readily envision collagen deposition disrupting this intricate network and pathway to access IL-7 as well as other growth factors and guidance cues, with devastating consequences to T cell survival and proliferation.
CONCLUSION
Lymphatic tissues are the primary site of HIV replication and as a result sustain significant architectural damage. There are significant functional sequelae with reduced numbers of CD4+ T cells, particularly naïve CD4+ T cells and likely impaired antigen response from changes in trafficking. These changes are progressive and do not appear to reverse with antiretroviral therapy alone. It is possible that therapies targeting TGFβ might inhibit or reverse this process and thus, aid efforts at immune reconstitution or even delay time to antiretroviral therapy. Human studies will be necessary to investigate this hypothesis.
ACKNOWLEDGEMENTS
The authors would like to thank Tim Leonard and Jacob Barthold for their assistance in figure preparation.
This work was supported by National Institutes of Health grants R01 AI48484 and AI056997 to A.T.H., T32 AI07421 to J.D.E., and Public Health P130-CA79458-01, 1RO1DE12934-01, MO1 RR00400, 2UO1 AI041535, RO1 AI54232-01A2, and R37 AI 28246. This project has also been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- 1.J. U. N. P. o. H. A (UNAIDS) 2006 Report on the global AIDS epidemic. 2006. [Google Scholar]
- 2.Martin M, Echevarria S, Leyva-Cobian F, Pereda I, Lopez-Hoyos M. Limited immune reconstitution at intermediate stages of HIV-1 infection during one year of highly active antiretroviral therapy in antiretroviral-naive versus non-naive adults. Eur J Clin Microbiol Infect Dis. 2001;20:871–879. doi: 10.1007/s100960100631. [DOI] [PubMed] [Google Scholar]
- 3.Gea-Banacloche JC, Clifford Lane H. Immune reconstitution in HIV infection. Aids. 1999;13 Suppl A:S25–S38. [PubMed] [Google Scholar]
- 4.Barbaro G, Barbarini G. HIV infection and cancer in the era of highly active antiretroviral therapy (Review) Oncol Rep. 2007;17:1121–1126. [PubMed] [Google Scholar]
- 5.Engels EA, Brock MV, Chen J, Hooker CM, Gillison M, Moore RD. Elevated incidence of lung cancer among HIV-infected individuals. J Clin Oncol. 2006;24:1383–1388. doi: 10.1200/JCO.2005.03.4413. [DOI] [PubMed] [Google Scholar]
- 6.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 7.Lewden C, Salmon D, Morlat P, Bevilacqua S, Jougla E, Bonnet F, Heripret L, Costagliola D, May T, Chene G. Causes of death among human immunodeficiency virus (HIV)-infected adults in the era of potent antiretroviral therapy: emerging role of hepatitis and cancers, persistent role of AIDS. Int J Epidemiol. 2005;34:121–130. doi: 10.1093/ije/dyh307. [DOI] [PubMed] [Google Scholar]
- 8.Palefsky JM, Holly EA, Efirdc JT, Da Costa M, Jay N, Berry JM, Darragh TM. Anal intraepithelial neoplasia in the highly active antiretroviral therapy era among HIV-positive men who have sex with men. Aids. 2005;19:1407–1414. doi: 10.1097/01.aids.0000181012.62385.4a. [DOI] [PubMed] [Google Scholar]
- 9.Brenchley JM, Price DA, Douek DC. HIV disease: fallout from a mucosal catastrophe? Nat Immunol. 2006;7:235–239. doi: 10.1038/ni1316. [DOI] [PubMed] [Google Scholar]
- 10.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clayton F, Snow G, Reka S, Kotler DP. Selective depletion of rectal lamina propria rather than lymphoid aggregate CD4 lymphocytes in HIV infection. Clin Exp Immunol. 1997;107:288–292. doi: 10.1111/j.1365-2249.1997.236-ce1111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, Dandekar S. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 14.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 15.Vajdy M, Veazey R, Tham I, deBakker C, Westmoreland S, Neutra M, Lackner A. Early immunologic events in mucosal and systemic lymphoid tissues after intrarectal inoculation with simian immunodeficiency virus. J Infect Dis. 2001;184:1007–1014. doi: 10.1086/323615. [DOI] [PubMed] [Google Scholar]
- 16.Finkel TH, Banda NK. Indirect mechanisms of HIV pathogenesis: how does HIV kill T cells? Curr Opin Immunol. 1994;6:605–615. doi: 10.1016/0952-7915(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 17.Gandhi RT, Chen BK, Straus SE, Dale JK, Lenardo MJ, Baltimore D. HIV-1 directly kills CD4+ T cells by a Fas-independent mechanism. J Exp Med. 1998;187:1113–1122. doi: 10.1084/jem.187.7.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herbein G, Van Lint C, Lovett JL, Verdin E. Distinct mechanisms trigger apoptosis in human immunodeficiency virus type 1-infected and in uninfected bystander T lymphocytes. J Virol. 1998;72:660–670. doi: 10.1128/jvi.72.1.660-670.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haase AT. Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu Rev Immunol. 1999;17:625–656. doi: 10.1146/annurev.immunol.17.1.625. [DOI] [PubMed] [Google Scholar]
- 20.Schacker TW, Nguyen PL, Beilman GJ, Wolinsky S, Larson M, Reilly C, Haase AT. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J Clin Invest. 2002;110:1133–1139. doi: 10.1172/JCI16413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burton GF, Conrad DH, Szakal AK, Tew JG. Follicular dendritic cells and B cell costimulation. J Immunol. 1993;150:31–38. [PubMed] [Google Scholar]
- 22.Kapasi ZF, Qin D, Kerr WG, Kosco-Vilbois MH, Shultz LD, Tew JG, Szakal AK. Follicular dendritic cell (FDC) precursors in primary lymphoid tissues. J Immunol. 1998;160:1078–1084. [PubMed] [Google Scholar]
- 23.Liu YJ, Johnson GD, Gordon J, MacLennan IC. Germinal centres in T-cell-dependent antibody responses. Immunol Today. 1992;13:17–21. doi: 10.1016/0167-5699(92)90199-H. [DOI] [PubMed] [Google Scholar]
- 24.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 25.MacLennan IC, Gray D. Antigen-driven selection of virgin and memory B cells. Immunol Rev. 1986;91:61–85. doi: 10.1111/j.1600-065x.1986.tb01484.x. [DOI] [PubMed] [Google Scholar]
- 26.Tew JG, Wu J, Qin D, Helm S, Burton GF, Szakal AK. Follicular dendritic cells and presentation of antigen and costimulatory signals to B cells. Immunol Rev. 1997;156:39–52. doi: 10.1111/j.1600-065x.1997.tb00957.x. [DOI] [PubMed] [Google Scholar]
- 27.Thorbecke G, Lerman SP. Germinal centers and their role in immune responses. Adv Exp Med Biol. 1976;73(PTA):83, 100. doi: 10.1007/978-1-4684-3297-8_8. [DOI] [PubMed] [Google Scholar]
- 28.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 29.Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gretz JE, Anderson AO, Shaw S. Cords, channels, corridors and conduits: critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol Rev. 1997;156:11–24. doi: 10.1111/j.1600-065x.1997.tb00955.x. [DOI] [PubMed] [Google Scholar]
- 31.Gretz JE, Kaldjian EP, Anderson AO, Shaw S. Sophisticated strategies for information encounter in the lymph node: the reticular network as a conduit of soluble information and a highway for cell traffic. J Immunol. 1996;157:495–499. [PubMed] [Google Scholar]
- 32.Gretz JE, Norbury CC, Anderson AO, Proudfoot AE, Shaw S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J Exp Med. 2000;192:1425–1440. doi: 10.1084/jem.192.10.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaldjian EP, Gretz JE, Anderson AO, Shi Y, Shaw S. Spatial and molecular organization of lymph node T cell cortex: a labyrinthine cavity bounded by an epithelium-like monolayer of fibroblastic reticular cells anchored to basement membrane-like extracellular matrix. Int Immunol. 2001;13:1243–1253. doi: 10.1093/intimm/13.10.1243. [DOI] [PubMed] [Google Scholar]
- 34.Sixt M, Kanazawa N, Selg M, Samson T, Roos G, Reinhardt DP, Pabst R, Lutz MB, Sorokin L. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005;22:19–29. doi: 10.1016/j.immuni.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 35.Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- 36.Willard-Mack CL. Normal structure, function, and histology of lymph nodes. Toxicol Pathol. 2006;34:409–424. doi: 10.1080/01926230600867727. [DOI] [PubMed] [Google Scholar]
- 37.Berzins SP, Boyd RL, Miller JF. The role of the thymus and recent thymic migrants in the maintenance of the adult peripheral lymphocyte pool. J Exp Med. 1998;187:1839–1848. doi: 10.1084/jem.187.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu YX, Chaplin DD. Development and maturation of secondary lymphoid tissues. Annu Rev Immunol. 1999;17:399–433. doi: 10.1146/annurev.immunol.17.1.399. [DOI] [PubMed] [Google Scholar]
- 39.Goldrath AW, Bevan MJ. Selecting and maintaining a diverse T-cell repertoire. Nature. 1999;402:255–262. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- 40.Surh CD, Sprent J. Homeostatic T cell proliferation: how far can T cells be activated to self-ligands? J Exp Med. 2000;192:F9–F14. doi: 10.1084/jem.192.4.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanchot C, Lemonnier FA, Perarnau B, Freitas AA, Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 42.Tough DF, Sprent J. Turnover of naive- and memory-phenotype T cells. J Exp Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.von Boehmer H, Hafen K. The life span of naive alpha/beta T cells in secondary lymphoid organs. J Exp Med. 1993;177:891–896. doi: 10.1084/jem.177.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okoye A, Meier-Schellersheim M, Brenchley JM, Hagen SI, Walker JM, Rohankhedkar M, Lum R, Edgar JB, Planer SL, Legasse A, Sylwester AW, Piatak M, Jr, Lifson JD, Maino VC, Sodora DL, Douek DC, Axthelm MK, Grossman Z, Picker LJ. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J Exp Med. 2007;204:2171–2185. doi: 10.1084/jem.20070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dai Z, Lakkis FG. Cutting edge: Secondary lymphoid organs are essential for maintaining the CD4, but not CD8, naive T cell pool. J Immunol. 2001;167:6711–6715. doi: 10.4049/jimmunol.167.12.6711. [DOI] [PubMed] [Google Scholar]
- 46.Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, Cyster JG, Luther SA. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8:1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 47.Haase AT. Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol. 2005;5:783–792. doi: 10.1038/nri1706. [DOI] [PubMed] [Google Scholar]
- 48.Miller CJ, Li Q, Abel K, Kim EY, Ma ZM, Wietgrefe S, La Franco-Scheuch L, Compton L, Duan L, Shore MD, Zupancic M, Busch M, Carlis J, Wolinsky S, Haase AT. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol. 2005;79:9217–9227. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Embretson J, Zupancic M, Ribas JL, Burke A, Racz P, Tenner-Racz K, Haase AT. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993;362:359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- 50.Fauci AS. Multifactorial nature of human immunodeficiency virus disease: implications for therapy. Science. 1993;262:1011–1018. doi: 10.1126/science.8235617. [DOI] [PubMed] [Google Scholar]
- 51.Haase AT, Henry K, Zupancic M, Sedgewick G, Faust RA, Melroe H, Cavert W, Gebhard K, Staskus K, Zhang ZQ, Dailey PJ, Balfour HH, Jr, Erice A, Perelson AS. Quantitative image analysis of HIV-1 infection in lymphoid tissue. Science. 1996;274:985–989. doi: 10.1126/science.274.5289.985. [DOI] [PubMed] [Google Scholar]
- 52.Pantaleo G, Fauci AS. Immunopathogenesis of HIV infection. Annu Rev Microbiol. 1996;50:825–854. doi: 10.1146/annurev.micro.50.1.825. [DOI] [PubMed] [Google Scholar]
- 53.Pantaleo G, Graziosi C, Demarest JF, Butini L, Montroni M, Fox CH, Orenstein JM, Kotler DP, Fauci AS. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 54.Pantaleo G, Graziosi C, Fauci AS. New concepts in the immunopathogenesis of human immunodeficiency virus infection. N Engl J Med. 1993;328:327–335. doi: 10.1056/NEJM199302043280508. [DOI] [PubMed] [Google Scholar]
- 55.Pantaleo G, Graziosi C, Fauci AS. The role of lymphoid organs in the pathogenesis of HIV infection. Semin Immunol. 1993;5:157–163. doi: 10.1006/smim.1993.1019. [DOI] [PubMed] [Google Scholar]
- 56.Cavert W, Notermans DW, Staskus K, Wietgrefe SW, Zupancic M, Gebhard K, Henry K, Zhang ZQ, Mills R, McDade H, Schuwirth CM, Goudsmit J, Danner SA, Haase AT. Kinetics of response in lymphoid tissues to antiretroviral therapy of HIV-1 infection. Science. 1997;276:960–964. doi: 10.1126/science.276.5314.960. [DOI] [PubMed] [Google Scholar]
- 57.Chun TW, Nickle DC, Justement JS, Meyers JH, Roby G, Hallahan CW, Kottilil S, Moir S, Mican JM, Mullins JI, Ward DJ, Kovacs JA, Mannon PJ, Fauci AS. Persistence of HIV in Gut-Associated Lymphoid Tissue despite Long-Term Antiretroviral Therapy. J Infect Dis. 2008 doi: 10.1086/527324. [DOI] [PubMed] [Google Scholar]
- 58.Schacker TW, Stevenson M, Brenchley J, Douek D, Haase AT. 15th Conference on Retroviruses and Opportunistic Infections. Boston, MA: 2008. Evidence for Persistent Viral Replication in Lymph Node and GALT of ARV-Treated Persons. [Google Scholar]
- 59.Racz P. Molecular, biologic, immunohistochemical, and ultrastructural aspects of lymphatic spread of the human immunodeficiency virus. Lymphology. 1988;21:28–35. [PubMed] [Google Scholar]
- 60.Tenner-Racz K. Human immunodeficiency virus associated changes in germinal centers of lymph nodes and relevance to impaired B-cell function. Lymphology. 1988;21:36–43. [PubMed] [Google Scholar]
- 61.Fauci AS, Pantaleo G, Stanley S, Weissman D. Immunopathogenic mechanisms of HIV infection. Ann Intern Med. 1996;124:654–663. doi: 10.7326/0003-4819-124-7-199604010-00006. [DOI] [PubMed] [Google Scholar]
- 62.Pantaleo G, Menzo S, Vaccarezza M, Graziosi C, Cohen OJ, Demarest JF, Montefiori D, Orenstein JM, Fox C, Schrager LK, et al. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. N Engl J Med. 1995;332:209–216. doi: 10.1056/NEJM199501263320402. [DOI] [PubMed] [Google Scholar]
- 63.O'Murchadha MT, Wolf BC, Neiman RS. The histologic features of hyperplastic lymphadenopathy in AIDS-related complex are nonspecific. Am J Surg Pathol. 1987;11:94–99. doi: 10.1097/00000478-198702000-00002. [DOI] [PubMed] [Google Scholar]
- 64.Estes JD, Baker JV, Brenchley JM, Khoruts A, Barthold JL, Bantle A, Reilly CS, Beilman G, George M, Douek DC, Haase AT, Schacker TW. Collagen Deposition Limits Immune Reconstitution in the Gut. The Journal of Infectious Diseases. 2008 doi: 10.1086/590112. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Estes JD, Wietgrefe S, Schacker T, Southern P, Beilman G, Reilly C, Milush JM, Lifson JD, Sodora DL, Carlis JV, Haase AT. Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor beta 1-positive regulatory T cells and begins in early infection. J Infect Dis. 2007;195:551–561. doi: 10.1086/510852. [DOI] [PubMed] [Google Scholar]
- 66.Schacker TW, Brenchley JM, Beilman GJ, Reilly C, Pambuccian SE, Taylor J, Skarda D, Larson M, Douek DC, Haase AT. Lymphatic tissue fibrosis is associated with reduced numbers of naive CD4+ T cells in human immunodeficiency virus type 1 infection. Clin Vaccine Immunol. 2006;13:556–560. doi: 10.1128/CVI.13.5.556-560.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schacker TW, Reilly C, Beilman GJ, Taylor J, Skarda D, Krason D, Larson M, Haase AT. Amount of lymphatic tissue fibrosis in HIV infection predicts magnitude of HAART-associated change in peripheral CD4 cell count. Aids. 2005;19:2169–2171. doi: 10.1097/01.aids.0000194801.51422.03. [DOI] [PubMed] [Google Scholar]
- 68.Zhang ZQ, Notermans DW, Sedgewick G, Cavert W, Wietgrefe S, Zupancic M, Gebhard K, Henry K, Boies L, Chen Z, Jenkins M, Mills R, McDade H, Goodwin C, Schuwirth CM, Danner SA, Haase AT. Kinetics of CD4+ T cell repopulation of lymphoid tissues after treatment of HIV-1 infection. Proc Natl Acad Sci U S A. 1998;95:1154–1159. doi: 10.1073/pnas.95.3.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 70.Border WA, Ruoslahti E. Transforming growth factor-beta in disease: the dark side of tissue repair. J Clin Invest. 1992;90:1–7. doi: 10.1172/JCI115821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mauviel A. Transforming growth factor-beta: a key mediator of fibrosis. Methods Mol Med. 2005;117:69–80. doi: 10.1385/1-59259-940-0:069. [DOI] [PubMed] [Google Scholar]
- 72.Verrecchia F, Mauviel A. Transforming growth factor-beta and fibrosis. World J Gastroenterol. 2007;13:3056–3062. doi: 10.3748/wjg.v13.i22.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Estes JD, Li Q, Reynolds MR, Wietgrefe S, Duan L, Schacker T, Picker LJ, Watkins DI, Lifson JD, Reilly C, Carlis J, Haase AT. Premature induction of an immunosuppressive regulatory T cell response during acute simian immunodeficiency virus infection. J Infect Dis. 2006;193:703–712. doi: 10.1086/500368. [DOI] [PubMed] [Google Scholar]
- 74.Katakai T, Hara T, Lee JH, Gonda H, Sugai M, Shimizu A. A novel reticular stromal structure in lymph node cortex: an immunoplatform for interactions among dendritic cells, T cells and B cells. Int Immunol. 2004;16:1133–1142. doi: 10.1093/intimm/dxh113. [DOI] [PubMed] [Google Scholar]
- 75.Katakai T, Hara T, Sugai M, Gonda H, Shimizu A. Lymph node fibroblastic reticular cells construct the stromal reticulum via contact with lymphocytes. J Exp Med. 2004;200:783–795. doi: 10.1084/jem.20040254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma B, Jablonska J, Lindenmaier W, Dittmar KE. Immunohistochemical study of the reticular and vascular network of mouse lymph node using vibratome sections. Acta Histochem. 2007;109:15–28. doi: 10.1016/j.acthis.2006.11.002. [DOI] [PubMed] [Google Scholar]