Abstract
Purpose
The International Neuroblastoma Risk Group (INRG) classification system was developed to establish a consensus approach for pretreatment risk stratification. Because the International Neuroblastoma Staging System (INSS) is a postsurgical staging system, a new clinical staging system was required for the INRG pretreatment risk classification system.
Methods
To stage patients before any treatment, the INRG Task Force, consisting of neuroblastoma experts from Australia/New Zealand, China, Europe, Japan, and North America, developed a new INRG staging system (INRGSS) based on clinical criteria and image-defined risk factors (IDRFs). To investigate the impact of IDRFs on outcome, survival analyses were performed on 661 European patients with INSS stages 1, 2, or 3 disease for whom IDRFs were known.
Results
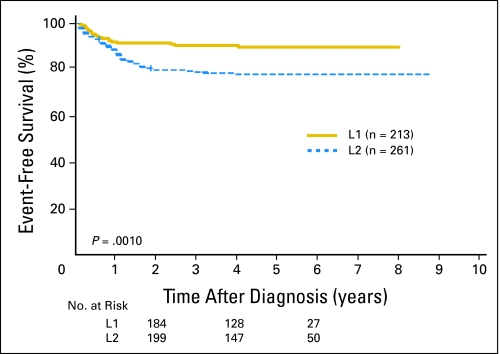
In the INGRSS, locoregional tumors are staged L1 or L2 based on the absence or presence of one or more of 20 IDRFs, respectively. Metastatic tumors are defined as stage M, except for stage MS, in which metastases are confined to the skin, liver, and/or bone marrow in children younger than 18 months of age. Within the 661-patient cohort, IDRFs were present (ie, stage L2) in 21% of patients with stage 1, 45% of patients with stage 2, and 94% of patients with stage 3 disease. Patients with INRGSS stage L2 disease had significantly lower 5-year event-free survival than those with INRGSS stage L1 disease (78% ± 4% v 90% ± 3%; P = .0010).
Conclusion
Use of the new staging (INRGSS) and risk classification (INRG) of neuroblastoma will greatly facilitate the comparison of risk-based clinical trials conducted in different regions of the world.
INTRODUCTION
The International Neuroblastoma Risk Group (INRG) classification system was developed to facilitate the comparison of risk-based clinical trials conducted in different regions of the world by defining homogenous pretreatment patient cohorts. As described in the companion article by Cohn and Pearson et al,1 the INRG classification system was based on survival tree regression analyses of data collected on 8,800 patients. Because the International Neuroblastoma Staging System (INSS) stage of locoregional tumors is based on the degree of surgical resection, this staging system is not suitable for the INRG pretreatment risk classification system. Therefore, the INRG Task Force1 (see Appendix, online only, for participants) developed a new staging system based on tumor imaging rather than extent of surgical resection.
The INSS was developed in 1986 after a meeting that was held to establish international consensus for a common staging system and response to therapy.2,3 Although many countries around the world adopted the INSS, difficulties have been encountered. For example, according to the INSS, the same tumor can be either stage 1 or 3 depending on the extent of surgical excision, making direct comparison of clinical trials based on INSS stages difficult.4 Furthermore, patients with localized disease who are observed because tumor regression is anticipated cannot be properly staged using INSS criteria.5 An additional limitation of the INSS is that assessment of lymph node involvement is necessary for proper staging. However, lymph node sampling is subject to the thoroughness of the individual surgeon, and the assessment of extra-regional lymph node involvement is difficult to apply uniformly.2-4
METHODS
Image-Defined Risk Factors
Since 1994, the International Society of Pediatric Oncology Europe Neuroblastoma Group (SIOPEN) has classified locoregional tumors as resectable or unresectable dependent on the absence or presence of “surgical risk factors,” but independent of INSS stage.6 Surgical risk factors are features detected on imaging that make safe, total tumor excision impracticable at the time of diagnosis.6,7 The SIOPEN principle for stratifying patients with locoregional tumors by imaging features was adopted by the INRG Task Force at a conference in Whistler, Canada, in 2005, and used in the design of the INRG Staging System (INRGSS). However, to avoid confusion with the INSS, the terms resectable and unresectable are not used in the INRG system.
The premise is that a staging system based on preoperative, diagnostic images will be more robust and reproducible than one based on operative findings and approach. Furthermore, because digital radiographs can be reviewed centrally, the images can be evaluated uniformly. As the surgical risk factors are based on radiographic images, it was decided to use the term “image-defined risk factors” (IDRFs), and consensus was reached for the IDRFs listed in Table 1. The IDRFs and the INRGSS are designed for use at the time of diagnosis, but they may also be used at reassessments during treatment. Although not needed for staging patients with disseminated disease, it is recommended that the IDRF status of the primary tumor be recorded in all patients (including patients with metastatic disease), so that the impact of IDRFs on surgical resection, surgical complications, and outcome can be prospectively evaluated in all patients.
Table 1.
Image-Defined Risk Factors in Neuroblastic Tumors
| Ipsilateral tumor extension within two body compartments |
| Neck-chest, chest-abdomen, abdomen-pelvis |
| Neck |
| Tumor encasing carotid and/or vertebral artery and/or internal jugular vein |
| Tumor extending to base of skull |
| Tumor compressing the trachea |
| Cervico-thoracic junction |
| Tumor encasing brachial plexus roots |
| Tumor encasing subclavian vessels and/or vertebral and/or carotid artery |
| Tumor compressing the trachea |
| Thorax |
| Tumor encasing the aorta and/or major branches |
| Tumor compressing the trachea and/or principal bronchi |
| Lower mediastinal tumor, infiltrating the costo-vertebral junction between T9 and T12 |
| Thoraco-abdominal |
| Tumor encasing the aorta and/or vena cava |
| Abdomen/pelvis |
| Tumor infiltrating the porta hepatis and/or the hepatoduodenal ligament |
| Tumor encasing branches of the superior mesenteric artery at the mesenteric root |
| Tumor encasing the origin of the coeliac axis, and/or of the superior mesenteric artery |
| Tumor invading one or both renal pedicles |
| Tumor encasing the aorta and/or vena cava |
| Tumor encasing the iliac vessels |
| Pelvic tumor crossing the sciatic notch |
| Intraspinal tumor extension whatever the location provided that: |
| More than one third of the spinal canal in the axial plane is invaded and/or the perimedullary leptomeningeal spaces are not visible and/or the spinal cord signal is abnormal |
| Infiltration of adjacent organs/structures |
| Pericardium, diaphragm, kidney, liver, duodeno-pancreatic block, and mesentery |
| Conditions to be recorded, but not considered IDRFs |
| Multifocal primary tumors |
| Pleural effusion, with or without malignant cells |
| Ascites, with or without malignant cells |
Abbreviation: IDRFs, image-defined risk factors.
Staging Investigations
Diagnosis.
In the INRG classification system, the diagnosis of neuroblastoma will be made using INSS criteria.3 A tissue diagnosis of neuroblastoma can be made by conventional histology (with or without immunohistology and increased urine or serum catecholamine or metabolites). A diagnosis can also be made if unequivocal tumor cells are seen in bone marrow samples and increased urine or serum catecholamines or metabolites are present.
Involvement of bone marrow.
Bone marrow involvement should be assessed by two aspirates and two biopsies from bilateral sites according to the recommendations of the INSS.3 Biopsy is not required for infants younger than 6 months of age. Bone marrow disease is determined by morphology on smears and biopsies. Biopsies should be complemented by immunohistochemical techniques. Immunocytologic and/or molecular techniques are also recommended to evaluate the presence of tumor cells in the bone marrow at the time of diagnosis, although the results of these assays are not used for staging (Beiske et al, manuscript in preparation on behalf of the INRG Task Force).
Required imaging studies.
Computed tomography (CT) and/or magnetic resonance imaging (MRI) with three-dimensional measurements and of sufficient quality to address IDRFs is mandatory for imaging the primary tumor. The presence or absence of each individual IDRF should be evaluated and recorded (Table 1). When possible, metastatic sites should also be measured by CT and/or MRI, as this information may be needed to evaluate treatment response.
Iodine-123 metaiodobenzylguanidine (MIBG) scintigraphy is mandatory, and it is recommended that the study is performed before tumor excision and according to the Standard Operating Procedure previously described.8 One unequivocal MIBG-positive lesion at a distant site is sufficient to define metastatic disease. A single equivocal lesion on MIBG requires confirmation by another imaging modality (plain radiographs, and if negative, MRI) and/or biopsy.
Technetium-99 bone scintigraphy is required only exceptionally, but in all age groups, if MIBG positivity of the primary tumor cannot be confirmed (ie, the primary tumor is removed or is not MIBG-avid). An isolated bone uptake should be confirmed by another imaging modality and/or biopsy.
Staging Definitions
The short-version definitions of the four INRGSS stages are listed in Table 2.
Table 2.
International Neuroblastoma Risk Group Staging System
| Stage | Description |
|---|---|
| L1 | Localized tumor not involving vital structures as defined by the list of image-defined risk factors and confined to one body compartment |
| L2 | Locoregional tumor with presence of one or more image-defined risk factors |
| M | Distant metastatic disease (except stage MS) |
| MS | Metastatic disease in children younger than 18 months with metastases confined to skin, liver, and/or bone marrow |
NOTE. See text for detailed criteria. Patients with multifocal primary tumors should be staged according to the greatest extent of disease as defined in the table.
Stage L1 tumors are localized tumors that do not involve vital structures as defined by the list of IDRFs (Table 1). The tumor must be confined within one body compartment, neck, chest, abdomen, or pelvis. The isolated finding of intraspinal tumor extension that does not fulfill the criteria for an IDRF (Table 1) is consistent with stage L1.
Stage L2 tumors are locoregional tumors with one or more IDRFs. The tumor may be ipsilaterally continuous within body compartments (ie, a left-sided abdominal tumor with left-sided chest involvement should be considered stage L2). However, a clearly left-sided abdominal tumor with right-sided chest (or vice versa) involvement is defined as metastatic disease.
Stage M is defined as distant metastatic disease (ie, not contiguous with the primary tumor) except as defined for MS. Nonregional (distant) lymph node involvement is metastatic disease. However, an upper abdominal tumor with enlarged lower mediastinal nodes or a pelvic tumor with inguinal lymph node involvement is considered locoregional disease. Ascites and a pleural effusion, even with malignant cells, do not constitute metastatic disease unless they are remote from the body compartment of the primary tumor.
Stage MS is metastatic disease in patients younger than 18 months (547 days) with metastases confined to skin, liver, and/or bone marrow. Bone marrow involvement should be limited to less than 10% of total nucleated cells on smears or biopsy. MIBG scintigraphy must be negative in bone and bone marrow. Provided there is MIBG uptake in the primary tumor, bone scans are not required. The primary tumor can be L1 or L2 and there is no restriction regarding crossing or infiltration of the midline.
Special Conditions
In addition to the IDRFs, and independent of the patient's INRGSS stage, three special conditions should be recorded: multifocal primary tumors, pleural effusion, and ascites (Table 1). Patients with multifocal primary tumors should be staged according to the greatest extent of disease as defined above (ie, stage L1, L2, M, or MS).
Relationship of INSS and INRG Stage
The INSS system is not in keeping with the INRG goal of a pretreatment classification system because the INSS assessment is made after the completion of the initial surgical procedure, and the INSS assessment is strongly dependent on the approach of the individual surgeon. To address these limitations, the INRGSS was developed. However, the survival tree regression analysis that forms the basis for the INRG classification system1 could not be performed in terms of INRGSS because the sample size of patients with known surgical risk factors (analogous to the IDRFs that define INRGSS) in the INRG database1 (< 850) was too small relative to patients with known INSS stage (> 8,500). Posthoc statistical analyses were therefore performed to determine whether it was reasonable to assign staging in terms of IDRFs of INRGSS instead of INSS, and if the prognostic ability of clinical stage was preserved if INRGSS was used. The analyses were restricted to patients with INSS stages 1, 2, or 3 disease because by definition, INSS stage 4 is equivalent to INRGSS M, and INSS stage 4S is very similar to INRGSS MS. Simon et al9 have previously demonstrated the prognostic value of using IDRFs to define stage in a retrospective review of German neuroblastoma studies. The only other available data that can be used to validate the clinical significance of IDRFs and the INRGSS are those from SIOPEN in the INRG database.1 The posthoc analysis of the SIOPEN data was performed in an attempt to validate the findings of the German study.
Statistical Considerations
Cross-tabulation of INRGSS and INSS was performed. The primary analytic end point for the predictive ability of INRGSS was event-free survival (EFS). Time to event was defined as time from diagnosis until time of first occurrence of relapse, progression, secondary malignancy, or death, or until time of last contact if none of these occurred. Univariate analyses were performed to assess the prognostic ability of INRGSS. Kaplan-Meier curves were generated, and curves were compared using log-rank test, with P values less than .05 considered statistically significant.10 EFS and overall survival (OS) values were reported at the 5-year time point ± SE (per Peto).11 It was not the goal of this analysis to compare outcome for INRGSS versus INSS (as was done in the study of Simon et al9).
RESULTS
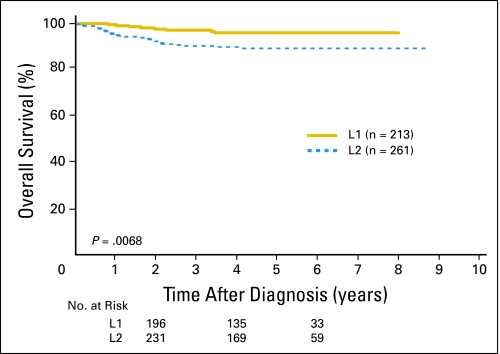
A total of 661 patients with INSS stage 1, 2, and 3 disease from SIOPEN met INRG eligibility criteria and had known data for IDRFs. Twenty-one percent of patients with INSS stage 1, 45% of patients with INSS stage 2, and 94% of patients with INSS stage 3 disease had IDRFs (ie, in total, 50% of all localized tumors were INRGSS stage L2; Table 3). The remainder of patients who had no IDRFs were classified as having INRGSS stage L1 disease. Of the 661 SIOPEN patients, 474 patients had available outcome data. Both INSS and INRGSS were found to be highly prognostic. The EFS for patients with INRGSS stage L1 disease (90% ± 3%, n = 213) was statistically significantly higher than for stage L2 (78% ± 4%, n = 261; P = .0010; Fig 1). The OS for patients with INRGSS stage L1 disease (96% ± 2%) was also significantly higher than for patients with INRGSS stage L2 disease (89% ± 3%; P = .0068; Fig 2). The EFS for patients with INSS stage 1 disease (92% ± 3%, n = 209) was statistically significantly higher than for patients with INSS stage 2 (78% ± 6%, n = 103; P = .0005) and INSS stage 3 disease (75% ± 5%, n = 162; P < .0001), whereas patients with INSS stage 2 and 3 disease had similar EFS (P = .6611). The OS rates for patients with INSS stage 1, 2, and 3 disease were respectively 98% ± 2%, 95% ± 3%, and 84% ± 4%.
Table 3.
Distribution of SIOPEN Patients by INRGSS Versus INSS
| INSS Stage | INRGSS L1
|
INRGSS L2
|
Total No. | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| 1 | 239 | 79 | 64 | 21 | 303 |
| 2 | 81 | 55 | 66 | 45 | 147 |
| 3 | 12 | 6 | 199 | 94 | 211 |
| Total | 332 | 50 | 329 | 50 | 661 |
Abbreviations: SIOPEN, International Society of Pediatric Oncology Europe Neuroblastoma Group; INRGSS, International Neuroblastoma Risk Group Staging System; INSS, International Neuroblastoma Staging System.
Fig 1.
Event-free survival curves for International Society of Pediatric Oncology Europe Neuroblastoma Group patients by International Neuroblastoma Risk Group Staging System stage L1 versus L2 (P = .0010; n = 474). The number of patients at risk for an event are shown along the curves at years 2, 4, and 6.
Fig 2.
Overall survival curves for International Society of Pediatric Oncology Europe Neuroblastoma Group patients by International Neuroblastoma Risk Group Staging System stage L1 versus L2 (P = .0068; n = 474). The number of patients at risk for death are shown along the curves at years 2, 4, and 6.
DISCUSSION
Because excision of the primary tumor is a prerequisite for assigning patients to INSS stages 1 and 2, and because it is possible to downstage patients by surgical treatment at diagnosis,4 the INSS is not suitable for pretreatment staging and risk assessment. A new clinical staging system (INRGSS) was, therefore, designed specifically to constitute one of seven prognostic factors in the INRG pretreatment classification system.1 In the INRGSS, locoregional disease is stratified into two stages instead of three (as in INSS). This decision was based on recognition of the increasing importance of biologic prognostic factors and the excellent OS rate for patients with nonmetastatic neuroblastomas.1,12-16 Although the INRGSS can be used as a separate and independent clinical staging system, its primary function is as a component of the INRG. The INRGSS is not intended to substitute for the INSS, and it is anticipated that most cooperative groups will continue to use INSS in parallel with INRGSS.
Data from European studies show that absence or presence of IDRFs at diagnosis has prognostic significance. Our posthoc analysis of SIOPEN data6 confirmed the results of Simon et al.9 In both studies, EFS was lower for patients with INRGSS stage L2 compared with L1 tumors, and the differences were highly statistically significant. These observations support the translation of EFS tree regression results (in terms of INSS stages) into the INRG classification system (in terms of INRGSS): INSS 1 → INRGSS L1; INSS 2 and 3 → INRGSS L2; INSS 4 → INRGSS M; and INSS 4S → INRGSS MS.1
Because the treatment effect of tumor excision is an inherent part of the INSS, the prognostic value of specific stages within INRGSS and INSS cannot be directly compared. For example, most readers would agree that a comparison between patients with INRGSS stage L1 and INSS stage 1 is actually a comparison between an untreated group of patients and a cohort in whom nearly all patients have already been cured. However, even if INRGSS is not intended to substitute for the INSS, the distribution of patients between the two systems is of interest. In the retrospective study of Simon et al,9 84% of 160 patients with INSS stage 1 disease met the criteria for INRGSS stage L1 (ie, no IDRFs), whereas only 16% of 139 patients with IDRFs (stage L2) had INSS stage 1 disease. Similarly, our posthoc statistical analyses of 661 SIOPEN patients, in whom the clinical impact of surgical risk factors (= IDRFs) was examined prospectively, confirm the results of Simon et al.9 In the data from SIOPEN (Table 3), 79% of patients with INSS stage 1 disease met the criteria for INRGSS stage L1, whereas 21% of patients with IDRFs (stage L2) had INSS stage 1 disease. In the SIOPEN LNESG1 study, 99% of 367 patients who met the criteria for INRGSS stage L1 underwent primary tumor excision (with one surgery-related death caused by renal failure). Among the 363 patients who underwent surgery, 75% had INSS stage 1 disease, 22% had INSS stage 2 disease, and 3% had INSS stage 3 disease. In 56% of 352 patients who had presence of one or more surgical risk factors (INRGSS stage L2), the initial surgical approach was limited to a biopsy; no attempt at primary tumor excision was made.6 Furthermore, both studies referred to above demonstrated that primary operations in patients with IDRFs were associated with significantly lower complete excision rates and greater risks of surgery-related complications.6,9
Recommendations on treatment are not part of the INRGSS, nor of the INRG. Treatment policies must be decided by the individual cooperative groups. However, a new staging and risk classification system cannot exclude possible treatment alternatives, as is the case with INSS and the treatment option of observation without surgery. Today, OS in localized neuroblastoma is more than 90%,1,12-16 and it can be assumed that a certain number of survivors have been overtreated. A main challenge in the years to come will be to maintain survival with reduced treatment. The INRGSS has been designed to permit uniform staging of all patients independent of the treatment alternatives contemplated.
The INRGSS differs from INSS in four important ways. First, it is based on preoperative imaging and IDRFs, not surgicopathologic findings. Second, the midline is not included in the staging criteria of the INRGSS. Third, lymph node status is not included in the staging of localized disease. Fourth, whereas INSS stage 4S has an upper age limit of 12 months, the Task Force decided to extend the age group for stage MS to patients younger than 18 months. The statistical basis for selecting a cutoff age of 18 months in INRG stages L2, M, and MS is presented and discussed in the companion article by Cohn and Pearson et al.1 In one German study, the 5-year EFS was 100% in eight patients aged 12 to 18 months with MYCN nonamplified tumors who, apart from age, had classical INSS stage 4S disease.17 The number of patients with “stage 4S disease aged 12 to 18 months” is small, but because the outcome in this patient cohort remains unclear, it is anticipated that the individual cooperative groups will give these patients special attention in prospective studies where careful stopping rules are included. Unlike INSS stage 4S, stage MS includes patients with primary tumors infiltrating the midline (INSS stage 3). The inclusion of all patients with stage L2 primaries is supported by the results of the SIOPEN 99.2 trial (B. De Bernardi, personal communication, February 2008). In this study, all 30 infants with INSS stage 4 disease having primary tumors corresponding to INSS stage 3 disease because of midline infiltration, and with stage 4S metastatic pattern, survived. Eight patients received no chemotherapy, and the remainder received only one or a few courses of chemotherapy to control symptoms. Only five of the patients had their primary tumor excised.
The effects of treatment on IDRFs are not known, although preliminary data from the SIOPEN Infant Neuroblastoma Study suggests that preoperative chemotherapy (or time) can decrease the incidence of IDRFs by 35% to 40%.18 It also remains unclear whether the risks of surgical complications are reduced by preoperative chemotherapy when delayed operations are performed in patients who have persistent IDRFs. The impact of individual IDRFs on outcome is currently not known, and the clinical significance of individual IDRFs will need to be analyzed in a larger series of patients to address these questions.
Although surgery is not required for INRGSS staging, the biologic characteristics of the tumor must be known to stratify patients according to the INRG pretreatment classification system.1 Image-guided core-needle biopsies are acceptable provided adequate material for the histologic and genetic studies are obtained. However, in many cases, complete or partial tumor excision may be a more rational way to obtain tissue for histologic categorization and genetic studies. In the latter case, it must be emphasized that the magnitude of the residual tumor does not influence the INRG stage. Even if completely excised at diagnosis, a localized tumor with (preoperative) one or more IDRFs will still be classified as an INRGSS stage L2.
The Task Force considered using a specific nomenclature to identify subgroups of patients with neuroblastoma with special features like multifocal primary tumors (because of the potential genetic implications of this diagnosis19,20). The experience with the INSS does not support a practice of subclassification within a staging system. Although the stage of patients with multifocal primary tumors in the INSS should be given a subscript letter M (stage 1M, stage 2AM, and so on),3 this subscript has not been widely accepted and only rarely used in published series. The Task Force, therefore, decided not to use subscripts in the INRGSS. This decision implies that patients with important special features not defined by the INRGSS have to be identified by other measures. It is recommended that data regarding the conditions listed in the last portion of Table 1 be collected.
Isolated pleural effusion and ascites are not considered IDRFs in the INRGSS. Although pleural disease is associated with reduced survival rates in patients with metastatic neuroblastoma,21,22 isolated pleural effusion or ascites is rare in patients with locoregional disease, and its impact on outcome is not clear. In a recent study of 31 patients with neuroblastoma having pleural effusion, none had INSS stage 1 disease and only one had stage 2 disease.23 It is assumed that the vast majority of patients with ascites also have either metastatic disease or the presence of IDRFs.
The extent of intraspinal tumor extension can range from a small tumor component bulging through one intervertebral foramen to a tumor occupying the majority of the spinal canal. In the SIOPEN studies, intraspinal tumor extension is considered a surgical risk factor if neurologic signs of spinal cord compression are present. However, because clinical signs are not image defined, in INRGSS, it was decided to consider intraspinal tumor extension an IDRF, provided one or more of the imaging criteria listed in Table 1 are present.
In conclusion, the INRGSS is a preoperative staging system that has been developed specifically for the INRG classification system. The extent of disease is determined by the presence or absence of IDRFs and/or metastatic tumor at the time of diagnosis, before any treatment. Use of this pretreatment staging system and the INRG classification system will facilitate the ability to compare results of risk-based clinical trials conducted in different regions of the world, and thereby, provide insight into optimal treatment strategies for patients with neuroblastic tumors.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Tom Monclair, Garrett M. Brodeur, Peter F. Ambros, Hervé J. Brisse, Giovanni Cecchetto, Keith Holmes, Michio Kaneko, Wendy B. London, Katherine K. Matthay, Jed G. Nuchtern, Dietrich von Schweinitz, Susan L. Cohn, Andrew D.J. Pearson
Financial support: Wendy B. London, Susan L. Cohn
Administrative support: Susan L. Cohn, Andrew D.J. Pearson
Collection and assembly of data: Tom Monclair, Wendy B. London, Thorsten Simon, Susan L. Cohn, Andrew D.J. Pearson
Data analysis and interpretation: Tom Monclair, Garrett M. Brodeur, Keith Holmes, Wendy B. London, Katherine K. Matthay, Thorsten Simon, Susan L. Cohn, Andrew D.J. Pearson
Manuscript writing: Tom Monclair, Garrett M. Brodeur, Hervé J. Brisse, Giovanni Cecchetto, Keith Holmes, Wendy B. London, Katherine K. Matthay, Jed G. Nuchtern, Susan L. Cohn, Andrew D.J. Pearson
Final approval of manuscript: Tom Monclair, Garrett M. Brodeur, Peter F. Ambros, Hervé J. Brisse, Giovanni Cecchetto, Keith Holmes, Michio Kaneko, Wendy B. London, Katherine K. Matthay, Jed G. Nuchtern, Dietrich von Schweinitz, Thorsten Simon, Susan L. Cohn, Andrew D.J. Pearson
Acknowledgments
INRG Task Force members: Susan L. Cohn, Andrew D.J. Pearson, Wendy B. London, Emanuele S.G. d'Amore, Andreas Faldum, Barbara Hero, Tomoko Iehara, David Machin, Veronique Mosseri, Michel Peuchmaur, Hiroyuki Shimada, Peter F. Ambros, Inge M. Ambros, Garrett M. Brodeur, Jerome Couturier, Michelle Haber, Javed Khan, John M. Maris, Akira Nakagawara, Gudrun Schleiermacher, Frank Speleman, Ruediger Spitz, Nadine Van Roy, Katherine K. Matthay, Klaus Beiske, Sue Burchill, Irene Cheung, Francesco Giammarile, Eiso Hiyama, Jean Michon, Robert C. Seeger, Barry Shulkin, Tom Monclair, Hervé Brisse, Giovanni Cecchetto, Keith S.J. Holmes, Michio Kaneko, Jed G. Nuchtern, Dietrich von Schweinitz, Frank Berthold, Victoria Castel, Robert P. Castleberry, Nai-Kong Cheung, Bruno De Bernardi, Helen Irving, Ruth Ladenstein, C. Patrick Reynolds, Jinhua Zhang, Julie R. Park, Roswitha Schumacher-Kuckelkorn, Thorsten Simon, Hidetaka Niizuma, Toby Trahair, Jennifer Forbeck, and John T. Kemshead. Participants in the INRG Meeting (September 17-19, 2005, Whistler, Vancouver, British Columbia, Canada), locations and group names, and roles are listed in Appendix Table A1, online only.
Appendix
Table A1.
The INRG Task Force: Participants of the INRG Meeting (Whistler, Canada, September 17-19, 2005)
| Name | Country and Cooperative Group | Role |
|---|---|---|
| Susan L. Cohn | United States, COG | Chair |
| Andrew D.J. Pearson | United Kingdom, SIOPEN | Chair |
| Statistics committee | ||
| Wendy B. London | United States, COG | Chair |
| Emanuele S.G. d'Amore | Italy, SIOPEN | |
| Andreas Faldum | Germany, GPOH | |
| Barbara Hero | Germany, GPOH | |
| Tomoko Iehara | Japan, JANB/JINCS | |
| David Machin | United Kingdom, SIOPEN | |
| Veronique Mosseri* | France, SIOPEN | |
| Michel Peuchmaur | France, SIOPEN | |
| Hiroyuki Shimada | United States, COG | |
| Biology committee | ||
| Peter F. Ambros | Austria, SIOPEN | Chair |
| Inge M. Ambros* | Austria, SIOPEN | |
| Garrett M. Brodeur | United States, COG | |
| Jerome Couturier | France, SIOPEN | |
| Michelle Haber | Australia | |
| Javed Khan | United States, COG | |
| John M. Maris | United States, COG | |
| Akira Nakagawara | Japan, JANB/JINCS | |
| Gudrun Schleiermacher | France, SIOPEN | |
| Frank Speleman* | Belgium, SIOPEN | |
| Ruediger Spitz | Germany, GPOH | |
| Nadine Van Roy | Belgium, SIOPEN | |
| Metastatic disease committee | ||
| Katherine K. Matthay | United States, COG | Chair |
| Klaus Beiske | Norway, SIOPEN | |
| Sue Burchill | United Kingdom, SIOPEN | |
| Irene Cheung | United States, COG | |
| Francesco Giammarile | France, SIOPEN | |
| Eiso Hiyama | Japan | |
| Jean Michon | France, SIOPEN | |
| Robert C. Seeger | United States, COG | |
| Barry Shulkin | United States, COG | |
| Surgery committee | ||
| Tom Monclair | Norway, SIOPEN | Chair |
| Hervé Brisse | France, SIOPEN | |
| Giovanni Cecchetto | Italy, SIOPEN | |
| Keith S.J. Holmes | United Kingdom, SIOPEN | |
| Michio Kaneko | Japan, JANB/JINCS | |
| Jed G. Nuchtern | United States, COG | |
| Dietrich von Schweinitz | Germany, GPOH | |
| Senior advisors | ||
| Frank Berthold | Germany, GPOH | |
| Victoria Castel | Spain, SIOPEN | |
| Robert P. Castleberry* | United States, COG | |
| Nai-Kong Cheung | United States, COG | |
| Bruno De Bernardi | Italy, SIOPEN | |
| Helen Irving | Australia, COG | |
| Ruth Ladenstein | Austria, SIOPEN | |
| C. Patrick Reynolds | United States, COG | |
| Jinhua Zhang | China | |
| Young investigators | ||
| Julie R. Park | United States, COG | |
| Roswitha Schumacher-Kuckelkorn | Germany, GPOH | |
| Thorsten Simon | Germany, GPOH | |
| Hidetaka Niizuma | Japan, JANB/JINCS | |
| Toby Trahair | Australia | |
| William Guy Forbeck Research Foundation | ||
| Jennifer Forbeck | United States | |
| John T. Kemshead | United Kingdom |
Abbreviations: COG, Children's Oncology Group; SIOPEN, International Society of Pediatric Oncology Europe Neuroblastoma Group; GPOH, German Pediatric Oncology and Hematology Group; JANB/JINCS, Japanese Advanced Neuroblastoma Study Group/Japanese Infantile Neuroblastoma Co-operative Study Group.
International Neuroblastoma Risk Group Task Force members not present at the Whistler meeting.
published online ahead of print at www.jco.org on December 1, 2008.
Supported by the William Guy Forbeck Research Foundation and Little Heroes Pediatric Cancer Research Fund; and Cancer Research UK and NHS funding to the NIHR Biomedical Research Centre (to A.D.J.P.).
Presented in part at the Advances in Neuroblastoma Research 12th Conference, May 17-20, 2006, Los Angeles, CA, and the International Society of Paediatric Oncology 38th Congress, September 18-21, 2006, Geneva, Switzerland.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Cohn SL, Pearson ADJ, London WB, et al: The International Neuroblastoma Risk Group (INRG) classification system: An INRG Task Force report. J Clin Oncol [epub ahead of print on December 1, 2008] [DOI] [PMC free article] [PubMed]
- 2.Brodeur GM, Seeger RC, Barrett A, et al: International criteria for diagnosis, staging and response to treatment in patients with neuroblastoma. J Clin Oncol 6:1874-1881, 1988 [DOI] [PubMed] [Google Scholar]
- 3.Brodeur GM, Pritchard J, Berthold F, et al: Revisions of the international criteria for neuroblastoma diagnosis, staging and response to treatment. J Clin Oncol 11:1466-1477, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Kushner BH, LaQuaglia MP, Kramer K, et al: Radically different treatment recommendations for newly diagnosed neuroblastoma: Pitfalls in assessment of risk. J Pediatr Hematol Oncol 26:35-39, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Hero B, Simon T, Spitz R, et al: Localized infant neuroblastomas often show spontaneous regression: Results of the prospective trials NB95-S and NB97. J Clin Oncol 26:1504-1510, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Cecchetto G, Mosseri V, De Bernardi B, et al: Surgical risk factors in primary surgery for localized neuroblastoma: The LNESG1 study of the European International Society of Pediatric Oncology Neuroblastoma Group. J Clin Oncol 23:8483-8489, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Holmes K, Mosseri V, Cecchetto, et al: Surgical risk factors (SRF) and outcome following primary surgery for localised neuroblastoma: Results of LNESG1. Pediatr Blood Cancer 49:433, 2007. (abstr O. 127) [Google Scholar]
- 8.Olivier P, Colarinha P, Fettich J, et al: Guidelines for radioiodinated MIBG scintigraphy in children. Eur J Nucl Med Mol Imaging 30:B45-B50, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Simon T, Hero B, Benz-Bohm G, et al: Review of image defined risk factors in localized neuroblastoma patients: Results of the GPOH NB97 trial. Pediatr Blood Cancer 50:965-969, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 11.Peto R, Peto J: Asymptotically efficient rank invariant test procedures. J Royal Stat Soc A 135:185-198, 1972 [Google Scholar]
- 12.Ikeda H, Iehara T, Tsuchida Y, et al: Experience with International Neuroblastoma Staging System and Pathology Classification. Br J Cancer 86:1110-1116, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubie H, Hartmann O, Michon J, et al: N-Myc gene amplification is a major prognostic factor in localized neuroblastoma: Results of the French NBL 90 study. J Clin Oncol 15:1171-1182, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Perez CA, Matthay KK, Atkinson JB, et al: Biologic variables in the outcome of stages I and II neuroblastoma treated with surgery as primary therapy: A Children's Cancer Group study. J Clin Oncol 18:18-26, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Evans AE, Silber JH, Shpilsky A, et al: Successful management of low-stage neuroblastoma without adjuvant therapies: A comparison of two decades, 1972 through 1981 and 1982 through 1992, in a single institution. J Clin Oncol 14:2504-2510, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Matthay KK, Perez C, Seeger RC, et al: Successful treatment of stage III neuroblastoma based on prospective biologic staging: A Children's Cancer Group study. J Clin Oncol 16:1256-1264, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Berthold F, Simon T, Mertens R, et al: Children with metastatic neuroblastoma between 12 and 18 months of age may represent “Biological stage 4S”. Presented at the Advances in Neuroblastoma Research Conference, Los Angeles, CA, May 17-20, 2006. (abstr 298)
- 18.Squire R, Sarnacki S, Haider N, et al: The outcome of surgical procedures at diagnosis in localised infant neuroblastoma, and the effect of chemotherapy on resectability: European infant neuroblastoma study. Pediatr Blood Cancer 49:402, 2007. (abstr O. 011) [Google Scholar]
- 19.Maris JM, Kyemba SM, Rebbeck TR, et al: Molecular genetic analysis of familial neuroblastoma. Eur J Cancer 33:1923-1928, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Hiyama E, Yokoyama T, Hiyama K, et al: Multifocal neuroblastoma: Biological behavior and surgical aspects. Cancer 88:1955-1963, 2000 [PubMed] [Google Scholar]
- 21.Cowie F, Corbett R, Ross Pinkerton C: Lung involvement in neuroblastoma: Incidence and characteristics. Med Pediatr Oncol 28:429-432, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Kammen BF, Matthay KK, Pacharn P, et al: Pulmonary metastases at diagnosis of neuroblastoma in pediatric patients: CT findings and prognosis. AJR 176:755-759, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Gupta H, Conrad J, Khoury JD, et al: Significance of pleural effusion in neuroblastoma. Pediatr Blood Cancer 49:906-908, 2007 [DOI] [PubMed] [Google Scholar]