Abstract
Tastes elicit innate behaviors critical for directing animals to ingest nutritious substances and reject toxic compounds, but the neural basis of these behaviors is not understood. Here, we use a neural silencing screen to identify neurons required for a simple Drosophila taste behavior, and characterize a neural population that controls a specific subprogram of this behavior. By silencing and activating subsets of the defined cell population, we identify the neurons involved in the taste behavior as a pair of motor neurons located in the subesophageal ganglion (SOG). The motor neurons are activated by sugar stimulation of gustatory neurons and inhibited by bitter compounds; however, experiments utilizing split-GFP detect no direct connections between the motor neurons and primary sensory neurons, indicating that further study will be necessary to elucidate the circuitry bridging these populations. Combined, these results provide a general strategy and a valuable starting point for future taste circuit analysis.
Introduction
Animals rely heavily on sensory cues to guide their behavior. In general, information about an organism’s environment is transformed into neural activity by peripheral sensory neurons that respond to stimuli such as touch, light, or chemicals. This information is relayed to neural circuits that process the information into a form that is read by motor programs directly driving behavior. It has been suggested that circuits may be assembled from simple “motifs” of a few synaptically connected neurons that are used repeatedly in information processing networks (Milo et al., 2002). Thus, studying simple circuits in model organisms may yield insight into neural processing in more complex nervous systems.
Studies of small neural networks in invertebrates, including the Aplysia gill withdrawal reflex, the lobster stomatogastric nervous system, the leech heartbeat and the C. elegans olfactory system, are providing fundamental insight into how neural connectivity and function dictate behavior and allow for behavioral plasticity. Much less is understood about neural processing as the scale of the neural circuit increases in complexity from tens of neurons to hundreds or thousands. The fly brain consists of about 100,000 neurons, a level of complexity midrange between the nervous system of C. elegans and mammals. The ability to couple molecular and genetic analyses with studies of cell activity and behavior in Drosophila provides a powerful approach to examine how a complex nervous system orchestrates behavior.
Much work remains to be done in mapping connectivity in the fly brain. The olfactory system of Drosophila is under heavy investigation, yet behavioral circuits are largely unmapped beyond the second order projection neurons (Datta et al., 2008; Jefferis et al., 2007; Marin et al., 2002; Wong et al., 2002). Understanding of the gustatory system is even more rudimentary; in this case only primary gustatory neurons have been identified (Thorne et al., 2004; Wang et al., 2004). Further mapping of taste circuits is an important goal that will lead to insight into how information that is critical to the animal’s survival – whether a substance is nutritious or toxic – is wired in the brain to produce reliable and appropriate behavioral responses.
Fruit flies detect taste compounds using specialized chemosensory bristles located on the proboscis, internal mouthparts, legs, wings and ovipositor (Singh, 1997; Stocker, 1994). Each chemosensory bristle is innervated by two to four gustatory neurons and a mechanosensory cell (Falk, 1976). Dendrites of gustatory receptor neurons (GRNs) extend to the bristle tip, allowing direct contact between receptor molecules on dendrites and chemicals in the environment. Taste information is relayed to the central nervous system by GRN axons, which project either directly to the subesophageal ganglion (SOG) of the fly brain or peripheral ganglia (Rajashekhar and Singh, 1994a; Stocker and Schorderet, 1981; Thorne et al., 2004; Wang et al., 2004).
Two striking characteristics make the fly gustatory system an ideal system for understanding how neural circuits transform sensory information into behavior. First, sensory neurons detect different taste qualities and mediate different behaviors, creating the basis for studying a simple case of sensory integration. GRNs appear to be separable into at least three distinct classes based on molecular and functional characterizations. Neurons expressing the Gr5a gene, one of 68 members in the putative gustatory receptor gene family, respond to sugars and elicit acceptance behavior (Dahanukar et al., 2007; Jiao et al., 2007; Marella et al., 2006; Slone et al., 2007; Wang et al., 2004). The enhancer trap E409-Gal4 marks a second neural population that responds to carbon dioxide and mediates acceptance (Fischler et al., 2007). By contrast, neurons expressing Gr66a respond to a wide array of bitter compounds and mediate avoidance behavior (Marella et al., 2006; Wang et al., 2004). Gr5a, E409 and Gr66a neurons send axons to distinct areas of the SOG, creating a map of taste quality at the first relay in the brain (Marella et al., 2006; Thorne et al., 2004; Wang et al., 2004). However, how this spatial map of taste quality is translated into behavior remains mysterious.
The second advantage of the gustatory system is that flies exhibit robust and quantitative taste behaviors that are readily amenable to analysis. The proboscis extension reflex (PER) is an attractive behavior to study the logic of taste processing in the fly brain because it involves a discrete and quantitative motor response that can be measured in individual animals (Dethier, 1976). PER is elicited by the detection of palatable substances by sensory neurons on the legs or labella. This leads to the coordinated contraction of muscles that drive extension of the proboscis, followed by opening of the labella (Dethier, 1976; Rajashekhar and Singh, 1994b). By contrast, detection of unpalatable substances sends inhibitory information to the SOG, and can stimulate the contraction of muscles that drive proboscis retraction (Dethier, 1976). The relative strength of palatable versus unpalatable tastes in a mixture will determine the probability that PER is initiated, illustrating that the fly brain integrates multiple sensory cues in making the choice of whether to extend (Dethier, 1976; Meunier et al., 2003; Wang et al., 2004). Furthermore, the observation that motor neurons driving PER also make connections in the SOG has been used to suggest that taste circuits may exist locally in this structure (Rajashekhar and Singh, 1994b; Singh, 1997).
We conducted a behavioral screen to identify components of the PER circuit. We focus on a pair of motor neurons that is activated by sugar taste detection and inhibited by bitter compounds, and is necessary and sufficient for a specific subprogram of PER. These neurons synapse on proboscis musculature and show broad dendritic fields in the SOG, but do not appear to make direct connections with GRNs. This study defines the first molecularly identified taste-selective neurons in the fly brain and opens the door to pursuing more detailed circuit analysis in this system.
Results
A screen to identify taste circuit neurons
To identify neurons involved in taste processing, we performed a genetic screen in which we silenced random neurons in the fly brain and examined the effect on taste behavior. To silence neural activity, an inward-rectifying potassium channel that prevents depolarization (Kir2.1; (Baines et al., 2001)) or the tetanus toxin light chain that prevents neurotransmitter release (TNT; (Sweeney et al., 1995)) was expressed in small numbers of neurons in the adult fly brain using specific Gal4 enhancer trap lines. Toxin expression was inhibited during development by ubiquitous expression of temperature sensitive Gal80, and then induced by transfer to 30°C two days prior to behavioral test (McGuire et al., 2004).
The proboscis extension reflex (PER) was used to test the flies’ ability to respond to sweet and bitter compounds. This behavior is rapid, specific, and does not require general coordination. In addition, visually monitoring the behavior of single flies enables the identification of subtle behavioral defects. Flies were stimulated with 100mM sucrose, 50mM sucrose and a mixture of 100mM sucrose and 75mM caffeine. 24 trials with control flies established that the response frequencies to these stimuli were 96% +/−5%, 64% +/− 9%, and 15% +/− 13% respectively (figure 1a).
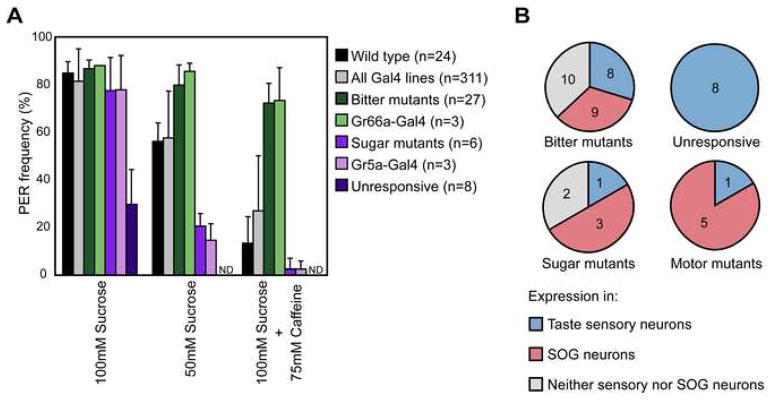
Figure 1. Summary of PER screen.
(A) Average response frequencies for different classes of mutants observed in screen. Each class represents the listed genotype crossed to UAS-KIR2.1, tub-Gal80ts. Mutants were designated based on a response frequency more than three standard deviations away from Wild type for one of the three conditions: 100mM sucrose for “unresponsive” mutants, 50mM sucrose for “sugar mutants”, and a mixture of 100mM sucrose and 75mM caffeine for “bitter mutants”.
(B) Expression analysis of Gal4 enhancer traps from different phenotypic classes. Gal4 expression was assayed by crossing to flies carrying UAS-CD8::GFP. Pie charts indicate the number of lines in each class showing expression in taste sensory neurons (blue), other cells in the SOG (pink), or only cells outside the SOG (gray).
Of 534 Gal-4 enhancer trap lines selected based on their expression in the fly brain, 311 Gal4 enhancer trap lines were viable after toxin expression. For each Gal4 line, ten flies were tested with two trials for each taste compound. On average, these lines showed a response profile very similar to control flies (figure 1a). To minimize false positives based on variation alone, we defined a behavioral mutant as one that had a response frequency greater than 3 standard deviations from the mean of control flies for one taste stimulus. Using this stringent cutoff, we identified 61 behavioral mutants in the primary screen, with 47 of the lines showing consistent behavioral defects in two re-tests. These lines were divided into classes based on the behavioral phenotype: those showing low response to 100mM sucrose were classified as “unresponsive”, those with a decreased response to 50mM sucrose were classified as “sugar mutants”, those with an increased response to the sucrose/caffeine mixture were classified as “bitter mutants” and those that showed a qualitatively altered behavior were called “motor mutants” (figure 1). In total, we identified 8 unresponsive mutants, 6 sugar mutants, 27 bitter mutants, and 6 motor mutants (supplemental table 1). The 6 motor mutants displayed very specific behavioral defects: three could not execute one or more of the specific movements required for proboscis extension, one could not retract the proboscis, one had difficulty ingesting liquid following proboscis extension, and one executed the behavior very slowly.
To gain insight into the identity of neurons affecting PER behavior in each of the lines, we further classified each line based on its Gal4 expression pattern. Since silencing Gr66a- or Gr5a-expressing gustatory sensory neurons leads to bitter mutant and sugar mutant phenotypes, respectively (figure 1a; Wang et al, 2004), we expected some of the behavioral mutants’ phenotypes to be caused by silencing of taste sensory neurons. Indeed, a subset of each behavioral class showed expression in taste sensory neurons (figure 1b). Interestingly, all of the unresponsive mutants showed taste neuron expression, suggesting that the severe phenotypes of this class may be achievable only by eliminating taste sensory detection in the periphery. One of the six sugar mutants showed sensory neuron expression and displayed a phenotype similar to silencing of Gr5a-Gal4 neurons (figure 1a). 8 of the 27 bitter mutant lines exhibited sensory expression, with behavioral phenotypes similar to silencing of Gr66a-Gal4 neurons (figure 1a).
The remaining mutants were roughly evenly divided between those with expression in SOG neurons and those without (figure 1b). Those with SOG expression are good candidates for including bona fide taste circuit neurons, since this area of the brain receives taste input and is presumed to include neurons involved in taste processing. The lines showing expression restricted to outside the SOG likely include neurons whose activity is required to modulate taste circuit activity or higher-order taste circuit neurons involved in levels of taste processing outside the SOG.
Although Gal4 expression patterns provide general information about candidate neurons that may participate in taste processing, the Gal4 lines label tens to thousands of neurons in the brain, only some of which are likely to participate in taste behaviors. The challenge ahead is to identify the specific neurons within each Gal4 line that contribute to behavior. We initially focused on one line, E49-Gal4, which displays a robust and specific motor defect, with the aim of isolating neurons causal for taste behaviors.
E49 neurons are required for a PER subprogram
PER involves several distinct and coordinated motions including lifting of the rostrum from the head, extension of the haustellum, tilting of the head towards the food source, and spreading of the labellar lobes, followed by retraction of the proboscis back to resting state once the stimulus is removed (Chabaud et al., 2006; Dethier, 1976; Singh, 1997; figure 2c and supplemental movie 1). The coordinated contraction of 12 pairs of muscles is required to fully execute this behavior (Rajashekhar and Singh, 1994b).
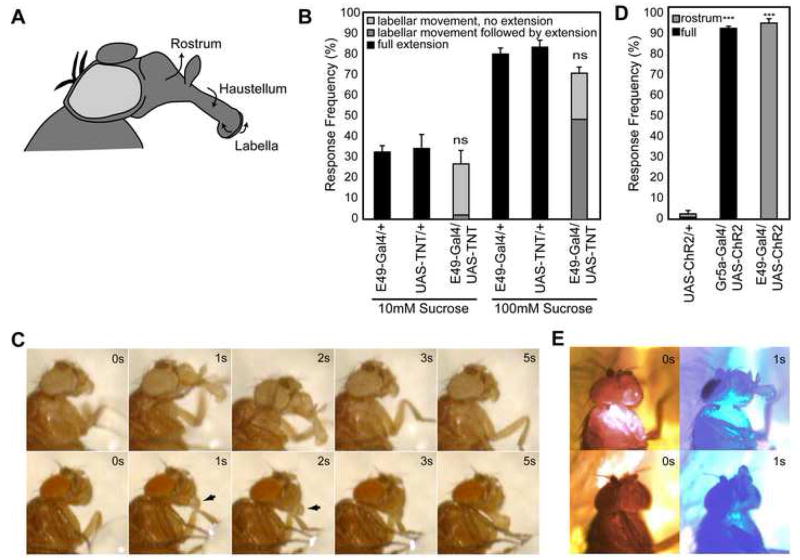
Figure 2. E49 neurons are necessary and sufficient for proboscis extension subprogram.
(A) Diagram of fly head with proboscis extended. The three segments of the proboscis are labeled, with arrows indicating their direction of movement during PER. The rostrum is extended and lifted upwards; the haustellum is extended by flipping downwards, away from its resting position against the rostrum; and the two lobes of the labellum are spread to allow food intake.
(B) Quantification (mean +/− s.e.m.) of PER phenotypes upon silencing of E49-Gal4 neurons. “Full extension” indicates movement of the rostrum, haustellum and labella. Flies expressing tetanus toxin driven by E49-Gal4 exhibit a specific deficit during extension. Frequencies are not significantly different (ns) between the three genotypes at either concentration. Values reflect 3 trials with 20–25 flies per trial per genotype.
(C) Time lapse photographs of wild-type (top row) and E49-Gal4, UAS-TNT (bottom row) flies following 100mM sucrose stimulation. Wild-type flies show full proboscis extension. E49-Gal4, UAS-TNT flies spread labella (arrows) and tilt head forward in the absence of other PER subprograms. Weak extension of the haustellum is observed after 5 seconds of stimulation. For movies, see supplemental materials (movies S1 and S2)
(D) Flies expressing ChR2 in Gr5a-expressing cells show robust full extension (black bars) in response to blue light (480 nm). Flies expressing ChR2 in E49-Gal4 neurons exhibit rostrum extension (gray bars) in response to blue light. n=45 flies for each genotype. Student t-test,*** = p<10−7.
(E) Time lapse photographs showing response of flies expressing ChR2 in Gr5a cells (top row) or E49-Gal4 neurons (bottom row) to intermittent exposure to red light (690 nm; 0s and 3s frames) and blue light (480 nm; 1s and 5s frames). For movies, see supplemental materials (movies S3 and S4).
E49-Gal4, UAS-TNT flies display a specific defect in the proboscis extension reflex. They respond to stimulation with two concentrations of sucrose at a frequency comparable to wild type, suggesting that they detect the sucrose stimulus normally (figure 2b). However, the nature of the response is different between wild-type and E49-Gal4, UAS-TNT flies; E49-Gal4, UAS-TNT flies fail to execute the full motor program. Specifically, they do not lift the rostrum from the head, although other movements remain intact, including tilting the head towards the sugar stimulus and spreading the labella (figure 2c and supplemental movie 2). These results indicate that E49-Gal4 drives expression in neurons required for a subprogram of PER behavior, and that silencing of these neurons allows continued execution of other independent subprograms.
E49 neurons are sufficient for PER subprogram
The neural silencing experiments argue that neurons marked by E49-Gal4 are necessary for proboscis extension. To determine whether activation of E49 neurons is sufficient to generate behavior, we used molecular genetic approaches to inducibly activate E49 neurons and examine the behavioral consequence.
Channelrhodopsin2 (ChR2) is a light-activated cation channel derived from the green alga Chlamydomonas renhardtii (Nagel et al., 2003). Expression of ChR2 causes neurons to rapidly depolarize in response to blue light (~480nm). ChR2 has been used effectively in C. elegans, Drosophila and mammals to precisely activate neural subsets and generate behaviors (Arenkiel et al., 2007; Nagel et al., 2005; Schroll et al., 2006; Zhang et al., 2007a; Zhang et al., 2007b). In control studies, flies expressing ChR2 in the sugar-sensing gustatory neurons display complete proboscis extension behavior to blue light, including head tilting, extension of the proboscis, and opening of the labella (figure 2 and supplemental movie 3), consistent with a recent study (Zhang et al., 2007b). By contrast, E49-Gal4, UAS-ChR2 flies respond to blue light by simply lifting the rostrum out of the head, causing the tip of the proboscis to point upwards (figure 2 and supplemental movie 4). This behavioral subprogram is executed in the absence of the others that normally accompany it during PER: extension of the haustellum, opening of the labella, and tilting of the head forward.
To confirm these results, we activated E49 neurons using a different exogenous, ligand-gated ion channel. VR1 is a mammalian cation channel from the TRP family that is gated by capsaicin, an ingredient in chili peppers, or temperatures above 45°C (Caterina et al., 1997; Marella et al., 2006). Thus, neurons ectopically expressing VR1 will depolarize when their temperature is transiently raised above 45°C. When a heated probe is brought in to close proximity of flies expressing VR1 in sugar-sensing gustatory neurons, these flies generate a complete proboscis extension behavior. Flies in which VR1 is expressed in E49 neurons, however, simply lift the rostrum of their proboscis in response to heat, in an isolated motion (supplemental figure 1).
Thus, the behavioral subprogram executed upon inducible activation of E49 neurons is precisely what was missing from PER when these neurons were silenced: lifting of the rostrum out of the head. This demonstrates that E49 neurons are both necessary and sufficient for this motor program. Furthermore, this program can be either executed or inhibited without affecting the other subprograms that are normally coordinated into one complete behavior.
Mosaic analysis reveals critical E49 cells as SOG motor neurons
The adult fly brain contains approximately 100,000 neurons, with different brain regions mediating different functions. Currently, little is known about the neural circuits controlling proboscis extension, except that gustatory sensory neurons (Singh, 1997; Thorne et al., 2004; Wang et al., 2004), motor neurons that synapse on proboscis muscles (Rajashekhar and Singh, 1994b), and candidate modulatory neurons (Wen et al., 2005; Wu et al., 2003) arborize in the brain region called the subesophageal ganglion (SOG). To identify neurons that mediate rostrum extension during PER, we initially characterized the expression pattern of E49-Gal4 in the nervous system.
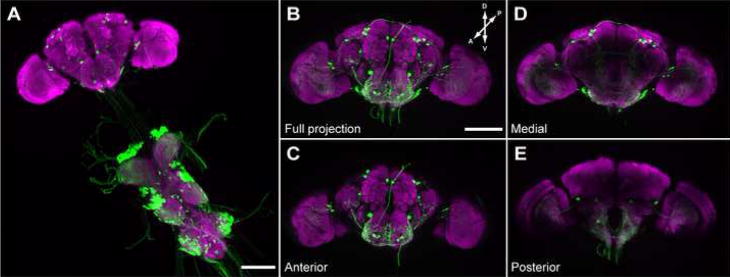
The brains and peripheral ganglia of flies expressing CD8::GFP under the control of E49-Gal4 were examined using immunofluorescence. These studies revealed that E49 drives expression in about 50 central brain neurons. Most appear to be interneurons, with some sending descending processes into the thoracic ganglia. There are also many interneurons with cell bodies in the thoracic ganglia, and a few extend ascending processes into the brain (figure 3). Very weak expression in bitter sensing gustatory neurons (defined by expression of Gr66a), and some olfactory receptor neurons was also observed (data not shown). In addition, significant labeling was seen in the SOG, both in cell bodies (about 20) and neuronal projections throughout the structure.
Figure 3. Expression pattern of E49-Gal4 in the central nervous system.
(A–E) Immunofluorescent detection of mCD8::GFP driven by E49-Gal4. Expression is seen in approximately 50 cells in the brain and numerous projections in the thoracic ganglia (A). Full (B), anterior (C), medial (D) and posterior (E) projections through the brain reveal E49-Gal4 expressing cells in these areas. Scale bars are 100μm.
To identify the neurons within the E49-Gal4 expression domain responsible for rostrum extension, we set out to restrict the activity of Gal4 by mosaic expression of the Gal4 repressor Gal80. We generated an excisable Gal80, in which a Gal80 gene driven by the tubulin promoter is flanked by FRT recombination sites (FLP-out Gal80), such that induction of the FLP recombinase causes stochastic excision of Gal80 via intrachromosomal recombination between the flanking FRT sites (figure 4a). This has the advantage of inducing gal80− cells post-mitotically, thereby directing expression in random combinations of cells without regard to lineage. We then generated flies carrying the following insertions: E49-Gal4; UAS-TNT to induce silencing; UAS-dsRed to mark gal80− cells; the FLP-out-Gal80; and a weak FLP source driven by heat shock promoter (hs-FLP). Following 35 minutes at 37°C, FLP induced loss of Gal80 in a small fraction of E49-Gal4 neurons per fly, thereby allowing expression of TNT and dsRed in only those cells. Among the 1549 resulting flies assayed for behavior, 11 animals showed a complete block in rostrum extension indistinguishable from the phenotype seen when all E49 neurons silenced. Additionally, 57 flies were identified that exhibited a partial phenotype with an obvious sidedness in their inability to lift the rostrum, manifested in extension of the proboscis downward and to one side. Both the full and partial phenotypes were considered impaired extension.
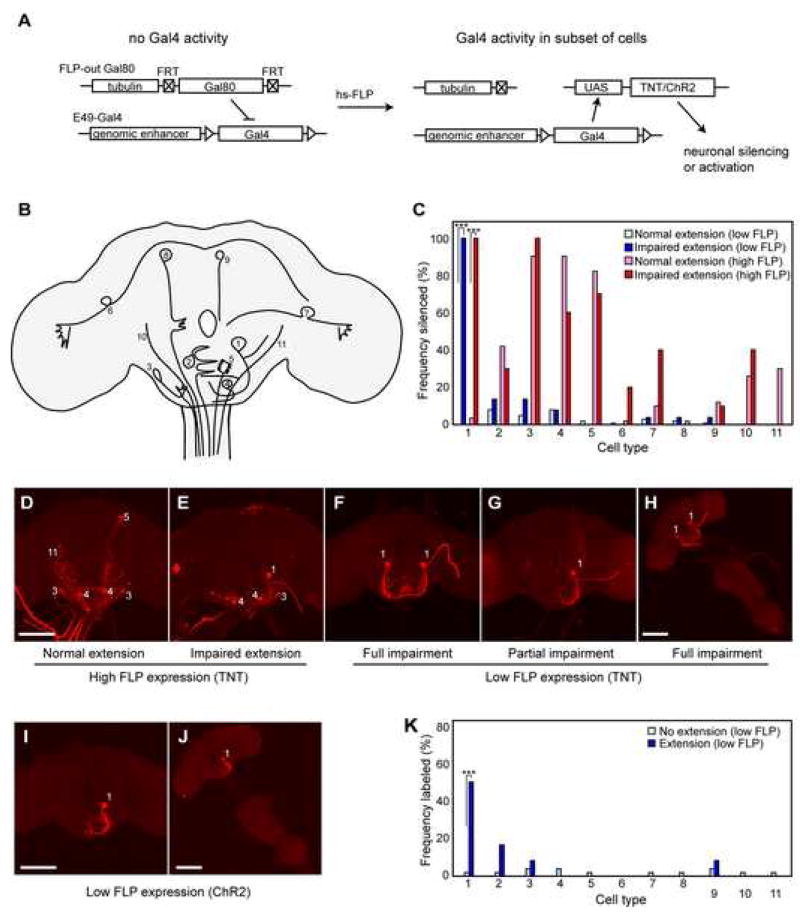
Figure 4. Mosaic analysis reveals neurons in E49-Gal4 population responsible for behavior.
(A) Mosaic silencing strategy using FLP-out Gal80. Prior to FLP expression, E49-Gal4 activity is repressed by ubiquitous expression of Gal80 repressor. Heat shock-driven FLP expression induces excision of the Gal80 cassette in a subset of cells, thereby allowing expression of tetanus toxin (UAS-TNT) and a visible marker (UAS-dsRed) (not illustrated) in those cells.
(B) Illustration of 11 cell types observed in FLP-out labeling during behavioral experiments.
(C) Frequencies of labeled (and therefore silenced) cell types observed in flies showing normal extension after low FLP expression (light blue bars; n=100 sampled from 1549 animals), impaired extension following low FLP expression (dark blue bars; n=68), normal extension following high FLP expression (pink bars; n=50 sampled from 220 animals), or impaired extension following high FLP expression (red bars; n=10), (genotypes: high FLP: hs-FLP; E49-Gal4/UAS-TNT; tub>Gal80>/UAS-dsRed; low FLP: tub>Gal80>; E49-Gal4/UAS-TNT; MKRS, hsFLP/UAS-dsRed). Frequencies represent the proportion of flies in each class where one or more cells of the given type have been silenced. Fisher’s exact test: *** = p<10−12. Cutoff for significance was set at 0.005 to produce an expected false positive rate of 5% of data sets (each including 11 cell types).
(D,E) Immunofluorescent detection of dsRed in example brains exposed to high FLP expression. The brain shown in (D) is from a fly showing normal extension; the brain in (E) is from a fly showing impaired extension. Numbers identify neuron types according to scheme shown in (B).
(F–H) Brains (F,G) or the complete CNS (H) of flies showing impaired PER following low FLP expression. Scale bars are 100μm.
(I,J) Representative images of brain (I) or full CNS (J) from flies showing rostrum extension in response to blue light following expression of dsRed (and hence ChR2) in neuron #1. Genotype: tub>Gal80>; E49-Gal4/UAS-ChR2; MKRS, hsFLP/UAS-dsRed.
(K) Frequency distributions of labeled (and therefore activated) cell types observed in flies showing no rostrum extension following exposure to blue light (light blue bars; n=50 sampled from 314 total) or extension of the rostrum (dark blue bars; n=12). Fisher’s exact test: *** = p<10−4. Cutoff for significance was set at 0.005 to produce an expected false positive rate of 5% of data sets (each including 11 cell types). From 314 animals screened, 12 showed extension of the rostrum. The false positives observed can be explained by the low level of background extension seen in the absence of Gal4 expression (figure 2).
The brains from the impaired extension group and normal group were examined for dsRed expression to identify silenced cells. Eleven different definable cell types were observed in genetic mosaic animals, and the frequencies of observing these cells in animals with normal or impaired PER were plotted (figure 4c). In the simplest model that there is one neuronal type responsible for the observed defect, one would expect the causal neuron to be very highly represented in the impaired class and occur very infrequently in the phenotypically normal animals. Indeed, only neuron #1, with a large cell body in the SOG, had a significantly biased distribution between the normal and impaired phenotypic classes, being silenced in 100% of impaired animals and 0% of normal animals (figure 4c). The 11 animals with a complete block in rostrum extension expressed dsRed (and hence TNT) in two bilaterally symmetric #1 neurons, while the 57 flies that exhibited sidedness in their inability to lift the rostrum showed expression in neuron #1 on only one side of the brain (figure 4). Many of the brains examined had 1–5 brain or ganglia neurons labeled in addition to neuron #1; however, no other brain neurons were present in the impaired group at a frequency higher than 14% (figure 4c). Furthermore, three flies were identified with an impaired PER phenotype that showed expression only in neuron #1 (figure 4h). Repeating the experiment with higher levels of FLP to increase the frequency of labeled neurons produced a similar result, with only silencing of neuron #1 correlating with the behavior despite the increased incidence of other silenced neural populations. In this case 10 of 250 flies assayed displayed impaired PER, with neuron #1 being silenced in 100% of impaired animals and only 4% or normal animals (figure 4c). Together, these mosaic data unequivocally demonstrate that neuron #1 is necessary for the PER subprogram underlying rostrum extension and that inactivation of both neurons is required to completely block this behavior.
We also performed an analogous experiment to determine the neurons within the E49-Gal4 expression domain sufficient to drive rostrum extension. In this case ChR2 expression was restricted to subsets of cells using the same FLP-out Gal80 approach and flies were tested for extension of their proboscis to light. Following low levels of FLP expression, 12 of 314 animals displayed rostrum extension upon stimulation with blue light. Only expression of ChR2 in neuron #1 correlated with rostrum extension, and animals in which only neuron #1 expressed ChR2 displayed extension to light (figure 4i–k). These data strongly implicate neuron #1 as the sole neuron responsible for the observed behavior and demonstrate that it is both necessary and sufficient to drive extension of the rostrum during PER.
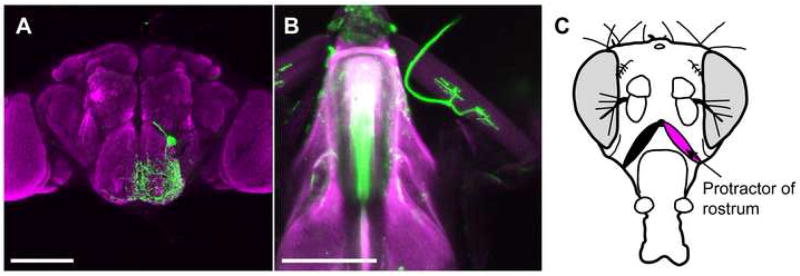
We performed genetic mosaic analyses to determine the anatomy of neuron #1. Single cell labeling of this neuron shows that it has broad dendritic arborizations in the anterior SOG (covering an area of approximately 75 × 80 μm), suggesting that it makes extensive synaptic connections in this structure (figure 5a). The axon from this neuron exits the brain through the pharyngeal nerve and synapses on a muscle associated with the proboscis called the protractor of rostrum (synonym: cibarial muscle 9 (Miller, 1950); figure 5b,c). Consistent with our behavioral analyses, the protractor of rostrum muscle has been previously implicated in lifting the proboscis out of the head capsule (Rajashekhar and Singh, 1994b).
Figure 5. E49 motor neurons synapse on proboscis muscle.
(A) Single E49 motor neuron labeled with mCD8::GFP. Pupae of the genotype yw,hs-flp; E49-Gal4; UAS> CD2,y+>mCD8::GFP were heat shocked for 10 minutes to remove the CD2 FLP-out cassette, and adult brains were stained with antibodies against GFP (green) and nc82 (magenta) to visualize neuropil. Scale bars are 100μm in (A) and (B).
(B) Synapse between E49 motor neuron and protractor of rostrum. Dissected proboscises from E49-Gal4, UAS-dsRed flies were fixed and stained with antibodies against RFP (green). Proboscis and muscles are visualized with autofluorescence (magenta).
(C) Diagram illustrating position of protractor of rostrum muscle.
E49 motor neurons are responsive to tastes
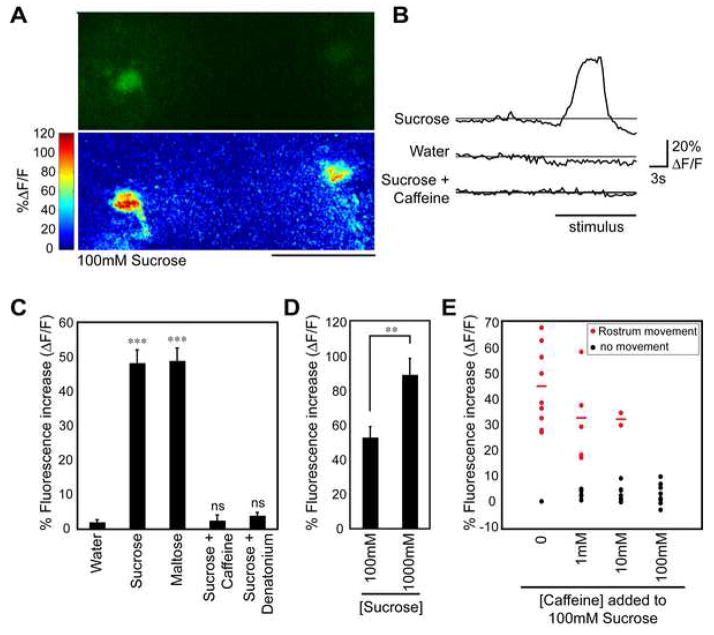
If E49 motor neurons mediate proboscis extension to taste compounds, one expectation is that they exhibit taste-induced activity. To measure motor neuron activity in response to tastants, we used the live preparation previously described for monitoring gustatory sensory activity in the fly brain (Marella et al., 2006). The high signal-to-noise calcium indicator G-CaMP (Nakai et al., 2001) was expressed in E49 cells and taste compounds were applied to the proboscis using a pipette. Changes in fluorescence were recorded using confocal microscopy, with a region of interest comprising the motor neuron cell body.
E49 motor neurons show robust activation in response to sugars delivered to the fly proboscis. High concentrations of sucrose (100mM) and maltose (200mM) induced fluorescence changes of approximately 50% (figure 6c). Interestingly, stimulation with 1M sucrose elicited almost twice the activity of 100mM sucrose (figure 6d). This indicates that information about stimulus intensity is maintained at the level of the motor neuron and suggests that perhaps a relatively simple feed-forward circuit underlies each PER subprogram. Additionally, adding bitter compounds to the sugar solution completely abrogated activation. The addition of either caffeine (50mM) or denatonium (1mM) to 100mM sucrose produced a response that was not significantly different from water alone. These experiments demonstrate that sugar detection exerts excitatory control on the motor neuron and bitter detection results in inhibition. This indicates that sweet and bitter sensory information is integrated in the taste circuit either at or before the level of the E49 motor neuron.
Figure 6. Taste cell stimulation activates E49 motor neurons.
(A) Images of G-CaMP fluorescence in E49 motor neurons. The top image is initial G-CaMP fluorescence, bottom is change in fluorescence (ΔF/F) to 100 mM sucrose. Fluorescence changes are color-coded differences (poststimulation minus prestimulation/prestimulation). Scale bar is 50 μm.
(B) Representative traces showing fluorescence changes of E49 motor neurons in response to stimulation with 100mM sucrose, water, or 100mM sucrose plus 50mM caffeine.
(C) Fluorescence changes in E49 motor neurons following stimulation with water, 100mM sucrose, 200mM maltose, 100mM sucrose plus 50mM caffeine, or 100mM sucrose plus 1mM denatonium. 6–8 flies were used for each condition. Values are mean +/− s.e.m. in this and subsequent panel. student t-test versus water: *** = p<10−4, ns = not significant
(D) Fluorescence changes in E49 motor neurons following stimulation with 100mM sucrose or 1M sucrose. 10 flies were used for each condition. Asterisks indicate significance by student t-test: ** p<0.01.
(E) Scatter plot of fluorescence changes seen in E49 neurons and the concomitant behavioral response following stimulation with 100mM sucrose mixed with varying concentrations of caffeine (n=10 trials for each condition). Red dots indicate trials in which rostrum extension was observed, black dots indicate no observed rostrum extension. Red lines indicate average fluorescence change among trials where a behavioral response was observed. Response frequency was significantly lowered by addition of 10mM and 100mM caffeine (p<0.01, Fisher’s exact test).
To examine the relationship between motor neuron activation and behavior, we used a camera to monitor rostrum movement while performing G-CaMP imaging of the E49 motor neurons. Upon stimulating flies with increasing concentrations of caffeine added to 100mM sucrose, we observed a perfect correlation between rostrum movement and motor neuron activity (figure 6e). As expected, the frequency of observing motor neuron activation decreased upon addition of increasing concentrations of caffeine, along with the frequency of rostrum extension. These data indicate that activity of the E49 motor neuron is a reliable correlate of taste behavior, and will serve as an invaluable tool in studying the flow of information in this potentially simple circuit.
E49 motor neurons do not directly connect to GRNs
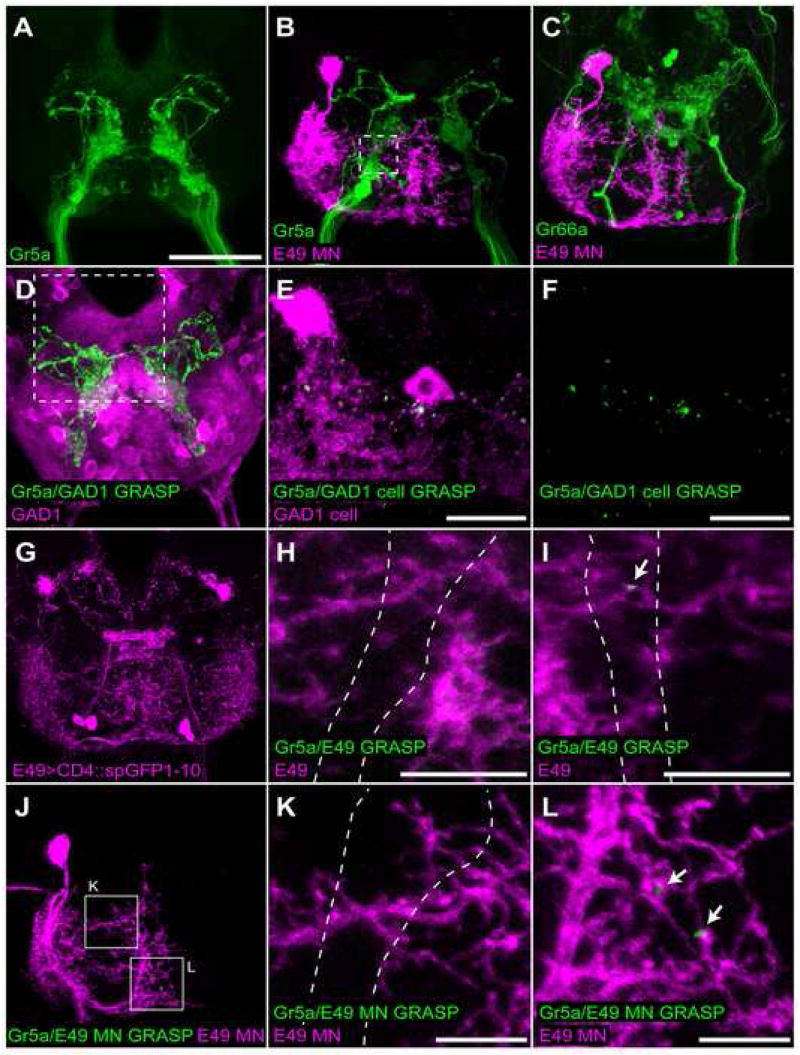
The observation that dendrites of proboscis motor neurons arborize extensively in the SOG, the same structure to which the axons of gustatory sensory neurons project, has been used to suggest that perhaps a monosynaptic circuit underlies PER (Singh, 1997). To address this possibility, we first examined the proximity of single labeled E49 motor neuron dendrites with GRN axons. Excision of the FLP-out Gal80 cassette was driven at low frequency in a genetic background containing E49-Gal4, a membrane-targeted red fluorescent protein (UAS-CD8::dsRed), and GFP driven by either the Gr5a or Gr66a promoter. We observed a very close proximity between E49 motor neuron dendrites and GRNs labeled by Gr5a-driven GFP, with the motor neuron dendrites filling the space around Gr5a axons (figure 8b). By contrast, no close association was seen with Gr66a axons, which project through open space within the E49 motor neuron dendritic field and arborize posteriorly (figure 8c). These data are consistent with the notion that the E49 motor neuron may be synaptically connected to sweet-sensing (Gr5a), but not bitter-sensing (Gr66a) gustatory neurons. To further examine this possibility, we adapted the split-GFP approach recently developed in C. elegans (Feinberg et al., 2008).
Figure 8. E49 motor neurons do not synapse on gustatory sensory neurons.
(A) Expression of CD2::GFP under the control of Gr5a-LexA::VP16 detected by immunofluorescence.
(B,C) Single labeled E49 motor neurons (magenta) and projections from Gr5a (B) or Gr66a (C) gustatory neurons (green). Genotypes are tub>Gal80>/Gr5a-LexA::VP16; E49-Gal4, UAS-CD8::dsRed; lexAop-CD2::GFP/MKRS, hsFLP and tub>Gal80>/Gr66a-Ires-GFP; E49-Gal4/UAS-CD8::dsRed; MKRS, hsFLP. Dashed box in (B) indicates size and approximate position of images presented in H and I.
(D) GRASP between Gr5a neurons expressing CD4::spGFP11 and GAD1 neurons expressing CD4::spGFP1-10 and CD8:dsRed (magenta). GRASP detected by immunofluorescence using monoclonal GFP antibody (green; Sigma). Genotype is GAD1-Gal4/lexAop-CD4::spGFP11; Gr5a-LexA::VP16/UAS-CD4::spGFP1-10. Dashed box indicates approximate position of images in E and F.
(E,F) GRASP between Gr5a neurons expressing CD4::spGFP11 and 2 GAD1 neurons expressing CD4:spGFP1-10. GRASP detected by immunofluorescence using monoclonal GFP antibody (green; Sigma). GAD1 cells expressing CD4::spGFP1-10 identified using rabbit polyclonal GFP antibody (magenta; Abcam). Genotype is Gr5a-LexA::VP16/tub>Gal80>; LexAop-CD4::spGFP11/GAD1-Gal4; UAS-CD4::spGFP1-10/MKRS, hsFLP.
(G) Expression of CD4::spGFP1-10 under control of E49-Gal4 detected by immunofluorescence against GFP1-10 (Abcam polyclonal antibody).
(H,I) Lack of GRASP observed between E49 neurons co-expressing CD4::spGFP1-10 and CD8::dsRed (magenta), and Gr5a neurons expressing CD4::spGFP11. Panels are representative images of E49 dendrites in Gr5a region. GRASP detected by immunofluorescence using polyclonal GFP antibody (green; Invitrogen). Arrow in (I) indicates infrequently observed GRASP signal. Dashes outline approximate position of Gr5a cells in each panel. Genotype is Gr5a-LexA::VP16/+; LexAop-CD4::spGFP11/E49-Gal4; UAS-CD4::spGFP1-10/UAS-CD8::dsRed.
(J–L) Lack of GRASP between Gr5a neurons expressing CD4::spGFP11 and a single labeled E49 motor neuron co-expressing CD4::spGFP1-10 and CD8::dsRed (magenta). GRASP detected by immunofluorescence using monoclonal GFP antibody (green; Sigma). (K) and (L) are enlargements of indicated areas from (J). Arrows in (L) show infrequently observed GRASP signals. Dashes outline approximate position of Gr5a cells in (K). Genotype is Gr5a-LexA::VP16/tub>Gal80>; LexAop-CD4::spGFP11/E49-Gal4, UAS-CD8::dsRed; UAS-CD4::spGFP1-10/MKRS, hsFLP.
Scale bar is 50 μm in (A) and applies to all panels not noted otherwise. Scale bars in (E.F) are 20 μm and (H,I,K,L) are 10 μm. “E49” indicates expression in all E49-Gal4 neurons, “E49 MN” indicates expression in a single E49 motor neuron.
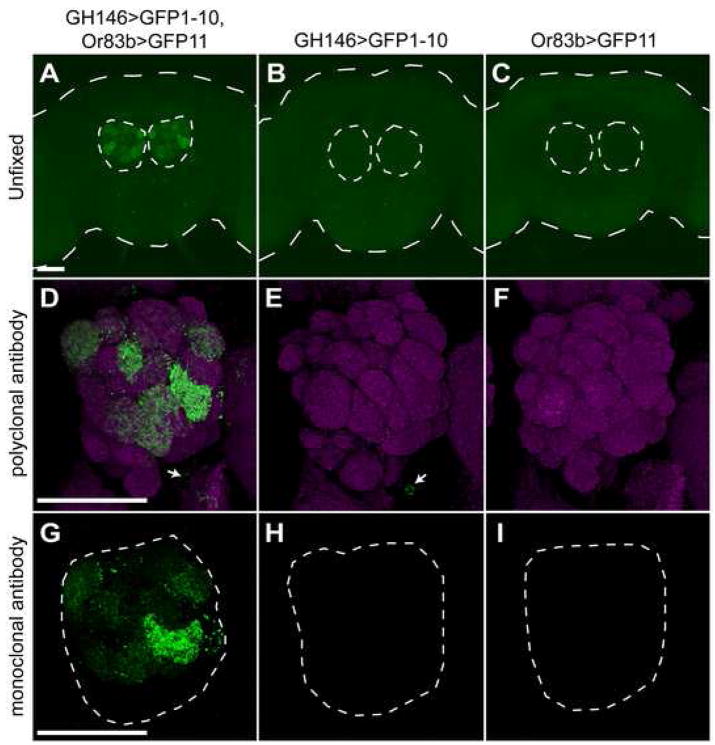
GFP reconstitution across synaptic partners (GRASP; (Feinberg et al., 2008)) is a method of detecting synaptic connections based on the expression of two halves of a split-GFP on the outer membrane of distinct neuronal populations. While neither half of GFP fluoresces individually, GFP is reconstituted trans-synaptically and exhibits fluorescence when the two neuron populations connect (Feinberg et al., 2008). To adapt GRASP to flies, we put one membrane-tethered split-GFP fragment under control of Gal4 (UAS-CD4::spGFP1-10) and the other under control of the LexA promoter (LexAop-CD4::spGFP11). Since neither fusion protein is specifically targeted to the synapse, we would expect to observe fluorescence upon cell-cell contact. This would include, but not be restricted to, synaptic connections. We tested fly GRASP using a known synaptic site in the olfactory system by driving expression in of CD4::spGFP11 and CD4::spGFP1-10 in olfactory receptor neurons (ORNs) and second-order projection neurons (PNs), respectively. As expected, we observed fluorescence in the ~30 GH146-positive antennal lobe glomeruli of animals expressing both constructs, but no fluorescence upon expression of either split-GFP alone (figure 7a–c).
Figure 7. Fly GRASP is detectable by GFP fluorescence and immunofluorescence.
Full brains (A–C) or one antennal lobe (D–I) were imaged from flies of the following genotypes: +/+; GH146-Gal4/LexAop-CD4:spGFP11; Or83b-LexA::VP16/UAS-CD4::spGFP1-10 (A,D,G), +/+; GH146-Gal4/LexAop-CD4::spGFP11; +/UAS-CD4::spGFP1-10 (B,E,H) and +/+; +/LexAop-CD4::spGFP11; Or83b-LexA::VP16/UAS-CD4::spGFP1-10 (C,F,I). Or83b-LexA::VP16 drives expression in 80% of olfactory sensory neurons, and GH146-Gal4 drives expression in second order projection neurons that project dendrites to about 30 glomeruli (Jefferis et al., 2007; Lai et al., 2008; Marin et al., 2002).
(A–C) Unfixed brains were imaged for GFP fluorescence. Long dashes outline brain and short dashes outline antennal lobes.
(D–F) The antennal lobes of fixed brains were imaged for immunofluorescence against GFP to detect GRASP (green; Invitrogen rabbit polyclonal antibody) and the neuropil (magenta; nc82). Arrows indicate weak detection of CD4::spGFP1-10 in PN cell bodies; this polyclonal antibody weakly recognized CD4::spGFP1-10 expressed alone.
(G–H) The antennal lobes of fixed brains were imaged for immunofluorescence against GFP to detect GRASP (green; mouse monoclonal antibody). No signal was observed in the absence of GFP reconstitution. Dashes outline antennal lobe. Scale bars are 50 μm.
Unexpectedly, we could also detect reconstitution of GFP in fixed tissue using immunofluorescence. Three different GFP antibodies raised against full-length GFP were tested for their ability to detect each split-GFP half and the reconstituted form. In two cases (Invitrogen polyclonal antibody and Sigma monoclonal antibody), robust immunofluorescence was observed in the antennal lobes of flies expressing both CD4::spGFP11 and CD4::spGFP1-10 (in ORNs and PNs respectively), but little or no signal upon expression of either half alone (figure 7). Although less sensitive than the polyclonal antibody, the monoclonal antibody showed no ability to detect either spGFP half alone, suggesting that it is perfectly specific for the reconstituted form (figure 7g–i). The third antibody tested (Abcam polyclonal antibody) detected non-reconstituted CD4::spGFP1-10 robustly and CD4::spGFP11 weakly, thereby providing a means to detect expression of these individual constructs (figure 8g). The observation that GRASP can be detected by immunofluorescence in fixed tissues is critical as it increases sensitivity over live imaging, reduces bleaching of fluorescence, and allows for co-staining of samples with other antibodies to visualize complete neuronal processes or other structures.
To examine possible connections with sugar-sensitive gustatory neurons, CD4::spGFP11 was expressed under the control of Gr5a-LexA::VP16. We first tested the ability to detect contact with these neurons by expressing CD4::spGFP1-10 under control of GAD1-Gal4. GAD1-Gal4 marks GABA-ergic neurons, which are known to make connections to sensory neurons in the olfactory system and are therefore good candidates for synaptic targets of taste neurons (Root et al., 2008; Stocker, 1994; Wilson et al., 2004). We detected extensive contact between Gr5a and GAD1 neurons, demonstrating that GRASP functions in this system (figure 8d). Furthermore, restricting CD4::spGFP1-10 to small numbers of neurons using the FLP-out Gal80 technique revealed that GRASP signals can be detected between Gr5a cells and a single contacting cell (figure 8e,f). It is not clear whether these signals represent synaptic or spurious contacts; however, they argue that the technique has the sensitivity to resolve single-cell contacts with Gr5a cells.
We then tested the possibility of contact between E49 and Gr5a neurons by expressing CD4::spGFP1-10 under control of E49-Gal4 and CD4::spGFP11 under control of Gr5a-LexA::VP16. No fluorescence was observed in unfixed brains under these conditions (data not shown). To increase sensitivity, we added expression of CD8::dsRed in E49 cells to visualize the motor neuron dendrites and examined GFP reconstitution by immunofluorescence. Once again we observed no evidence for synaptic connections between E49 motor neurons and Gr5a-expressing cells (figure 8h). Infrequently, we detected reconstituted GFP at points of contact between E49 motor neurons and Gr5a cells (0–2 instances per motor neuron; figure 8i), indicating that detection of contact was possible. However, the infrequency of these contacts relative to the size of the dendritic field and the large number of Gr5a cells (~30 per side), combined with their lack of consistent position from brain to brain argues that they do not represent functional synapses, but rather incidental points of contact between the neurons. Similar data were obtained when FLP-out Gal80 was used to restrict expression of CD4::spGFP1-10 and CD8::dsRed to single E49 motor neurons, strongly suggesting that Gr5a sensory neurons do not contact E49 motor neurons (figure 8j–l). We cannot completely rule out the possibility that synapses between E49 motor neurons and Gr5a gustatory neurons exist below the level of detection offered by GRASP. However, based on the observed sensitivity of GRASP in our tests and in other organisms (Feinberg et al., 2008), we find this possibility unlikely.
Discussion
Understanding how the brain translates sensory information into behavior requires knowledge of neurons involved in this processing. To know how flies perceive tastes, we must move beyond identification of the sensory neurons involved and identify higher order taste circuit components. Here, we present the results of a behavioral screen that identified 47 Gal4 lines driving expression in neurons required for normal proboscis extension behavior. By analyzing one of these lines, we describe the first molecularly identified taste circuit neurons in the fly brain. The bilateral pair of E49 motor neurons is activated in response to sugars, inhibited by inclusion of bitter compounds, and is both necessary and sufficient to generate a subprogram of the proboscis extension reflex.
It has long been known that in the fly, both primary taste neuron projections and motor neurons innervating proboscis musculature reside in the SOG (Rajashekhar and Singh, 1994a; Rajashekhar and Singh, 1994b; Singh, 1997; Stocker and Schorderet, 1981). This led to the prediction that local circuits controlling taste behaviors may be restricted to this structure (Scott, 2005; Singh, 1997). However, questions about the structure and activity of these putative circuits awaited the identification of molecular handles to both identify and manipulate circuit components. In the last few years, much progress has been made in characterizing the first order taste neurons: Gr5a, Gr66a, NP1017-Gal4, and E409-Gal4 were identified as markers for sensory neurons that detect sugars, bitter compounds, water, and carbon dioxide, respectively (Fischler et al., 2007; Inoshita and Tanimura, 2006; Marella et al., 2006; Thorne et al., 2004; Wang et al., 2004). These studies have demonstrated a map of taste quality in the SOG and provided the tools to monitor and manipulate the activity of these neurons. For instance, activation of Gr5a cells is sufficient to generate attractive taste behaviors, including PER ((Zhang et al., 2007b); this study).
Identifying the E49 motor neurons allows us to begin to answer an important question: how does taste neuron activity impinge on motor output? We addressed this question here by asking how the activity of E49 neurons is affected by taste detection. The simplest possibility would be that PER is a reflexive behavior in which activation of sugar cells elicits contraction of extensor muscles in the same way touch stimulates gill withdrawal muscles in Aplysia (Kupfermann et al., 1970). Similarly, bitters could stimulate contraction of retractor muscles to counteract the extension behavior, as suggested previously (Thompson, 1977). However, the observation that E49 neurons are activated by sugars and this activation is inhibited by the inclusion of bitter compounds demonstrates that E49 neurons do not simply receive attractive taste information. Instead, integration of sweet and bitter information must occur upstream of or at the level of the E49 neurons themselves. We also observed that activation of E49 neurons was not an “all or nothing” decision. Increasing the concentration of the sugar stimulus increased the magnitude of the motor neuron response, demonstrating that information about stimulus intensity is maintained throughout this taste circuit. More complex questions about the relationship between sensory input and motor output can now be pursued. For instance: how do the activities of other taste neuron classes affect E49 neuron activity? How do learned associations affect the relationships between sensory detection and motor activity? Do changes in internal states such as hunger affect these relationships? The ability to simultaneously monitor sensory and motor activity in the SOG while examining proboscis extension behavior in a single fly provides the opportunity to directly dissect the relationship between cell activity and behavior.
The identification of E49 motor neurons also provides a valuable starting point for identifying other gustatory circuit components. We have shown the utility of GRASP in flies by detecting cell-cell contact at synapses in the olfactory system, and demonstrated that GFP reconstitution can be monitored by both live imaging and immunofluorescence. This technique was then used to answer an important question: whether PER is driven by simple monosynaptic connections of sensory to motor neurons. The observation that E49 neurons do not directly synapse onto Gr5a gustatory neurons implies that there are higher order neurons in this taste circuit that are yet unidentified. There is a wide array of possibilities for the general structure of this circuit. On one end of the spectrum, taste information could be transmitted from sensory to motor neurons with minimal processing via a simple network of interneurons restricted to the SOG. However, it is also possible that taste sensory information is relayed to decision-making circuits higher in the brain, where it is integrated with information from other senses and about the internal state of the fly before sending a motor command back to the SOG. With the remaining behavioral mutants from our PER screen as a starting point, it should be possible to use the same techniques demonstrated here to comprehensively map the inputs to E49 neurons and the outputs of taste sensory neurons. Such knowledge will give valuable insight into the neural architecture of the PER circuit and provide the tools for dissecting how taste information is processed in the fly.
Materials and Methods
Experimental Animals
Drosophila stocks were maintained on standard cornmeal/agar/molasses medium at 25°C. w1118 strains were used for transgene injections. P element-mediated germline transformations were performed using standard techniques (Genetic Services Inc.) The following lines were used: E49-Gal4 (from Ulrike Heberlein’s Gal4 collection); UAS-TNT (Sweeney et al., 1995); UAS-KIR2.1 (Baines et al., 2001); tub-Gal80ts (McGuire et al., 2004); UAS-ChR2::YFP (a gift from Steven Stowers); UAS-mCD8::GFP (Lee and Luo, 1999); UAS-mCD8::dsRed (Ye et al., 2007); UAS-dsRed, tub>Gal80> (see below); hs-FLP, MKRS (Bloomington stock center); UAS> CD2,y+>-mCD8::GFP (Wong et al., 2002); UAS-G-CaMP (Wang et al., 2003); Or83b-LexA::VP16 (Lai and Lee, 2006); UAS-CD2::GFP (Lai and Lee, 2006); Gr5a-LexA::VP16 (see below); UAS-CD4::spGFP1-10 (see below); LexAop-CD4::spGFP11 (see below).
Generation of transgenes
Details of the construction of ptub-FRT-Gal80-FRT (ptub>Gal80>), UAS-CD4::spGFP1-10, LexAop-CD4::spGFP11 and Gr5a-LexA:VP16 are described in supplemental materials.
Proboscis Extension Reflex
PER was performed as described previously (Wang et al., 2004), with the exception that flies were not prescreened for a response to water. Movies and still images were acquired using a digital camera (Canon S300) and a Nikon stereomicroscope.
For the PER screen, each Gal4 line was crossed to UAS-KIR2.1, tub-Gal80. 2–3 day old females were collected and incubated on food at 30°C for 32h and then with only water for 16h. For each genotype, 10 water-satiated flies were stimulated twice with 100mM sucrose, 50mM sucrose and a mixture of 100mM sucrose and 75mM caffeine.
Inducible activation
Flies expressing ChR2 were raised on standard medium supplemented with 1mM all trans retinal. 1–2 day old flies were starved 16h on 1mM retinal and mounted on myristic acid according to PER techniques. Extension assay was performed by exposing flies for 3s to light passed through the GFP filter set and 20X objective on a Zeiss PASCAL confocal microscope. Frequencies were calculated using 3 exposures per fly. Movies and still images were taken under a Zeiss Lumar stereomicroscope using a uEye camera (UI-1220C; Imaging Development Systems). Illumination for movies was achieved using the Polychrome V monochromator (Till Photonics). Flies were subjected to alternating exposures of blue (480nm; 3s) and red (690nm; 3s) light.
Immunohistochemistry
Staining and imaging was performed as described previously (Wang et al., 2004). Antibodies were used at the following dilutions: rabbit anti-GFP (1:1000; Invitrogen, cat# A11122), rabbit anti-GFP (1:800; Abcam, cat# ab290), mouse anti-GFP (1:100; Sigma, cat# G6539), rabbit anti-RFP (1:200; Chemicon), nc82 (1: 50), rat anti-CD8 (1:100; Invitrogen, cat# RM2200). All images shown are collapsed confocal stacks of 1 μm optical slices.
Mosaic analysis of E49-Gal4
Silencing
To generate large numbers of gal80− cells, flies of the genotype hs-FLP; E49-Gal4/UAS-TNT; tub>Gal80>/UAS-dsRed were raised at 25°C. For small numbers of gal80− cells, flies of the genotype tub>Gal80>; E49-Gal4/UAS-TNT; MKRS, hs-FLP/UAS-dsRed were raised at 25°C and subjected to a 35min heat shock at 37°C during pupal stages. Flies were collected at 0–2 days old and prepared for PER as previously described (Wang et al., 2004). Multiple stimulations of each fly with 100mM sucrose were used to separate flies into phenotypic classes: those showing normal PER were classified as “normal”, those showing movement of the labella or head but impaired rostrum extension were classified as “impaired”, and those showing no detectable response were discarded as the non-responders generally observed in PER assays (~5–10%). The identities of inactivated neurons were assessed by immunohistochemistry against dsRed as described above.
Activation
Flies of the genotype tub>Gal80>; E49-Gal4/UAS-ChR2::YFP; MKRS, hs-FLP/UAS-dsRed were raised at 25°C and subjected to a 35min heat shock at 37°C as described above for silencing experiments. Exposure to light was performed as described above for experiments using entire Gal4 pattern. Under the conditions of our experiment it was difficult to assess any sidedness to the motor response that may have resulted from activating only one of the bilateral #1 neurons.
G-CaMP imaging
Imaging studies were performed as previously described (Marella et al., 2006) with the exception that the proboscis was pinned in a position only partly extended from the head, thereby allowing for further extension upon stimulation. Analysis and pseudo-color image production were performed as described previously (Marella et al., 2006), with the exception color-coded fluorescence changes were created by calculating the percent change in fluorescence ((post stimulation − prestimulation)/prestimulation) rather than the absolute change. The region of interest used for measuring fluorescence changes encompassed one of the E49 motor neuron cell bodies in each fly studied.
Under these conditions, stimulation with water alone was not observed to elicit a motor neuron response, despite the fact that water can drive PER in a thirsty fly (Inoshita and Tanimura, 2006). This could result from the change in flies’ ion balance caused by the imaging preparation, or from a generally lowered response frequency under these conditions.
To correlate cellular responses with behavior, a web camera (Envision V-Cam II) was used to monitor rostrum movement while performing G-CaMP imaging of the E49 motor neurons. Since flies were free to move somewhat during stimulation, G-CaMP fluorescence changes up to 10% were observed from movement of the preparation alone. Thus we can only reliably designate any change above 10% as neuronal activity.
Supplementary Material
Acknowledgments
We thank Ulrike Heberlein for the Gal4 enhancer trap collection; Cori Bargmann and Evan Feinberg for sharing GRASP constructs prior to publication; Tzumin Lee for LexA reagents; Steven Stowers, Sebastian Rumpf, and the Bloomington stock center for flies; Claire Wyart, Gautam Agarwal, and Chris Rodgers for technical assistance; Sunanda Marella for preliminary studies of sugar cell light activation; and the Scott lab for comments and discussion. MDG is a Damon Runyon Fellow supported by the Damon Runyon Cancer Research Foundation (DRG-1947-07). This research was also supported by a grant from the NIDCD 1R01DC006252 and the John Merck Scholars Program (KS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arenkiel BR, Peca J, Davison IG, Feliciano C, Deisseroth K, Augustine GJ, Ehlers MD, Feng G. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron. 2007;54:205–218. doi: 10.1016/j.neuron.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines RA, Uhler JP, Thompson A, Sweeney ST, Bate M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J Neurosci. 2001;21:1523–1531. doi: 10.1523/JNEUROSCI.21-05-01523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chabaud MA, Devaud JM, Pham-Delegue MH, Preat T, Kaiser L. Olfactory conditioning of proboscis activity in Drosophila melanogaster. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192:1335–1348. doi: 10.1007/s00359-006-0160-3. [DOI] [PubMed] [Google Scholar]
- Dahanukar A, Lei YT, Kwon JY, Carlson JR. Two Gr genes underlie sugar reception in Drosophila. Neuron. 2007;56:503–516. doi: 10.1016/j.neuron.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Vasconcelos ML, Ruta V, Luo S, Wong A, Demir E, Flores J, Balonze K, Dickson BJ, Axel R. The Drosophila pheromone cVA activates a sexually dimorphic neural circuit. Nature. 2008;452:473–477. doi: 10.1038/nature06808. [DOI] [PubMed] [Google Scholar]
- Dethier VG. The Hungry Fly. Cambridge, MA; Harvard University Press; 1976. [Google Scholar]
- Falk R, Bleiser-Avivi N, Atidia J. Labellar taste organs of Drosophila melanogaster. J Morphol. 1976;150:327–341. doi: 10.1002/jmor.1051500206. [DOI] [PubMed] [Google Scholar]
- Feinberg EH, Vanhoven MK, Bendesky A, Wang G, Fetter RD, Shen K, Bargmann CI. GFP Reconstitution Across Synaptic Partners (GRASP) defines cell contacts and synapses in living nervous systems. Neuron. 2008;57:353–363. doi: 10.1016/j.neuron.2007.11.030. [DOI] [PubMed] [Google Scholar]
- Fischler W, Kong P, Marella S, Scott K. The detection of carbonation by the Drosophila gustatory system. Nature. 2007;448:1054–1057. doi: 10.1038/nature06101. [DOI] [PubMed] [Google Scholar]
- Inoshita T, Tanimura T. Cellular identification of water gustatory receptor neurons and their central projection pattern in Drosophila. Proc Natl Acad Sci U S A. 2006;103:1094–1099. doi: 10.1073/pnas.0502376103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferis GS, Potter CJ, Chan AM, Marin EC, Rohlfing T, Maurer CR, Jr, Luo L. Comprehensive maps of Drosophila higher olfactory centers: spatially segregated fruit and pheromone representation. Cell. 2007;128:1187–1203. doi: 10.1016/j.cell.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Moon SJ, Montell C. A Drosophila gustatory receptor required for the responses to sucrose, glucose, and maltose identified by mRNA tagging. Proc Natl Acad Sci U S A. 2007;104:14110–14115. doi: 10.1073/pnas.0702421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfermann I, Castellucci V, Pinsker H, Kandel E. Neuronal correlates of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970;167:1743–1745. doi: 10.1126/science.167.3926.1743. [DOI] [PubMed] [Google Scholar]
- Lai SL, Awasaki T, Ito K, Lee T. Clonal analysis of Drosophila antennal lobe neurons: diverse neuronal architectures in the lateral neuroblast lineage. Development. 2008;135:2883–2893. doi: 10.1242/dev.024380. [DOI] [PubMed] [Google Scholar]
- Lai SL, Lee T. Genetic mosaic with dual binary transcriptional systems in Drosophila. Nat Neurosci. 2006;9:703–709. doi: 10.1038/nn1681. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Marella S, Fischler W, Kong P, Asgarian S, Rueckert E, Scott K. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 2006;49:285–295. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Marin EC, Jefferis GS, Komiyama T, Zhu H, Luo L. Representation of the glomerular olfactory map in the Drosophila brain. Cell. 2002;109:243–255. doi: 10.1016/s0092-8674(02)00700-6. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Mao Z, Davis RL. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE 2004. 2004 doi: 10.1126/stke.2202004pl6. pl6. [DOI] [PubMed] [Google Scholar]
- Meunier N, Marion-Poll F, Rospars JP, Tanimura T. Peripheral coding of bitter taste in Drosophila. J Neurobiol. 2003;56:139–152. doi: 10.1002/neu.10235. [DOI] [PubMed] [Google Scholar]
- Miller A. The internal anatomy and histology of the imago of Drosophila melanogaster. In: Demerec M, editor. Biology of Drosophila. New York: Hafner; 1950. pp. 420–531. [Google Scholar]
- Milo R, Shen-Orr S, Itzkovitz S, Kashtan N, Chklovskii D, Alon U. Network motifs: simple building blocks of complex networks. Science. 2002;298:824–827. doi: 10.1126/science.298.5594.824. [DOI] [PubMed] [Google Scholar]
- Nagel G, Brauner M, Liewald JF, Adeishvili N, Bamberg E, Gottschalk A. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol. 2005;15:2279–2284. doi: 10.1016/j.cub.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nat Biotechnol. 2001;19:137–141. doi: 10.1038/84397. [DOI] [PubMed] [Google Scholar]
- Rajashekhar KP, Singh RN. Neuroarchitecture of the tritocerebrum of Drosophila melanogaster. J Comp Neurol. 1994a;349:633–645. doi: 10.1002/cne.903490410. [DOI] [PubMed] [Google Scholar]
- Rajashekhar KP, Singh RN. Organization of motor neurons innervating the proboscis musculature in Drosophila melanogaster meigen (Diptera: Drosophilidae) Int J Insect Morphol & Embryol. 1994b;23:225–242. [Google Scholar]
- Root CM, Masuyama K, Green DS, Enell LE, Nassel DR, Lee CH, Wang JW. A presynaptic gain control mechanism fine-tunes olfactory behavior. Neuron. 2008;59:311–321. doi: 10.1016/j.neuron.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroll C, Riemensperger T, Bucher D, Ehmer J, Voller T, Erbguth K, Gerber B, Hendel T, Nagel G, Buchner E, Fiala A. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr Biol. 2006;16:1741–1747. doi: 10.1016/j.cub.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Scott K. Taste recognition: food for thought. Neuron. 2005;48:455–464. doi: 10.1016/j.neuron.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Singh RN. Neurobiology of the gustatory systems of Drosophila and some terrestrial insects. Microsc Res Tech. 1997;39:547–563. doi: 10.1002/(SICI)1097-0029(19971215)39:6<547::AID-JEMT7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Slone J, Daniels J, Amrein H. Sugar receptors in Drosophila. Curr Biol. 2007;17:1809–1816. doi: 10.1016/j.cub.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker RF. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- Stocker RF, Schorderet M. Cobalt filling of sensory projections from internal and external mouthparts in Drosophila. Cell Tissue Res. 1981;216:513–523. doi: 10.1007/BF00238648. [DOI] [PubMed] [Google Scholar]
- Sweeney ST, Broadie K, Keane J, Niemann H, O’Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- Thompson AJ. The mechanics of proboscis extension by the blowfly Phormia regina Meigen. Canadian Journal of Zoology. 1977;55:602–606. [Google Scholar]
- Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr Biol. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112:271–282. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Wen T, Parrish CA, Xu D, Wu Q, Shen P. Drosophila neuropeptide F and its receptor, NPFR1, define a signaling pathway that acutely modulates alcohol sensitivity. Proc Natl Acad Sci U S A. 2005;102:2141–2146. doi: 10.1073/pnas.0406814102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Turner GC, Laurent G. Transformation of olfactory representations in the Drosophila antennal lobe. Science. 2004;303:366–370. doi: 10.1126/science.1090782. [DOI] [PubMed] [Google Scholar]
- Wong AM, Wang JW, Axel R. Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell. 2002;109:229–241. doi: 10.1016/s0092-8674(02)00707-9. [DOI] [PubMed] [Google Scholar]
- Wu Q, Wen T, Lee G, Park JH, Cai HN, Shen P. Developmental control of foraging and social behavior by the Drosophila neuropeptide Y-like system. Neuron. 2003;39:147–161. doi: 10.1016/s0896-6273(03)00396-9. [DOI] [PubMed] [Google Scholar]
- Ye B, Zhang Y, Song W, Younger SH, Jan LY, Jan YN. Growing dendrites and axons differ in their reliance on the secretory pathway. Cell. 2007;130:717–729. doi: 10.1016/j.cell.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: optical technologies for probing neural signals and systems. Nat Rev Neurosci. 2007a;8:577–581. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
- Zhang W, Ge W, Wang Z. A toolbox for light control of Drosophila behaviors through Channelrhodopsin 2-mediated photoactivation of targeted neurons. Eur J Neurosci. 2007b;26:2405–2416. doi: 10.1111/j.1460-9568.2007.05862.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.