Abstract
We report the synthesis and characterization of amino acid ester based chiral ionic liquids, derived from L- and D-alanine tert butyl ester chloride. The synthesis was accomplished via an anion metathesis reaction between commercially available L-and D-alanine tert butyl ester chloride using a variety of counterions such as lithium bis (trifluoromethane) sulfonimide, silver nitrate, silver lactate, and silver tetrafluoroborate. Both enantiomeric forms were obtained as confirmed by bands of opposite sign in the circular dichroism spectra. The L- and D-alanine tert butyl ester bis (trifluoromethane) sulfonimide were obtained as liquids at room temperature and intriguingly exhibited the highest thermal stability (up to 263°C). In addition, the ionic liquids demonstrated enantiomeric recognition ability as evidenced by splitting of racemic Mosher's sodium salt signal using a liquid state 19F nuclear magnetic resonance (NMR) and fluorescence spectroscopy. The L- and D-alanine tert butyl ester chloride resulted in solid salts with nitrate, lactate, and tetrafluoroborate anions. This illustrates the previously observed tunability of ionic liquid synthesis, resulting in ionic liquids of varying properties as a function of varying the anion.
Keywords: chiral ionic liquids, chiral selectors, synthesis, chiral recognition
INTRODUCTION
Ionic liquids (ILs), commonly termed room temperature ionic liquids (RTILs), are a class of organic salts. These molecules typically contain an organic cation with delocalized charge and a bulky inorganic anion. Interest in RTILs continues to grow because of their potential as greener solvent alternatives to conventional environmentally damaging organic solvents.1 In addition, ILs have unique properties such as lack of measurable vapor pressure, high thermal stability, and recyclability.1-5 Such environmental-friendly properties make ILs relatively benign solvents for cleaner processes to minimize toxic chemical wastes which have become a priority for chemical industries.6 Room temperature ILs have been used in various applications such as replacing conventional organic solvents in organic synthesis,7 solvent extractions,8 electrochemical reactions,9,10 liquid-liquid extractions,11 and in enzymatic reactions.12 In addition, analytical applications of ILs such as their use as buffers in capillary electrophoresis,13 as stationary phases in gas chromatography14,15 as well as high performance liquid chromatography,16 and enhancement of sensitivity in thermal lens measurement have also been investigated.17 A review of several analytical applications of ILs has been reported by Baker et al.18 In addition, Baker et al. have developed an optical sensor based on an ionic liquid.19
Analysis of chiral molecules is very important since different enantiomers of a chiral racemic drug may display different properties.20 For example, one enantiomer of a chiral drug may have the desired medicinal properties, while the other enantiomer may be harmful. Various chiral selectors, such as cyclodextrins, molecular micelles, antibody, and crown ethers have been widely used because of their chiral recognition abilities.21-24 However, the use of many current chiral selectors is often limited because of low solubility, difficult organic syntheses, instability at high temperature, as well as high cost. In addition, many currently available chiral selectors require the use of another solvent and sometimes more than one solvent system if the analyte and the chiral selector are not soluble in the same solvent. Therefore, there is a need for the development of new chiral selectors that can be used simultaneously as solvent and chiral selector. Thus, the use of chiral ILs have gained popularity since they can be used as chiral solvents for asymmetric induction in synthesis.25 Chiral ILs can also serve as chiral stationary phases in chromatography as demonstrated by Ding and Armstrong in capillary electrophoresis.26 Tran and coworkers have also recently used chiral ILs for determining the enantiomeric composition of pharmaceutical products.27,28
To our knowledge, the first chiral ionic liquid, 1-butyl-3-methyl imidazolium lactate was reported by Seddon and his coworkers in 1999.29 This chiral ionic liquid, with a chiral anion, was prepared via anion metathesis using 1-butyl-3-methyl imidazolium chloride and sodium (S)-2-hydroxypropionate. This ionic liquid also afforded good endo/exo selectivity in a Diels-Alder reaction. Recently, ILs with chiral carboxylates have been synthesized by Allen et al.30 This synthesis was achieved in water by reacting tetrabutylammonium hydroxide with the corresponding amino acids or chiral carboxylic acids. In 2002, Wasserscheid et al. synthesized chiral ILs from chiral starting materials affording many different chiral ILs in good yields.31 A novel imidazolium based chiral ionic liquid with planar chirality was synthesized by Saigo and coworkers in 2002.32 However, this ionic liquid could only be obtained as a racemic mixture and required further separation of the enantiomers before investigating its applications. Other chiral ILs based on imidazolium,33,34 ephedrinium,35 and pyridinium36 cations have been synthesized.33-36 However, chiral precursors for ILs are often used in a multistep synthesis. A detailed review of chiral ionic liquid synthesis has been reported.37 Tao et al. have also successfully prepared chiral ILs from amino acids and their corresponding methyl and ethyl esters.38 These chiral ILs from amino acid esters had low melting points compared with those derived from amino acids. Other interesting amino acid derived chiral ILs have also been synthesized recently.39-41 Ding et al. have successfully synthesized chiral ILs via a simple anion exchange between a commercially available halide salt with N-lithiotrifluoromethanesulfonimide.42 This approach provides an attractive one step synthesis with a chiral precursor, while allowing the product to be easily purified by washing with water. The same approach was employed by Tran and Oliveira to prepare chiral ILs.27 The variety and applications of these ILs suggest that other chiral ILs need to be explored in order to obtain ILs for different applications.
Natural materials are often used as precursors for preparing chiral ILs in multiple step synthesis. As an example, the use of amino acids for preparation of imidazolium cations is clear evidence of such use.33 Chiral induction in some reactions may be required to afford chiral ILs, mostly in one enantiomeric form. In addition, the few commercially available chiral ILs are often very expensive. The high cost, combined with the difficulty in chiral ionic liquid synthesis, has limited their extensive study and applications.
The primary objective of this study was to synthesize and characterize both enantiomeric forms of new chiral ILs from commercially available amino acid ester chlorides. The presence of a chiral center in the precursor further simplifies the synthesis, alleviating the need for asymmetric induction. In addition, esterification reduces hydrogen bonding of amino acids affording low melting point ILs.38 By varying the anions, the synthesis of ILs can be tailored, resulting in ILs of different properties that may be used for various applications. In addition, this study reports an investigation of the enantiomeric recognition ability of a variety of chiral amino acid ester based ILs. The synthesis of chiral ILs was accomplished via a simple anion metathesis reaction and characterization was performed using nuclear magnetic resonance (NMR), thermal gravimetric analysis (TGA), differential scanning calorimetry (DSC), circular dichroism (CD), and elemental micro-analysis. The relatively simple synthetic procedure and the presence of chiral centers in the precursors is a tremendous advantage for our approach. Finally, the chiral recognition ability of L- and D-alanine tert-butyl ester bis (trifluoromethane) sulfonimide ionic liquid was evaluated using 19F NMR with a racemic Mosher's sodium salt substrate and fluorescence spectroscopy with some chiral fluorescent analytes.
EXPERIMENTAL PROCEDURES
Chemicals and Materials
L- and D-alanine tert butyl ester hydrochloride [L- and DAlaC4Cl)], bis(trifluoromethane) sulfonimide lithium salt (LiNTf2), silver tetrafluoroborate (AgBF4), silver lactate (AgLac), silver nitrate (AgNO3), 2-methoxy-2-(trifluoromethyl) phenylacetic acid (Mosher's acid), and methanol (ACS certified) were purchased from Sigma Aldrich Chemicals (Milwaukee, WI). In addition, enantiomerically pure R- and S-enantiomers of warfarin, naproxen and 2,2,2-trifluoroanthrylethanol (TFAE) were purchased from Sigma Aldrich Chemicals (Milwaukee, WI). All chemicals were used as received.
General Instrumental Methods
The NMR spectra were recorded in d6-DMSO on a Bruker-250 MHz instrument with tetramethyl silane (TMS) as an internal standard. Melting point (Tm) was determined by differential scanning calorimeter using a thermal analysis instrument TA SDT2960 at a scanning rate of 5°C min-1. Thermal decomposition temperature (Tdec) of ILs was determined with a thermal analysis instrument 2950 TGA HR V6.1A (module TGA 1000°C). The heating rate for TGA was 5°C min-1 under nitrogen from 25 to 300°C. A Jasco-710 spectropolarimeter was used to obtain the CD spectra of our ILs. Steady-state fluorescence measurements were recorded at room temperature by use of a Spex Fluorolog-3 spectrofluorimeter (model FL3-22TAU3; Jobin Yvon, Edison, NJ) equipped with a 450-W xenon lamp and R928P photomultiplier tube (PMT) emission detector. Fluorescence emission spectra were collected in a 4-mm quartz fluorescence cuvet with slit widths set for entrance exit bandwidths of 4 nm on both excitation and emission monochromators for warfarin, 2 nm for TFAE, and 1.5 nm for naproxen, respectively. Fluorescence for warfarin, TFAE, and naproxen were respectively monitored at excitation wavelengths of 306, 365, and 280 nm. In addition, all fluorescence spectra were blank subtracted before data analysis.
19F NMR Experiment
Racemic Mosher's sodium salt was prepared from Mosher's acid by reacting with an equivalent of sodium hydroxide in water and the salt was dried under vacuum before 19F NMR measurement. The racemic Mosher's sodium salt (8.38 mg, 0.03 mmol) and 129.07 mg (0.3 mmol) of L- or D-AlaC4NTf2 were dissolved in 0.75 ml of d6 DMSO. The mixture was shaken vigorously for 10 min on an orbital shaker before recording 19F NMR spectra.
Synthesis of L- and D-Alanine tert Butyl Ester Bis (trifluoromethane) Sulfonimide AlaC4NTf2
0.5 g (2.75 mmol) of L-or D-alanine tert butyl ester chloride was dissolved in water. An equimolar amount (0.79 g, 2.75 mmol) of bis (trifluoromethane) sulfonimide lithium salt was dissolved separately in water. The two solutions were mixed and stirred for 2 h at room temperature. The mixture resulted in two layers, of which the lower layer was separated and dried under vacuum overnight. This resulted in 0.94 g (79% yield) of colorless ionic liquid. The decomposition temperature (Tdec) of this ionic liquid was found to be 263°C by use of TGA measurements. 1H NMR (250 MHz, d6 DMSO) δ (ppm) 8.19 (s, 3H), 3.97 (q, 1H), 1.45 (s, 9H), 1.36 (d, J = 7.0 Hz, 3H). 13C NMR δ (ppm) 170.12, 83.66, 49.19, 28.32, 16.69. Anal. Calcd. for C9H16N2O6S2F6: C, 25.35; H, 3.78; N, 6.57. Found: C, 25.07; H, 3.98; N, 6.52.
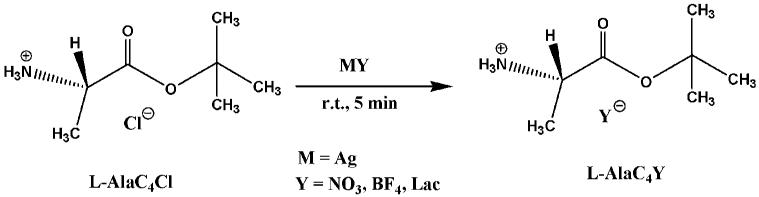
Synthesis of L- and D-Alanine tert Butyl Ester of Nitrate, Tetrafluoroborate, and Lactate
Representative procedure: Synthesis of L-alanine tert butyl ester nitrate
0.1 g (0.55 mmol) of L-AlaBuCl was dissolved in methanol. An equimolar amount of silver nitrate (0.0935 g, 0.55 mmol) was suspended separately in methanol. The two solutions were mixed and stirred, and the precipitate was then filtered. The product was evaporated in vacuo and purified by crystallizing in methanol/ether to obtain 0.08 g (77% yield) of the product L-AlaC4NO3 as white crystals (Tm, 104°C). This ionic liquid was thermally stable up to 130°C. 1H NMR (250 MHz, d6 DMSO) δ (ppm) 8.36 (s, 3H), 3.92 (q, 1H), 1.44 (s, 9H), 1.35 (d, J = 7.0 Hz, 3H). 13C NMR δ (ppm) 170.05, 83.51, 49.18, 28.36. Anal Calcd. for C7H16N2O5: C, 40.38; H, 7.75; N, 13.45. Found: C, 44.97; H, 8.53; N, 9.03.
L-alanine tert butyl ester tetrafluoroborate
Yield: 80%. Tm, 93°C; Tdec, 125°C. 1H NMR (250 MHz, d6 DMSO) δ (ppm) 8.18 (s, 3H), 3.94 (q,1H) 1.45 (s,9H), 1.36 (d, J = 7Hz, 3H). 13C NMR δ (ppm) 170.13, 83.68, 49.19, 28.38, 16.74. Anal. Calcd. for C7H16NO2BF4: C, 36.08; H, 6.92; N, 6.01. Found: C, 35.71; H, 7.31; N, 6.13.
L-alanine tert butyl ester lactate
Yield: 86%. Tm, 114°C; Tdec, 132°C. 1H NMR (250 MHz, d6 DMSO) δ (ppm) 5.53 (s, 3H), 3.79 (q, 1H), 3.62 (q, 1H), 1.40 (s, 9H), 1.25 (d, J = 7 Hz, 3H) 1.15 (d, J = 6.75, 3H). 13C NMR δ (ppm) 209.50, 178.31, 173.10, 110.00, 81.97, 67.28, 49.91, 28.46, 21.87, 18.98. Anal Calcd. for C10H21NO5: C, 51.05; H, 8.99; N, 5.95. Found: C, 50.85; H, 8.96; N, 5.96.
RESULTS AND DISCUSSION
Synthesis and Characterization of a New Amino Acid Ester Chiral Ionic Liquid (L-or D-AlaC4NTf2)
The synthesis of both enantiomeric forms of alanine tert-butyl ester bis (trifluoromethane) sulfonimide was accomplished via anion metathesis reaction of the corresponding amino acid ester chloride and bis (trifluoromethyl) sulfonylimide lithium salt (Scheme 1). The reaction proceeded well in water with good yield (79%) of L-or D-alanine tert-butyl ester bis (trifluoromethyl) sulfonylimide (L-or D-AlaC4NTf2). The 1H NMR (Fig. 1) and 13C NMR (see Fig. 2) of the ionic liquid was consistent with the chemical structure of AlaC4NTf2. The NMR spectra obtained for other ILs were very similar to that of AlaC4NTf2. The similarity in NMR spectra was expected since only the anions were varied. The alanine tert-butyl ester bis (trifluoromethyl) sulfonylimide ionic liquid was found to be a desirable liquid at room temperature and stable up to 263°C, as indicated by thermal gravimetric analysis (TGA) measurement (Fig. 3A).
Scheme 1.
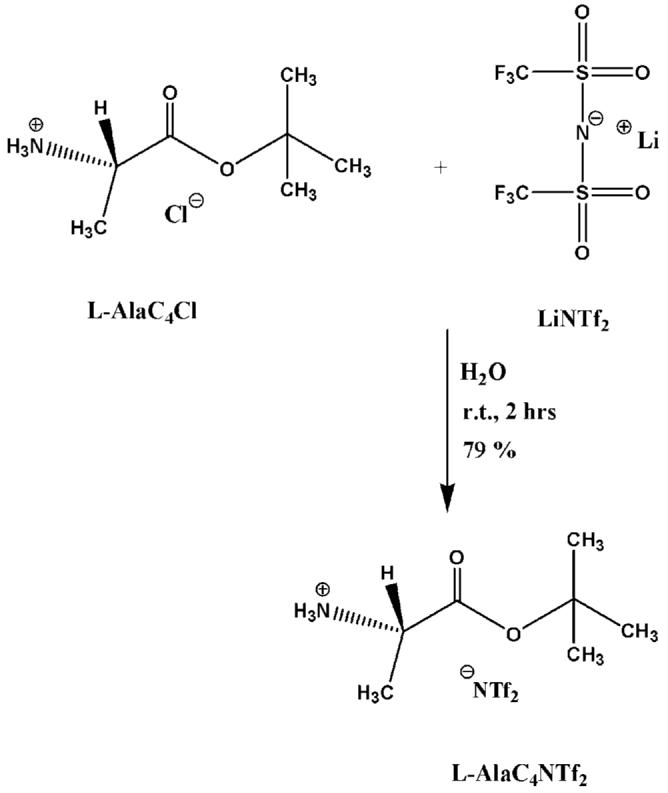
Synthesis of L-AlaC4NTf2.
Fig. 1.
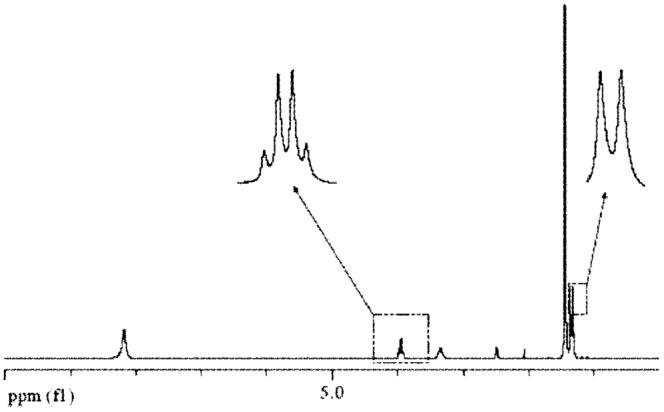
Proton (1H) NMR spectrum of L-AlaC4NTf2 in d6 DMSO with tetramethyl silane (TMS) as an internal standard at room temperature.
Fig. 2.
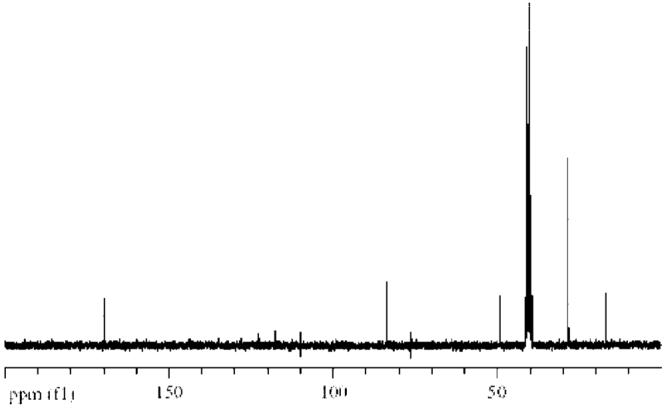
Carbon-13 (13C) NMR spectrum of L-AlaC4NTf2 in d6 DMSO with tetramethyl silane (TMS) as an internal standard at room temperature.
Fig. 3.
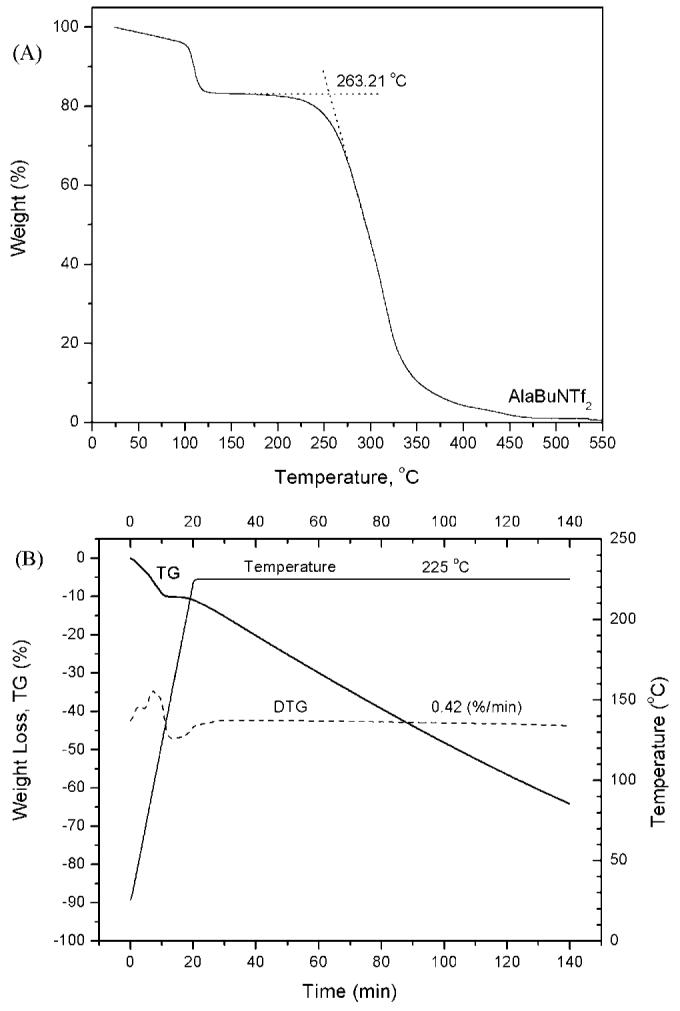
Thermal gravimetric analysis of L-AlaC4NTf2 with a heating rate of 58°C min-1 under nitrogen (A) from 25 to 3008°C, and (B) from 25 to 2258°C, then isothermally at 2258°C for 2 h.
The ionic liquid L-AlaC4NTf2 was also heated at 225°C for 2 h to verify and confirm whether decomposition occurs at a lower temperature. From Figure 3B, it seems that at this isothermal plateau (225°C), there is a constant rate loss of 0.42%/min for the span of 120 min. The constancy of the weight loss rate might be an indication that a physical phenomenon (e.g. evaporation), rather than a chemical decomposition process, is occurring. The rather low decomposition temperature for the BF4 salt (125°C), is still uncertain and may not be solely due to the cation instability upon moderate heating. This is because as much as the cation might not be very stable at moderate heating, the same cation had relatively higher stability (263°C) with the NTf2 anion.
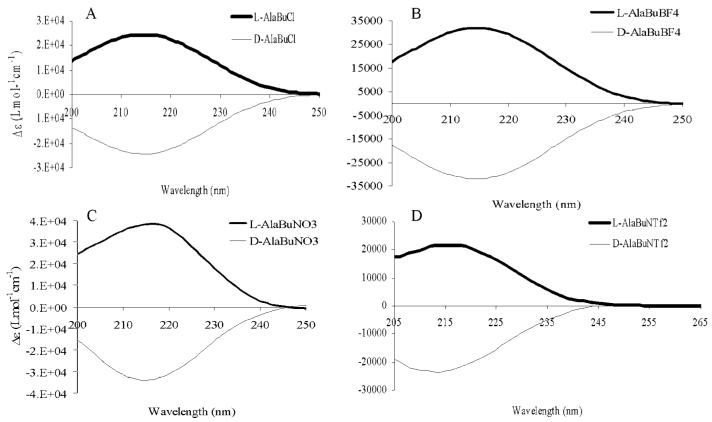
The high thermal stability of alanine tert-butyl ester bis (trifluoromethyl) sulfonylimide makes it a preferred chiral selector and chiral solvent for reactions at high temperature or as a coating in gas chromatography. It is indeed extremely important that the chiral center in the precursor be retained in the final ionic liquid product. According to Jodry and Mikami, some imidazolium based chiral ILs will sometimes undergo racemization after synthesis.34 The possibility of racemization in the synthesized ILs was investigated by use of circular dichroism (CD) measurements of the ionic liquid products and their precursors. Examples of the CD bands obtained for both enantiomeric forms of the ILs are as shown in Figure 4. As expected, the CD bands of the precursors were in the same direction as those of the ionic liquid products, confirming retention of configuration. In addition, the opposite CD bands confirmed that the L-and D-configurations of the ILs had been retained (see Fig. 4).
Fig. 4.
Circular dichroism spectra of L-and D-(A) AlaBuCl, (B) AlaBuBF4,(C) AlaBuNO3,and (D) AlaBuNTf2 at room temperature.
Chiral Recognition Study of ILs Using 19F NMR
As previously noted, L- and D-alanine tert-butyl ester bis (trifluoromethane) sulfonimide are liquids at room temperature. It was therefore interesting to investigate their ability to act both as solvent and chiral selector. The chiral recognition ability of L- and D-alanine tert-butyl ester bis (trifluoromethane) sulfonimide was investigated by use of 19F NMR and racemic Mosher's sodium salt. In this experiment, various solvents such as methylene chloride, deuterium oxide, dimethyl sulfoxide (DMSO) as well as chloroform were examined. However, d6-DMSO demonstrated good solubility as compared with other solvents investigated. The results of the 19F NMR study for enantiomeric recognition ability of L- and D-alanine tert-butyl ester bis (trifluoromethane) sulfonimide are shown in Figure 5. The diastereomeric interactions lead to a shift in the 19F NMR signal of the racemic Mosher's sodium salt. In addition, the 19F NMR signal of the racemic substrate was split by both enantiomeric forms of the ionic liquid demonstrating their enantiomeric recognition (see Fig. 5). This confirms that this ionic liquid can be a suitable chiral selector for various applications such as determination of enantiomeric composition of chiral molecules of pharmaceutical, biomedical, and environmental interest.
Fig. 5.
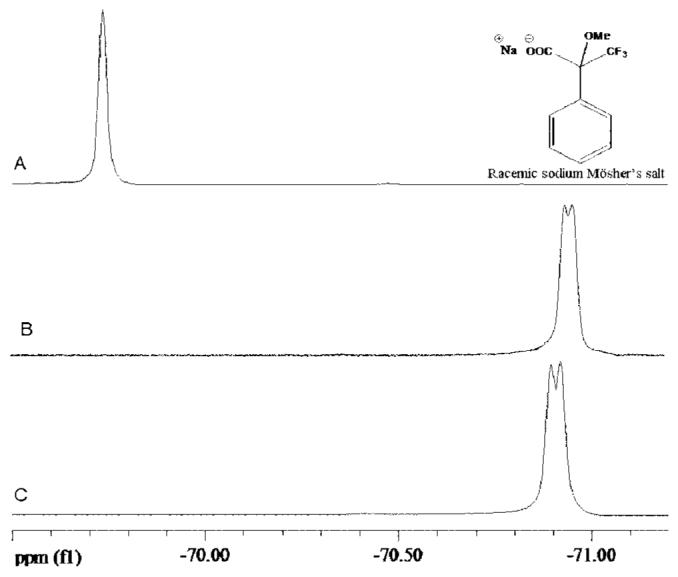
19F NMR spectra of (A) racemic sodium Mösher's salt; and a mixture of the racemic sodium Mösher's salt with (B) D-AlaC4NTf2,and (C) L-AlaC4NTf2 at room temperature.
Chiral Recognition of ILs Using Fluorescence Spectroscopy
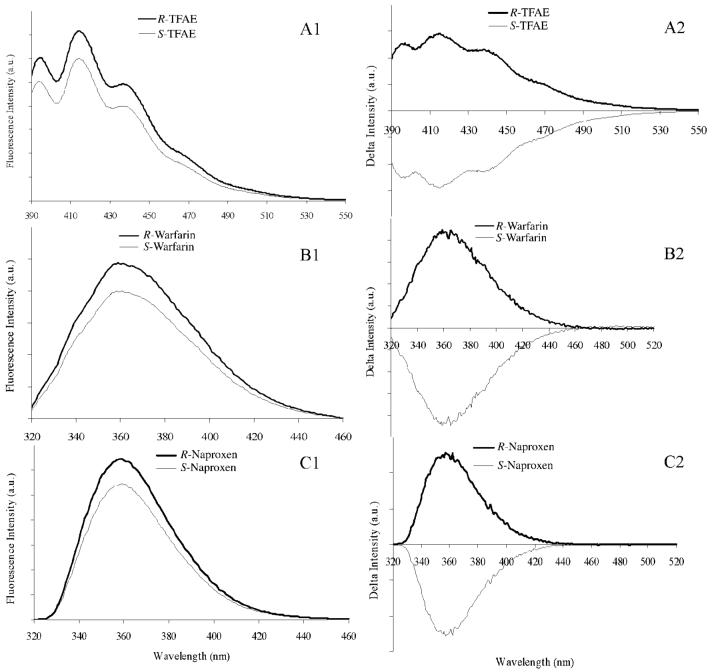
Steady-state fluorescence spectroscopy was further used to evaluate the chiral recognition ability and enantio-selectivity of the chiral ILs on 2,2,2-trifluoroanthrylethanol (TFAE), warfarin, and naproxen chiral analytes. The choice of these chiral analytes in this study was due to their fluorescence properties; furthermore, they are of environmental and pharmaceutical interest. For instance, warfarin is an anticoagulant drug used for the treatment of thromboembolic diseases and is also generally used as a pesticide, while naproxen is used as an anti-infammatory drug.
The emission spectra of the 10 μM R- and S-enantiomers of TFAE, warfarin, and naproxen analytes in the presence of L-AlaC4NTf2 chiral ILs are, respectively, shown in Figures 6A1, B1, and C1. The intensity of emission of the R-enantiomers obtained in the presence of ionic liquid solvent and chiral selector is noted to be higher than that of the S-enantiomers for all three analytes investigated. A difference in emission intensity of R- and S-enantiomers in the presence of chiral selectors is due to different diastereomeric interactions between the enantiomers and the IL chiral selectors. Such spectral shifts have been widely reported and associated with enantioselectivity of chiral selectors as a result of diastereomeric complexes.43-45
Fig. 6.
Fluorescence emission and mean centered spectral plots of 10 μM R-and S-(A) TFAE, (B) warfarin, and (C) naproxen enantiomers in the presence of L-AlaC4NTf2 chiral ionic liquid. The emission spectra of TFAE, warfarin, and naproxen were monitored at excitation wavelength of 365, 306, and 280 nm, respectively, at room temperature.
Finally, a mean-centered plot of emission spectra was used to gain better insight into the enantiomeric selectivity and chiral recognition ability of the chiral ILs. The mean centered plot of the emission spectra depicted in Figures 6A1, B1, and C1 are shown in Figure 6A2, B2, and C2, respectively. The spectra were obtained by subtracting the spectrum of R- and S-enantiomer in the presence of chiral selectors from the R- and S-mean spectra. It is of great importance to note that R- and S- enantiomers have opposite mean spectra, further demonstrating the chiral recognition ability and enantio-selectivity of the ionic liquid chiral selector.
Synthesis and Characterization of Novel Amino Acid Ester Chiral ILs (L-or D-AlaC4NO3, AlaC4BF4, AlaC4Lac)
A similar anion metathesis reaction used for the synthesis of AlaC4NTf2 was employed for the synthesis of AlaC4NO3, AlaC4BF4, and AlaC4Lac. However, the reaction was performed in methanol at a shorter time of 5 min (Scheme 2). The silver chloride precipitate was filtered off in each case affording good yields of the respective chiral ILs after removing methanol in vacuo.
Scheme 2.
Synthesis of AlaC4NO3, AlaC4BF4, AlaC4Lac.
Alanine tert-butyl ester nitrate (AlaC4NO3) crystallized to form a white solid at room temperature. AlaC4BF4 was obtained as a colorless greasy solid, whereas AlaC4Lac forms clear needle-like crystals at room temperature. The fact that solid ILs were obtained upon changing the anion demonstrates the tunability of the ILs synthesized in this study. By varying the anion or cation, different ILs with different properties is obtained for various applications. These results illustrate that bis (trifluoromethane) sulfonimide is probably a poorly coordinating anion with an ala-nine tert butyl ester cation. The poor crystal packing between the anion and cation results in ionic liquid. Del Po' polo describes this as frustrated packing between a bulky inorganic anion and cation leading to lower melting point ILs.46 Their findings are in agreement with our results that bis (trifluoromethane) sulfonimide being the largest of the anions yielded an ionic liquid product that was liquid at room temperature. The other smaller anions such as nitrate afforded ILs that was solid at room temperature.
CONCLUSION
In summary, we have successfully synthesized a series of new chiral ILs in both enantiomeric forms using a simple metathesis reaction between the chiral chloride ester salt and the corresponding anion sources. Alanine tert-butyl ester bis (trifluoromethane) sulfonylimide is desirably liquid at room temperature and is thermally stable up to 263°C. It can therefore be used in high temperature reactions or as a chiral selector in gas chromatography. Furthermore, the ILs presented here has the same chiral configuration as the chloride salt precursors indicating that enantiomeric salts were obtained upon anion metathesis. Both enantiomeric forms of alanine tert-butyl ester bis (trifluoromethane) sulfonylimide ionic liquid demonstrated enantiomeric recognition of racemic Mosher's sodium salt. This is an advantage since the ionic liquid can serve both as solvent and chiral selector, alleviating the need for use of environmentally damaging solvents to dissolve the analyte. As an example, this compound could probably be used to provide chiral selectivity in the determination of enantiomeric composition of pharmaceutical products and in chiral separations. While the solid ILs could not be used for this purpose, we believe that their synthesis and characterization is a step towards exploring their potential applications.
ACKNOWLEDGMENTS
The authors acknowledge Dr. Gary Baker for helpful discussions.
Contract grant sponsors: National Science Foundation, Phillip West Endowment.
LITERATURE CITED
- 1.Welton T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem Rev. 1999;99:2071–2083. doi: 10.1021/cr980032t. [DOI] [PubMed] [Google Scholar]
- 2.Levillain J, Dubant G, Abrunhosa I, Gulea M, Gaumont AC. Synthesis and properties of thiazoline based ionic liquids derived from the chiral pool. Chem Commun. 2003:2914–2915. doi: 10.1039/b308814f. [DOI] [PubMed] [Google Scholar]
- 3.Tran CD, Lacerda SHP. Determination of binding constants of cyclodextrins in room-temperature ionic liquids by near-infrared spectrometry. Anal Chem. 2002;74:5337–5341. doi: 10.1021/ac020320w. [DOI] [PubMed] [Google Scholar]
- 4.Tran CD, Lacerda SHP, Oliveira D. Absorption of water by room-temperature ionic liquids: effect of anions on concentration and state of water. Appl Spectrosc. 2003;57:152–157. doi: 10.1366/000370203321535051. [DOI] [PubMed] [Google Scholar]
- 5.Mele A, Tran CD, Lacerda SHP. The structure of a room-temperature ionic liquid with and without trace amounts of water: the role of C_H…O and C_H…F interactions in 1-n-butyl-3-methylimidazolium tetrafluoroborate. Angew Chem Int Ed Engl. 2003;42:4364–4366. doi: 10.1002/anie.200351783. [DOI] [PubMed] [Google Scholar]
- 6.Huddleston JG, Willauer HD, Swatloski RP, Rogers RD. Room temperature ionic liquids as novel media for `clean' liquid-liquid extraction. Chem Commun. 1998:1765–1766. [Google Scholar]
- 7.Dupont J, de Souza RF, Suarez PAZ. Ionic liquid (molten salt) phase organometallic catalysis. Chem Rev. 2002;102:3667–3692. doi: 10.1021/cr010338r. [DOI] [PubMed] [Google Scholar]
- 8.Dietz ML, Dzielawa JA. Ion-exchange as a mode of cation transfer into room-temperature ionic liquids containing crown ethers: implications for the `greenness' of ionic liquids as diluents in liquid-liquid extraction. Chem Commun. 2001:2124–2125. doi: 10.1039/b104349h. [DOI] [PubMed] [Google Scholar]
- 9.Compton DL, Laszlo JA. Direct electrochemical reduction of hemin in imidazolium-based ionic liquids. J Electroanal Chem. 2002;520:71–78. [Google Scholar]
- 10.Quinn BM, Ding Z, Moulton R, Bard AJ. Novel electrochemical studies of ionic liquids. Langmuir. 2002;18:1734–1742. [Google Scholar]
- 11.Kazarian SG, Briscoe BJ, Welton T. Combining ionic liquids and supercritical fluids: in situ ATR-IR study of CO2 dissolved in two ionic liquids at high pressures. Chem Commun. 2000:2047–2048. [Google Scholar]
- 12.Laszlo JA, Compton DL. α-Chymotrypsin catalysis in imidazolium-based ionic liquids. Biotechnol Bioeng. 2001;75:181–186. doi: 10.1002/bit.1177. [DOI] [PubMed] [Google Scholar]
- 13.Mwongela SM, Numan A, Gill NL, Agbaria RA, Warner IM. Separation of achiral and chiral analytes using polymeric surfactants with ionic liquids as modifiers in micellar electrokinetic chromatography. Anal Chem. 2003;75:6089–6096. doi: 10.1021/ac034386i. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong DW, He L, Liu YS. Examination of ionic liquids and their interaction with molecules, when used as stationary phases in gas chromatography. Anal Chem. 1999;71:3873–3876. doi: 10.1021/ac990443p. [DOI] [PubMed] [Google Scholar]
- 15.Anderson JL, Armstrong DW. High-stability ionic liquids. A new class of stationary phases for gas chromatography. Anal Chem. 2003;75:4851–4858. doi: 10.1021/ac0345749. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q, Baker GA, Baker SN, Colon LA. Surface confined ionic liquid as a stationary phase for HPLC. Analyst. 2006;131:1000–1005. doi: 10.1039/b607337a. [DOI] [PubMed] [Google Scholar]
- 17.Tran CD, Challa S, Franko M. Ionic liquids as an attractive alternative solvent for thermal lens measurements. Anal Chem. 2005;77:7442–7447. doi: 10.1021/ac0512913. [DOI] [PubMed] [Google Scholar]
- 18.Baker GA, Baker SN, Padey S, Bright F. An analytical view of ionic liquids. Analyst. 2005;130:800–808. doi: 10.1039/b500865b. [DOI] [PubMed] [Google Scholar]
- 19.Baker GA, Baker SN, McClensky TM. Noncontact two-color luminescence thermometry based on intramolecular luminophore cyclization within an ionic liquid. Chem Commun. 2003:2932–2933. doi: 10.1039/b310459c. [DOI] [PubMed] [Google Scholar]
- 20.Caldwell J. Importance of stereospecific bioanalytical monitoring in drug development. J Chromatogr A. 1996;719:3–13. doi: 10.1016/0021-9673(95)00465-3. [DOI] [PubMed] [Google Scholar]
- 21.Fanali S. Enantioselective determination by capillary electrophoresis with cyclodextrins as chiral selectors. J Chromatogr A. 2000;875:89–122. doi: 10.1016/s0021-9673(99)01309-6. [DOI] [PubMed] [Google Scholar]
- 22.Desiderio C, Fanali S. Chiral analysis by capillary electrophoresis using antibiotics as chiral selector. J Chromatogr A. 1998;807:37–56. doi: 10.1016/s0021-9673(98)00061-2. [DOI] [PubMed] [Google Scholar]
- 23.Hyun MH, Jin JS, Lee W. Liquid chromatographic resolution of race-mic amino acids and their derivatives on a new chiral stationary phase based on crown ether. J Chromatogr A. 1998;822:155–161. [Google Scholar]
- 24.Fakayode SO, Williams AA, Busch MA, Busch KW, Warner IM. The use of poly(sodium N-undecanoyl-L-leucylvalinate), poly(sodium N-undecanoyl-L-leucinate) and poly(sodium N-undecanoyl-L-valinate) surfactants as chiral selectors for determination of enantiomeric composition of samples by multivariate regression modeling of fluorescence spectral data. J Fluoresc. 2006;16:659–670. doi: 10.1007/s10895-006-0104-x. [DOI] [PubMed] [Google Scholar]
- 25.Baudequin C, Baudoux J, Levillain J, Cahard D, Gaumont A-C, Plaquevent JC. Ionic liquids and chirality: opportunities and challenges. Tetrahedron: Asymmetry. 2003;14:3081–3093. [Google Scholar]
- 26.Ding J, Armstrong W. Chiral ionic liquids as stationary phases in gas chromatography. Anal Chem. 2004;76:6819–6822. doi: 10.1021/ac049144c. [DOI] [PubMed] [Google Scholar]
- 27.Tran CD, Oliveira D, Yu S. Chiral ionic liquid that functions as both solvent and chiral selector for the determination of enantiomeric compositions of pharmaceutical products. Anal Chem. 2006;78:1349–1356. doi: 10.1021/ac051897r. [DOI] [PubMed] [Google Scholar]
- 28.Tran CD, Oliveira D. Fluorescence determination of enantiomeric composition of pharmaceuticals via use of ionic liquid that serves as both solvent and chiral selector. Anal Biochem. 2006;356:51–58. doi: 10.1016/j.ab.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 29.Earle MJ, McCormac PB, Seddon KR. Diels-Alder reactions in ionic liquids. Green Chem. 1999;1:23–25. [Google Scholar]
- 30.Allen CR, Richard PL, Ward AJ, van de Water LGA, Masters AF, Maschmeyer T. Facile synthesis of ionic liquids possessing chiral carboxylates. Tetrahedron Lett. 2006;47:7367–7370. [Google Scholar]
- 31.Wassercheid P, Boősmann A, Bolm C. Synthesis and properties of ionic liquids derived from the `chiral pool.'. Chem Commun. 2002:200–201. doi: 10.1039/b109493a. [DOI] [PubMed] [Google Scholar]
- 32.Ishida Y, Miyauchi H, Saigo K. Design and synthesis of a novel imidazolium-based ionic liquid with planar chirality. Chem Commun. 2002:2240–2241. doi: 10.1039/b206495b. [DOI] [PubMed] [Google Scholar]
- 33.Bao WL, Wang ZM, Li YX. Synthesis of chiral ionic liquids from natural amino acids. J Org Chem. 2003;68:591–593. doi: 10.1021/jo020503i. [DOI] [PubMed] [Google Scholar]
- 34.Jodry JJ, Mikami K. New chiral imidazolium ionic liquids: 3D-network of hydrogen bonding. Tetrahedron Lett. 2004;45:4429–4431. [Google Scholar]
- 35.Vo-Thanh G, Pegot B, Loupy A. Solvent-free microwave-assisted preparation of chiral ionic liquids from (—)-N-methylephedrine. Eur J Org Chem. 2004:1112–1116. [Google Scholar]
- 36.Patrascu C, Sugisaki C, Mintogaud C, Marty JD, Génisson Y, Viguerie NL. New pyridinium chiral ionic liquids. Heterocycles. 2004;63:2033–2041. [Google Scholar]
- 37.Ding J, Armstrong W. Chiral ionic liquids: synthesis and applications. Chirality. 2005;17:281–292. doi: 10.1002/chir.20153. [DOI] [PubMed] [Google Scholar]
- 38.Tao G-H, He L, Sun N, Kou Y. New generation ionic liquids: cations derived from amino acids. Chem Commun. 2005:3562–3564. doi: 10.1039/b504256a. [DOI] [PubMed] [Google Scholar]
- 39.Ni B, Garre S, Headley AD. Design and synthesis of fused-ring chiral ionic liquids from amino acid derivatives. Tetrahedron Lett. 2007;48:1999–2002. [Google Scholar]
- 40.Ni B, Headley AD. Novel imidazolium chiral ionic liquids that contain a urea functionality. Tetrahedron Lett. 2006;47:7331–7334. [Google Scholar]
- 41.Luo S-P, Xu D-Q, Yue H-D, Wang L-P, Yang W-L, Xu Z-H. Synthesis and properties of novel chiral-amine-functionalized ionic liquids. Tetrahedron: Asymmetry. 2006;17:2028–2033. [Google Scholar]
- 42.Ding J, Desikan V, Han X, Xiao TL, Ding R, Jenks WS, Armstrong DW. Use of chiral ionic liquids as solvents for the enantioselective photoisomerization of dibenzobicyclo [2.2.2] octatrienes. Org Lett. 2005;7:335–337. doi: 10.1021/ol047599i. [DOI] [PubMed] [Google Scholar]
- 43.Smith VK, Ndou TT, Warner IM. Spectroscopic study of the interaction of catechin with α-, β-, and γ-cyclodextrins. J Phys Chem. 1994;98:8627–8631. [Google Scholar]
- 44.Dotsikas Y, Kontopanou E, Allagiannis C, Loukas YL. Interaction of 6-p-toluidinylnaphthalene-2-sulphonate with β-cyclodextrin. J Pharm Biomed Anal. 2000;23:997–1003. doi: 10.1016/s0731-7085(00)00392-7. [DOI] [PubMed] [Google Scholar]
- 45.Macdonald SA, Hieftje GM. Use of shift reagents to determine enantiomers by near-infrared analysis. Appl Spectrosc. 1996;50:1161–1164. [Google Scholar]
- 46.Del Po'polo MG, Voth GA. On the structure and dynamics of ionic liquids. J Phys Chem B. 2004;108:1744–1752. [Google Scholar]