Abstract
Alcohol consumption in neonatal rats produces cerebellar damage and is widely used to model 3rd-trimester human fetal alcohol exposure. Neonatal “binge-like” exposure to high doses of alcohol (5 g/kg/day or more) impairs acquisition of eyeblink classical conditioning (EBC), a cerebellar-dependent Pavlovian motor learning task. We have recently found impairments in interstimulus interval (ISI) discrimination – a complex task variant of EBC - in adult rats following postnatal day (PD) 4–9 alcohol exposure at doses of 3, 4, and 5 g/kg/day. Because robust developmental differences in conditioned response (CR) generation and CR latency measures are present between untreated juveniles and adults in this task, we sought to extend alcohol findings to juvenile rats (PD30). Five neonatal treatment groups were used: (1) undisturbed controls, (2) sham intubation controls, (3) 3 g/kg/day of alcohol (blood alcohol concentration {BAC} = 139.9 mg/dl), (4) 4 g/kg/day of alcohol (BAC = 237.3 mg/dl), or (5) 5 g/kg/day of alcohol (BAC = 301.8 mg/dl). Intubations occurred over PD4-9. ISI discrimination training in juveniles (PD30-33) revealed dose-dependent CR deficits in all three alcohol-exposed groups relative to controls. Contrary to expected outcomes, CR latency measures were not significantly affected as a function of neonatal treatment. Comparison of these findings with our recent study in adults suggests that alcohol-induced impairments in ISI discrimination EBC may be greater in adults relative to juveniles. The present findings provide further evidence that ISI discrimination may provide greater sensitivity to functional deficits resulting from moderate levels of neonatal alcohol exposure relative to single-cue EBC paradigms.
Keywords: Fetal alcohol syndrome, eyeblink conditioning, neonatal alcohol, juvenile rat
11. Introduction
Prenatal alcohol exposure in humans is among the leading preventable causes of mental retardation in the Western world (Abel and Sokol, 1986). The term fetal alcohol spectrum disorders (FASD) has been applied to characterize the range of deficits present in individuals prenatally exposed to alcohol with or without symptoms required for a diagnosis of fetal alcohol syndrome (FAS; Hoyme et al., 2005; Manning and Hoyme, 2007; Riley and McGee, 2005). FASD is associated with widespread brain damage and impairments in a variety of domains, including executive functioning, motor coordination, spatial memory, and social functioning (Hamilton et al., 2003; Mattson et al., 1998; Riley and McGee, 2005; Roebuck et al., 1998; Schonfeld et al., 2001). The extent of brain damage and corresponding cognitive and behavioral deficits is due to the duration, dosage, and developmental timing of alcohol exposure (Maier et al., 1996) in addition to interactions with maternal and genetic factors (Riley and McGee, 2005; Warren and Li, 2005). Isolation of contributing variables through use of animal models has enhanced our understanding of the neurobehavioral consequences of alcohol exposure at critical development periods (Cronise et al., 2001; Tran and Kelly, 2003; West et al., 1989, 1990).
Findings of behavioral and cognitive impairments in humans prenatally exposed to alcohol levels lower than those associated with full-blown FAS (Jacobson et al., 1993; Jacobson and Jacobson, 1994; Larroque and Kaminski, 1998; Sood et al., 2001) have highlighted the importance of identifying dose thresholds in animal models. The neonatal period in rats is a time of rapid brain growth and maturation (termed the ‘brain growth spurt’; Dobbing and Sands, 1979) that is particularly susceptible to alcohol-induced brain damage (Goodlett et al., 2000; Livy et al., 2003; Marcussen et al., 1994; Tran and Kelly, 2003). Therefore, this animal model of FASD may be well-suited to identify functional dose thresholds.
“Binge-like” alcohol delivery over the neonatal period in rats at levels capable of producing sufficiently high peak blood alcohol concentrations (BACs; usually 200+ mg/dl) produces substantial cerebellar neuronal loss (Bonthius and West, 1990, 1991; Goodlett et al., 1998) and correlated motor learning impairments (Goodlett and Lundahl, 1996; Thomas et al, 1996, 1998). The cerebellum is particularly susceptible to alcohol-induced neuronal loss over postnatal days (PD) 4–9, a period in the rat that models 3rd trimester alcohol exposure in humans (Goodlett and Lundahl, 1996; Green et al., 2002b; Maier et al., 1999; Marcussen et al., 1994; Tran et al., 2005). These findings are largely consistent with those of cerebellar damage and impairments in cerebellar-dependent behaviors (i.e., motor coordination) in humans prenatally exposed to alcohol (Archibald et al., 2001; Bookstein et al., 2006; Connor et al., 2006; O’Hare et al., 2005; Roebuck et al., 1998; Sowell et al., 1996). However, because of the extensive involvement of peripheral mechanisms, direct links between alcohol-induced brain damage and behavioral impairments are difficult to ascertain in tasks that involve multi-joint coordination.
Eyeblink classical conditioning (EBC), a cerebellar-dependent form of associative learning that utilizes discrete stimuli and an easily-measurable behavior, may be ideal for cross-species investigation of causal relationships between alcohol-induced brain damage and behavior (Goodlett et al., 2000; Green, 2004; Stanton and Freeman, 1994). In EBC, a conditioned stimulus (CS) that does not initially evoke a blink (e.g., tone or light) precedes (typically by less than one second) and (usually) coterminates with an airpuff or periocular shock unconditioned stimulus (US) sufficient to produce an unconditioned blink response (UR). After repeated contingent CS-US pairings, the CS elicits a learned blink (conditioned response {CR}) that occurs immediately prior to US onset. EBC has been extensively characterized at the behavioral level in rabbits (Gormezano et al., 1983; Schneiderman et al., 1962) and requires the functional integrity of a well-defined cerebellar-brainstem circuit (Christian and Thompson, 2003). The well known behavioral, developmental, and neurobiological properties of EBC appear to be similar across species, including rats (Freeman & Nicholson, 2004; Freeman et al., 2005a; Rogers et al., 2001; Skelton, 1988; Stanton & Freeman, 2000) and humans (Gerwig et al., 2007; Ivkovich et al., 2000a; Woodruff-Pak and Steinmetz, 2000).
Binge-like alcohol exposure at high doses (5 g/kg/day or more) over PD4-9 impairs acquisition of EBC in post-weanling, juvenile, and adult rats (Brown et al., 2007, 2008; Green et al., 2000, 2002a, b; Lindquist et al., 2007; Stanton and Goodlett, 1998; Tran et al., 2005, 2007). These impairments appear to reflect deficits in learning rather than performance (Green et al., 2000; Stanton and Goodlett, 1998; Tran et al., 2007) and are correlated with cerebellar neuronal loss (Green et al., 2002b; Tran et al., 2005) and impaired cerebellar electrophysiological activity (Green et al., 2002a). This rodent model can be translated to the human condition, as children with FAS are also impaired in acquisition of EBC (Coffin et al., 2005; Jacobson et al., 2008).
Until recently, EBC deficits have not been observed following neonatal exposure to moderate alcohol levels. Green et al. (2006) demonstrated impairments in single-cue EBC following condensed exposure to moderate alcohol levels (2 and 3 g/kg/day) over PD2-11. When alcohol exposure is limited to PD4-9, however, doses below 5 g/kg/day have failed to produce deficits in single-cue EBC (Goodlett et al., 2007; Stanton et al., 2000) even though similar neonatal exposure scenarios (< 5 g/kg/day) are sufficient to produce damage to cerebellar regions critical for EBC (Bonthius and West, 1990, 1991; Goodlett and Lundahl, 1996; Goodlett et al., 1997, 1998; Marcussen et al., 1994). Recent work from our laboratory involving testing of adult rats (Brown et al., 2008) indicates that more complex task variants of EBC (e.g., interstimulus interval – ISI - discrimination) may provide enhanced sensitivity to functional impairments resulting from PD4-9 exposure of alcohol doses below 5 g/kg/day.
Single-cue EBC using auditory or visual CSs emerges gradually over PD17-24 in rats (Paczkowski et al., 1999; Stanton et al., 1992, 1998) and requires the functional integrity of cerebellar-brainstem circuitry necessary for adult EBC (Freeman et al., 1995a, b; Freeman and Nicholson, 2004; Freeman et al., 2005b). In post-weanling/juvenile and adult rats, short delay single-cue EBC produces more robust and earlier-timed CRs than long delay single-cue EBC (Ivkovich et al., 2000b; Tran et al., 2007). ISI discrimination EBC, which consists of two distinct CSs (tone; light) reinforced at two different CS-US intervals (280 and 880 ms), emerges over a more protracted developmental period than single-cue EBC (Brown et al., 2006). When training commences at PD 23 or 30, conditioning to the long CS (e.g., tone) is strongly influenced by the short CS (e.g., light)-US pairing, as evidenced by earlier peak latencies, enhanced CR amplitudes, and increased frequency of double-peaked CRs to the long CS relative to age-matched controls that do not receive paired short CS-US presentations. However, ISI discrimination EBC in adults does not demonstrate the magnitude of influence of short CS conditioning upon conditioning to the long CS observed in younger subjects (Brown et al., 2006, Experiment 3). Consequently, CRs to the long CS in adults occur closer to US onset relative to post-weanling/juveniles. Training in an ISI discrimination EBC task in rabbits reveals patterns of anterior lobe Purkinje cell electro-physiological activity associated with response inhibition during early portions of the CS period and CR production later in the CS period, closer to US onset (Green and Steinmetz, 2005). These electrophysiological response patterns are consistent with theories of cerebellar cortical control of CR timing in EBC (Medina et al., 2000; Nores et al., 2000). Increases in demands on cerebellar cortical functions required for appropriate timing of two distinct CSs may contribute to the protracted developmental emergence of ISI discrimination relative to single-cue EBC.
The present study attempted to extend our previous findings of impaired conditioning in ISI discrimination following neonatal exposure to alcohol doses below 5 g/kg/day. Specifically, we sought to determine alcohol dose thresholds on acquisition of ISI discrimination during the juvenile period in rats, a stage in which developmental modification of conditioning in this task is ongoing (Brown et al., 2006). We predicted that PD4-9 binge-like exposure of 3, 4, or 5 g/kg/day of alcohol via intragastric intubation would produce dose-dependent EBC deficits in juveniles (PD30-33) trained in ISI discrimination, similar to our recent findings in adults (Brown et al., 2008). Alcohol-induced effects on conditioning were expected in the form of impaired CR generation as well as in later-timed CRs to the long CS relative to controls. CR onset and peak latencies were later-timed (to the long CS) in alcohol-treated adults trained in ISI discrimination, particularly in the high dose group (5g/kg/day; Brown et al., 2008). Because CR timing to the long CS in ISI discrimination undergoes substantial developmental changes beyond the juvenile period, later-timed CRs to the long CS were expected in the present study following exposure to alcohol doses (3 and 4g/kg/day) lower than those found to alter CR timing in adults.
2. Results
A total of 27 animals were excluded from analyses due to: excessively low UR percentages and/or amplitudes (3 females {F}, 7 males {M}), electrode displacement (2 F, 1 M), or excessive spontaneous blink rates/noise during the pre-CS baseline period (9 F, 5 M). The distribution of excluded animals was generally even as a function of neonatal treatment. The extensive amount of training in the present study (12 sessions) relative to many previous reports using this freely-moving rodent preparation (6 sessions) contributed to the loss of subjects. Further analyses include the remaining 95 subjects (Group UD N = 20: F, Light short CS+ [6]; F, Tone short CS+ [4]; M, Light short CS+ [5]; M, Tone short CS+ [5]; Group SI N = 18: F, Light short CS+ [5]; F, Tone short CS+ [4]; M, Light short CS+ [4]; M, Tone short CS+ [5]; Group 3 g/kg/day N = 21: F, Light short CS+ [6]; F, Tone short CS+ [5]; M, Light short CS+ [5]; M, Tone short CS+ [5]; Group 4 g/kg/day N = 19: F, Light short CS+ [4]; F, Tone short CS+ [3]; M, Light short CS+ [6]; M, Tone short CS+ [6]; Group 5 g/kg/day N = 17: F, Light short CS+ [4]; F, Tone short CS+ [3]; M, Light short CS+ [5]; M, Tone short CS+ [5]).
2.1. Body weight and blood alcohol concentrations (BACs)
Consistent with our previous reports (Brown et al., 2008), substantial weight gain was present in all groups between PD4 and PD9, though Group 5 g/kg/day experienced mild growth retardation during the intubation period (see Table 1). These effects were supported statistically by a significant main effect of days [F(1, 75) = 1834.623, p < .01], and a significant interaction of Treatment × Days [F(4, 75) = 8.478, p < .01]. Newman-Keuls post hoc tests indicated that while there were no differences in body weights at PD4 (all ps > .6), significant group differences were present at PD9. Body weights at PD9 for Group 5 g/kg/day were significantly lower than all other groups (ps < .01), while those for Group 3 g/kg/day were significantly higher than Groups SI and 5 g/kg/day (ps < .01), and approaching significance for Group UD (p < .08). There were no significant weight differences across Groups UD, SI, or 4 g/kg/day (ps > .24). Significant group differences were no longer present at the time of testing (p > .8).
Table 1.
Mean (± SEM) Body Weights (g) Across the Neonatal Treatment Period and at Session 1 (PD30) of Eyeblink Conditioning (EBC) Training (M = males; F = females), and Blood Alcohol Concentrations (BACs; collected on PD4).
| Body Weight (grams) | BAC mg/dl) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dose | PD4 | PD5 | PD6 | PD7 | PD8 | PD9 | Session 1 M, PD30) | Session 1 F, PD30) | PD4 |
| UD | 11.1 ± 0.4 | N/A | N/A | N/A | N/A | 20.1 ± 0.8 | 93.1 ± 4.6 | 92.1 ± 1.8 | N/A |
| SI | 11.2 ± 0.2 | 12.8 ± 0.2 | 14.5 ± 0.3 | 16.2 ± 0.3 | 17.8 ± 0.3 | 19.6 ± 0.4 | 100.3 ± 2.5 | 90.3 ± 2.4 | N/A |
| 3 g | 10.8 ± 0.2 | 12.7 ± 0.3 | 14.6 ± 0.3 | 16.6 ± 0.3 | 18.9 ± 0.3 | 21.1 ± 0.4 | 102.4 ± 2.7 | 87.6 ± 2.3 | 139.9 ± 8.6 |
| 4 g | 10.9 ± 0.2 | 12.2 ± 0.3 | 14.0 ± 0.3 | 15.8 ± 0.4 | 18.0 ± 0.4 | 20.3 ± 0.5 | 98.0 ± 2.8 | 87.1 ± 3.4 | 237.3 ± 17.5 |
| 5 g | 11.4 ± 0.2 | 11.8 ± 0.2 | 13.2 ± 0.3 | 14.6 ± 0.3 | 16.3 ± 0.4 | 18.1 ± 0.5 | 96.5 ± 3.0 | 88.3 ± 2.9 | 301.8 ± 29.1 |
UD: Undisturbed Controls; SI: Sham-intubated Controls; 3 g: 3 g/kg/day of alcohol over postnatal days (PD) 4–9; 4 g: 4 g/kg/day of alcohol over PD4-9; 5 g: 5 g/kg/day of alcohol over PD4-9
BACs were available for 49 of the 57 alcohol-exposed subjects (6 missing from Group 3 g/kg/day, 1 missing each from Groups 4 and 5 g/kg/day; see Table 1 for mean BACs). An ANOVA of BACs revealed a significant main effect of treatment [F(2, 37) = 13.798, p < .01], with post hoc analyses indicating significant differences across groups (5 g > 4 g > 3 g; ps < .05).
2.2. Behavioral measures
Behavioral data are reported combined across sex and CS modality. Significant interactive effects involving treatment condition with sex occurred infrequently and were inconsistent across measures (CR percentage, peak amplitude, and latency), CSs (short and long), and trial types (CS-US trials and CS-alone trials). There were no significant interactive effects involving CS modality and treatment condition. In contrast, the reported effects involving treatment (alone, or interactive only with sessions) were robust and consistent across CR measures, CSs, and trial types. No significant treatment effects were present in CR latency or double peak measures (see Table 2), contrary to what is found in adults trained under these conditions (Brown et al., 2008).
Table 2.
Mean (± SEM) Double Peak (DP) Conditioned Response (CR) Percentages (Taken from CS-alone Trials for Short – 280 ms CS-US interval - and Long – 880 ms CS-US interval - CSs Following Probe Trials after Session 12), CR Onset Latencies (CL), and CR Peak Latencies (CPL; Taken from CS-alone Trials for Short and Long CS-alone Trials from Sessions 7–12; expressed in ms)
| Dose | Short CS | Long CS | ||||
|---|---|---|---|---|---|---|
| DP% | CL | CPL | DP% | CL | CPL | |
| UD | 43.1 ± 5.7 | 179.6 ± 7.0 | 293.4 ± 18.1 | 52.2 ± 7.1 | 290.0 ± 26.8 | 572.9 ± 21.2 |
| SI | 34.4 ± 7.8 | 192.9 ± 8.3 | 309.8 ± 14.0 | 37.2 ± 9.0 | 316.8 ± 23.1 | 591.2 ± 32.8 |
| 3 g | 30.5 ± 8.4 | 199.0 ± 6.5 | 320.5 ± 17.3 | 36.5 ± 8.5 | 355.8 ± 33.6 | 608.3 ± 33.3 |
| 4 g | 33.5 ± 5.6 | 201.4 ± 8.6 | 329.2 ± 16.8 | 34.1 ± 8.7 | 334.7 ± 31.1 | 605.5 ± 26.0 |
| 5 g | 21.4 ± 5.7 | 191.1 ± 6.0 | 330.4 ± 10.4 | 28.1 ± 9.1 | 371.9 ± 39.7 | 634.5 ± 26.1 |
UD: Undisturbed Controls; SI: Sham-intubated Controls; 3 g: 3 g/kg/day of alcohol over postnatal days (PD) 4–9; 4 g: 4 g/kg/day of alcohol over PD4-9; 5 g: 5 g/kg/day of alcohol over PD4-9
To streamline results reporting, consistent main or interactive effects that do not involve the factor of treatment are only briefly discussed here. Consistent with previous reports (Brown et al., 2006, 2008), startle responding was more robust to the tone CS while conditioning was generally more robust with light as the CS.
2.2.1 Performance measures - - startle (SR) and unconditioned response (UR) amplitudes
Startle response (SR) amplitudes were reported from short CS trials at Session 1. UR amplitudes were reported from the first 10 long-CS+ trials from Session 1 to avoid confounding from CR-UR summation effects, which can cause UR amplitudes to change across sessions as CR incidence and amplitude increases. SR and UR amplitudes did not significantly differ across groups as a function of neonatal treatment (Group UD: SR amp = 4.2 ± 1.6/UR amp = 723.7 ± 46.2; Group SI: SR amp = 6.8 ± 2.1/UR amp = 765.5 ± 43.3; Group 3 g/kg/day: SR amp = 4.9 ± 1.8/UR amp = 706.5 ± 54.6; Group 4 g/kg/day: SR amp = 2.6 ± 1.1/UR amp = 750.0 ± 50.7; Group 5 g/kg/day: SR amp = 2.3 ± 1.4/UR amp = 684.4 ± 56.8). Consistent with previous studies (Brown et al., 2007, 2008; Tran et al, 2007), this suggests that sensory processing of the CS and US and the ability to elicit the eyeblink reflex was equivalent across groups.
2.2.2. Eyeblink conditioning (EBC)
A robust dose-dependent effect of neonatal alcohol exposure was evident in conditioning at both short and long CSs. Alcohol-induced impairments were observed primarily early in training in the CR percentage measure, while impairments in CR peak amplitude typically extended into later stages of training. Groups 3 g/kg/day and 4 g/kg/day performed at intermediate levels between that of control subjects (Groups UD and SI) and the high-dose group (5 g/kg/day). Adaptive CRs were less impaired, suggesting that group differences were primarily reflective of early portions of the CR period for the long CS.
2.2.2a CR percentage
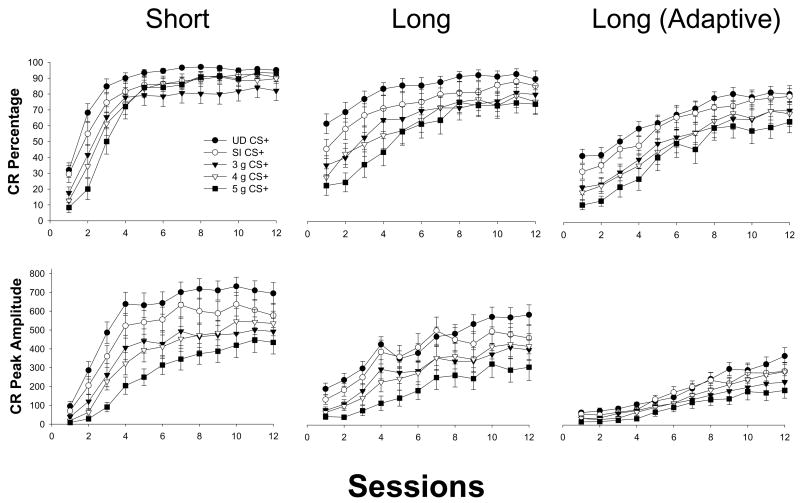
Neonatal binge alcohol exposure produced deficits in the rate of conditioning to the short and long CS as measured by the percentage of trials containing a CR (see Figure 2, top). Significant main effects of treatment [F(4, 75) = 3.009, p < .03, short CS; F(4, 75) = 3.068, p < .03, long CS] and sessions [F(11, 825) = 318.802, p < .01, short CS; F(11, 825) = 109.911, p < .01, long CS] were present, as were significant interactions of Treatment × Sessions [F(44, 825) = 4.295, p < .01, short CS; F(44, 825) = 1.975, p < .01, long CS]. Newman-Keuls post hoc analyses of these interactions indicated that Group 5 g/kg/day was impaired relative to controls primarily early in training, and impairments were more substantial to the long CS (5 g < UD and SI at sessions 1–3 [short CS]; 5 g < UD at all sessions, 5 g < SI at sessions 1–5, 7 [long CS], ps < .05). A similar pattern emerged in the group contrasts between Groups 3 and 4 g/kg/day relative to controls, though impairments were generally less prevalent (4 g < UD and SI at sessions 1–3 [short CS]; 3 g < UD at sessions 1–3, 6–9, 3 g < SI at sessions 1–2 [short CS]; 4 g < UD at sessions 1–10, 4 g < SI at sessions 1–5 [long CS]; 3 g < UD at sessions 1–5, 7–10; 3 g < SI at session 2 [long CS], ps < .05). Differences between alcohol-exposed groups were similar across CSs (5 g < 4 g at sessions 2–3 [short and long CS]; 5 g < 3 g at sessions 1–3 [short CS]; 5 g < 3 g at sessions 1–4 [long CS]; ps < .05 – there were no significant differences between Groups 3 and 4 g/kg/day at any session). Controls differed at only one session at each CS (SI < UD at session 2 [short CS]; SI < UD at session 1 [long CS], ps < .05), suggesting that group effects in conditioning were minimally influenced by the intubation procedure per se. Learning was evident in all five groups as evidenced by significantly greater performance at the end of training compared to the start of training (within each group: session 12 > session 1; ps < .01).
Figure 2.
Mean (± SEM) percentage of total CR frequency (top row) and CR peak amplitude (bottom row) from CS+ trials for subjects trained in interstimulus interval (ISI) discrimination -left column: CRs for the short CS (280 ms CS-US interval); middle column: CRs for the long CS (880 ms CS-US interval); right column: adaptively-timed CRs (CRs occurring within the last 200 ms prior to US onset) for the long CS. CR peak amplitude is measured in arbitrary units. For both the short (left column) and long CS (middle column), consistent and robust dose-dependent deficits in CR generation were observed in alcohol-exposed subjects (3g, 4g, and 5g) relative to controls (UD and SI). Alcohol-induced CR impairments were less robust for adaptively-timed CRs to the long CS (right column). UD = Undisturbed Controls; SI = Sham-intubated Controls; 3 g = 3 g/kg/day of alcohol; 4 g = 4 g/kg/day of alcohol; 5 g = 5 g/kg/day of alcohol.
2.2.2b. CR peak amplitude
Alcohol exposure produced deficits in conditioning as measured by CR peak amplitude (Figure 2, bottom). ANOVA revealed significant main effects of treatment [F(4, 75) = 5.944, p < .01, short CS; F(4, 75) = 3.651, p < .01, long CS] and sessions [F(11, 825) = 208.049, p < .01, short CS; F(11, 825) = 97.899, p < .01, long CS]. A significant interaction of Treatment × Sessions [F(44, 825) = 2.050, p < .01] was observed only in the short CS (p > .57 for the long CS). For the treatment main effect for the long CS, only Group 5 g/kg/day differed significantly from controls (5 g < UD and SI, ps < .05). Post hoc analyses of the Treatment × Sessions interaction (short CS) indicated that Group 5 g/kg/day was impaired relative to controls for most of training (5 g < UD and SI at sessions 2–12, ps < .05). Groups 3 and 4 g/kg/day were slightly less impaired relative to controls (3 g and 4 g < UD at sessions 2–12; 4 g < SI at sessions 2–7; 3 g < SI at sessions 7–8, 10, ps < .05). Group 5 g/kg/day was impaired relative to the lower-dosed groups (5 g < 3 g and 4 g at sessions 3–5; 5 g < 3 g at session 7, p < .05), while Groups 3 g/kg/day and 4 g/kg/day did not differ. Controls differed significantly only at session 9 (SI < UD, p < .05). Evidence for learning was present in all five groups (session 12 > session 1; ps < .01).
2.2.2c. Adaptive CR measures (long CS)
Alcohol-induced impairments in adaptively-timed CRs (CRs occurring within 200 ms of US onset) for the long CS were less severe than impairments observed in short and long CRs spanning the entire CS-US interval (see Figure 2, right panels). ANOVA revealed the expected main effect of sessions for both measures [F(11, 825) = 152.532, p < .01, CR percentage; F(11, 825) = 98.807, p < .01, CR peak amplitude], though a main effect of treatment was present only in the CR percentage measure [F(4, 75) = 2.852, p < .03; F(4, 75) = 2.306, p < .07 for CR peak amplitude, possibly reflecting a “floor effect”]. There were no significant interactive effects involving treatment in either measure. Post hocs of the treatment main effect revealed significant differences only between Groups UD and 5 g/kg/day (5 g < UD, p < .05; difference between 5 g and SI approaching significance, p < .07).
2.3. Summary of findings
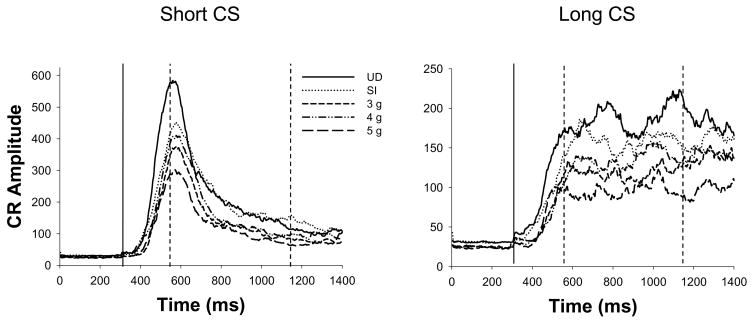
Neonatal binge alcohol exposure produced CR acquisition impairments in a dose-dependent fashion. 3 g/kg/day of alcohol produced mild CR impairments in juveniles (PD30-33) trained in ISI discrimination, consistent with our previous findings in adults (Brown et al., 2008). Doses of 4 and 5 g/kg/day of alcohol impaired CR acquisition, also consistent with previous reports from our laboratory using task variants that are more complex than single-cue EBC (Brown et al., 2007, 2008). Treatment differences in adaptively-timed CRs to the long CS were less robust than those observed in short and long CRs spanning the entire CS-US interval, suggesting that alcohol-induced impairments to long CS conditioning are driven primarily by early portions of the CR. Neither CR latency nor CR double peak measures were significantly impacted by neonatal alcohol exposure, contrary to findings with adults (Brown et al., 2008). Inspection of CR topography (see Figure 3) illustrates early-onset CRs across groups and evidence of double-peaked CRs to the long CS, consistent with our previous findings with post-weanlings/juveniles trained in ISI discrimination (Brown et al., 2006, Experiments 1–2). In summary, these findings support our previous claims of enhanced sensitivity of EBC ISI discrimination to functional deficits resulting from exposure to moderate alcohol doses over PD4-9. However, there was no clear indication that treatment effects in juvenile rats in the present study were larger than in adult rats, indeed they may have been smaller (see Brown et al., 2008).
Figure 3.
Tracings of integrated EMG activity averaged across all short- (280 ms CS-US interval; left) and long-CS-alone probe trials (880 ms CS-US interval; right) following session 12. The solid vertical line represents the onset of the CS, and the two dashed vertical lines represent the onset of the short and long US (time expressed in ms). Note differences in scaling across graphs. CR amplitudes are diminished in alcohol-exposed subjects (3g, 4g, and 5g) relative to controls (UD and SI). However, the timing of the CR does not appear to differ as a function of neonatal treatment. CRs for the short CS (left) peak precisely at the point of US onset (280 ms after CS onset), while CRs for the long CS demonstrate an early peak that appears to correspond to the short US onset, and a second peak that occurs closer to US onset for the long CS (880 ms after CS onset).
3. Discussion
The present data support and extend previous findings of impaired EBC in rats following heavy binge-like alcohol exposure over PD4-9 (Brown et al., 2007, 2008; Green et al., 2000; 2002a, b; Lindquist et al., 2007; Stanton and Goodlett, 1998; Tran et al., 2005, 2007). Consistent with a previous report from our laboratory (Brown et al., 2008), deficits are present in ISI discrimination EBC following PD4-9 exposure to an alcohol dose as low as 3 g/kg/day, suggesting that this task may display enhanced sensitivity to the effects of neonatal alcohol exposure relative to single-cue EBC paradigms (Goodlett et al., 2007; Stanton et al., 2000). Contrary to outcomes with adults trained in this task, CR timing was not significantly altered as a consequence of neonatal alcohol exposure in the present study (see Table 2). Conditioning to the long CS suggested a strong degree of cross-modal influence of the short CS-US, as reflected by a high percentage of double-peaked CRs and early onset and early peak latencies (see Table 2; Figure 3), similar to previous findings in untreated post-weanling/juvenile rats trained in ISI discrimination (Brown et al., 2006, Experiments 1–2).
Neonatal alcohol exposure differentially affects aspects of conditioning in ISI discrimination as a function of age of training. Later-onset and later-peaked CRs to the long CS are present in alcohol-exposed subjects relative to controls trained in ISI discrimination in adulthood (Brown et al., 2008), while significant alcohol-related CR timing effects were absent in the present study. Additionally, there is evidence that conditioning in adults is more severely impaired as a result of neonatal alcohol exposure relative to juveniles. For example, adults exposed to 5 g/kg/day of alcohol over PD4-9 display no evidence of learning as reflected by the CR peak amplitude measure to the long CS (Brown et al., 2008). Juveniles experiencing identical neonatal treatment show substantial improvement in conditioning across sessions in all measures (see Figure 2). Deficits in short (280 ms ISI) and long delay (880 ms ISI) single-cue EBC appear to be similar in magnitude in juvenile and adult rats following PD4-9 binge-like exposure to a high dose of alcohol (5.25 g/kg/day; Tran et al., 2007), suggesting that this differential sensitivity as a function of age of training may be task-specific.
Ontogenetic changes in conditioning to the long CS may account for these differential effects. CRs to the long CS in juveniles (PD30) trained in ISI discrimination display characteristics typical of conditioning with short CS-US intervals, including earlier-timed CRs and enhanced CR amplitudes relative to controls not receiving paired short CS-US presentations (Brown et al., 2006, Experiments 1–2). Adults, on the other hand, do not demonstrate a similar magnitude of cross-modal influence of short CS (e.g., tone)-US conditioning on conditioning to the long CS (e.g., light) in this task (Brown et al., 2006, Experiment 3). Recent data from our laboratory supports these findings, as a direct, within-experiment comparison of ISI discrimination EBC in PD23, PD30, and adult rats (PD70+) revealed enhanced amplitude and earlier-timed CRs to the long CS at both younger ages relative to adults (unpublished findings). While juveniles do not demonstrate complete generalization across CSs, the enhanced influence of the short CS may contribute to alcohol-related effects to the long CS in juveniles that resemble alcohol-related effects to short CS conditioning in adults trained in ISI discrimination. If so, this would presumably make responding to the long CS less sensitive to alcohol in juvenile rats. Consistent with this proposal, CR timing is unaffected and CR acquisition, though impaired relative to controls, improves across sessions for the short CS in ISI discrimination training in alcohol-exposed adults, a pattern similar to that observed with long CS conditioning in alcohol-exposed juveniles. Therefore, while conditioning is impaired in alcohol-exposed juveniles in the present study, the as-yet unidentified processes underlying enhanced cross modal processing in juvenile rats trained in ISI discrimination may attenuate alcohol-induced impairments. Investigation of brain regions involved in EBC that receive converging auditory and visual input may help elucidate the mechanisms responsible for these developmental changes.
Cerebellar cortical functions may contribute to cross-modal transfer effects in EBC. The cerebellar cortex receives auditory and visual sensory input (Brodal, 1967; Glickstein, 1997; Snider and Stowell, 1944) and is hypothesized to govern features of the CR (e.g., amplitude and timing; Bao et al., 2002; Garcia and Mauk, 1998; Garcia et al., 1999; Medina et al., 2000; Ohyama and Mauk, 2001; Perrett et al., 1993) that transfer across sensory modalities as reflected by ISI discrimination training (Brown et al., 2006). However, most aspects of cerebellar cortical Purkinje cell structure (Altman and Bayer, 1997) as well as relevant synaptic connections (Puro and Woodward, 1977a, b) and molecular profiles (Metzger and Kapfhammer, 2003) achieve ‘adult-like’ levels by the 3rd – 4th postnatal weeks, prior to the approximate period of observed developmental changes in ISI discrimination EBC (beyond the 4th postnatal week). Though these findings do not rule out the possibility of developmental changes in other cerebellar cortical neuronal populations that may contribute to cross-modal transfer effects, the absence of a clear link between the developmental time course of cerebellar cortical Purkinje cell structure and function and developmental cross-modal transfer effects in ISI discrimination training indicates that essential cross-modal plasticity may instead (or additionally) occur upstream and/or downstream of the cerebellar cortex. The deep nuclei of the cerebellum receives multimodal CS input (c.f., Tracy et al., 2001) and may modulate relevant multimodal CS information via feedback upon pontine nuclei (see Clark et al., 1997). Developmental changes in cerebello-pontine interactions, as well as in other structures normally involved in EBC (e.g., hippocampus; inferior colliculus) may also contribute to cross-modal transfer effects. Further experimentation is required to identify neural substrates of these developmental changes.
Previous studies investigating the effects of PD4-9 alcohol exposure on EBC during the post-weanling/juvenile period have reported deficits in CR generation similar to those reported here. Consistent with the present findings, CRs in young rats are impaired – but not abolished –as a result of PD4-9 exposure to alcohol doses as high as 5.25 g/kg/day (Brown et al., 2007; Tran et al., 2005, 2007). The unique feature of the present study relative to previous reports involving post-weanling/juveniles are findings of EBC impairments following alcohol exposure to doses as low as 3 g/kg/day. The neural mechanisms underlying the enhanced sensitivity of ISI discrimination to neonatal alcohol exposure are currently unknown, however. It is well-established that the cerebellar cortex is normally involved in EBC (Chen et al., 1996; Garcia et al., 1999; Lavond and Steinmetz, 1989; Nolan and Freeman, 2006; Yeo and Hardiman, 1992), and learning-related cerebellar cortical electrophysiological activity has been demonstrated in an ISI discrimination task in adult rabbits (Green and Steinmetz, 2005). The number of cerebellar cortical neurons and/or extent of cerebellar cortical regions required for ISI discrimination may exceed that required for simpler, single-cue EBC tasks. The degree of brain damage following ‘moderate’ neonatal alcohol exposure may therefore be sufficient to produce deficits in complicated task variants of EBC that would not be observed with training in less complex tasks.
While the low dose used in the present study (3 g/kg/day) is moderate relative to levels typically required to produced functional impairments in EBC (5 g/kg/day), BACs produced by our low-dose exposure (~140 mg/dl) are considerably higher than mean BACs (less than 85 mg/dl) required to produce anatomical and neurochemical alterations in rodent prenatal exposure models (Sutherland et al., 1997; Swartzwelder et al., 1988; Wigal and Amsel, 1990; Zhou et al., 2001). Impairments in learning have also been reported following early developmental alcohol exposure producing BACs less than 40 mg/dl (Lochry and Riley, 1980; Savage et al., 2002; Vaglenova and Petkov, 1998). Alcohol-related spatial learning impairments in Savage et al. (2002) were observed with training in a complex variant of the Morris water maze, but not in the standard procedure, thus supporting our proposal that increasing task complexity may reveal functional deficits resulting from moderate prenatal/neonatal alcohol exposure. Modifications to ISI discrimination (e.g., longer ISIs; inclusion of ‘trace’ intervals between CS offset and US onset) may therefore reveal deficits at doses even lower than 3 g/kg/day.
Assessing functional dose thresholds using EBC tasks may be advantageous for a number of reasons. Since the essential neural circuitry underlying EBC is well-characterized, a greater understanding of how alcohol-induced brain damage translates to functional impairments can be obtained. Knowledge of mechanisms of alcohol-induced brain damage may promote useful amelioration/rehabilitative strategies for affected individuals (see Goodlett et al., 2005). Furthermore, EBC has been identified as an indicator of prenatal alcohol exposure in humans (Coffin et al., 2005; Jacobson et al., 2008). Improving diagnostic screening methods is a goal of current FASD research (see Caprara et al., 2007; Chudley et al., 2007), as early identification of fetal alcohol exposure is critical for achieving optimal outcomes (see Streissguth et al., 2004). The compatibility of EBC across development (Herbert et al., 2003; Ivkovich et al., 1999, 2000a; Little et al., 1984) suggests that a reliable behavioral phenotype resulting from prenatal alcohol exposure may emerge through EBC training in human infants considered at-risk for FASD.
4. Experimental Procedure
4.1. Subjects
The subjects were 122 Long Evans rats (58 F, 64 M) derived from 23 litters (9 undisturbed litters, 14 dosed litters). Breeders were obtained from Harlan Laboratories (Frederick, MD) and mated overnight at the animal housing colony of the Office of Laboratory Animal Medicine at the University of Delaware. Gestational day (GD) 0 was defined as the day following overnight breeding. Dams were housed in 45 × 24 × 21 cm clear Polypropylene cages in a facility maintained on a 12:12-hr light-dark cycle (lights on at 7:00 a.m) that operated in accordance with NIH guidelines. The date of birth was designated as PD0 (GD22). On PD3, litters were culled to 8 pups (usually 4 F, 4 M). Pups were weaned on PD21 and housed in groups of same-sex littermates in 45 × 24 × 17 cm cages and continuously supplied with rat chow and water. Following surgery rats were single-housed for the remainder of the experiment.
4.2. Alcohol Dosing
Alcohol was delivered in a binge-like fashion via intragastric intubation over PD4-9 (Goodlett et al., 1997, 1998). All dosing procedures were identical to those described in Brown et al. (2008) and are standard in the literature (see Green et al., 2000, 2002a, see Green et al., b; Tran et al., 2005, 2007). Each intubated litter comprised 2 subjects per dosing treatment (SI; 3, 4, or 5 g/kg/day of alcohol), with sex usually balanced within each treatment group. Subjects in the undisturbed group (Group UD) were untreated over this period and were derived from different litters. Group UD was not handled during the neonatal period save for collection of individual body weights on PD4 and on PD9. Two to four pups (1–2 F, 1–2 M) were sampled from each undisturbed litter. In all cases, no more than one same-sex littermate was assigned to a particular experimental condition (treatment × behavioral group). For intubations, PE10 tubing was lubricated with corn oil and gently passed over the tongue and down the esophagus into the stomach. Alcohol-exposed subjects received a custom milk formula (made according to the method of West et al., 1984) mixed with alcohol, while sham intubated controls (Group SI) received the same intubation exposure but without infusion of formula. Each intubation lasted 15 – 20 sec. Pups were kept on a heating pad (GE model #E12107) set at the lowest temperature while separated from their dam (for approximately 20 minutes) and showed minimal signs of distress during intubations. Daily intubations began prior to 11 a.m, and intubations always started within 1 hour (at most) of the start time from the previous day.
Alcohol was delivered across two daily intubations administered at 6.80% (Group 3 g/kg/day; two intubations of 1.5 g/kg), 9.06% (Group 4 g/kg/day; two intubations of 2 g/kg), or 11.33% (Group 5 g/kg/day; two intubations of 2.5 g/kg) v/v. Intubations were separated by 2 hours (+/− 5 minutes), consistent with inter-dosing intervals used in previous studies (Goodlett et al., 1997, 1998; Green et al., 2000). Condensed alcohol exposures produce greater brain damage relative to similar total alcohol levels delivered over more extended periods (Bonthius and West, 1990, 1991). Formula was delivered in a volume of 0.02778 ml/g body weight (1/36 of the weight in grams of each pup). Pups were returned to the dam for the interval between each intubation. Two hours after the second intubation on PD4, a 20-ul blood sample was collected in a heparinized capillary tube from a small tail-clip of each intubated pup, including SI controls. Immediately following blood collection, alcohol-dosed subjects received an intubation of the milk formula without alcohol, followed by another milk intubation two hours later for a total of 4 intubations (2 of alcohol/milk, 2 of milk alone). The additional calories of the supplementary milk feedings are given during the peak period of intoxication in order to maintain growth rates of alcohol-dosed subjects near those of controls (Goodlett et al., 1998). Group SI received the same number of intubations at the same relative time points. Intubations on PD5-9 occurred as on PD4, with the exception that (1) blood samples were not collected, and (2) only one milk-only intubation (two hours after the second alcohol dose) was administered.
4.3. Blood Alcohol Concentration (BAC) Analysis
Blood samples from alcohol-exposed subjects (Groups 3, 4 and 5 g/kg/day) were centrifuged and plasma was separated and stored at −20°C. As is conventional in the literature, samples from control subjects were not analyzed because no alcohol is detected (Brown et al., 2007; 2008). BACs were determined with an Analox GL5 Analyzer (Analox Instruments, Lunenburg, MA).
4.4. Surgery
On PD28 subjects were anesthetized with an intraperitoneal injection of a ketamine/xylazine cocktail (87 mg/kg ketamine/13 mg/kg xylazine in an injection volume of .65–.8 ml/kg). An injection of a dilute buprenex solution (0.06 mg/ml) was delivered subcutaneously 5–10 minutes after the initial ketamine/xylazine injection. A custom-built electrode (Plastics One, Roanoke, VA) containing both differential electromyograph (EMG) electrodes (implanted in the left upper eyelid muscle to record eyelid movement) and a bipolar stimulating electrode (implanted immediately caudal to the left eye; used to deliver the shock US) was implanted as described previously (Brown et al., 2006, 2008; Stanton et al., 1992; Stanton and Freeman, 2000). A separate wire connected to the electrode was placed at the back of the neck (under the skin) and served as a ground. Electrode connectors were secured to the skull with dental acrylic and two 15-mm strips of galvanized steel wires were implanted onto the skull to form an anchor for the dental acrylic. Immediately post-surgery, subjects were housed in cages that were temporarily heated on one side by an electric heating pad maintained at the lowest setting.
4.5. Apparatus
The conditioning apparatus consisted of sixteen animal chambers (BRS/LVE, Laurel, MD) lined with sound-absorbing foam, as described previously (Stanton and Freeman, 1994; available from JSA Designs, Raleigh, NC). Within each chamber animals were kept in stainless steel wire mesh cages measuring 22 × 22 × 26 cm. Each chamber was equipped with a fan which produced ‘background’ noise (< 60 dB), a house light (15W), and two speakers for delivery of the auditory CS. The present study used a 70 dB, 2.8 kHz tone as the auditory CS and activation of the house light (against the dark background) as the visual CS. The US was produced by a constant-current, 60-Hz square wave stimulator (World Precision Instruments, Sarasota, FL). Headstages were connected to wire leads that allowed subjects to move freely about the chamber.
4.6. Design and Procedures
4.6.1. Subject assignment
Groups were counterbalanced with respect to their experimental chamber and for surgeon. For a given sex within each treatment group, post-surgery assignment to the modality/ISI condition (tone = short CS+/light = long CS+, or light = short CS+/tone = long CS+) was further balanced according to body weights, the “puff-test” score, and when available, BACs. Same-sex littermates in the same treatment group were assigned to different conditions (one assigned to tone = short CS+; other assigned to light = short CS+).
4.6.2. Training
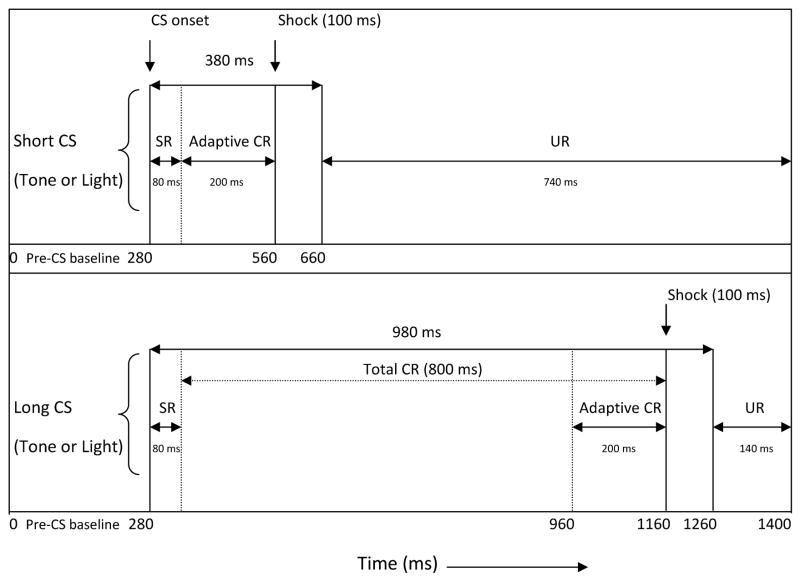
Subjects were placed in the training apparatus on PD29 and connected to the recording equipment for 1–2 minutes for a “puff-test” to confirm EMG recording quality (see Brown et al., 2006, 2007). The puff-test consisted of blowing a brief burst of air into the rat’s left eye, with the integrated EMG signal produced by the blink appearing on an oscilloscope. On PD30, ISI discrimination training commenced (as described in Brown et al., 2006, 2008; see Figure 1 for an illustration of the basic training procedure). Subjects were given 10 minutes to acclimate to the chamber before the start of Session 1. Each session consisted of 100 trials: 50 trials of a 380 ms CS, and 50 trials of a 980 ms CS (CS modality was counterbalanced within each group.) Both CSs preceded and coterminated with a 2-mA, 100 ms periocular-shock US, producing ISIs of 280 and 880 ms, respectively. Trials were presented in a pseudorandom order (no more than three consecutive trials of the same CS modality). The intertrial interval varied randomly around an average of 30 sec (range: 18–42 sec.). Within a block of 10 trials, each CS was presented 5 times and paired with the US on 4 trials (the 5th trial was CS presented alone). Training consisted of 3 sessions per day over PD30-33 (12 total sessions), with an interval of 5 hours (+/−30 minutes) between the start of each session. The start time of training sessions ranged between 8 and 9:30 a.m each day, and daily sessions typically began within 30 minutes of the start time of the previous day. Immediately following session 12, a “probe” session (20 trials) was run in which the US reinforcement schedule was changed from 4/5 trials to 1/5 trials. CS-alone probe trials are sampled for production of “tracings” used to provide a visual representation of CR topography and for assessment of double-peaked CRs. The trial sequence was otherwise identical to that in the regular sessions.
Figure 1.
Illustration of stimuli used in the interstimulus interval (ISI) discrimination procedure. All CSs preceded, overlapped, and coterminated with a 100 ms shock US. The CS durations were 380 ms (short CS) and 980 ms (long CS) with ISIs between CS and US onset of 280 and 880 ms, respectively. Adaptive CRs were recorded from the last 200 ms of the CS period prior to US onset. The short and long CSs were of different sensory modalities (one CS was a tone, the other CS was a light; reprinted from Brown et al., 2008).
4.7. Data Analysis
Data analysis was identical to that described in Brown et al. (2008). EMG signals were rectified, integrated and sampled in 3.5 ms bins during the 1400 ms epoch of each trial type. Each trial was divided into five time periods (see Figure 1): (1) a 280 ms pre-CS baseline period; (2) a startle response (SR; also termed “alpha response”) period reflecting the first 80 ms after CS onset; (3) total conditioned response (CR) period; (4) adaptive CR period; and (5) UR period.
The pre-CS baseline period (280 ms) is typically devoid of robust responding. Spontaneous blinks are infrequent, and when present usually reach relatively small peak amplitudes. Trials with excessively high spontaneous blink amplitudes were not included in analyses. Subjects exhibiting a high percentage of spontaneous blinks during the pre-CS period (30% or more; also termed “noise”) were excluded from analyses. The SR period measures non-associative “startle” reactions (or “alpha responses”) to the CS (see Gormezano, 1966). SRs are reflexive eyelid movements to the CS that are typically smaller than CRs. SRs occur equally in unpaired controls and are therefore excluded from consideration as true CRs that develop from a learned CS-US relationship. This measure is useful in identifying basic sensory deficits.
CRs reflect the learned CS-US relationship. The “total” CR period represents the period immediately following the SR period and lasts until US onset. For the short CS, this period begins immediately after the 80 ms SR period and lasts until the point of US onset (280 ms). Therefore the total CR period is 200 ms for short CS trials. For long CS trials, the total CR period represents the 800 ms period immediately following the SR period until US onset (880 ms). The total CR period for the long CS is of interest since any cross-modal influence of short CS-US training on long CS conditioning would likely be reflected in this period (e.g., early onset and enhanced amplitude CRs). Adaptive CR measures emphasize CRs that anticipate the time of US onset. The adaptive CR period only represents the 200 ms CR period that immediately precedes US onset – for the short CS this is the same as the total CR period.
The UR period represents EMG activity that occurs between the offset of the US to the end of the trial. The 100 ms of US presentation was not recorded so as to avoid stimulation “artifact” resulting from shock delivery. For the short CS, the UR period is 740 ms; for the long CS the UR period is 140 ms. URs provide a reliable indication of detection of the shock US, and production of the desired blink response. Therefore, low URs may suggest that a particular group was unable to adequately (1) detect the shock US, and/or (2) produce the desired blink response.
The threshold for registering an EMG response was set 40 arbitrary units above the average baseline amplitude during the pre-CS period (Skelton, 1988). CR peak amplitude measures counted trials in which CRs were not present as “zero amplitude” (sometimes termed CR “magnitude”, e.g., Garcia et al., 2003; Gormezano, 1966). CR percentage and peak amplitude are reported from paired CS-US trials (the pattern of data was virtually identical across CS-US and CS-alone trials). CR percentage is the most commonly reported index of learning in classical conditioning tasks, but the CR peak amplitude measure may be more sensitive to cerebellar cortical insult (Freeman et al., 1995a; Garcia et al., 1999; Perrett et al., 1993). Data reported from long-CS trials refer to the entire 800 ms CR period unless stated otherwise. This period precedes and encompasses the 200 ms “adaptive” CR period (see Figure 1). On CS-alone test trials, CR sampling periods extended to the end of the trial and therefore included the period designated as the “US period” on paired trials.
CR topography was presented as composite tracings from CS-alone probe trials following Session 12. Illustrations of CR topography have been useful in providing a visual representation of the cross-modal influence of short CS-US conditioning on long CS conditioning in this task (Brown et al., 2006). Double peaked CRs were also analyzed from probe CS-alone trials by two investigators (MAB and HBD) using the criteria defined in Brown et al. (2006). Briefly, CRs for double peak analysis were defined by an initial increase of at least 100 EMG units followed by a decrease of more than 50 units and a second increase of at least 100 units. The incidence of double peaked CRs provides additional indication of cross-modal transfer between short and long CSs in this task. If conditioning of the short CS-US has a strong influence on how the long CS is processed, a preponderance of double-peaked CRs may be expected during long CS trials –one peak occurring near the point of US onset corresponding to short CS trials and a second peak present closer to US onset for long CS trials (see Brown et al., 2006).
CR latency measures were taken from CS-alone trials from Sessions 7–12. CR latency was measured from CS-alone trials to sample the extended CR period without interference from the US. CR latency was analyzed from the latter half of training in order to allow sufficient CR sampling (which is not always possible given the low CR percentages present early in training). CR onset latency reflects the point at which the first CR within a trial crosses the threshold for registering a response. CR peak latency reflects the time point at which the CR reaches its maximum amplitude. The timing of the CR in EBC typically corresponds strongly with the interval between CS and US onset (or ISI) in well-trained subjects (Smith, 1968). As indicated above, early onset and/or early peaked CRs to the long CS (e.g., tone) in ISI discrimination provide an indication of enhanced cross-modal influence of the short CS (e.g., light).
4.8. Statistical analysis
Data were statistically analyzed via analysis of variance (ANOVA) and Newman-Keuls post hoc tests, with significance levels set at p < 0.05. Statistical analyses were performed using Statsoft Statistica software. Short and long CSs were analyzed separately. ANOVA involved the between-subjects factors of dosing condition (UD, SI, 3 g/kg/day of alcohol, 4 g/kg/day of alcohol, 5 g/kg/day of alcohol), sex (male, female), and modality (tone, light), and the within-subjects factor of sessions. Body weights were analyzed across all 5 treatment conditions at PD4 and PD9, and from Session 1 of training (PD30).
Acknowledgments
This research was supported by NIH grant 1-R01-AA11945 and NIAAA grant 1-F31-AA16250-01. The authors thank Nicole Buzin and Nathen Murawski for technical assistance. The authors also thank Dr. Charles Goodlett, Jessie McKay, and Tiffany Mullen for supplying milk formula used for dosing.
Footnotes
Abbreviations: ANOVA, analysis of variance; BAC, blood alcohol concentration; CR, conditioned response; CS, conditioned stimulus; EBC, eyeblink classical conditioning; EMG, electromyogram; FAS, fetal alcohol syndrome; FASD, fetal alcohol spectrum disorders; Group 3g/4g/5g, dosed with 3/4/5 g/kg/day of alcohol; Group SI, sham-intubated control; Group UD, undisturbed control; GD, gestational day; ISI, interstimulus interval; mA, milliamp; PD, postnatal day; UR, unconditioned response; US, unconditioned stimulus
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel EL, Sokol RJ. Fetal alcohol syndrome is now leading cause of mental retardation. Lancet. 1986;2:1222. doi: 10.1016/s0140-6736(86)92234-8. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the cerebellar system in relation to its evolution, structure, and functions. CRC Press; Boca Raton: 1997. [Google Scholar]
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol. 2001;43:148–154. [PubMed] [Google Scholar]
- Bao S, Chen L, Kim JJ, Thompson RF. Cerebellar cortical inhibition and classical eyeblink conditioning. Proc Natl Acad Sci U S A. 2002;99:1592–1597. doi: 10.1073/pnas.032655399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthius DJ, West JR. Alcohol-induced neuronal loss in developing rats: increased brain damage with binge exposure. Alcohol, Clin Exp Res. 1990;14:107–118. doi: 10.1111/j.1530-0277.1990.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, West JR. Permanent neuronal deficits in rats exposed to alcohol during the brain growth spurt. Teratology. 1991;44:147–163. doi: 10.1002/tera.1420440203. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Streissguth AP, Connor PD, Sampson PD. Damage to the human cerebellum from prenatal alcohol exposure: the anatomy of a simple biometrical explanation. Anatom Rec. 2006;289b:195–209. doi: 10.1002/ar.b.20114. [DOI] [PubMed] [Google Scholar]
- Brodal A. Anatomical studies of cerebellar fibre connections with special reference to problems of functional localization. Prog Brain Res. 1967;25:135–173. doi: 10.1016/S0079-6123(08)60964-4. [DOI] [PubMed] [Google Scholar]
- Brown KL, Pagani JH, Stanton ME. The ontogeny of interstimulus interval (ISI) discrimination of the conditioned eyeblink response in rats. Behav Neurosci. 2006;120:1057–1070. doi: 10.1037/0735-7044.120.5.1057. [DOI] [PubMed] [Google Scholar]
- Brown KL, Calizo LH, Goodlett CR, Stanton ME. Neonatal alcohol exposure impairs acquisition of eyeblink conditioned responses during discrimination learning and reversal in weanling rats. Dev Psychobiol. 2007;49:243–257. doi: 10.1002/dev.20178. [DOI] [PubMed] [Google Scholar]
- Brown KL, Calizo LH, Stanton ME. Dose dependent deficits in dual interstimulus interval classical eyeblink conditioning tasks following neonatal binge alcohol exposure in rats. Alcohol, Clin Exp Res. 2008;32:277–293. doi: 10.1111/j.1530-0277.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- Caprara DL, Nash K, Greenbaum R, Rovet J, Koren G. Novel approaches to the diagnosis of fetal alcohol spectrum disorder. Neurosci Biobehav Rev. 2007;31:254–260. doi: 10.1016/j.neubiorev.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Chen L, Bao S, Lockard JM, Kim JJ, Thompson RF. Impaired classical eyeblink conditioning in cerebellar-lesioned and Purkinje cell degeneration (pcd) mutant mice. J Neurosci. 1996;16:2829–2838. doi: 10.1523/JNEUROSCI.16-08-02829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem. 2003;11:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Chudley AE, Kilgour AR, Cranston M, Edwards M. Challenges of diagnosis in fetal alcohol syndrome and fetal alcohol spectrum disorder in the adult. Am J Med Genet C Semin Med Genet. 2007;145C:261–272. doi: 10.1002/ajmg.c.30140. [DOI] [PubMed] [Google Scholar]
- Clark RE, Gohl EB, Lavond DG. The learning-related activity that develops in the pontine nuclei during classical eyeblink conditioning is dependent on the interpositus nucleus. Learn Mem. 1997;3:532–544. doi: 10.1101/lm.3.6.532. [DOI] [PubMed] [Google Scholar]
- Coffin JM, Baroody S, Schneider K, O’Neill J. Impaired cerebellar learning in children with prenatal alcohol exposure: a comparative study of eyeblink conditioning in children with ADHD and dyslexia. Cortex. 2005;41:389–398. doi: 10.1016/s0010-9452(08)70275-2. [DOI] [PubMed] [Google Scholar]
- Connor PD, Sampson PD, Streissguth AP, Bookstein FL, Barr HM. Effects of prenatal alcohol exposure on fine motor coordination and balance: a study of two adult samples. Neuropsychologia. 2006;44:744–751. doi: 10.1016/j.neuropsychologia.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Cronise K, Marino MD, Tran TD, Kelly SJ. Critical periods for the effects of alcohol exposure on learning in rats. Behav Neurosci. 2001;115:138–145. doi: 10.1037/0735-7044.115.1.138. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Barone S, Stanton ME. Disruption of cerebellar maturation by an antimitotic agent impairs the ontogeny of eyeblink conditioning in rats. J Neurosci. 1995a;15:7301–7314. doi: 10.1523/JNEUROSCI.15-11-07301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Carter CS, Stanton ME. Early cerebellar lesions impair eyeblink conditioning in developing rats: differential effects of unilateral lesions on postnatal day 10 or 20. Behav Neurosci. 1995b;109:893–902. doi: 10.1037//0735-7044.109.5.893. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Nicholson DA. Developmental changes in the neural mechanisms of eyeblink conditioning. Behav Cogn Neurosci Rev. 2004;3:3–13. doi: 10.1177/1534582304265865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Halverson HE, Poremba A. Differential effects of cerebellar inactivation on eyeblink conditioned excitation and inhibition. J Neurosci. 2005a;25:889–895. doi: 10.1523/JNEUROSCI.4534-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Rabinak CA, Campolattaro MM. Pontine stimulation overcomes developmental limitations in the neural mechanisms of eyeblink conditioning. Learn Mem. 2005b;12:255–259. doi: 10.1101/lm.91105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia KS, Mauk MD. Pharmacological analysis of cerebellar contributions to the timing and expression of conditioned eyelid responses. Neuropharmacology. 1998;37:471–480. doi: 10.1016/s0028-3908(98)00055-0. [DOI] [PubMed] [Google Scholar]
- Garcia KS, Steele PM, Mauk MD. Cerebellar cortex lesions prevent acquisition of conditioned eyelid responses. J Neurosci. 1999;19:10940–10947. doi: 10.1523/JNEUROSCI.19-24-10940.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia KS, Mauk MD, Weidemann G, Kehoe EJ. Covariation of alternative measures of responding in rabbit (Oryctolagus cuniculus) eyeblink conditioning during acquisition training and tone generalization. Behav Neurosci. 2003;117:292–303. doi: 10.1037/0735-7044.117.2.292. [DOI] [PubMed] [Google Scholar]
- Gerwig M, Kolb FP, Timmann D. The involvement of the human cerebellum in eyeblink conditioning. Cerebellum. 2007;6:38–57. doi: 10.1080/14734220701225904. [DOI] [PubMed] [Google Scholar]
- Glickstein M. Mossy-fibre sensory input to the cerebellum. Prog Brain Res. 1997;114:251–259. doi: 10.1016/s0079-6123(08)63368-3. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Lundahl KR. Temporal determinants of neonatal alcohol-induced cerebellar damage and motor performance deficits. Pharmacol Biochem Behav. 1996;55:531–540. doi: 10.1016/s0091-3057(96)00248-1. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Peterson SD, Lundahl KR, Pearlman AD. Binge-like alcohol exposure of neonatal rats via intragastric intubation induces both Purkinje cell loss and cortical astrogliosis. Alcohol, Clin Exp Res. 1997;21:1010–1017. [PubMed] [Google Scholar]
- Goodlett CR, Pearlman AD, Lundahl KR. Binge neonatal alcohol intubations induce dose-dependent loss of Purkinje cells. Neurotoxicol Teratol. 1998;20:285–292. doi: 10.1016/s0892-0362(97)00102-5. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Stanton ME, Steinmetz JE. Alcohol-induced damage to the developing brain: functional approaches using classical eyeblink conditioning. In: Woodruff-Pak DS, Steinmetz JE, editors. Eyeblink Classical Conditioning: Animal Models. Vol. 2. Kluwer Academic Publishers; Boston: 2000. pp. 135–153. [Google Scholar]
- Goodlett CR, Horn KH, Zhou FC. Alcohol teratogenesis: mechanisms of damage and strategies for intervention. Exp Biol Med. 2005;230:394–406. doi: 10.1177/15353702-0323006-07. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Mullen TA, McKay JS. Deficits in adult Pavlovian eyeblink conditioning induced by binge alcohol exposure in neonatal rats are dose-dependent. Alcohol, Clin Exp Res. 2007;31:184A. [Google Scholar]
- Gormezano I. Classical conditioning. In: Sidowski JB, editor. Experimental Methods and Instrumentation in Psychology. McGraw-Hill; New York: 1966. pp. 385–420. [Google Scholar]
- Gormezano I, Kehoe EJ, Marshall BS. Twenty years of classical conditioning with the rabbit. Prog Psychobio Physiol Psychol. 1983;10:197–275. [Google Scholar]
- Green JT, Rogers RF, Goodlett CR, Steinmetz JE. Impairment in eyeblink classical conditioning in adult rats exposed to ethanol as neonates. Alcohol, Clin Exp Res. 2000;24:438–447. [PubMed] [Google Scholar]
- Green JT, Johnson TB, Goodlett CR, Steinmetz JE. Eyeblink classical conditioning and interpositus nucleus activity are disrupted in adult rats exposed to ethanol as neonates. Learn Mem. 2002a;9:304–320. doi: 10.1101/lm.47602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JT, Tran TD, Steinmetz JE, Goodlett CR. Neonatal ethanol produces cerebellar deep nuclear cell loss and correlated disruption of eyeblink conditioning in adult rats. Brain Res. 2002b;956:302–311. doi: 10.1016/s0006-8993(02)03561-8. [DOI] [PubMed] [Google Scholar]
- Green JT. The effects of ethanol on the developing cerebellum and eyeblink classical conditioning. Cerebellum. 2004;3:178–187. doi: 10.1080/14734220410017338. [DOI] [PubMed] [Google Scholar]
- Green JT, Steinmetz JE. Purkinje cell activity in the cerebellar anterior lobe after rabbit eyeblink conditioning. Learn Mem. 2005;12:260–269. doi: 10.1101/lm.89505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JT, Arenos JD, Dillon CJ. The effects of moderate neonatal ethanol exposure on eyeblink conditioning and deep cerebellar nuclei numbers in the rat. Alcohol. 2006;39:135–150. doi: 10.1016/j.alcohol.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Kodituwakku P, Sutherland RJ, Savage DD. Children with fetal alcohol syndrome are impaired at place learning but not cued navigation in a virtual Morris water task. Behav Brain Res. 2003;143:85–94. doi: 10.1016/s0166-4328(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Herbert JS, Eckerman CO, Stanton ME. The ontogeny of human learning in delay, long-delay, and trace eyeblink conditioning. Behav Neurosci. 2003;117:1196–1210. doi: 10.1037/0735-7044.117.6.1196. [DOI] [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 Institute of Medicine criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivkovich D, Collins KL, Eckerman CO, Krasnegor NA, Stanton ME. Classical delay eyeblink conditioning in 4- and 5-month-old human infants. Psychol Sci. 1999;10:4–8. [Google Scholar]
- Ivkovich D, Eckerman CO, Krasnegor NA, Stanton ME. Using eyeblink conditioning to assess neurocognitive development. In: Woodruff-Pak DS, Steinmetz JE, editors. Eyeblink Classical Conditioning: Applications in Humans. Vol. 1. Kluwer Academic Publishers; Boston: 2000a. pp. 119–142. [Google Scholar]
- Ivkovich D, Paczkowski CM, Stanton ME. Ontogeny of delay versus trace eyeblink conditioning in the rat. Dev Psychobiol. 2000b;36:148–160. doi: 10.1002/(sici)1098-2302(200003)36:2<148::aid-dev6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Sokol RJ, Martier SS, Ager JW, Kaplan-Estrin MG. Teratogenic effects of alcohol on infant development. Alcohol, Clin Exp Res. 1993;17:174–183. doi: 10.1111/j.1530-0277.1993.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Prenatal alcohol exposure and neurobehavioral development – where is the threshold? Alcohol Health Res World. 1994;18:30–36. [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme E, Robinson LK, Khaole N, Jacobson JL. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol, Clin Exp Res. 2008;32:365–372. doi: 10.1111/j.1530-0277.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- Larroque B, Kaminski M. Prenatal alcohol exposure and development at preschool age: main results of a French study. Alcohol, Clin Exp Res. 1998;22:295–303. doi: 10.1111/j.1530-0277.1998.tb03652.x. [DOI] [PubMed] [Google Scholar]
- Lavond DG, Steinmetz JE. Acquisition of classical conditioning without cerebellar cortex. Behav Brain Res. 1989;33:113–164. doi: 10.1016/s0166-4328(89)80047-6. [DOI] [PubMed] [Google Scholar]
- Lindquist DH, Sokoloff G, Steinmetz JE. Ethanol-exposed neonatal rats are impaired as adults in classical eyeblink conditioning at multiple unconditioned stimulus intensities. Brain Res. 2007;1150:155–156. doi: 10.1016/j.brainres.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little AH, Lipsitt LP, Rovee-Collier C. Classical conditioning and retention of the infant’s eyelid response: effects of age and interstimulus interval. J Exp Child Psychol. 1984;37:512–524. doi: 10.1016/0022-0965(84)90074-2. [DOI] [PubMed] [Google Scholar]
- Livy DJ, Miller EK, Maier SE, West JR. Fetal alcohol exposure and temporal vulnerability: effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol Teratol. 2003;25:447–458. doi: 10.1016/s0892-0362(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Lochry EA, Riley EP. Retention of passive avoidance and T-maze escape in rats exposed to alcohol prenatally. Neurobehav Toxicol. 1980;2:107–115. [Google Scholar]
- Maier SE, Chen WJ, West JR. The effects of timing and duration of alcohol exposure on development of the fetal brain. In: Abel EL, editor. Fetal Alcohol Syndrome: From Mechanism to Prevention. CRC Press; Boca Raton: 1996. pp. 27–50. [Google Scholar]
- Maier SE, Miller JA, Blackwell JM, West JR. Fetal alcohol exposure and temporal vulnerability: regional differences in cell loss as a function of the timing of binge-like alcohol exposure during brain development. Alcohol, Clin Exp Res. 1999;23:726–734. doi: 10.1111/j.1530-0277.1999.tb04176.x. [DOI] [PubMed] [Google Scholar]
- Manning MA, Hoyme HE. Fetal alcohol spectrum disorders: a practical clinical approach to diagnosis. Neurosci Biobehav Rev. 2007;31:230–238. doi: 10.1016/j.neubiorev.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Marcussen BL, Goodlett CR, Mahoney JC, West JR. Developing rat Purkinje cells are more vulnerable to alcohol-induced depletion during differentiation than during neurogenesis. Alcohol. 1994;11:147–156. doi: 10.1016/0741-8329(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Delis DC, Jones KL. Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome. Neuropsychology. 1998;12:146–153. doi: 10.1037//0894-4105.12.1.146. [DOI] [PubMed] [Google Scholar]
- Medina JF, Garcia KS, Nores WL, Taylor NM, Mauk MD. Timing mechanisms in the cerebellum: testing predictions of a large-scale computer simulation. J Neurosci. 2000;20:5516–5525. doi: 10.1523/JNEUROSCI.20-14-05516.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger F, Kapfhammer JP. Protein kinase C: its role in activity-dependent Purkinje cell dendritic development and plasticity. Cerebellum. 2003;2:206–214. doi: 10.1080/14734220310016150. [DOI] [PubMed] [Google Scholar]
- Nolan BC, Freeman JH. Purkinje cell loss by OX7-saporin impairs acquisition and extinction of eyeblink conditioning. Learn Mem. 2006;13:359–365. doi: 10.1101/lm.168506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nores WL, Medina JF, Steele PM, Mauk MD. Relative contributions of the cerebellar cortex and cerebellar nucleus to eyelid conditioning. In: Woodruff-Pak DS, Steinmetz JE, editors. Eyeblink Classical Conditioning: Animal Models. Vol. 2. Kluwer Academic Publishers; Boston: 2000. pp. 205–229. [Google Scholar]
- O’Hare ED, Kan E, Yoshii J, Mattson SN, Riley EP, Thompson PM, Toga AW, Sowell ER. Mapping cerebellar vermal morphology and cognitive correlates in prenatal alcohol exposure. Neuroreport. 2005;16:1285–1290. doi: 10.1097/01.wnr.0000176515.11723.a2. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Mauk MD. Latent acquisition of timed responses in cerebellar cortex. J Neurosci. 2001;21:682–690. doi: 10.1523/JNEUROSCI.21-02-00682.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paczkowski C, Ivkovich D, Stanton ME. Ontogeny of eyeblink conditioning using a visual conditional stimulus. Dev Psychobiol. 1999;35:253–263. doi: 10.1002/(sici)1098-2302(199912)35:4<253::aid-dev1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Perrett SP, Ruiz BP, Mauk MD. Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. J Neurosci. 1993;13:1708–1718. doi: 10.1523/JNEUROSCI.13-04-01708.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puro DG, Woodward DJ. Maturation of evoked climbing fiber input to rat cerebellar Purkinje cells: I. Exp Brain Res. 1977a;28:85–100. doi: 10.1007/BF00237088. [DOI] [PubMed] [Google Scholar]
- Puro DG, Woodward DJ. Maturation of evoked mossy fiber input to rat cerebellar Purkinje cells: II. Exp Brain Res. 1977b;28:427–441. doi: 10.1007/BF00235721. [DOI] [PubMed] [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med. 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Roebuck TM, Simmons RW, Mattson SN, Riley EP. Prenatal exposure to alcohol affects the ability to maintain postural balance. Alcohol, Clin Exp Res. 1998;22:252–258. [PubMed] [Google Scholar]
- Rogers RF, Britton GB, Steinmetz JE. Learning-related interpositus activity is conserved across species as studied during eyeblink conditioning in the rat. Brain Res. 2001;905:171–177. doi: 10.1016/s0006-8993(01)02532-x. [DOI] [PubMed] [Google Scholar]
- Savage DD, Becher M, de la Torre AJ, Sutherland RJ. Dose-dependent effects of prenatal ethanol exposure on synaptic plasticity and learning in mature offspring. Alcohol, Clin Exp Res. 2002;26:1752–1758. doi: 10.1097/01.ALC.0000038265.52107.20. [DOI] [PubMed] [Google Scholar]
- Schneiderman N, Fuentes I, Gormezano I. Acquisition and extinction of the classically conditioned eyelid response in the albino rabbit. Science. 1962;136:650–652. doi: 10.1126/science.136.3516.650. [DOI] [PubMed] [Google Scholar]
- Schonfeld AM, Mattson SN, Lang AR, Delis DC, Riley EP. Verbal and nonverbal fluency in children with heavy prenatal alcohol exposure. J Stud Alcohol. 2001;62:239–246. doi: 10.15288/jsa.2001.62.239. [DOI] [PubMed] [Google Scholar]
- Skelton RW. Bilateral cerebellar lesions disrupt conditioned eyelid responses in unrestrained rats. Behav Neurosci. 1988;102:586–590. doi: 10.1037//0735-7044.102.4.586. [DOI] [PubMed] [Google Scholar]
- Smith MC. CS-US interval and US intensity in classical conditioning of the rabbit’s nictitating membrane response. J Comp Physiol Psychol. 1968;66:679–687. doi: 10.1037/h0026550. [DOI] [PubMed] [Google Scholar]
- Snider RS, Stowell A. Receiving areas of the tactile, auditory, and visual systems in the cerebellum. J Neurophysiol. 1944;7:331–357. [Google Scholar]
- Sood B, Delaney-Black V, Covington C, Nordstrom-Klee B, Ager J, Templin T, Janisse J, Martier S, Sokol RJ. Prenatal alcohol exposure and childhood behavior at age 6 to 7 years: I. dose-response effect. Pediatrics. 2001;108:1–9. doi: 10.1542/peds.108.2.e34. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Jernigan TL, Mattson SN, Riley EP, Sobel DF, Jones KL. Abnormal development of the cerebellar vermis in children prenatally exposed to alcohol: size reduction in lobules I-V. Alcohol Clin Exp Res. 1996;20:31–34. doi: 10.1111/j.1530-0277.1996.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Freeman JH, Skelton RW. Eyeblink conditioning in the developing rat. Behav Neurosci. 1992;106:657–665. doi: 10.1037//0735-7044.106.4.657. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Freeman JH. Eyeblink conditioning in the developing rat: an animal model of learning in developmental neurotoxicology. Environ Health Perspec. 1994;102:131–139. doi: 10.1289/ehp.94102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton ME, Fox GD, Carter CS. Ontogeny of the conditioned eyeblink response in rats: acquisition or expression? Neuropharmacology. 1998;37:623–632. doi: 10.1016/s0028-3908(98)00072-0. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Goodlett CR. Neonatal ethanol exposure impairs eyeblink conditioning in weanling rats. Alcohol, Clin Exp Res. 1998;22:270–275. [PubMed] [Google Scholar]
- Stanton ME, Freeman JH. Developmental studies of eyeblink conditioning in the rat. In: Woodruff-Pak DS, Steinmetz JE, editors. Eyeblink Classical Conditioning: Animal Models. Vol. 2. Kluwer Academic Publishers; Boston: 2000. pp. 17–49. [Google Scholar]
- Stanton ME, Tran T, Robinette BL, Goodlett CR. Neonatal alcohol exposure impairs eyeblink conditioning in weanling rats: dose-response effects. Soc for Neurosci Abst. 2000;483.1 [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K, Young JK. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. Dev Behav Pediatr. 2004;25:228–238. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, McDonald RJ, Savage DD. Prenatal exposure to moderate levels of ethanol can have long-lasting effects on hippocampal synaptic plasticity in adult offspring. Hippocampus. 1997;7:232–238. doi: 10.1002/(SICI)1098-1063(1997)7:2<232::AID-HIPO9>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Farr KL, Wilson WA, Savage DD. Prenatal exposure to ethanol decreases physiological plasticity in the hippocampus of the adult rat. Alcohol. 1988;5:121–124. doi: 10.1016/0741-8329(88)90008-0. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Wasserman EA, West JR, Goodlett CR. Behavioral deficits induced by bingelike exposure to alcohol in neonatal rats: importance of developmental timing and number of episodes. Dev Psychobiol. 1996;29:433–452. doi: 10.1002/(SICI)1098-2302(199607)29:5<433::AID-DEV3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Goodlett CR, West JR. Alcohol-induced Purkinje cell loss depends on developmental timing of alcohol exposure and correlates with motor performance. Dev Brain Res. 1998;105:159–166. doi: 10.1016/s0165-3806(97)00164-8. [DOI] [PubMed] [Google Scholar]
- Tracy J, Britton GB, Steinmetz JE. Comparison of single unit responses to tone, light, and compound conditioned stimuli during rabbit classical eyeblink conditioning. Neurobiol Learn Mem. 2001;76:253–267. doi: 10.1006/nlme.2001.4024. [DOI] [PubMed] [Google Scholar]
- Tran TD, Kelly SD. Critical periods for ethanol-induced cell loss in the hippocampal formation. Neurotoxicol Teratol. 2003;25:519–528. doi: 10.1016/s0892-0362(03)00074-6. [DOI] [PubMed] [Google Scholar]
- Tran TD, Jackson HD, Horn KH, Goodlett CR. Vitamin E does not protect against neonatal ethanol-induced cerebellar damage or deficits in eyeblink classical conditioning in rats. Alcohol, Clin Exp Res. 2005;29:117–129. doi: 10.1097/01.alc.0000150004.53870.e1. [DOI] [PubMed] [Google Scholar]
- Tran TD, Stanton ME, Goodlett CR. Binge-like ethanol exposure during the early postnatal period impairs eyeblink conditioning at short and long CS-US intervals in rats. Dev Psychobiol. 2007;49:589–605. doi: 10.1002/dev.20226. [DOI] [PubMed] [Google Scholar]
- Vaglenova J, Petkov VV. Fetal alcohol effects in rats exposed pre- and postnatally to a low dose of ethanol. Alcohol, Clin Exp Res. 1998;22:697–703. [PubMed] [Google Scholar]
- Warren KR, Li TK. Genetic polymorphisms: impact on the risk of fetal alcohol spectrum disorders. Birth Defects Res A Clin Mol Teratol. 2005;73:195–203. doi: 10.1002/bdra.20125. [DOI] [PubMed] [Google Scholar]
- West JR, Hamre KM, Pierce DR. Delay in brain growth induced by alcohol in artificially reared rat pups. Alcohol. 1984;1:213–222. doi: 10.1016/0741-8329(84)90101-0. [DOI] [PubMed] [Google Scholar]
- West JR, Goodlett CR, Bonthius DJ, Pierce DR. Manipulating peak blood alcohol concentrations in neonatal rats: review of an animal model for alcohol-related developmental effects. Neurotoxicology. 1989;10:347–365. [PubMed] [Google Scholar]
- West JR, Goodlett CR, Bonthius DJ, Hamre KM, Marcussen BL. Cell population depletion associated with fetal alcohol brain damage: mechanisms of BAC-dependent cell loss. Alcohol, Clin Exp Res. 1990;14:813–818. doi: 10.1111/j.1530-0277.1990.tb01820.x. [DOI] [PubMed] [Google Scholar]
- Wigal T, Amsel A. Behavioral and neuroanatomical effects of prenatal, postnatal, or combined exposure to ethanol in weanling rats. Behav Neurosci. 1990;104:116–126. doi: 10.1037//0735-7044.104.1.116. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Steinmetz JE. Past, present, and future of human eyeblink classical conditioning. In: Woodruff-Pak DS, Steinmetz JE, editors. Eyeblink Classical Conditioning: Applications in Humans. Vol. 1. Kluwer Academic Publishers; Boston: 2000. pp. 1–17. [Google Scholar]
- Yeo CH, Hardiman MJ. Cerebellar cortex and eyeblink conditioning: a reexamination. Exp Brain Res. 1992;88:623–638. doi: 10.1007/BF00228191. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Zhang JK, Goodlett CR, Li TK. Prenatal alcohol exposure retards the migration and development of serotonin in fetal C57BL mice. Dev Brain Res. 2001;126:147–155. doi: 10.1016/s0165-3806(00)00144-9. [DOI] [PubMed] [Google Scholar]