Abstract
Acid-degradable particles containing a model protein antigen, ovalbumin, were prepared from a polyurethane with acetal moieties embedded throughout the polymer, and characterized by dynamic light scattering and transmission electron microscopy. The small molecule degradation by-product of the particles was synthesized and tested in vitro for toxicity indicating an LC50 of 12,500 μg/ml. A new liquid chromatography-mass spectrometry technique was developed to monitor the in vitro degradation of these particles. The degradation by-product inside RAW macrophages was at its highest level after 24 hours of culture and was efficiently exocytosed until it was no longer detectable after four days. When tested in vitro, these particles induced a substantial increase in the presentation of the immunodominant ovalbumin-derived peptide SIINFEKL in both macrophages and dendritic cells. In addition, vaccination with these particles generated a cytotoxic T-lymphocyte response that was superior to both free ovalbumin and particles made from an analogous but slower-degrading acid-labile polyurethane polymer. Overall, we present a fully degradable polymer system with non-toxic by-products, which may find use in various biomedical applications including protein-based vaccines.
Keywords: Vaccination, microparticles, acid-degradable materials, polyacetal, CTL, LCMS
Introduction
Prophylactic treatment of viral infections with attenuated viruses has been successfully employed for many diseases including polio (1), smallpox (2), and measles (3). Attenuated viruses infect the host in a limited manner, resulting in the presentation of viral peptide fragments on major histocompatibility complex class I (MHC I) molecules. Presentation of antigens via the MHC I pathway leads to an effective CD8+ cytotoxic T-cell lymphocyte (CTL) response, resulting in protective immunity against certain viruses (4). However, using attenuated or inactivated viruses as vaccine formulations is unacceptable for many diseases such as HIV or hepatitis C due to the safety risks inherent to the treatment. Recombinant viral proteins are an attractive alternative to attenuated viruses and have been used successfully to generate high antibody titers in the treatment of diseases such as hepatitis B (5). The efficacy of recombinant protein-based vaccines is frequently limited due to their inability to deliver antigens directly to the MHC I presentation pathway, resulting in a limited CTL response.
Several methods have been developed to increase recombinant protein presentation via the MHC I pathway including genetically modifying bacterial toxins to possess CTL epitopes (6), fusion of antigens with heat shock proteins (7), and adsorbing proteins on the surface of iron oxide or polystyrene beads (8,9). Delivery of proteins in particles capable of being phagocytosed by antigen presenting cells represents a robust method of generating CTL responses against any recombinant protein. Expanding on earlier work done on conjugating proteins to insoluble carriers, we have developed acid-labile particles that encapsulate proteins in a crosslinked polyacrylamide-based hydrogel (10-13). Upon phagocytosis, these particles quickly degrade under the acidic conditions found in endo- and lysosomal compartments, resulting in the release of the encapsulated protein. We have found that MHC I presentation is dramatically increased with such acid-degradable particle systems when compared to free protein and non-degradable microgels (14).
Although our acid-labile polyacrylamide particles have been successful as a proof of concept system, biocompatibility issues with the high molecular weight polyacrylamide by-products may necessitate the use of fully acid-degradable materials. Polymers that can break down completely into low molecular weight by-products have a distinct advantage over our prior system since they should be easily cleared from the body once fully degraded. Several polymers of this type have previously been reported including poly(β-amino) esters (15-17), poly(ketal)s (18) and poly(acetal)s (19), all of which degrade faster at the acidic pH of 5.0-6.0 than at physiological pH (7.4). However, the complete degradation of these polymers into small molecule products typically occurs on the order of several days to a few weeks, which may increase their toxicity. We recently generated a library of polyurethanes that possess dimethyl acetal moieties in the polymer backbone, which hydrolyze fully under acidic conditions to yield small molecule diols and acetone (Figure 1A) (20). Further exploring the utility of these acid-degradable materials, we made microparticles out of polymer 1 and evaluated their potential as carriers for protein-based vaccines. Herein we demonstrate that particles made from polymer 1 are more effective in generating an immune response compared to free protein and analogous particles prepared from a slower degrading polymer. Finally, to analyze the fate of intracellular degradation products (Figure 1B), a chromatographic technique was developed to study the localization of diol 2. Together, these studies demonstrate that particles made from polymer 1 are promising materials for vaccine applications due to their acid-sensitivity, ability to induce an antigen-specific CTL response in vivo, and innocuous degradation products.
Figure 1.
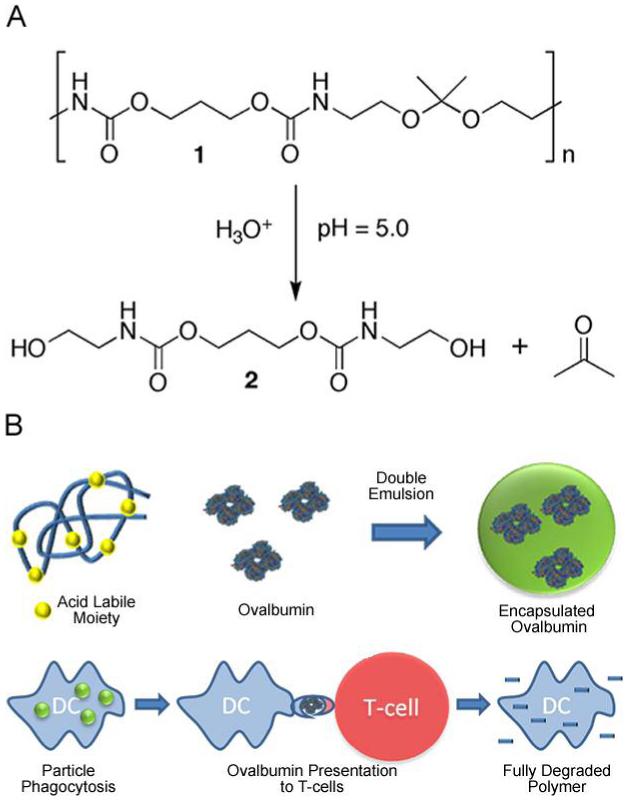
(A) Under acidic conditions (pH 5.0) polymer 1 degrades into acetone and compound 2. (B) OVA is encapsulated in acid-degradable microparticles made from polymer 1 via a double emulsion method. OVA-containing particles are phagocytosed by dendritic cells resulting in antigen presentation to CD8+ T-cells. Following phagocytosis, polymer 1 is fully degraded into small molecules.
Experimental Section
General Procedures and Materials
Reagents and materials were purchased from commercial sources and were used without further purification unless otherwise noted. 1H NMR spectra were recorded at 400 MHz and 13C spectra were recorded at 100 MHz. Chemical shifts (ppm) are reported relative to tetramethylsilane and coupling constants are reported in Hz. High-resolution fast atom bombardment mass spectrometry (FAB-HRMS) analysis was performed at the UC Berkeley mass spectrometry facility. Elemental analyses were obtained from the UC Berkeley analytical facility. Water (dd-H2O) for buffers and particle washing steps was purified to a resistance of 18 MΩ using a NANOpure purification system (Barnstead, USA). When used in the presence of acetal containing materials, dd-H2O was rendered basic (pH 8) by the addition of triethylamine (TEA) (approximately 0.01%). Fluorescence measurements were obtained on a Spectra Max Gemini XS (Molecular Devices, USA), usage courtesy of Prof. Jonathan Ellman. RAW 309 cells were obtained from ATCC (Manassas, VA) and grown according to ATCC’s directions. Liquid chromatography-electrospray mass spectrometry (LC-MS) analysis was performed using an API 150EX system (Applied Biosystems, USA) equipped with a Turbospray source, an 1100 series LC pump (Agilent), and Analyst software (version 1.4.2, Applied Biosystems). LC experiments were conducted using a Jupiter 5μ C18 300Å (Phenomenex, USA) reversed phase column (2.0 mm × 150 mm) at a flow rate of 0.25 ml/min with a MeCN:ddH2O (0.1% ammonium formate, pH = 7.7) gradient. Diol 6 was added to every sample to be analyzed by LC-MS at a fixed final concentration of 45.5 μg/mL.
Polymers 1 and 3 as well as compounds 4 and 5 were prepared as described previously (20)
Diol 2. Bis(para-nitrophenyl carbonate) 4 (0.50 g, 1.2 mmol) was dissolved in CH2Cl2 (20 mL) and cannulated into a solution of ethanolamine (0.45 mL, 7.4 mmol) in CH2Cl2 (20 mL) at 0 °C. The resulting solution was stirred and allowed to warm to rt over 6 h. The reaction mixture was concentrated and the residue was purified by column chromatography eluting with 3% followed by 10% MeOH in CH2Cl2 to yield diol 2 (214 mg, 69%) as a white solid. MP: 118-119 °C. IR (cm-1): 3323 (br), 1695, 1546, 1275. 1H NMR (400 MHz, MeOH-d4): δ 1.93 (m, 2H), 3.21 (t, J = 6, 4H), 3.57 (t, J = 6, 4H), 4.12 (m, 4H). 13C NMR (100 MHz, MeOH-d4): δ 30.1, 44.3, 62.0, 62.7, 159.3. Calcd: [M+H]+ (C9H19N2O6) m/z = 251.1243. Found FAB-HRMS: [M+H]+ m/z = 251.1239. Anal. Calcd. for C9H18N2O6: C, 43.20; H, 7.25; N, 11.19. Found: C, 43.09; H, 7.33; N, 11.04.
Diol 6 was prepared according to the same procedure used for diol 2 except bis(para-nitrophenol carbonate) 5 was used as the starting material to yield 6 (87%) as a white solid. MP: 72-73 °C. IR (cm-1): 3332 (br), 1695, 1548, 1267. 1H NMR (400 MHz, MeOH-d4): δ 0.98 (d, J = 7, 3H), 2.11 (m, 1H), 3.20 (t, J = 6, 4H), 3.57 (t, J = 6, 4H), 3.98 (d, J = 5, 4H). 13C NMR (100 MHz, MeOH-d4): δ 14.1, 34.6, 44.3, 62.0, 67.5, 159.3. Calcd: [M+H]+ (C10H21N2O6) m/z = 265.1400. Found FAB-HRMS: [M+H]+ m/z = 265.1398. Anal. Calcd. for C10H20N2O6: C, 45.45; H, 7.63; N, 10.60. Found: C, 45.16; H, 7.71; N, 10.32.
Production of OVA-Loaded Microparticles
Acid-degradable microparticles were prepared using a double emulsion water/oil/water (w/o/w) evaporation method similar to that described by Bilati et al. (21). Briefly, ovalbumin (OVA, 10 mg) was dissolved in phosphate buffered saline (PBS, pH 7.4, 50 μL). Polymer 1 or 3 (200 mg) was dissolved in CH2Cl2 (1 mL) and added to the OVA solution. This mixture was then emulsified by sonicating for 30 s on ice using a probe sonicator (Branson Sonifier 450) with an output setting of 3 and a duty cycle of 10%. This primary emulsion was added to an aqueous solution of poly(vinyl alcohol) (PVA, MW = 13,000 - 23,000 g/mol, 87-89% hydrolyzed) (2 mL, 3% w/w in PBS) and sonicated for an additional 30 s on ice using the same settings. The resulting double emulsion was immediately poured into a second PVA solution (10 ml, 0.3% w/w in PBS) and stirred for 4 h allowing the organic solvent to evaporate. The particles were then washed five times using tangential flow filters (TFF, Spectrum Laboratories, USA) with a surface area of 11 cm2 and a pore size of 0.05 μm. To stabilize the suspensions of the particles, a solution of sucrose in water (10% w/v) was added as a cryoprotectant. The particles were then frozen and lyophilized.
Quantification of Encapsulated OVA
Particles containing OVA were suspended at a concentration of 2 mg/mL in an acetate buffer (0.3 M, pH 5.0) and incubated at 37 °C under gentle agitation for 3 d using a Thermomixer R heating block (Eppendorf). After the particles had been fully degraded, the resulting solution was analyzed for protein content using the fluorescamine reagent and a microplate assay as described by Lorenzen et al. (22). Empty particles were degraded in a similar fashion and used to determine a background fluorescence level. The results were compared to a standard curve and the mass of OVA encapsulated was calculated. The protein loading was found to be 2 ± 0.28 % and the loading efficiency was 40 ± 0.5%.
Transmission Electron Microscopy
An aqueous suspension of particles (10 μL, approximately 2 mg/mL) was deposited on a carbon coated copper grid and allowed to sit undisturbed for 3 min. The excess solution was blotted off and the grid allowed to air-dry. Specimens were observed on a FEI Technai 12 TEM equipped with a Gatan CCD camera using an accelerating voltage of 120 kV.
Particle Size Analysis by Dynamic Light Scattering
Particle size distributions and average particle diameters were determined by dynamic light scattering using a Nano ZS (Malvern Instruments, United Kingdom). Particles were suspended in dd-H2O (pH 8) at a concentration of 1 mg/mL and three measurements were taken of the resulting dispersions. Results are presented as a volume distribution in Figure 2C.
Figure 2.
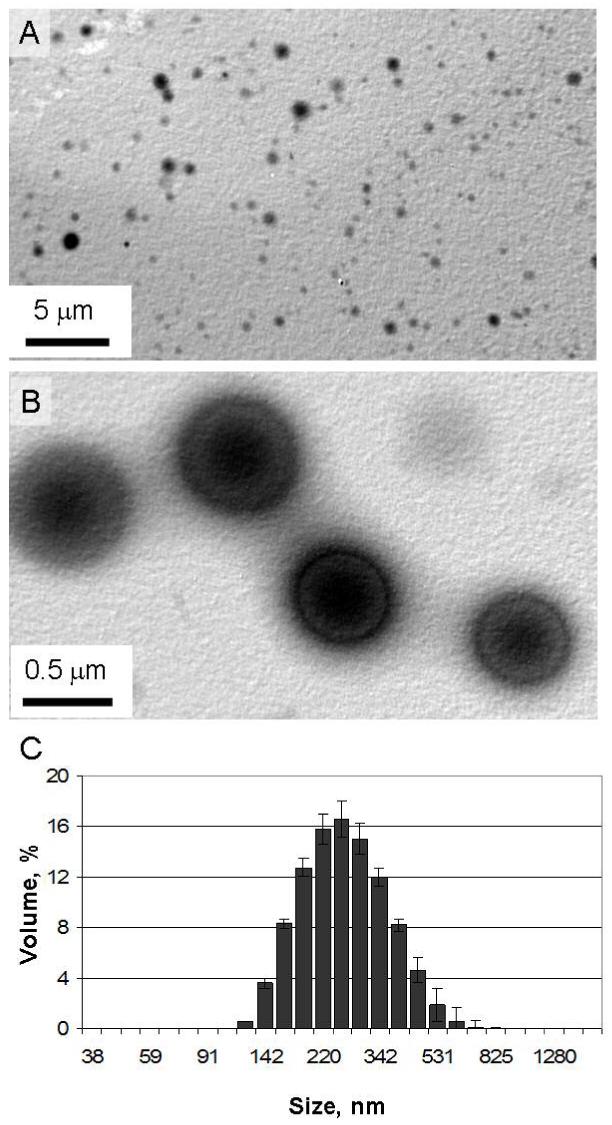
(A) TEM images of particles made from polymer 1. (B) Magnified image of larger particles selected for their high contrast. (C) Volume distribution of polymer 1 particles with an average diameter of 249 nm, a size suitable for phagocytosis by dendritic cells.
Bone Marrow Derived Dendritic Cells
Bone marrow derived dendritic cells (BMDCs) were isolated in manner similar to a previously reported protocol (23). Two to four month old female C57BL/6 mice (Jackson Laboratory) were sacrificed and the femurs and tibias were isolated. The bone marrow was flushed out with PBS using a 25-gauge needle. The cells were passed through a cell strainer and cultured with DMEM medium supplemented with FBS (10% v/v), penicillin (100 units/ml), streptomycin (100 μg/ml), L-glutamine (2 mM, GIBCO/BRL), GM-CSF and IL-4 (both 5 ng/mL, Peprotech). After six days of culture in a 24-well plate seeded at 1 million cells/ml, the BMDCs were removed from the wells, immuno-isolated using CD11c magnetic beads per the manufacturer’s directions (Miltenyi Biotech) and transferred into a 96-well plate at 5 × 104 cells per well.
MHC Class I Presentation (B3Z) Assay
B3Z cells (a generous gift of Prof. N. Shastri, University of California, Berkeley), a CD8+ T-cell hybridoma engineered to produce β-galactosidase when its T-cell receptor engages an OVA257-264:Kb complex (24,25) were maintained in RPMI 1640 (Invitrogen, USA) supplemented with 10% fetal bovine serum, 2 mM Glutamax, 50 mM 2-mercaptoethanol, 1 mM sodium pyruvate, 100 U/ml penicillin and 100 mg/ml streptomycin. 1×104 RAW macrophages or 5×104 BMDCs were seeded overnight in a 96 well plate and subsequently incubated with OVA-containing particles or free OVA. After 6 h, the cells were washed and 1×105 B3Z cells were added and cocultured for an additional 16 h. The medium was removed and 100 μL of CPRG buffer (91 mg of chlorophenol red β-D-galactopyranoside (Roche, USA), 1.25 mg of NP40 (EMD Sciences, USA), and 857 mg MgCl2 in1 L of PBS) was added to each well. After 30 min, the absorbance at 595 nm was measured using a microplate reader. The results are presented as the mean of triplicate cultures ± 95% confidence intervals.
Immunization and CTL assay
Immunization, in vitro stimulation and an in vitro CTL assay were based on previously described procedures (26-28). On day -14 and -7, mice were immunized subcutaneously at the base of the tail with either PBS, 50 μg OVA or particles containing an equivalent amount of protein made from polymer 1 or polymer 3. On day 0, spleens were harvested, mechanically disassociated and RBCs were lysed with RBC lysis buffer. 5×106 cells/ml from each spleen were cultured with 2.5×106 syngeneic spleen cells/ml pulsed with 5 μg/ml SIINFEKL. After five days of stimulation, effector T-cells in varying concentrations were cocultured with 60,000 target cells (syngeneic spleen cells pulsed with 10 μg/ml SIINFEKL). Target cell death was determined by the release of lactate dehydrogenase using the CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega, USA) per the manufacturer’s instructions. The results are presented as the mean of triplicate cultures ± 95% confidence intervals.
Toxicity of Compound 2
Compound 2 was incubated with 10,000 RAW macrophages for 24 h at various concentrations. After 24 h, compound 2 was removed, the cells were washed twice with medium and incubated overnight. Cell viability was measured the following day using CellTiter 96AQueousOne Solution Cell Proliferation Assay MTS (Promega, USA) per the manufacturer’s instructions. The results are normalized to untreated cells and presented as the mean of triplicate cultures ± 95% confidence intervals.
Cellular Degradation of Acid-Labile Particles Analyzed by Liquid Chromatography-Mass Spectrometry
Particles prepared from polymer 1 were incubated at a concentration of 2 mg/ml in a 96 well plate with either RAW macrophages (40,000 cells/well) or cell culture medium. Untreated macrophages (incubated without particles) were cultured in parallel to serve as a negative control. After 2 days, the 96 well plate was removed from the incubator and immediately frozen. The cells were lysed by 5 freeze/thaw (alternating -80 °C/rt) cycles. The lysed macrophage and medium samples were then centrifuged (12,000 x g) at 4 °C for 1 h to remove cellular debris and residual particles. The supernatant was removed and EtOH (35 mL, cooled to -80 °C, and with 1% triethylamine to avoid acid-catalyzed hydrolysis of acetal containing compounds) was added to precipitate proteins and nucleic acids. The resulting solutions were then centrifuged (15,000 x g) at 4 °C for 1 h. The supernatant was removed and concentrated using a rotary evaporator. The resulting residue was dissolved in PBS (1 mL), diluted 100 fold, and analyzed for the presence of diol 2 using LC-MS. Results are presented as the mean of triplicate measurements ± 95% confidence intervals.
Intracellular Degradation and Exocytosis of Compound 2 by Liquid Chromatography Mass Spectrometry
Particles prepared from polymer 1 were incubated at a concentration of 2 mg/ml in a 96 well plate with RAW macrophages (60,000 cells/well). After 24 h, the cells were trypsinized, washed 3 times with PBS to remove any residual particles and plated in T-25 flasks. The macrophages were then cultured for 1, 2 or 4 days. Untreated macrophages (incubated without particles for 4 days) were cultured in parallel to serve as a negative control. After these time points, the medium was removed from the cells, centrifuged (12,000 x g) at 4 °C for 1 h to remove any residual particles and the supernatant frozen for further analysis. After removal of the medium, the cells were rinsed with PBS, dislodged from the T-25 flask using a cell scraper, washed twice with PBS and finally suspended in 1 ml of PBS. The cells were then lysed by 5 freeze/thaw (alternating -80 °C/rt) cycles and centrifuged (12,000 x g) at 4 °C for 1 h to remove cellular debris and any residual particles. This supernatant as well as the samples prepared from the medium were then directly analyzed by LC-MS. Results are presented as the mean of triplicate measurements ± 95% confidence intervals.
Results and Discussion
Particle Formation and Characterization
We have previously reported the synthesis of a library of polyurethane polymers with tunable acid-sensitivity (20). These step-growth polymers were prepared by reactions involving an acetal-containing diamine monomer and a library of bis(p-nitrophenyl carbamate/carbonate) or diisocyanate monomers. The degradation half-life at pH 5.0 of microparticles prepared from this family of polyacetals was highly dependent on the hydrophobicity of the constituent polymer from which the particles were prepared. In general, we found that the hydrophilic polymers degraded significantly faster than their more hydrophobic counterparts. However, polymers that were too hydrophilic were incapable of generating particles via standard emulsion techniques (21). In balancing degradability versus processability, we chose polymer 1 as a suitable material for protein-based vaccine applications due to its ability to form stable microparticles via a double emulsion procedure and a reasonably short protein release half-life (approximately 15 hours at pH 5.0 and 37 °C) from these particles.
Microparticles encapsulating a model antigen, OVA, were made by a standard double emulsion protocol (21). After the final emulsification step and evaporation of the organic solvent, the particles were isolated using tangential flow filtration (TFF) (29). Prior to lyophilization of an aqueous suspension of the particles, a 10 wt% solution of sucrose was added as a cryprotectant to prevent particle aggregation. The OVA loading efficiency was found to be 25% and the particle recovery was 42% based on the masses of starting OVA and polymer 1, respectively.
Microparticles prepared from polymer 1 were characterized by transmission electron microscopy (TEM) and dynamic light scattering (DLS). According to these data (Figure 2 A-C), the particles were between 100-500 nm in size with an average diameter of 249 ± 5 nm. Particles of this size have been shown to be suitable for targeting DCs in vitro and in vivo (30,31), however, there is still some debate in the literature over the optimal particle size for inducing class I peptide presentation (8,32).
In Vitro Macrophage and Dendritic Cell MHC I Presentation of a Model Antigen
In order for a vaccine to generate an effective CTL immune response, antigen presenting cells (APCs) must efficiently present pathogen-derived peptides on MHC I molecules to CD8+ T-cells. A useful in vitro method for quantifying T-cell presentation is the B3Z assay (24). B3Z cells are a line of T-cells, which have been transfected with the β-galactosidase gene controlled by the nuclear factor of activated T-cells element of the human interleukin 2 enhancer. Due to the specificity of this assay, only upon engagement of APCs presenting SIINFEKL, the immunodominant peptide sequence of OVA on H-2Kb, B3Z cells produce β-galactosidase. The resulting β-galactosidase activity, which correlates directly with SIINFEKL presentation, can be colorimetrically detected. We first evaluated free OVA and OVA-loaded particles prepared from polymer 1 for their ability to stimulate T-cells by incubating them with RAW macrophages. B3Z cells were then co-cultured with the macrophages and the resulting presentation of SIINFEKL was measured (Figure 3A). Compared to free OVA, the use of particles made from polymer 1 led to a 28 fold increase in T-cell activation. Overall, particles prepared from polymer 1 dramatically increased the ability of macrophages to activate T-cells in vitro.
Figure 3.
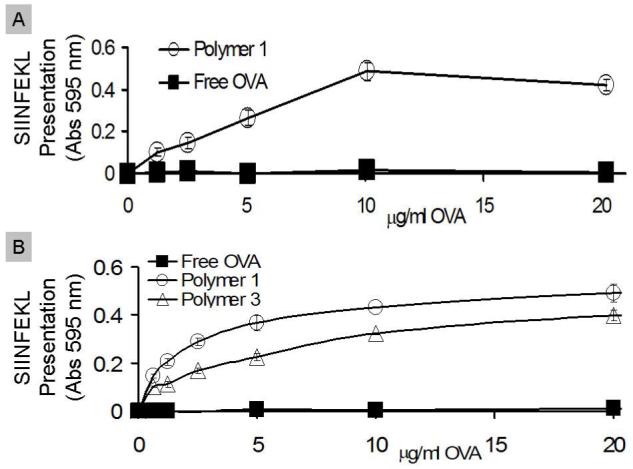
Free OVA (filled squares), polymer 1 particles (open circles), or polymer 3 particles (open triangles) were cultured with (A) RAW macrophages or (B) dendritic cells for six hours. After six hours, antigen presenting cells were cultured with B3Z cells for 16 hours and SIINFEKL presentation was measured.
To further study in vitro T-cell activation, free OVA and OVA-loaded acid-degradable particles made from polymer 1 were tested in BMDCs in the same manner as with the macrophages above (Figure 3B). Particles made from polymer 3, which has a similar structure to polymer 1 (Figure 4) but slower degradation kinetics at pH 5.0, were tested in parallel. Polymer 1 induced the best presentation of SIINFEKL, followed by polymer 3, and finally soluble OVA. This trend correlates well with the protein release half-lives of these two polymers, which have been previously reported (20). The importance of pH-sensitivity in vaccine carriers in the context of efficient MHC I presentation has previously been examined in DCs (33,34). Our results further support findings that pH-sensitivity is an important factor in determining the extent of MHC I presentation in DCs.
Figure 4.
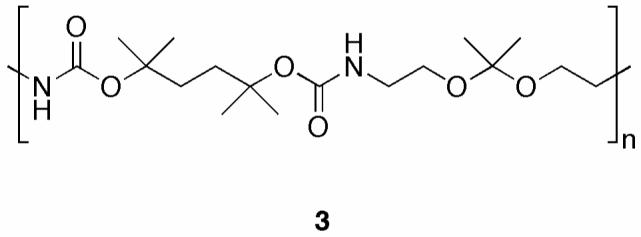
Structure of polymer 3, a more hydrophobic analog of polymer 1.
Ex Vivo CTL Cytotoxicity Assay
Encouraged by the promising in vitro T-cell activation results, we next tested the ability of particles made from polymer 1 to evoke a CTL response in vivo. To this end, mice were vaccinated twice (day -14 and day -7) with 50 μg of OVA, or an equivalent amount of OVA encapsulated in polymer 1, or polymer 3 microparticles. On day 0, spleens were removed, stimulated in vitro for 5 days, and assayed for CTL activity (Figure 5). Similar to our findings with the B3Z assay, polymer 1 particles induced the highest level of CTL activity. We found no statistically significant CTL response for OVA-loaded particles prepared from polymer 3. These data further suggest that the degradation half-life and protein release half-life of pH-sensitive materials is a critical parameter governing the generation of CTL responses in vivo. For future studies, we anticipate being able to increase in vivo CTL responses by vaccinating with more particles, using adjuvants, or by increasing the degradation kinetics by fine tuning the structural features and hydrophobicity of the parent polymer.
Figure 5.
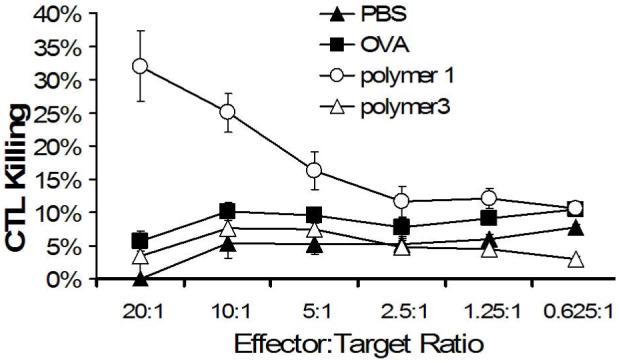
Mice were vaccinated on day -14 and day -7 with PBS (filled triangles), free OVA (filled squares), polymer 1 particles (open circles), and polymer 6 particles (open triangles). On day 0, spleen cells were removed, restimulated in vitro for five days then tested for CTL activity.
In vitro Toxicity of Compound 2
Because polymer 1 has the novel property of degrading into acetone (a non-toxic metabolic intermediate) and small molecule 2, we synthesized (Scheme 1) and tested the toxicity of this compound in vitro (Figure 6). Diol 2 was synthesized in one step through the reaction of an excess of ethanolamine with bis(para-nitrophenyl carbonate) 4. Diol 6, which is identical to diol 2 except for the addition of a single methyl group, was also synthesized by a similar route to serve as an internal standard for LC-MS studies (see below).
Scheme 1.
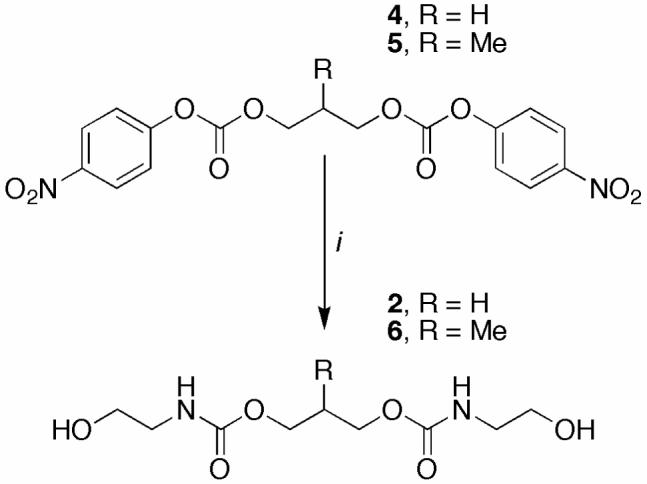
Synthesis of diols 2 and 6
(i) ethanolamine, CH2Cl2, 0 °C → rt.
Figure 6.
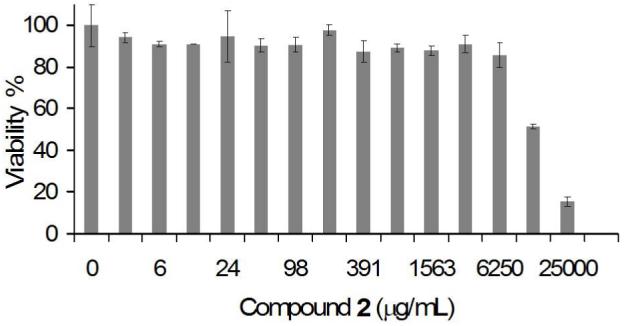
Viability of RAW macrophages incubated with compound 2 as determined by the MTS assay. Two fold dilutions of compound 2 were cultured for 24 hours with RAW macrophages. The cells were then washed, allowed to rest for an additional 24 hours and tested for cell viability.
To study the toxicity of diol 2, serial dilutions of this compound were incubated with macrophages for 24 hours. After one day of rest, we tested the viability of the cells using an MTS assay and determined that the LC50 of compound 2 in RAW macrophages was approximately 12,500 μg/ml. This value is similar to that reported by Claes et al. for the degradation products of FDA approved polymers such as poly(L, DL-lactide) (PLA) and poly(L-lactide-co-glycolide) (PLGA), which were not toxic up to 15,000 μg/ml (35). However, unlike those of PLGA, the by-products of polymer 1 should not lead to an acidic microenvironment (36). Shenderova et al. measured the pH microenvironment of PLGA particles suspended in buffer to be 1.8. In some instances, this low pH may be unsuitable for cells, as well as the protein and drug payloads typically encapsulated in these particles. Therefore a possible advantage of polymer 1 over PLGA is that it should preserve a neutral microenvironment inside particles, which may be a beneficial feature for pH-sensitive cargoes.
LC-MS Detection of Compound 2 from In Vitro Degradation Experiments
We have previously shown that polymer 1 hydrolyzes to yield compound 2 when incubated in pH 5.0 buffer for three days at 37 °C (20). Because they degrade into highly water soluble small molecules, the by-products from polymer 1 should be easily eliminated from the body via renal filtration (37). In addition, we have previously investigated the uptake and degradation of polymer 1 particles via confocal microscopy. These studies suggested that polymer 1 particles may be degraded by phagocytic cells (20). However, to obtain direct evidence that polymer 1 particles are effectively degraded inside APCs, we needed to develop a method to detect the presence of compound 2 in cell culture. Hua et al. were able to detect the degradation products derived from PLGA in vivo by labeling the intact polymer with 125I and measuring the distribution of the radioactive signal (38). However, radiolabeling is a process that can significantly alter the properties of the native polymer and subsequently affect the biodistribution. In an method providing an alternative to radiolabeling, Yoo et al. were able to measure the degradation of PLGA in buffered solutions using HPLC (39). Based on the retention times of lactic and glycolic acids, they were able to study the time dependent degradation of PLGA incubated at various pH values. However, this method is not suitable for our purposes due to the heavy signal contamination arising from proteins, nucleic acids, and a variety of small molecules (such as antibiotics and nutrients) present in cells and cell culture media.
To circumvent the issue of contamination in samples obtained from cells, we developed an LC-MS technique to study the degradation of particles made from polymer 1 in vitro. To detect the degradation product of polymer 1, we first synthesized diols 2 and 6 (see Scheme 1 and discussion above) and optimized a method to detect them using LC-MS. When analyzed as solutions in PBS and using neutral mobile phases (to minimize background hydrolysis of acetal-containing compounds present in subsequent samples), diols 2 and 6 were both ionized primarily as their sodium adducts and found to have similar retention times (Supplementary Figure S1). In analogy to the work of Fent et al. (40), we developed a semi-quantitative LC-MS technique for the detection of diol 2 wherein a fixed amount of compound 6 was spiked into each sample. After analysis, we extracted chromatograms of the intensity of masses corresponding to the sodium adducts (m/z = [M+Na]+) of 2 or 6 versus time. To normalize the data, the area under the curve of diol 2 was divided by the area under the curve of diol 6 for each sample. To test our method, serial two-fold dilutions of compound 2 were spiked with 45.5 μg/mL of compound 6 (Supplementary Figure S2) and analyzed by LC-MS. The correlation coefficient for the dependence of the normalized area of diol 2 on the concentration was found to be 0.99. Based on these data, this method was deemed suitable for analyzing the relative amounts of compound 2 in each sample.
After developing our LC-MS method, we next attempted to detect an increase in diol 2 when particles prepared from polymer 1 were cultured in the presence of RAW macrophages. In this experiment, polymer 1 particles were incubated with RAW macrophages at a concentration of 2 mg/ml. To account for background acetal hydrolysis at pH 7.4, particles were also incubated in cell culture medium under identical conditions. Macrophages cultured without particles served as a negative control. After 48 hours the cells were lysed in the cell culture medium, and analyzed using LC-MS (Figure 7A). To increase the sensitivity of the assay, the cell lysate and supernatant were measured together. Using the LC-MS method described above, the extracted normalized area for compound 2 was seven fold higher when particles were incubated with macrophages compared to particles incubated in medium alone (Figure 7B). These data suggest that the presence of phagocytic cells led to a greater degree of degradation of particles prepared from polymer 1 compared to the background hydrolysis of these acid-sensitive materials.
Figure 7.
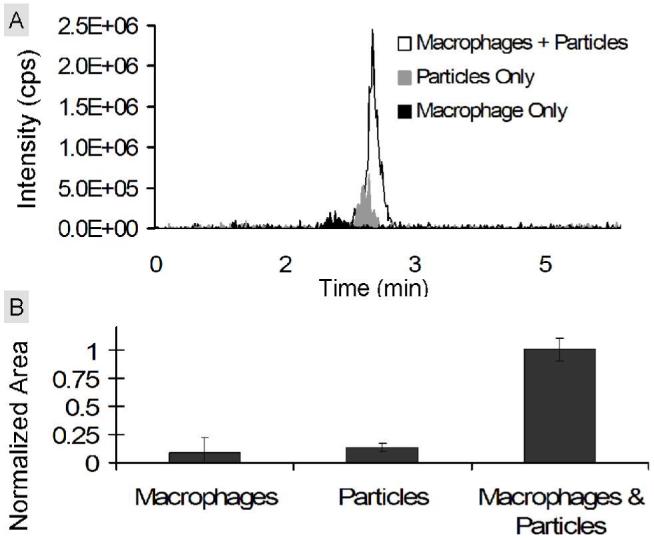
Detection of compound 2 in vitro. RAW macrophages were cultured with polymer 1 particles for 48 hours. Polymer 1 particles incubated in medium and macrophages alone served as controls. (A) Representative extracted chromatograms for compound 2 of RAW macrophages incubated with particles (white), macrophages alone (black), or polymer 1 particles incubated in medium alone (gray). (B) Average areas calculated from the extracted chromatograms of compound 2 in each sample. For each sample, the area for compound 2 was divided by the area of an internal standard (compound 6) and normalized with respect to the maximum average area (macrophage & particle sample).
After confirming that we were able to detect diol 2 in a mixture of cell culture medium and cell lysate, we next wanted to explore the kinetics of removal (exocytosis) of compound 2 from macrophages pulsed with polymer 1 particles. For this experiment, RAW macrophages were cultured with particles at a concentration of 2 mg/ml. After 24 hours, the cells were washed extensively to remove residual particles and cultured for an additional zero, one, two or four days in fresh medium. After the additional culture time, the cells were separated from their medium. Samples prepared from the cell lysate or the collected medium were analyzed using LC-MS (Figure 8A-C) and the values from each group were normalized with respect to their respective maximum values. We found the highest concentration of diol 2 inside the cell on day 0. The amount of compound 2 on subsequent days continued to decrease, and by day four the concentration was near background levels. In contrast, the concentration of compound 2 in the cell culture medium samples followed an opposite trend. Specifically, we found the lowest concentration of diol 2 on day zero with the highest concentration on day four. Based on these findings, we conclude that once particles made from polymer 1 are internalized by macrophages, they are broken down and efficiently secreted. These findings make polymer 1 an exceptional candidate as a material for drug delivery applications due to its rapid clearance from the cell. Once exocytosed from the cell, the extremely water soluble compound 2 should be easily removed from the body via the renal system. Future experiments will explore the in vivo toxicity of compound 2 and whether this molecule is effectively cleared from the body. Furthermore, the LC-MS method developed in this paper could also be applied to study the degradation of other frequently used biodegradable polymers used in in vitro and in vivo applications.
Figure 8.
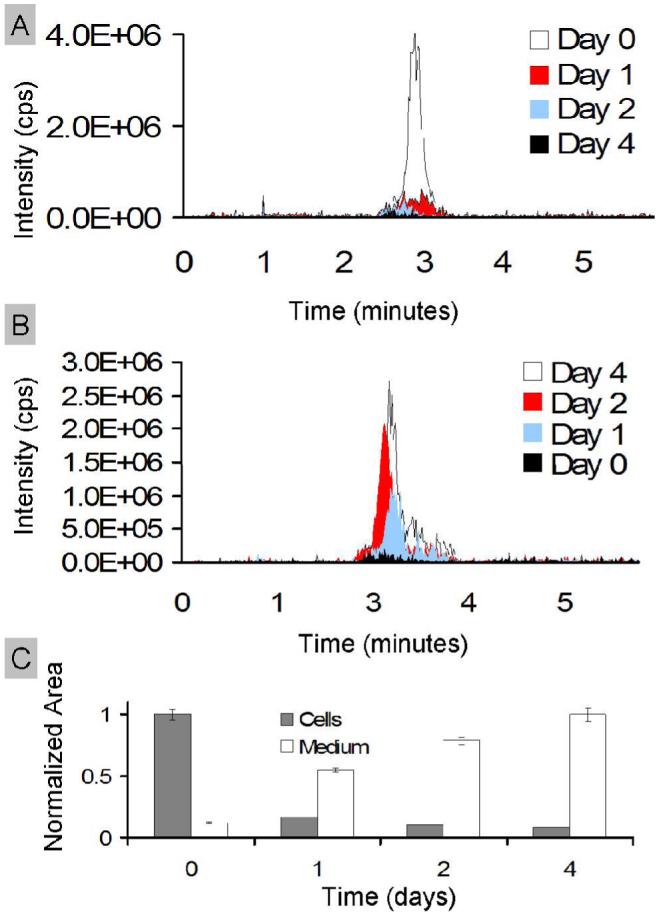
Exocytosis kinetics of compound 2 in vitro. RAW macrophages were cultured with polymer 1 particles for 24 hours. Cells were then washed and allowed to grow for an additional zero, one, two, or four days. (A) Representative extracted chromatograms for compound 2 inside RAW macrophages. (B) Representative extracted chromatograms for compound 2 in the cell culture medium. (C) Average areas calculated from the chromatograms of compound 2 in each sample. The area for compound 2 was divided by the area of the internal standard (compound 6) for each sample and then normalized with respect to the maximum value for each group. For cells the maximum value was on day zero and for the medium the maximum value was on day four.
Conclusions
We have shown that protein-loaded particles made from polymer 1 increase T-cell presentation in vitro, generate a CTL response in vivo and degrade into small innocuous by-products. Together, these findings suggest that polymer 1 is a suitable material for vaccines and other in vivo therapeutic applications.
Supplementary Material
ACKNOWLEDGMENT
We thank the National Institutes of Health (Grant RO1 EB005824) and the NIH Program of Excellence in Nanotechnology (1 U01 HL080729-01) for support of this work. We also thank Ann Fisher in the MCB Cell Culture Facility for her help and Patrick Holder for his LC-MS expertise.
REFERENCES
- 1.Chumakov K, Ehrenfeld E, Wimmer E, Agol VI. Vaccination against polio should not be stopped. Nat. Rev. Microbiol. 2007;5(12):952–8. doi: 10.1038/nrmicro1769. [DOI] [PubMed] [Google Scholar]
- 2.Parrino J, Graham BS. Smallpox vaccines: Past, present, and future. J. Allergy Clin. Immunol. 2006;118(6):1320–6. doi: 10.1016/j.jaci.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffin DE, Pan CH, Moss WJ. Measles vaccines. Front. Biosci. 2008;13:1352–70. doi: 10.2741/2767. [DOI] [PubMed] [Google Scholar]
- 4.Moron G, Dadaglio G, Leclerc C. New tools for antigen delivery to the MHC class I pathway. Trends Immunol. 2004;25(2):92–7. doi: 10.1016/j.it.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Kundi M. New hepatitis B vaccine formulated with an improved adjuvant system. Expert Rev Vaccines. 2007;6(2):133–40. doi: 10.1586/14760584.6.2.133. [DOI] [PubMed] [Google Scholar]
- 6.Carbonetti NH, Tuskan RG, Lewis GK. Stimulation of HIV gp120-specific cytolytic T lymphocyte responses in vitro and in vivo using a detoxified pertussis toxin vector. AIDS Res. Hum. Retroviruses. 2001;17(9):819–27. doi: 10.1089/088922201750252016. [DOI] [PubMed] [Google Scholar]
- 7.Susumu S, Nagata Y, Ito S, Matsuo M, Valmori D, Yui K, Udono H, Kanematsu T. Cross-presentation of NY-ESO-1 cytotoxic T lymphocyte epitope fused to human heat shock cognate protein 70 by dendritic cells. Cancer Sci. 2008;99(1):107–12. doi: 10.1111/j.1349-7006.2007.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovacsovics-Bankowski M, Clark K, Benacerraf B, Rock KL. Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc. Natl. Acad. Sci. U. S. A. 1993;90(11):4942–6. doi: 10.1073/pnas.90.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chersi A, Galati R, Accapezzato D, Francavilla V, Barnaba V, Butler RH, Tanigaki N. Responses of peptide-specific T cells to stimulation with polystyrene beads carrying HLA class I molecules loaded with single peptides. J. Immunol. Methods. 2004;291(12):79–91. doi: 10.1016/j.jim.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Murthy N, Thng YX, Schuck S, Xu MC, Frechet JM. A novel strategy for encapsulation and release of proteins: hydrogels and microgels with acid-labile acetal cross-linkers. J. Am. Chem. Soc. 2002;124(42):12398–9. doi: 10.1021/ja026925r. [DOI] [PubMed] [Google Scholar]
- 11.Murthy N, Xu M, Schuck S, Kunisawa J, Shastri N, Frechet JM. A macromolecular delivery vehicle for protein-based vaccines: acid-degradable protein-loaded microgels. Proc. Natl. Acad. Sci. U. S. A. 2003;100(9):4995–5000. doi: 10.1073/pnas.0930644100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwon YJ, James E, Shastri N, Frechet JM. In vivo targeting of dendritic cells for activation of cellular immunity using vaccine carriers based on pH-responsive microparticles. Proc Natl Acad Sci U S A. 2005;102(51):18264–8. doi: 10.1073/pnas.0509541102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon YJ, Standley SM, Goodwin AP, Gillies ER, Frechet JM. Directed antigen presentation using polymeric microparticulate carriers degradable at lysosomal pH for controlled immune responses. Mol Pharm. 2005;2(1):83–91. doi: 10.1021/mp0498953. [DOI] [PubMed] [Google Scholar]
- 14.Standley SM, Kwon YJ, Murthy N, Kunisawa J, Shastri N, Guillaudeu SJ, Lau L, Frechet JM. Acid-degradable particles for protein-based vaccines: enhanced survival rate for tumor-challenged mice using ovalbumin model. Bioconjug. Chem. 2004;15(6):1281–8. doi: 10.1021/bc049956f. [DOI] [PubMed] [Google Scholar]
- 15.Shenoy D, Little S, Langer R, Amiji M. Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs. 1. In vitro evaluations. Mol. Pharm. 2005;2(5):357–66. doi: 10.1021/mp0500420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shenoy D, Little S, Langer R, Amiji M. Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs: part 2. In vivo distribution and tumor localization studies. Pharm. Res. 2005;22(12):2107–14. doi: 10.1007/s11095-005-8343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shenoy D, Fu W, Li J, Crasto C, Jones G, DiMarzio C, Sridhar S, Amiji M. Surface functionalization of gold nanoparticles using hetero-bifunctional poly(ethylene glycol) spacer for intracellular tracking and delivery. Int. J. Nanomedicine. 2006;1(1):51–7. doi: 10.2147/nano.2006.1.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heffernan MJ, Murthy N. Polyketal nanoparticles: a new pH-sensitive biodegradable drug delivery vehicle. Bioconjug. Chem. 2005;16(6):1340–2. doi: 10.1021/bc050176w. [DOI] [PubMed] [Google Scholar]
- 19.Tomlinson R, Klee M, Garrett S, Heller J, Duncan R, Brocchini S. Pendent chain functionalized polyacetals that display pH-dependent degradation: A platform for the development of novel polymer therapeutics. Macromolecules. 2002;35:473–480. [Google Scholar]
- 20.Paramonov SE, Bachelder EM, Beaudette TT, Standley SM, Lee CC, Dashe J, Frechet JM. Fully Acid-degradable biocompatible polyacetal microparticles for drug delivery. Bioconjug. Chem. 2008;19(4):911–9. doi: 10.1021/bc7004472. [DOI] [PubMed] [Google Scholar]
- 21.Bilati U, Allemann E, Doelker E. Sonication parameters for the preparation of biodegradable nanocapsules of controlled size by the double emulsion method. Pharm. Dev. Technol. 2003;8(1):1–9. doi: 10.1081/pdt-120017517. [DOI] [PubMed] [Google Scholar]
- 22.Lorenzen a., Kennedy SW. A Fluorescence-Based Protein Assay for Use with a Microplate Reader. Analytical Biochemistry. 1993;214(1):346–348. doi: 10.1006/abio.1993.1504. [DOI] [PubMed] [Google Scholar]
- 23.Abdi K, Singh N, Matzinger P. T-cell control of IL-12p75 production. Scand. J. Immunol. 2006;64(2):83–92. doi: 10.1111/j.1365-3083.2006.01767.x. [DOI] [PubMed] [Google Scholar]
- 24.Karttunen J, Sanderson S, Shastri N. Detection of rare antigen-presenting cells by the lacZ T-cell activation assay suggests an expression cloning strategy for T-cell antigens. Proc. Natl. Acad. Sci. U. S. A. 1992;89(13):6020–4. doi: 10.1073/pnas.89.13.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karttunen J, Shastri N. Measurement of ligand-induced activation in single viable T cells using the lacZ reporter gene. Proc. Natl. Acad. Sci. U. S. A. 1991;88(9):3972–6. doi: 10.1073/pnas.88.9.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee KY, Chun E, Seong BL. Investigation of antigen delivery route in vivo and imune-boosting effects mediated by pH-sensitive liposomes encapsulated with K(b)-restricted CTL epitope. Biochem. Biophys. Res. Commun. 2002;292(3):682–8. doi: 10.1006/bbrc.2002.6711. [DOI] [PubMed] [Google Scholar]
- 27.Mandal M, Lee KD. Listeriolysin O-liposome-mediated cytosolic delivery of macromolecule antigen in vivo: enhancement of antigen-specific cytotoxic T lymphocyte frequency, activity, and tumor protection. Biochim. Biophys. Acta. 2002;1563(12):7–17. doi: 10.1016/s0005-2736(02)00368-1. [DOI] [PubMed] [Google Scholar]
- 28.Wang D, Molavi O, Lutsiak ME, Elamanchili P, Kwon GS, Samuel J. Poly(D,L-lactic-co-glycolic acid) microsphere delivery of adenovirus for vaccination. J. Pharm. Pharm. Sci. 2007;10(2):217–30. [PubMed] [Google Scholar]
- 29.Dalwadi G, Benson HA, Chen Y. Comparison of diafiltration and tangential flow filtration for purification of nanoparticle suspensions. Pharm. Res. 2005;22(12):2152–62. doi: 10.1007/s11095-005-7781-z. [DOI] [PubMed] [Google Scholar]
- 30.Hirota K, Hasegawa T, Hinata H, Ito F, Inagawa H, Kochi C, Soma G, Makino K, Terada H. Optimum conditions for efficient phagocytosis of rifampicin-loaded PLGA microspheres by alveolar macrophages. J. Control Release. 2007;119(1):69–76. doi: 10.1016/j.jconrel.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Foged C, Brodin B, Frokjaer S, Sundblad A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int. J. Pharm. 2005;298(2):315–22. doi: 10.1016/j.ijpharm.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 32.Fifis T, Gamvrellis A, Crimeen-Irwin B, Pietersz GA, Li J, Mottram PL, McKenzie IF, Plebanski M. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J Immunol. 2004;173(5):3148–54. doi: 10.4049/jimmunol.173.5.3148. [DOI] [PubMed] [Google Scholar]
- 33.Haining WN, Anderson DG, Little SR, von Bergwelt-Baildon MS, Cardoso AA, Alves P, Kosmatopoulos K, Nadler LM, Langer R, Kohane DS. pH-triggered microparticles for peptide vaccination. J. Immunol. 2004;173(4):2578–85. doi: 10.4049/jimmunol.173.4.2578. [DOI] [PubMed] [Google Scholar]
- 34.Hu Y, Litwin T, Nagaraja AR, Kwong B, Katz J, Watson N, Irvine DJ. Cytosolic delivery of membrane-impermeable molecules in dendritic cells using pH-responsive core-shell nanoparticles. Nano. Lett. 2007;7(10):3056–64. doi: 10.1021/nl071542i. [DOI] [PubMed] [Google Scholar]
- 35.Ignatius AA, Claes LE. In vitro biocompatibility of bioresorbable polymers: poly(L, DL-lactide) and poly(L-lactide-co-glycolide) Biomaterials. 1996;17(8):831–9. doi: 10.1016/0142-9612(96)81421-9. [DOI] [PubMed] [Google Scholar]
- 36.Shenderova A, Burke TG, Schwendeman SP. The acidic microclimate in poly(lactide-co-glycolide) microspheres stabilizes camptothecins. Pharm. Res. 1999;16(2):241–8. doi: 10.1023/a:1018876308346. [DOI] [PubMed] [Google Scholar]
- 37.Caliceti P, Veronese FM. Pharmacokinetic and biodistribution properties of poly(ethylene glycol)-protein conjugates. Adv. Drug Deliv. Rev. 2003;55(10):1261–77. doi: 10.1016/s0169-409x(03)00108-x. [DOI] [PubMed] [Google Scholar]
- 38.Hua N, Sun J. Body distribution of poly(D: ,L: -lactide-co-glycolide) copolymer degradation products in rats. J. Mater. Sci. Mater. Med. 2008 doi: 10.1007/s10856-008-3460-z. [DOI] [PubMed] [Google Scholar]
- 39.Yoo JY, Kim JM, Seo KS, Jeong YK, Lee HB, Khang G. Characterization of degradation behavior for PLGA in various pH condition by simple liquid chromatography method. Biomed. Mater. Eng. 2005;15(4):279–88. [PubMed] [Google Scholar]
- 40.Fent KW, Jayaraj K, Ball LM, Nylander-French LA. Quantitative monitoring of dermal and inhalation exposure to 1,6-hexamethylene diisocyanate monomer and oligomers. J. Environ. Monit. 2008;10(4):500–7. doi: 10.1039/b715605g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


