Abstract
Background
Several decades of research link childhood parental loss with risk for major depression and other forms of psychopathology. A large body of preclinical work on maternal separation and some recent studies of humans with childhood parental loss have demonstrated alterations of hypothalamic-pituitary-adrenal (HPA) axis function which could predispose to the development of psychiatric disorders.
Methods
Eighty-eight healthy adults with no current Axis I psychiatric disorder participated in this study. Forty-four participants experienced parental loss during childhood, including 19 with a history of parental death and 25 with a history of prolonged parental separation. The loss group was compared to a matched group of individuals who reported no history of childhood parental separation or childhood maltreatment. Participants completed diagnostic interviews and questionnaires and the dexamethasone/corticotropin-releasing hormone (Dex/CRH) test. Repeated measures general linear models were used to test the effects of parental loss, a measure of parental care, sex, and age on the hormone responses to the Dex/CRH test.
Results
Parental loss was associated with increased cortisol responses to the test, particularly in males. The effect of loss was moderated by levels of parental care; participants with parental desertion and very low levels of care had attenuated cortisol responses. ACTH responses to the Dex/CRH test did not differ significantly as a function of parental loss.
Conclusions
These findings are consistent with the hypothesis that early parental loss induces enduring changes in neuroendocrine function.
Keywords: childhood parental loss, parental death, depression, cortisol, HPA axis
INTRODUCTION
Psychosocial stressors, such as childhood parental loss, have long figured prominently in theories and research on the pathogenesis of major depression and related disorders (1–8). Neurobiological systems that regulate stress reactivity are likely involved in the vulnerability to psychiatric disorders following exposure to childhood parental loss. Corticotropin-releasing hormone (CRH) and the hypothalamic-pituitary-adrenal (HPA) axis are activated in response to stress and are thought to play an important role in the pathophysiology of major depression (10–12), and other stress-related psychiatric disorders (13–17). A large body of work in rodents and non-human primates demonstrates that early maternal separation results in changes in brain circuitry regulating stress reactivity, mood, and behavior, with associated exaggeration or attenuation of HPA axis activity (22). The direction and pattern of such HPA alterations may depend on the nature and timing of the stressor (22–24).
Unlike other forms of childhood stress, parental death, and to a lesser degree parental desertion, is a discrete and objective event that may be minimally influenced by recall bias or subjective judgments. However, as with other forms of childhood adversity, the study of parental loss is complicated because 1) the nature and timing of such losses is highly variable, 2) individuals with parental loss may suffer from psychiatric disorders which can have associated changes in HPA axis function, and 3) parental loss may be linked to other risk factors for psychiatric disorders, which may themselves contribute to HPA axis dysfunction, such as poverty and childhood maltreatment.
Children who experienced permanent or long-term separations from parents or parental death have been found to have increases in basal salivary cortisol concentrations (25, 26) and cortisol nonsuppression in the dexamethasone-suppression test (27), but decreased morning cortisol concentrations are seen in some cases of separation (25) and in studies of institutionalized children (28–30). Two recent studies of university students with less severe forms of loss have found attenuation of the cortisol response to corticotropin releasing hormone (CRH) stimulation in subjects with a childhood history of parental divorce (31) and a decreased awakening cortisol response in students with a history of either parental separation/divorce or death of a very close friend or relative (32). However, reports of adults with a history of childhood parental death have found increased basal (33, 34) or psychosocial stress-induced cortisol concentrations (35, 36). Leucken and colleagues found that this effect was moderated by the rearing environment such that participants with parental loss who reported low levels of care by the surviving parent (35) or childhood maltreatment (36) had elevated cortisol responses to the speech stressor. Only a few studies have controlled for factors which are associated with parental loss and may themselves be associated with abnormalities in neuroendocrine function, including current and past psychiatric disorders (26, 31, 33, 34), relationships with parents (33, 35, 36), socioeconomic status (31), and childhood maltreatment (31, 36).
In the present study we administered the dexamethasone/CRH (Dex/CRH) test, a sensitive probe of HPA axis function (39), to healthy adults with a history of childhood parental loss, and assessed effects of potential confounding factors. We hypothesized that parental loss would be associated with elevated ACTH and cortisol responses and that the quality of parental care would moderate these effects.
METHODS AND MATERIALS
Subjects
Eighty-eight adults with no current Axis I psychiatric disorder participated in this study. Flyers and advertisements on the Internet and in local newspapers for several thematically and methodologically related studies advertised for 1) healthy adults, and 2) individuals with a history of early parental loss, and 3) adults with a history of early-life stress. Participants were included in the present study if they met the inclusion and exclusion criteria detailed below. Subjects underwent physical and neurological examinations, an electrocardiogram, and laboratory studies for complete blood count, electrolytes, thyroid stimulating hormone, urine toxicology, and urinalysis. Subjects were excluded if they worked night shifts, met criteria for a current Axis I psychiatric disorder, or if they had one or more of the following conditions: acute or unstable medical illness, a history of brain injury, seizure disorder, endocrine disease, or substance abuse. Also excluded were individuals undergoing treatment with drugs which might influence HPA axis function, including psychotropics, beta blockers, angiotensin-converting enzyme inhibitors, ketoconazole, metyrapone, and corticosteroids. Subjects were free of these medications for at least two weeks (6 weeks for fluoxetine) prior to participation. Oral contraceptives were allowed. All subjects gave voluntary written informed consent to participate in this study, which was approved by the Butler Hospital Institutional Review Board.
Forty-four individuals who had experienced either parental death (N=19, Parental Death group) or prolonged separation or desertion of a parent (N=25, Parental Desertion group) before the age of 18 were considered to have parental loss (Loss group). Participants were considered to have prolonged parental separation or desertion if they identified a parent as having left them without attempting to contact them or responding to attempts at contact for at least six months. Table 1 shows the details of the loss characteristics for these groups.
Table 1.
Loss characteristics
|
Death (N=19) | |
| Age at loss, Mean (SD) | 9.2 (4.5) |
| Range | 3–17 |
| Sex of Loss Parent, N (%) | |
| Mother | 2 (10.5) |
| Father | 17 (89.5) |
| Type of Death, N (%) | |
| Illness/Disease | 16 (84.2) |
| Accident | 2 (10.5) |
| Suicide | 1 (5.3) |
|
Desertion (N=25) | |
| Age at loss, Mean (SD) | 5.2 (4.8) |
| Range | 0–14 |
| Sex of Loss Parent, N (%) | |
| Mother Only | 4 (16.0) |
| Father Only | 13 (52.0) |
| Both | 6 (24.0) |
| Father + Stepfather | 2 (8.0) |
| Circumstance at Time of Loss, N (%) | |
| Following Divorce | 8 (32.0) |
| At Birth | 5 (20.0) |
| Unknown | 12 (48.0) |
| Duration of Separation, N (%) | |
| Permanent | 22 (88.0) |
| Non-Permanent | 3 (12.0) |
Note. Death from illness or disease included heart disease, n=4; cancer, n=4; liver disease, n=2; vascular disease, n=2; “natural causes,” n=2; blood disorder, n=1; renal failure, n=1. Permanent desertion included 1 subject who was adopted from Korea 6 months after birth. Nonpermanent desertion included 1 which occurred at age 2 and lasted 4 years, 1 which occurred at age 13 for 6 months, and 1 with an 8-month separation from the mother at age 9, followed by separation from the father with twice yearly contact thereafter.
The Loss group was matched with respect to sex to 44 subjects with no childhood parental separation, including divorce (No Loss group). In order to minimize the likelihood of childhood stress in this group, participants who reported a history of childhood neglect or abuse were not included in this group. A Parental Loss variable was created that included three categories: Parental Death, Parental Desertion, and No Loss. Demographic characteristics for all subjects are presented in Table 2.
Table 2.
Subject characteristics.
| No Loss (N=44) | Death (N= 19) | Desertion (N=25) | |
|---|---|---|---|
| Subject Age, Year, Mean (SD), | 27.3 (9.2) | 28.3 (9.3) | 32.4 (11.2) |
| Range | 18–55 | 19–45 | 19–52 |
| Sex; N Female (%) | 29 (65.9) | 11 (57.9) | 17 (68.0) |
| Race, N (%) | |||
| White | 37 (84.1) | 18 (94.7) | 18 (72.0) |
| Black | 1 (2.3) | 0 | 3 (12.0) |
| Asian | 2 (4.5) | 0 | 1 (4.0) |
| Native American | 0 | 0 | 1 (4.0) |
| Other | 3 (6.8) | 1 (5.3) | 2 (8.0) |
| Not Reported | 1 (2.3) | 0 | 0 |
| Socioeconomic Adversity, N (%) | 2 (4.5) | 1 (5.3) | 12 (48.0) † |
| Multiple Loss, N, (%) | 0 | 1 (5.3) | 6 (24.0) † |
| Foster Care, N, (%) | 0 | 0 | 4 (16.0) ** |
| Reported Abuse/Neglect, N (%) | |||
| Emotional Abuse | 0 | 3 (15.8) ** | 12 (48.0) † |
| Physical Abuse | 0 | 1 (5.3) | 6 (24.0) † |
| Sexual Abuse | 0 | 3 (15.8) ** | 7 (28.0) † |
| Emotional Neglect | 0 | 5 (26.3) † | 10 (40.0) † |
| Physical Neglect | 0 | 2 (10.5) * | 8 (32.0) † |
| Compound Stress, N (%) | 0 | 3 (15.8) ** | 10 (40.0) † |
Note: Multiple Loss refers to subjects who reported the loss of a parent plus the loss of at least one additional caregiver.
Abuse or neglect considered present when the Childhood Trauma Questionnaire subscale score reached threshold for “moderate” or “severe.”
Compound stress considered present when three or more of eight stressors were reported: multiple loss, foster care, socioeconomic adversity, and reported abuse or neglect on each of the 5 types of maltreatment.
Analysis of Variance with post hoc Tukey’s HSD tests and Chi Square tests were used to compare groups. Symbols refer to comparisons with the No Loss group,
p < .05,
p < .01;
p < .001.
The Desertion and Death groups were significantly different from each other with respect to Emotional Abuse (p < .05) and Socioeconomic Adversity (p < .005). There were trend-level differences between the Desertion and Death groups on Multiple Loss, Foster Care, Physical Neglect, and Compound Stress (p < 1.0).
Measures
Socioeconomic Adversity
Socioeconomic adversity in the childhood home was determined if participants scored in the adverse direction on either of the following true/false statements: 1) I grew up in an area of high crime and, 2) My family was generally financially stable when I was growing up, and all of my basic needs (food, shelter, and clothing) were met during my childhood.
The Structured Clinical Interview for DSM-IV (SCID; (40))
Current and lifetime history of Axis I psychiatric diagnoses were assessed using the SCID for DSM-IV.
The Childhood Trauma Questionnaire (CTQ;(41))
The 28-item version of the CTQ was used (42) to ensure that the No Loss comparison subjects did not have a history of childhood maltreatment (defined as a moderate or severe score on any of the CTQ subscales including emotional abuse, physical abuse, sexual abuse, emotional neglect and physical neglect).
Parental Bonding Instrument (PBI; (43))
The PBI, which assesses the childhood experience of parental care and overprotection for each parent, has good reliability and validity (44). The care dimension was used for the present study because previous work has identified low parental care and neglect as important determinants of psychiatric and neuroendocrine sequelae following childhood parental loss (4, 5, 35). Low scores on the care subscales may be indicative of neglect; maternal care and paternal care on the PBI are highly negatively correlated with the maternal neglect and paternal neglect subscales of the Childhood Experience of Care and Abuse Questionnaire (r=−.80 and r=−.70, respectively, 56). Average ratings for the mother and father were calculated (parental care). Scores for only the surviving parent were used for four participants with a history of parental loss who did not have information on the loss parent..
The following additional self-report measures were included to further characterize the groups: (1) Inventory of Depressive Symptomatology-Self Report (IDS-SR; (45)), (2) State-Trait Anxiety Questionnaire (STAI; (46)), (3) Perceived Stress Scale (PSS; (47)), and (4) the Tridimensional Personality Questionnaire (TPQ;(48)).
The Dex/CRH Test
On a subsequent visit, subjects completed the Dex/CRH test as follows. On the night before the test, a single oral dose of dexamathasone 1.5 mg was self-administered at 11:00 p.m. The following day, participants arrived at 12:00 p.m., were given lunch, and queried about whether they had experienced any somatic symptoms, stressors or changes in usual habits over the preceding week. Participants who reported any significant aberrations had the visit rescheduled. Topical anesthetic cream [EMLA (lidocaine 2.5% and prilocaine 2.5%)] was applied to the subject’s forearm between 12:30 and 12:45 p.m. At 1:00 p.m. an indwelling intravenous (IV) catheter was inserted in the forearm by a research nurse with extensive experience with IV catheter placement. Subjects then remained in a semi-recumbent position throughout the procedure except to use the bathroom. They were permitted to read or watch preselected films that did not contain emotionally-charged material. Vital signs were monitored throughout the test. At 2:30, 3:30 and 4:30 p.m., subjects completed visual analogue rating scales (VAS) that assessed the degree to which they felt a number of mood states, including “anxious,” “depressed,” “fearful,” “irritable,” “nervous” and “sad.” All scales were anchored from 0 = not at all to 100 = most ever.
At 3 p.m., CRH 100 μg (corticorelin ovine triflutate, Acthrel®, Ferring Pharmaceuticals, Inc.) reconstituted in 2 ml 0.9% sodium chloride was infused intravenously over 30 seconds. Blood samples were drawn at 2:59 p.m. and every 15 minutes thereafter until 5:00 p.m. Samples were immediately stored on ice, centrifuged within 45 minutes, and then stored at −80° C for assay of ACTH and cortisol. ACTH assay was performed on samples drawn at 2:59 p.m., 3:30 p.m., 4:00 p.m., 4:30 p.m., and 5:00 p.m. Plasma ACTH was assayed in duplicate 200 ul plasma samples using an immunoradiometric assay (49) according to the manufacturer’s instructions (Scantibodies Laboratory, Santee, CA). The minimum detectable ACTH concentration was 2 pg/ml, and the intra- and inter-assay coefficients of variation for this series of assays were 4.6% and 5.3%, respectively. Cortisol assays were performed on samples from 2:59 p.m., 3:30 p.m., 3:45 p.m., 4:00 p.m., 4:15 p.m., and 5:00 p.m. The GammaCoat cortisol I-125 coated-tube radioimmunoassay (RIA) kit (INCSTAR Corp., Stillwater, Minn.) was used to measure cortisol at each time point. The intra-assay and inter-assay CVs observed for quality assessment samples (5 and 20 ug/dl) were less than 5% and 10%, respectively.
Statistical Analyses
Analyses were conducted using SPSS 14.0 for Windows. All analyses were two-tailed with alpha set to 0.05. ACTH and cortisol values, as well as age and IDS-SR, were positively skewed and were therefore log-transformed. Parental Care was negatively skewed and was therefore log-transformed after subtracting individual values from the highest score plus one. Repeated measures general linear models were used to determine whether there were changes in ACTH, cortisol, and mood state over time in the Dex/CRH test. For all general linear models, Wilks’ Lambda was used for multivariate tests, and Mauchly’s Test of Sphericity followed by Huynh-Feldt corrections was used for within-subjects effects. Some of the VAS scales showed a change in mood state during the test, so we created a composite variable to use as a covariate in later analyses. “Anxious distress” was composed of the mean of the VAS scales “anxious,” “nervous,” and “irritable”. The area under the curve (AUC) for this variable was computed.
To test the main hypotheses, repeated measures general linear models were used to predict ACTH and cortisol responses to the Dex/CRH test over time with the following independent variables: age, sex, socioeconomic adversity, Parental Loss, and Parental Care. Additional models tested effects of potential covariates.
RESULTS
Participant Characteristics
Characteristics of the participants according to loss group are shown in Tables 1 and 2. Participants with parental desertion were younger on average at the time of loss (t= 2.78, df= 42, p<.01) and were more likely than those with parental death to report a history of emotional abuse and socioeconomic adversity (Table 2).
Neuroendocrine and Mood Changes in the Dex/CRH Test
The general linear models testing neuroendocrine changes over time in the Dex/CRH test were significant for both ACTH and cortisol (F(4, 76)=62.1, p<.001; F(5, 83)=114.9, p<.001 respectively). While there were no changes in the VAS items “sad” or “depressed” over time in the test, the items “anxious,” “fearful,” “irritable,” and “nervous” increased in response to the Dex/CRH test (p’s < .05). The composite variable “anxious distress” also increased in the test; however this variable did not vary as a function of Loss group.
Parental Loss and Neuroendocrine Response to the Dex/CRH Test
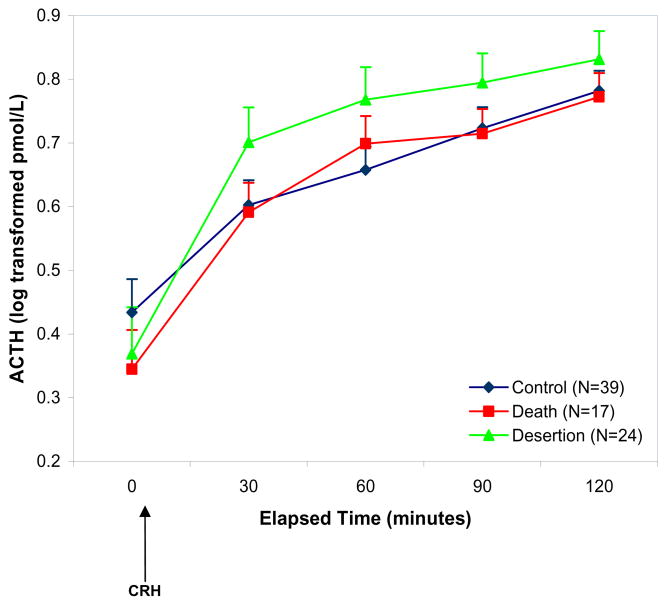
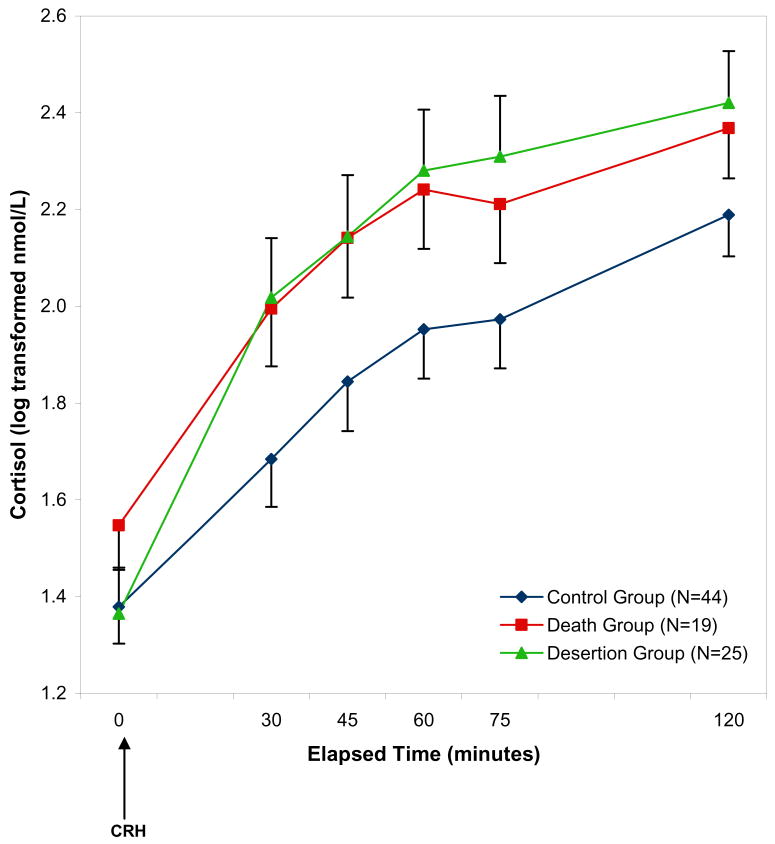
For ACTH, there were no significant main effects or interactions of the Parental Loss variable in the repeated measures model predicting ACTH over time (p’s >.08; Figure 1). For cortisol, there were significant within subjects effects of Parental Loss group and parental care, and interactions of Loss group with Parental Care. In addition, there were significant between subjects effects of Parental Care and an interaction of Parental Loss by sex (Table 3). Figure 2 shows that there were increased cortisol responses in the Death and Desertion groups in comparison with the No Loss group. The interaction between Loss group and sex was due to the effect of loss being more pronounced among males. Post-hoc analyses were conducted to characterize the nature of the within-subjects interaction of Loss group and parental care as described below.
Figure 1. ACTH responses to the Dex/CRH test according to Loss Group.
Note: CRH refers to corticotrophin-releasing hormone. The repeated measures general linear model testing the effects of parental loss and parental care predicting ACTH concentrations was not significant.
Table 3.
General linear model predicting cortisol concentrations in the Dex/CRH test.
| df | F | p | |
|---|---|---|---|
| Within Subjects | |||
| Time × Loss | 5, 193 | 3.16 | .008 |
| Time × Care | 3, 193 | 5.42 | .002 |
| Time × Loss × Sex | 5, 193 | .986 | .429 |
| Time × Loss × Care | 5, 193 | 2.60 | .025 |
| Time × Loss × Sex × Care | 8, 193 | 1.17 | .320 |
| Between Subjects | |||
| Loss | 2, 74 | 1.44 | .244 |
| Care | 1, 74 | 6.35 | .014 |
| Loss × Sex | 2, 74 | 3.39 | .039 |
| Loss × Care | 2, 74 | .975 | .382 |
| Loss × Care × Sex | 3, 74 | 2.31 | .083 |
Note. Time refers to time during the Dex/CRH test. Loss refers to the Parental Loss grouping which includes the No Loss, Parental Death, and Parental Desertion groups. Sex refers to sex of the participant.
Figure 2. Plasma cortisol responses to the Dex/CRH test according to Loss Group.
Note: This figure depicts the significant effect of parental loss over time on cortisol concentrations in the repeated measures general linear model (F(5, 193)= 3.16, p=.008; see Table 2 for additional effects in this model).
First, within-group analyses testing the effects of parental care on cortisol over time were performed. These showed a significant effect of parental care over time in the Desertion group (p<.01), a marginal effect in the Death group (p=.06), and no effect in the No Loss group (p=.83). Lower parental care was associated with lower cortisol concentrations in the Loss groups. To further explore this interaction effect between Loss group (a categorical variable) and parental care (a continuous variable), a post-hoc analysis was conducted using a categorical variable that encompassed the loss and care variables as follows. Parental care was dichotomized at the median of 23 for the Loss groups (because the association with parental care was found only in the Loss groups), and participants in each Loss group were categorized as to whether they high or low on the parental care variable (Loss/Care groups: Desertion/Low Care N=16, Desertion/High Care N=9, Death/Low Care N=6, Death/High Care N=13). The No Loss participants were all grouped together; 43 of the 44 scored above the Loss-group median for parental care and the remaining subject scored only one half point below the median (i.e., consistent with a “high care” designation). Analysis of variance (ANOVA) with post-hoc Tukey HSD tests of scores on parental care for these five groups revealed that in addition to the expected significant differences on parental care between the high and low Care groups, the Desertion/Low Care group had significantly lower parental care scores than the Death/Low Care group (Table 4). An additional model testing this grouping as a predictor of cortisol response to the Dex/CRH test controlling for age, sex, and socioeconomic adversity, revealed a significant within-subjects effect over time (F(10, 197)=2.16, p=.02) and a significant between-subjects effect (F(4, 76)=2.72, p=.04). There was no interaction with gender. Figure 3 shows that the Desertion/Low Care group had the lowest cortisol concentrations, and the remaining loss groups had elevated cortisol concentrations in the Dex/CRH test.
Table 4.
Parental care scores according to the Loss/Care grouping.
| PBI Care Scores |
Compound Stress |
|||
|---|---|---|---|---|
| Loss/Care Group | Range | Mean | SD | N (%) |
| No Loss (High Care) (N=44) | 22.5– 36.0 | 30.8 | 3.0 a | 0 |
| Death High Care (N=13) | 23.5– 36.0 | 30.0 | 3.2 a | 1 (8.0) |
| Desertion High Care (N=9) | 24.0– 34.5 | 28.6 | 4.0 a | 1 (11.0) |
| Death Low Care (N=6) | 13.0– 23.0 | 17.8 | 3.6 b | 2 (33.0)* |
| Desertion Low Care (N=16) | 2.5– 19.5 | 12.4 | 6.3 c | 9 (56.0)** |
Note. Care scores represent the average of maternal and paternal care scores on the PBI. The Loss/Care groups were compared using ANOVA with post hoc Tukey HSD tests. Groups with different letters were significantly different from each other. Letters b and c differed from each other at p<.05 and differed from those marked a at p<.001.
Compound Stress was considered present when three or more of eight stressors were reported: multiple loss, foster care, socioeconomic adversity, and reported abuse or neglect on each of the 5 types of maltreatment. Chi square analyses were conducted to compare the Loss/Care groups on Compound Stress. Due to small Ns, the three High Care groups were considered together, and were found to differ from the Death Low Care group (*p<.005) and the Desertion Low Care Group (**p<.001).
Figure 3. Cortisol response to the Dex/CRH test according to Loss/Care grouping.
Note: A repeated measures general linear model controlling for age, sex, and SES revealed a significant within-subjects effect of Loss/Care group over time (F(10, 197)=2.16, p=.02) and a significant between-subjects effect of Loss/Care group (F(4, 76)=2.72, p=.04).
A large proportion of the participants in the Desertion group had multiple stressors, termed “compound stress” in addition to low levels of parental care (Tables 2 and 4), however, an exploratory analysis of the effects of this variable on cortisol response was not significant (p=.24).
Subclinical Symptoms, Stress, and History of Psychiatric Disorder
Table 5 shows the scores on the measures of subclinical symptomatology and perceived stress according to loss group. In comparison with the No Loss group, the Loss groups had significantly higher scores on several scales despite not meeting the criteria for an Axis I psychiatric disorder. Table 6 shows the frequencies of past Axis I disorders according to Loss group. The Parental Death subjects had significantly more lifetime Axis I disorders than the No Loss group (p=.001), but this difference did not reach significance for the Parental Desertion group (p=.06).
Table 5.
Subject characteristics: ratings of subclinical symptoms and stress [Mean (SD)].
| No Loss (N=44) | Death (N= 19) | Desertion (N=25) | |
|---|---|---|---|
| Depressive Symptoms | 6.3 (5.1) | 13.2 (7.9) ‡ | 10.5 (6.6) † |
| State Anxiety | 27.5 (7.1) | 31.9 (8.6) * | 30.2 (8.4) |
| Trait Anxiety | 28.2 (6.2) | 34.5 (8.3) † | 31.8 (8.1) * |
| Perceived Stress | 15.1 (5.9) | 19.8 (9.4) * | 19.1 (6.1) ** |
| Novelty Seeking | 21.1 (4.2) | 20.1 (6.9) | 18.4 (5.2) |
| Harm Avoidance | 8.9 (4.4) | 13.3 (3.7) † | 10.5 (5.8) |
| Reward Dependence | 17.3 (3.5) | 16.0 (3.5) | 14.0 (3.8) * |
| Childhood Maltreatment | 5.3 (0.3) | 7.2 (2.6) ‡ | 10.3 (3.9) ‡ |
| Parental Care | 30.8 (3.0) | 26.2 (6.7) ‡ | 18.2 (9.6) ‡ |
| Parental Overprotection | 8.2 (4.3) | 10.4 (6.5) | 13.0 (8.8) † |
Note: Depressive Symptoms refer to scores on the IDS-SR, State and Trait Anxiety measured with the STAI, Perceived Stress assessed with the PSS, Novelty Seeking, Harm Avoidance, and Reward Dependence assessed with the TPQ, Childhood Maltreatment refers to the CTQ total score, Parental Care and Overprotection measured with the PBI. Symbol denotes significant differences from No Loss group.
p<.05,
p≤.01,
p<.005, and
p≤.001.
The Desertion and Death Groups differed significantly only with respect to Child Maltreatment (p=.001) and Parental Care (p<.005).
Table 6.
History of lifetime Axis I psychiatric disorders [N (%)].
| No Loss (N=44) | Death (N= 19) | Desertion (N=25) | |
|---|---|---|---|
| Major Depressive Disorder | 5 (11.4) | 7 (36.8) | 4 (16) |
| Depressive Disorder NOS | 0 | 0 | 2 (8) |
| Post Traumatic Stress Disorder | 1 (2.3) | 0 | 1 (4) |
| Other Anxiety Disorder | 1 (2.3) | 2 (10.5) | 2 (8) |
| Substance Use Disorder | 3 (6.8) | 2 (10.5) | 3 (12) |
| Adjustment Disorder | 0 | 3 (15.8) | 1 (4) |
| Any Disorder | 7 (15.9) | 11 (57.9) * | 9 (36.0) |
Note: None of the participants had a current Axis I disorder. Due to small Ns, statistical analysis of group differences conducted only for “Any Disorder.” Symbol denotes significant difference from the No Loss group,
p=.001.
There was a trend for the Parental Desertion group to have more lifetime Axis I disorders than the No Loss group but this did not reach significance (p=.06) No other significant differences were found.
Effect of Parental Loss Controlling for Potential Confounds
Additional models were conducted to control for the factors that differed between the groups and might account for the association of parental loss with cortisol response. These models tested the effects of the anxious distress VAS variable, history of Axis I disorder, childhood maltreatment on the CTQ, subclinical depressive symptoms, perceived stress, harm avoidance, novelty seeking, reward dependence, and trait anxiety. The effects of Parental Loss group and parental care remained after controlling for each of these factors.
Given that we included a broad range of loss experiences, further analyses were conducted to determine whether characteristics of the loss, such as age at the time of parental loss, might influence the findings. In repeated measures general linear models, age at loss was not a significant predictor of ACTH or cortisol responses to the test, even after controlling for current age, gender, and socioeconomic adversity (p=.40 and p=.38, respectively). Next, we attempted to make the Parental Death and Desertion groups more homogeneous by including only those subjects with Parental Death due to illness/disease and those with permanent Parental Desertion (see Table 1). The subject who was adopted at age 6 months was also excluded from these analyses since this may have differed from other forms of separation/desertion. The findings for ACTH and cortisol were unchanged, and the effects of the Loss/Care variable also remained. An additional model was tested which excluded the five control subjects with a history of major depression. In this model, there were again no effects of Loss group or parental care on ACTH and the effects and interactions for cortisol, and the effect of the Loss/Care grouping remained.
Finally, we conducted further analyses aimed at determining whether oral contraceptive (OC) use altered our findings. Of the 57 female participants, 25 were taking OCs, including 16 of the 29 (55%) in the control group, five of nine (56%) in the Death High Care group, zero of two females (0%) in the Death Low Care group, one of five females (20%) in the Desertion High Care group, and three of nine (25%) in the Desertion Low Care group. Women using OCs had significantly higher cortisol (p=.01), but not ACTH curves, than women not on OCs. However, after excluding women on OCs, there continued to be no significant effects of Loss group or care on ACTH, and the prediction of cortisol concentration with Loss group and care and their interactions, as well as the Loss/Care variable, remained significant.
DISCUSSION
In this study we found that childhood parental loss is associated with alterations in adult neuroendocrine function and that this effect was moderated by the reported quality of parental relationships. Both childhood parental loss and HPA axis abnormalities have been implicated in the etiology of mood and anxiety disorders. Findings from the current study are consistent with the hypothesis that childhood parental loss may lead to altered neuroendocrine reactivity. The findings were not accounted for by effects of current or past Axis I psychiatric disorders, current depressive or anxious symptomatology, perceived stress, temperament, socioecononomic adversity, mood changes during the test, or reported childhood maltreatment.
The overall finding that parental loss was associated with increased cortisol responses to the Dex/CRH test is consistent with prior reports of increased cortisol concentrations in subjects with a history of childhood parental death (26, 27, 33–36). However studies of other forms of parental separation, most of which have involved institutionalized children, have usually found attenuated cortisol concentrations (28–31). Other forms of childhood adversity have also been linked to both increases (14, 57) and decreases (32, 55) in basal and provoked cortisol concentrations. It has been speculated that characteristics of the stressor such as the type, duration, intensity, or developmental timing may determine the direction of HPA axis abnormalities.
Our findings highlight how the specific nature of the stressor may determine whether cortisol reactivity is exaggerated or attenuated. While overall we found that parental loss was associated with increased cortisol concentrations in the Dex/CRH test, the interaction with parental care indicated a more complex relationship. Our post-hoc analysis of this interaction revealed that subjects with parental desertion and very low levels of parental care showed attenuation of their cortisol response. More than half of our participants with desertion and low care had compound stressors including socioeconomic adversity, multiple losses and foster care, and other forms of childhood maltreatment. This is in contrast to the group with parental death and low care in which there were fewer subjects with compound stress and the levels of care were not as low. The attenuation of cortisol response in subjects with parental desertion and very low levels of parental care is consistent with findings of low basal salivary cortisol concentrations in some studies of orphanages (28–30). An expanding literature in animals and humans indicates that other forms of chronic stress or deprivation may lead to dampened HPA axis reactivity (38, 50–51, 54–55). Although the mechanism for such hypo-reactivity is unknown, it has been postulated that chronic HPA axis activation could lead to counter-regulatory mechanisms such as receptor down-regulation, increased negative feedback sensitivity, and reduced biosynthesis or depletion of hormones at various levels of the HPA axis (38, 52). However, given that we did not find significant effects for ACTH, changes in sensitivity or morphology of the adrenal cortex are more consistent with the present findings.
These findings contrast with those of a study by Luecken (35), in which university students with a history of death of one parent during childhood had increased cortisol levels following a speech stressor only if they reported low levels of care on the PBI by the surviving parent. In addition to the difference in the nature of the HPA challenge used, differences in sample composition are likely to account for these discrepant findings. In the study by Luecken all subjects were university students, parental death but not desertion was studied, and the level of parental care on the PBI did not differ for the loss and control groups.
Our finding that, overall, the neuroendocrine effect of parental loss was greatest in males should be interpreted in light of the fact that in most cases, the loss parent was the father. This is consistent with findings by Flinn and colleagues (1996) in which abnormalities of basal salivary cortisol concentrations were seen in father-absent males, but not females. It is possible that the effect of paternal loss is most stressful for males. Alternatively, males and females may have different patterns of neuroendocrine sequelae of parental loss, with males having alterations of adrenocortical sensitivity, glucocorticoid receptor signaling or other regulatory components of the HPA axis, whereas effects in females could depend on other social or psychological influences. Kendler and colleagues (53) recently found that childhood parental loss was a more potent predictor of risk factors for depressive episodes in males compared to females. Age at the time of parental loss could also influence neuroendocrine and behavioral outcomes, although we did not find a significant effect of age at loss or duration of loss in our analyses. While some investigators have found that the likelihood of developing a mood disorder following childhood parental loss is greater when the loss occurs during early childhood (1), others have not found such an association (6).
Limitations of this study include the possibility that the sample may not be representative of the general population given the modest sample size and the recruitment method which included advertisements for subjects with a history of childhood stress. While childhood neglect may sometimes result from parental death and other forms of maltreatment such as physical and sexual abuse may be linked to some cases of parental desertion, our method of recruitment likely resulted in a higher percent with childhood maltreatment than in the general population. In addition, we included a broad range of loss experiences so that specific effects of more homogeneous experiences may have been obscured. The cross-sectional design and retrospective nature of the measures employed limit our conclusions regarding causal effects of childhood parental loss. It is also possible that other factors, such as additional contextual factors or risk genes which might co-vary with parental loss, could explain our neuroendocrine findings.
In conclusion, results of this study indicate that individuals who experienced childhood parental loss have alterations in cortisol responses to the Dex/CRH test. This study demonstrates the importance of assessing multiple sources of early-life adversity when modeling effects on neuroendocrine function.
Acknowledgments
The authors thank Kelly Colombo, B.A. for her assistance with data management, and John P. Carvalho, B.A., Margaret C. Wyche, B.S., and Sandra B. Tavares, R.N., B.S.N. for their clinical work with research participants, and Carl Sikkema, B.S. for performance of the ACTH assays. Supported by a Young Investigator Award from NARSAD (ART), 1 K23 MH067947 (ART), R01 MH068767-01 (LLC), and the Department of Veterans Affair (CWW).
Footnotes
No therapeutic pharmaceutical or device products were utilized in this research protocol. Acthrel™ was provided at a discounted price by Ferring Pharmaceuticals.
FINANCIAL DISCLOSURES
The authors report the following biomedical financial interests over the last two years. Drs. Tyrka, Price, and Carpenter have received grant/research support from the National Institutes of Health, the United States Department of the Interior, the United States Department of Defense, Sepracor, Pfizer, Cyberonics and Medtronic. Dr. Tyrka has received honoraria for continuing medical education from Wyeth. Dr. Price has received speakers’ bureau honoraria from AstraZeneca and has served as a consultant for Gerson Lehrman, Bolt International, BioVid, Boston Scientific, Wiley, Oxford University Press, and Springer. Dr. Carpenter has served as a consultant or on the advisory board for Abbot, Cyberonics, Novartis, Pfizer, and Wyeth, and has received travel support from Neuronetics. Dr. Carpenter has received honoraria for continuing medical education from Wyeth, and Cyberonics, and spearkers’ bureau honoraria for AstraZeneca, Pfizer, and Cyberonics. Ms. Wier, Ms. Ross, Dr. Wilkinson, and Dr. Anderson report no biomedical financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H, et al. Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry. 1999;4(2):163–172. doi: 10.1038/sj.mp.4000473. [DOI] [PubMed] [Google Scholar]
- 2.Barnes GE, Prosen H. Parental death and depression. J Abnorm Psychol. 1985;94(1):64–69. doi: 10.1037//0021-843x.94.1.64. [DOI] [PubMed] [Google Scholar]
- 3.Birtchnell J. Depression in relation to early and recent parent death. Br J Psychiatry. 1970;116(532):299–306. doi: 10.1192/bjp.116.532.299. [DOI] [PubMed] [Google Scholar]
- 4.Canetti L, Bachar E, Bonne O, Agid O, Lerer B, Kaplan De-Nour A, et al. The impact of parental death versus separation from parents on the mental health of Israeli adolescents. Compr Psychiatry. 2000;41(5):360–368. doi: 10.1053/comp.2000.9002. [DOI] [PubMed] [Google Scholar]
- 5.Harris T, Brown GW, Bifulco A. Loss of parent in childhood and adult psychiatric disorder: the role of lack of adequate parental care. Psychol Med. 1986;16(3):641–659. doi: 10.1017/s0033291700010394. [DOI] [PubMed] [Google Scholar]
- 6.Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. Childhood parental loss and adult psychopathology in women. A twin study perspective. Arch Gen Psychiatry. 1992;49(2):109–116. doi: 10.1001/archpsyc.1992.01820020029004. [DOI] [PubMed] [Google Scholar]
- 7.Kendler KS, Sheth K, Gardner CO, Prescott CA. Childhood parental loss and risk for first-onset of major depression and alcohol dependence: the time-decay of risk and sex differences. Psychol Med. 2002;32(7):1187–1194. doi: 10.1017/s0033291702006219. [DOI] [PubMed] [Google Scholar]
- 8.Oakley Browne MA, Joyce PR, Wells JE, Bushnell JA, Hornblow AR. Disruptions in childhood parental care as risk factors for major depression in adult women. Aust N Z J Psychiatry. 1995;29(3):437–448. doi: 10.3109/00048679509064952. [DOI] [PubMed] [Google Scholar]
- 9.Hafner RJ, Roder MJ. Agoraphobia and parental bereavement. Aust N Z J Psychiatry. 1987;21(3):340–344. doi: 10.3109/00048678709160930. [DOI] [PubMed] [Google Scholar]
- 10.Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160(1):1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 11.Ehlert U, Gaab J, Heinrichs M. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: the role of the hypothalamus-pituitary-adrenal axis. Biol Psychol. 2001;57(1–3):141–152. doi: 10.1016/s0301-0511(01)00092-8. [DOI] [PubMed] [Google Scholar]
- 12.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23(5):477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 13.Rasmusson AM, Lipschitz DS, Wang S, Hu S, Vojvoda D, Bremner JD, et al. Increased pituitary and adrenal reactivity in premenopausal women with posttraumatic stress disorder. Biol Psychiatry. 2001;50(12):965–977. doi: 10.1016/s0006-3223(01)01264-1. [DOI] [PubMed] [Google Scholar]
- 14.Rinne T, de Kloet ER, Wouters L, Goekoop JG, DeRijk RH, van den Brink W. Hyperresponsiveness of hypothalamic-pituitary-adrenal axis to combined dexamethasone/corticotropin-releasing hormone challenge in female borderline personality disorder subjects with a history of sustained childhood abuse. Biol Psychiatry. 2002;52(11):1102–1112. doi: 10.1016/s0006-3223(02)01395-1. [DOI] [PubMed] [Google Scholar]
- 15.Tafet GE, Feder DJ, Abulafia DP, Roffman SS. Regulation of hypothalamic-pituitary-adrenal activity in response to cognitive therapy in patients with generalized anxiety disorder. Cogn Affect Behav Neurosci. 2005;5(1):37–40. doi: 10.3758/cabn.5.1.37. [DOI] [PubMed] [Google Scholar]
- 16.Yehuda R. Clinical relevance of biologic findings in PTSD. Psychiatr Q. 2002;73(2):123–133. doi: 10.1023/a:1015055711424. [DOI] [PubMed] [Google Scholar]
- 17.Young EA, Tolman R, Witkowski K, Kaplan G. Salivary cortisol and posttraumatic stress disorder in a low-income community sample of women. Biol Psychiatry. 2004;55(6):621–626. doi: 10.1016/j.biopsych.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 18.McCleery JM, Goodwin GM. High and low neuroticism predict different cortisol responses to the combined dexamethasone--CRH test. Biol Psychiatry. 2001;49(5):410–415. doi: 10.1016/s0006-3223(00)01056-8. [DOI] [PubMed] [Google Scholar]
- 19.Tyrka AR, Mello AF, Mello MF, Gagne GG, Grover KE, Anderson GM, et al. Temperament and hypothalamic-pituitary-adrenal axis function in healthy adults. Psychoneuroendocrinology. 2006;31(9):1036–1045. doi: 10.1016/j.psyneuen.2006.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tyrka AR, Wier LM, Anderson GM, Wilkinson CW, Price LH, Carpenter LL. Temperament and response to the Trier Social Stress Test. Acta Psychiatr Scand. 2007;115(5):395–402. doi: 10.1111/j.1600-0447.2006.00941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zobel A, Barkow K, Schulze-Rauschenbach S, Von Widdern O, Metten M, Pfeiffer U, et al. High neuroticism and depressive temperament are associated with dysfunctional regulation of the hypothalamic-pituitary-adrenocortical system in healthy volunteers. Acta Psychiatr Scand. 2004;109(5):392–399. doi: 10.1111/j.1600-0447.2004.00313.x. [DOI] [PubMed] [Google Scholar]
- 22.Pryce CR, Ruedi-Bettschen D, Dettling AC, Weston A, Russig H, Ferger B, et al. Long-term effects of early-life environmental manipulations in rodents and primates: Potential animal models in depression research. Neurosci Biobehav Rev. 2005;29(4–5):649–674. doi: 10.1016/j.neubiorev.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Mathew SJ, Coplan JD, Smith EL, Scharf BA, Owens MJ, Nemeroff CB, et al. Cerebrospinal fluid concentrations of biogenic amines and corticotropin-releasing factor in adolescent non-human primates as a function of the timing of adverse early rearing. Stress. 2002;5(3):185–193. doi: 10.1080/1025389021000010521. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol. 2001;13(3):419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- 25.Flinn MV, Quinlan RJ, Decker SA, Turner MT, England BG. Male-Female Differences in Effects of Parental Absence on Glucocorticoid Stress Response. Human Nature. 1996;7(2):125–162. doi: 10.1007/BF02692108. [DOI] [PubMed] [Google Scholar]
- 26.Pfeffer CR, Altemus M, Heo M, Jiang H. Salivary cortisol and psychopathology in children bereaved by the September 11, 2001 terror attacks. Biological Psychiatry. 2007;61(8):957–965. doi: 10.1016/j.biopsych.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 27.Weller EB, Weller RA, Fristad MA, Bowes JM. Dexamethasone suppression test and depressive symptoms in bereaved children: a preliminary report. J Neuropsychiatry Clin Neurosci. 1990;2(4):418–421. doi: 10.1176/jnp.2.4.418. [DOI] [PubMed] [Google Scholar]
- 28.Carlson M, Earls F. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Ann N Y Acad Sci. 1997;807:419–428. doi: 10.1111/j.1749-6632.1997.tb51936.x. [DOI] [PubMed] [Google Scholar]
- 29.Fisher PA, Gunnar MR, Dozier M, Bruce J, Pears KC. Effects of therapeutic interventions for foster children on behavioral problems, caregiver attachment, and stress regulatory neural systems. Ann N Y Acad Sci. 2006;1094:215–225. doi: 10.1196/annals.1376.023. [DOI] [PubMed] [Google Scholar]
- 30.Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from romanian orphanages. Dev Psychopathol. 2001;13(3):611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- 31.Bloch M, Peleg I, Koren D, Aner H, Klein E. Long-term effects of early parental loss due to divorce on the HPA axis. Horm Behav. 2007;51(4):516–523. doi: 10.1016/j.yhbeh.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Meinlschmidt G, Heim C. Decreased cortisol awakening response after early loss experience. Psychoneuroendocrinology. 2005;30(6):568–576. doi: 10.1016/j.psyneuen.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Breier A, Kelsoe JR, Jr, Kirwin PD, Beller SA, Wolkowitz OM, Pickar D. Early parental loss and development of adult psychopathology. Arch Gen Psychiatry. 1988;45(11):987–993. doi: 10.1001/archpsyc.1988.01800350021003. [DOI] [PubMed] [Google Scholar]
- 34.Nicolson NA. Childhood parental loss and cortisol levels in adult men. Psychoneuroendocrinology. 2004;29(8):1012–1018. doi: 10.1016/j.psyneuen.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Luecken L. Parental caring and loss during childhood and adult cortisol responses to stress. Psychology and Health. 2000;15:841–851. [Google Scholar]
- 36.Luecken LJ, Appelhans BM. Early parental loss and salivary cortisol in young adulthood: the moderating role of family environment. Dev Psychopathol. 2006;18(1):295–308. doi: 10.1017/S0954579406060160. [DOI] [PubMed] [Google Scholar]
- 37.Carpenter LL, Tyrka AR, McDougle CJ, Malison RT, Owens MJ, Nemeroff CB, et al. Cerebrospinal fluid corticotropin-releasing factor and perceived early-life stress in depressed patients and healthy control subjects. Neuropsychopharmacology. 2004;29(4):777–784. doi: 10.1038/sj.npp.1300375. [DOI] [PubMed] [Google Scholar]
- 38.Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25(1):1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 39.Heuser I, Yassouridis A, Holsboer F. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. J Psychiatr Res. 1994;28(4):341–356. doi: 10.1016/0022-3956(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 40.First MB, Spitzer RL, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 41.Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. J Am Acad Child Adolesc Psychiatry. 1997;36(3):340–348. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 42.Bernstein DPFL. Childhood trauma questionnaire: A retrospective self-report manual. San Antonio: The Psychological Corporation; 1998. [Google Scholar]
- 43.Parker G, Tupling H, Brown LB. A Parental Bonding Instrument. British Journal of Medical Psychology. 1979;52:1–10. [Google Scholar]
- 44.Parker G. The Parental Bonding Instrument. A decade of research. Soc Psychiatry Psychiatr Epidemiol. 1990;25(6):281–282. doi: 10.1007/BF00782881. [DOI] [PubMed] [Google Scholar]
- 45.Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26(3):477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- 46.Spielberger C. Manual for the State-Trait Anxiety Inventory STAI (Form Y) 1983 [Google Scholar]
- 47.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 48.Cloninger CR, Przybeck TR, Svrakic DM. The Tridimensional Personality Questionnaire: U.S. normative data. Psychol Rep. 1991;69(3 Pt 1):1047–1057. doi: 10.2466/pr0.1991.69.3.1047. [DOI] [PubMed] [Google Scholar]
- 49.Wilkinson CW, Raff H. Comparative evaluation of a new immunoradiometric assay for corticotropin. Clin Chem Lab Med. 2006 doi: 10.1515/CCLM.2006.113. in press. [DOI] [PubMed] [Google Scholar]
- 50.Ladd CO, Owens MJ, Nemeroff CB. Persistent changes in corticotropin-releasing factor neuronal systems induced by maternal deprivation. Endocrinology. 1996;137(4):1212–1218. doi: 10.1210/endo.137.4.8625891. [DOI] [PubMed] [Google Scholar]
- 51.Ostrander MM, Ulrich-Lai YM, Choi DC, Richtand NM, Herman JP. Hypoactivity of the hypothalamo-pituitary-adrenocortical axis during recovery from chronic variable stress. Endocrinology. 2006;147(4):2008–2017. doi: 10.1210/en.2005-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30(10):1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 53.Kendler KS, Baker JH. Genetic influences on measures of the environment: a systematic review. Psychol Med. 2006:1–12. doi: 10.1017/S0033291706009524. [DOI] [PubMed] [Google Scholar]
- 54.Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rythym: Potential indices of risk in human development. Dev Psychopathol. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- 55.Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, et al. Decreased Adrenocorticotropic Hormone and Cortisol Responses to Stress in Healthy Adults Reporting Significant Childhood Maltreatment. Biological Psychiatry. 2007;62(10):1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith N, Lam D, Bifulco A, Checkley S. Childhood Experience of Care and Abuse Questionnaire (CECA.Q) Validation of a screening instrument for childhood adversity in clinical populations. Soc Psychiatry Psychiatr Epidemiol. 2002;37:572–579. doi: 10.1007/s00127-002-0589-9. [DOI] [PubMed] [Google Scholar]
- 57.Heim C, Newport J, Heit S, Graham YP, Wilcox M, Bonsall R, et al. Pituitary-Adrenal and Autonomic Responses to Stress in Women After Sexual and Physical Abuse in Childhood. JAMA. 2000;284(5):592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]