Abstract
OBJECTIVE
The objective of the study was to test the null hypothesis that continuing vigorous weight-bearing exercise throughout pregnancy has no discernible long-term effect on indices of fitness and/or cardiovascular risk.
STUDY DESIGN
This was a follow-up observational study of the fitness and cardiovascular risk profile of 39 women conducted on the General Clinical Research Center at the University of Vermont. Data were analyzed using the paired Student t test, analysis of variance, and linear regression.
RESULTS
Women who voluntarily maintain their exercise regimen during pregnancy continue to exercise over time at a higher level than those who stop. Over time they also gain less weight (3.4 vs 9.9 kg), deposit less fat (2.2 vs 6.7 kg), have increased fitness, and have a lower cardiovascular risk profile than those who stop.
CONCLUSION
Women who continue weight-bearing exercise during pregnancy maintain their long-term fitness and have a low cardiovascular risk profile in the perimenopausal period.
Keywords: cardiovascular risk, exercise, fitness, pregnancy
Although continuing regular weight-bearing exercise throughout pregnancy and adulthood maintains fitness and/or lowers cardiovascular risk,1–6 the specific long-term effects of continuing regular weight-bearing exercise during pregnancy are unknown. The purpose of the current study was to examine this question in a longitudinal study of a cohort of women initially studied serially before, during, and for 1 year after pregnancy 18–20 years ago.3,7–9 The null hypothesis tested was that continuing a vigorous weight-bearing exercise regimen during pregnancy has no discernible long-term effect on indices of fitness and/or cardiovascular risk in the perimenopausal period. Other aspects of long-term outcome were also examined (musculoskeletal, genitourinary, psychosocial, etc) and will be reported separately.
MATERIALS AND METHODS
Study sample
We attempted to contact the first 52 women who participated in our earlier observational studies of weight-bearing exercise (running, aerobics, and cross-country skiing) during pregnancy (26 who continued to exercise regularly throughout pregnancy and 26 who did not).3,7–9 Four were lost to follow-up and 9 decided not to participate.
The data obtained from the remaining 39 women form the basis for this report. All had exercised regularly and had their fitness evaluated prior to and shortly after their index pregnancy that occurred 18–20 years ago. Twenty had continued a vigorous program of weight-bearing exercise throughout their pregnancy (EX group), whereas the remaining 19 either stopped entirely or reduced their overall exercise volume by at least 75% before the 12th week of pregnancy (NOEX group). All resumed a regular recreational exercise program after the pregnancy and 11 women in the EX group had had serial cardiac ultrasound evaluations performed before, during, and after their index pregnancy.10
At the time the original studies were carried out, human subject concerns prevented us using the randomized approach we utilized in recent work.11 Thus, the women self-selected their training regimens both before and during pregnancy, and outcomes were compared between the group of women who continued to exercise at or above 50% of prepregnancy training levels throughout pregnancy and the group of women who stopped.
Study protocol
The 3-day study protocol was approved by the Institutional Review Boards of the University of Vermont and the Metro-Health Medical Center and the Scientific Review Committee of The University of Vermont General Clinical Research Center. Each participant gave her informed written consent prior to participation, and all studies were conducted using the General Clinical Research Center and the athletic facilities at the University of Vermont.
On the first study day, each subject’s height and nude weight were initially determined using a metabolic balance scale and a stadiometer followed by a general history, physical examination, and detailed dietary assessment. Then assessments of flexibility using a sit-and-reach device (Novel Products, Rockton, IL), goniometer, and ruler; body composition using Harpenden skinfold calipers (5-site skinfold thicknesses)12 and a dual-energy x-ray absorptiometer (DEXA) (Lunar, Madison, WI); and a self-assessment of overall body image (on a scale of 1–10) were carried out. Finally, after a 5 to 10 minute warm-up period, maximum aerobic capacity (VO2max) was determined using a modified Astrand, fixed speed, progressive grade, treadmill protocol with a breath-by-breath indirect calorimetry system (Sensor Medics, Yorba Linda, CA) and electrocardiogram monitoring. A detailed description may be found elsewhere.13
All subjects met the following 3 criteria denoting a valid measurement of VO2max: the pulse rate was within ± 5 beats/min of the age-predicted maximum heart rate; the respiratory exchange ratio exceeded 1.05; and there was a plateau in the oxygen consumption over the final minute of the test.
On the second study day, resting heart rate and blood pressure were recorded every 5–10 minutes over a 45 minute time interval using palpation, a stopwatch, a stethoscope, and a mercury manometer zeroed at the level of the left ventricle (midaxillary line). The first and fifth Korotkoff sounds were used to denote systolic and diastolic blood pressure. 14 Mean arterial pressure was calculated as diastolic pressure plus one third of the pulse pressure. During the last 30 minutes, concurrent left ventricular chamber dimensions and wall thickness were estimated according to the guidelines published by the American Society of Echocardiography using ultrasound to obtain a long-axis, real-time view and M-mode tracing with a 5 MHz linear array transducer (Acuson, Malvern, PA).10,15–17 Over that time interval a minimum of 12 and a maximum of 24 separate estimates were obtained at various phases of the respiratory cycle, and average values were used to calculate left ventricular volumes and mass.15–17 The within-subject variability of these parameters around the mean ranged between ± 2% and ± 10%.
End-systolic and end-diastolic volumes were calculated using the Teichholtz equation for a prolate ellipsoid (the product of cube of the dimension and a constant [7.2] divided by the sum of the dimensions and a constant [2.6]).16 Stroke volume was calculated as the difference between end-diastolic and end-systolic volumes; cardiac output as the product of stroke volume and heart rate; and systemic vascular resistance in peripheral vascular resistance units (mean arterial pressure −4 mm Hg divided by cardiac output in milliliters per second). The latter was also converted and expressed in the more commonly used units of dyne per centimeters per second − 5 by multiplying by a constant.
Once the resting measurements were finished, the subjects changed into their exercise apparel, warmed up, and completed a timed 2 mile walk/run on the track of their choice (indoor or outdoor). They were instructed to complete the course as fast as they could, and both split times (first vs second mile) and physical signs of increasing stress were used to assess compliance.
On the third study day, after having eaten a standard 55% carbohydrate, low-glycemic diet for 2.5 days, fasting bloods were drawn for a lipid profile and glucose and insulin levels. This was followed by a 2 hour, 75 g oral glucose tolerance test.
Data analysis
Initially the current values were compared with both population-based norms12,17–19 and the values obtained prior to the index pregnancy using either analysis of variance or a paired t test. Then both the current values and the magnitude of the changes over the 18–20 year interval were compared between the EX and NOEX groups using analysis of variance. Finally, the data were reexamined based on current individual training volumes using both analysis of variance and linear regression to determine whether current exercise performance introduced significant bias. Statistix software (Statistix, Tallahassee, FL) was used for all the analyses. The level of significance was set at P < .05, and all data are presented as the mean ± SEM.
RESULTS
Subjects
The 9 women who declined participation did so for 2 reasons: time constraints and/or embarrassment about their current physical condition. The former was the major reason for all 4 of the women in the EX group, and the latter was the major reason for 4 of the 5 in the NOEX group. The 4 EX women who chose not to participate were not significantly different from the 20 who completed the protocol in terms of prepregnancy weight, current weight, and activity levels at both time points. The 5 NOEX women who chose not to participate were similar to the 19 who completed the protocol in terms of prepregnancy weight and activity but were currently significantly heavier and more sedentary.
Current age and parity were similar in both groups. EX women had a median age of 48 years (mean, 48 ± 1) and a median parity of 2 (range, 1–3). NOEX women had a median age of 49 years (mean, 49 ± 1) and a median parity of 2 (range, 1–3). All were nonsmokers and had no history of other substance abuse other than social ingestion of alcohol in moderation since the index pregnancy. Approximately 80% of the women in both groups continued to work outside the home, and most ate either a mixed or high glycemic diet (about 75%).
Training regimens then and now
Prior to the index pregnancy, more than 75% of the women in both groups ran, cross-country skied, and/or performed aerobics 5 or more times a week for 30 minutes or more each session at an intensity that exceeded 65% of their VO2max. The remainder exercised 3–5 times a week for 30 minutes or more each session at a mean intensity that exceeded 50% of their VO2max. During pregnancy the women in the EX group voluntarily continued to exercise at or above 50% of their prepregnancy exercise volume and all had returned to near prepregnancy exercise levels (greater than 75%) by 6 months after the birth.3–5
Seventy-nine percent of the NOEX group voluntarily stopped completely in the periconceptional period. Another 16% voluntarily stopped completely by the 10th week, and the remaining woman voluntarily reduced her training regimen to less than 25% of her prepregnancy volume by the 10th week. All except 1 woman in the NOEX group had returned to near prepregnancy levels (greater than 75%) by 6 months after the birth.3 She experienced a musculoskeletal injury that delayed her return to prepregnancy exercise levels until 10 months after the birth.
At the time of current study, the women in the EX group exercised at 82 ± 7% of their prepregnancy level, which was significantly higher (P < .01) than that of the women in the NOEX group (52 ± 11%). However, the range of performance in both groups was similar (EX group, 10–150%; NOEX group, 5–125%).
Body composition and body image
Weight prior to the index pregnancy was not significantly different between the EX (60.0 ± 1.7 kg) and the NOEX (60.8 ± 1.7 kg) groups. However, current weight (63.5 ± 2 kg vs 70.8 ± 3.9 kg), change in weight from prepregnancy levels (3.4 ± 0.9 vs 9.9 ± 3.0 kg), and the weight for age percentile (30 ± 4 vs 41 ± 5) were significantly less (P < .05 to < .002) in the EX group. Likewise, prepregnancy body mass indices (BMIs) were not significantly different between groups (21.2 ± 0.5 vs 22.2 ± 0.5 mg/m2) but current BMI (22.1 ± 0.5 vs 25.8 ± 1.1 mg/m2), change in BMI (0.8 ± 0.4 vs 3.6 ± 1.3 mg/m2), and BMI age-adjusted population percentile (22nd ± 3 vs 40th ± 5) were significantly less in the EX group (P < .01).
Current percent body fat estimates by skinfold thicknesses and DEXA were 9% and 8%less in the EX vs the NOEX group (P < .001) as were the age-adjusted percentiles for triceps (13 ± 3 vs 31 ± 6) and subscapular (11 ± 3 vs 41 ± 6) skinfold thicknesses. The calculated change in fat mass (based on 5-site skinfold thicknesses) from prepregnancy levels was also significantly less (P < .004) in the EX group (2.2 ± 0.7 vs 6.7 ± 1.4 kg).
Whole-body, total hip, and femoral neck bone densities were not significantly different between the groups. Age-adjusted percentiles in the EX group were 113 ± 2, 112 ± 2, and 114 ± 3, respectively. They were 112 ± 2, 109 ± 2, and 110 ± 3 in the NOEX group.
Overall self-assessed body image was significantly higher (P < .01) in the EX (7 ± 1) vs the NOEX group (5 ± 1).
Flexibility
All aspects of flexibility were virtually identical in the 2 groups. Total body flexion (sit and reach) was 5 ± 2 cm, and total body extension was 45 ± 4 degrees. Lateral flexion was 37 ± 3 degrees bilaterally, thigh flexion greater than 90 degrees, and thigh extension 35 ± 3 degrees. Upper extremity range of motion and shoulder girdle motion were above average in all subjects.
Cardiopulmonary fitness then and now
Although there were no significant between-group differences in weight-adjusted VO2max values prior to the index pregnancy in the earlier observational study,3,9 there were slight but significant between-group differences (EX group, 54 ± 1 mL/kg per minute, NOEX group, 50 ± 2 mL/kg per minute, P<.02) in the current follow-up of 39 of its earliest participants. However, in these 39 women, there were no between-group differences in either prepregnancy absolute VO2max values (EX group, 3.25 ± 0.86 L/min; NOEX group, 3.08 ± 0.93 L/min) or the age-adjusted VO2max percentiles (EX group, 98th ± 1; NOEX group, 98th ± 1). Thus, despite the between-group difference in weight-adjusted VO2max, all 39 women in the current follow-up sample were extremely fit at the time of their initial prepregnancy evaluation.
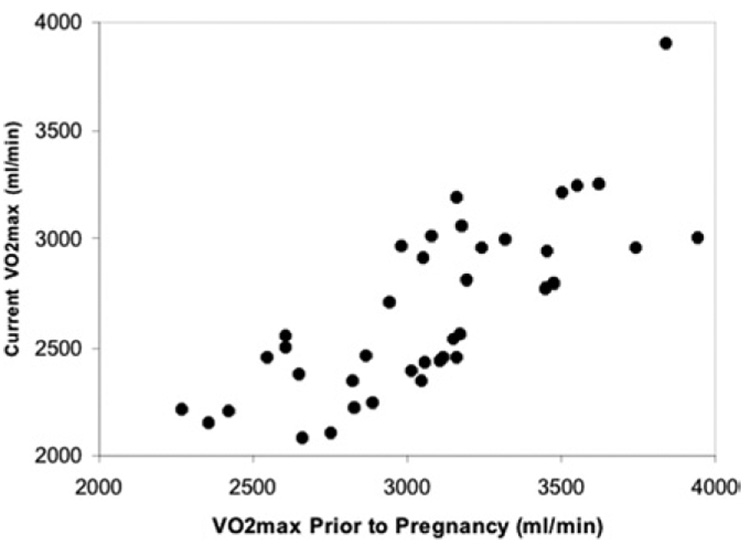
As illustrated in Figure 1, current absolute VO2max values were directly related to that present prior to pregnancy (r2 = 0.6326, P < .0001). The relationship for the entire 39 women is described by the equation: current absolute VO2max ( milliliters per minute) = 282 (milliliters per minute) + 0.775 absolute VO2max value prior to pregnancy (milliliters per minute). There were no significant between-group differences in the change in absolute VO2max over time. The r2 values (EX group, 0.6279; NOEX group, 0.6200), the slope of the relationship (EX group, 0.773; NOEX group, 0.782), the percent change over time (EX group, 13 ± 2%; NOEX group, 13 ± 2%), and the calculated absolute change per year (EX group, 22 ± 3 ml/min per year; NOEX group, 21 ± 3 ml/min per year) were virtually identical.
FIGURE 1. VO2max now and then.
The relationship between absolute VO2max now and that present prior to pregnancy is shown. The equation describing the relationship is y = 282 + 0.775x, and the r2 is 0.6326.
However, because of the large between-group differences in interval weight gain noted in the previous text, the current weight-adjusted VO2max values were significantly higher in the EX group (EX group, 44 ± 1 ml/kg per minute vs NOEX group, 38 ± 1 ml/kg per minute, P ± .002), and the relationship between the weight-adjusted VO2max values then and now was not significant in either group (EX group, r2 = 0.0998, P = .15, and NOEX group, r2 = 0.0120, P=.68). Nonetheless, all the individual weight-corrected values in both groups were well above average, with most women still achieving values that were greater than the 90th percentile for age (EX group, 98th ± 1, and NOEX group, 93rd ± 1). Maximum pulse rates were also similar in the 2 groups (179 ± 2 beats/min and 181 ± 3 beats/min).
Compliance with the 2 mile run instructions were present in all subjects as judged by evidence of extreme fatigue in the last lap and second mile split time, which either equaled or exceeded that for the first. The 2 mile run times were significantly (P < .005) shorter in the EX group (17minutes and 6 seconds ± 46 seconds vs 19 minutes and 44 seconds± 54 seconds). Eight women in each group ran/walked on the indoor track and the remainder on the outdoor track. This between-group balance in performance site ruled out the possibility that environmental differences between the 2 facilities played a role in the significantly faster 2 mile times in the EX group.
Cardiovascular function and risk
Resting heart rate and its age-adjusted percentile were significantly lower (P < .0003) in the EX group (55±1 vs 64±2 beats/min and 15th±2 vs 41st±7), and end diastolic volume was significantly larger (114 ± 4 vs 99 ± 4 mL) in the EX group. However, resting systolic and diastolic blood pressures were identical in the 2 groups (111 and 66 mm Hg), and the small between-group differences in cardiac output (2.29 vs 2.86 L/m2), total peripheral vascular resistance (1500 vs 1535 dyne/cm per second−5), and left ventricular mass (92 vs 82 g/m2) were not significant.
Cholesterol levels and age-adjusted percentiles were significantly lower (P< 0.02) in the EX group (169±4 vs 190± 9 mg/dL and 14th ± 3 vs 36th ± 9). The mean high-density lipoprotein level was identical in the 2 groups (60 mg/dL), so the between-group difference in total cholesterol was entirely due to a significantly lower low-density lipoprotein level in the EX group (93 ± 5 vs 113 ± 6 mg/dL)
Changes in cardiovascular parameters over time in 11 subjects
There were no significant changes from prepregnancy levels over time in resting heart rate (60 ± 1 vs 62 ± 2 beats/min), mean arterial blood pressure (75 ± 2 vs 77 ± 2 mm Hg), or cardiac output (2.43 ± 0.08 vs 2.46 ± 0.1 L/min per square meter). However, left ventricular end-diastolic volume (111 ± 5 vs 102 ± 6 mL) was significantly greater now (P < .01) and the current value for total peripheral resistance (1290±40 vs 1490± 70 dyne/cm per second−5) was significantly less (P < .005) as well.
Parameters of insulin resistance
Glucose and insulin responses to fasting and 75 g of glucose were quite varied in both groups. As a result, the between-group differences were not statistically significant. Fasting glucose and insulin levels in the EX and NOEX groups were 74±3 vs 82±3 mg/dL and 8±1 vs 9± 1 µU/mL, respectively. Likewise the hepatic insulin sensitivity index and the composite insulin sensitivity index in the EX and NOEX groups were 0.77±0.1 vs 0.63 ± 0.08 and 27 ± 6 vs 18 ± 4, respectively.20
Confounding effect of current individual training regimens
As noted earlier, the women who exercised during pregnancy currently exercised at a higher level than those who discontinued exercise during pregnancy. As a result, when the data were reanalyzed based on current individual training regimens, some of the fitness and cardiovascular risk parameters were more favorable in those who had continued to exercise above 50% of their prepregnancy levels. The 50% level was used because that was the training regimen cutoff point used during the index pregnancy to include a woman in the EX group.
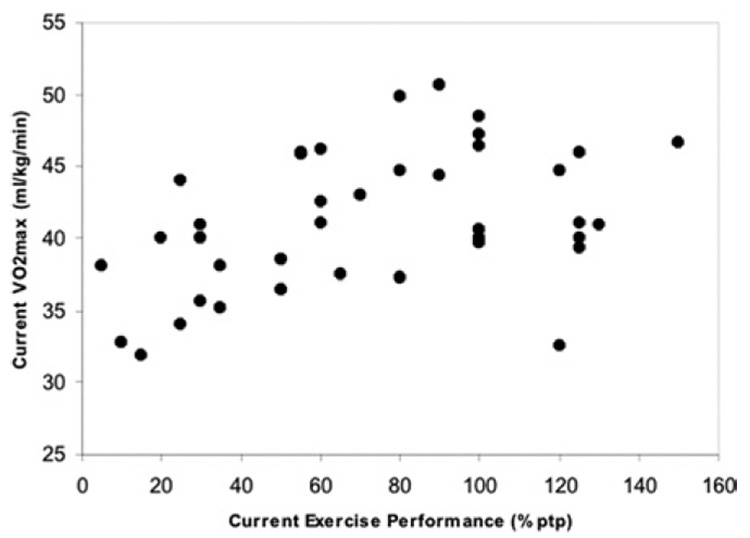
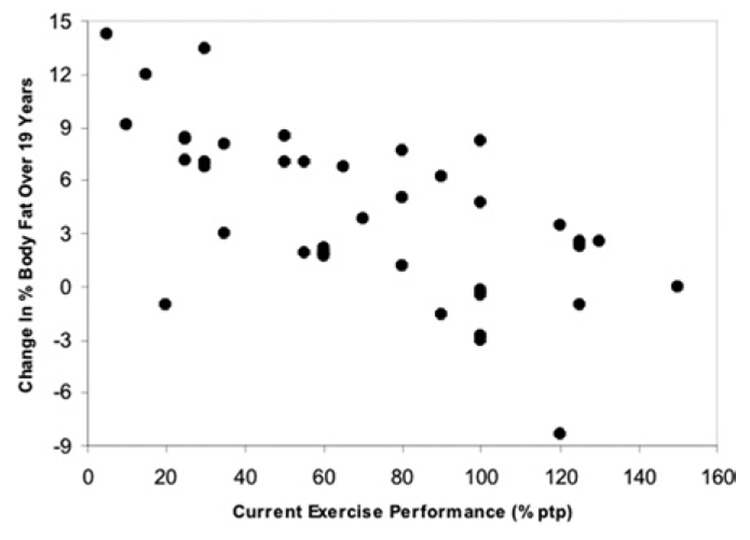
Linear regression of fitness and cardiovascular parameters against current individual training volume (expressed as a percentage of prepregnancy levels) confirmed the confounding role of current individual training volume for many but not all of these differences. Two of these, the relationship between it and both current weight-corrected VO2max (r2 = 0.1563, P < .02) and the change over time in fat mass (r2 = 0.3385, P<.0001), are shown in Figure 2 and Figure 3. Note that the relationship with current weight-adjusted VO2max is truly linear only up to 100% of the training volume performed prior to pregnancy (r2 for these women = 0.3522, P < .0005), whereas the relationship with the change in fat mass over time is linear throughout. There were no between-group differences for the relationship between current training volume and current VO2max, but the relationship with the change in percent body fat over time was significantly higher in the NOEX group (r2 = 0.4763).
FIGURE 2. Current exercise training volume and current weight-adjusted VO2max.
The relationship between current exercise training volume and current weight-adjusted VO2max is shown. Current exercise training volume is expressed as a percentage of the subject’s training volume prior to pregnancy (%ptp). The equation describing the overall relationship is y = 37.8 + 0.048x, and the r2 is 0.1563. However, if only the linear portion of the relationship is analyzed (current exercise volume between 5% and 100%), the r2 is 0.3522.
FIGURE 3. Current exercise training volume and the change in percent body fat over time.
The relationship between current exercise training volume (% ptp) and the change in percent body fat over time is shown. The equation describing the relationship is y = 9.2 – 0.07x, and the r2 is 0.3385.
Current training volume was also related to multiple other fitness parameters. The r2 values for the relationship between current training volume and the changes over time in weight (0.16) and BMI (0.18) were significant (P < .05) as were those for current weight percentile (r2 < 0.34), current percent body fat (r2 = 0.29), current BMI percentile (r2 = 0.17), current skinfold percentile (r2 = 0.23), 2 mile run time (r2 = 0.14), resting heart rate (r2 = 0.18), and left ventricular mass (r2 = 0.11). However, this was not the case for current absolute VO2max, blood pressure, left ventricular end diastolic dimension, total peripheral resistance, total cholesterol, or low-density lipoprotein levels.
COMMENT
These data are the first to describe the long-term fitness characteristics of women who continued to exercise throughout pregnancy, and the findings are similar to those reported for active women in general.1,2 The major new finding is that continuing exercise during pregnancy appears to be a marker that identifies women who spontaneously maintain a fairly vigorous recreational exercise regimen for at least 18–20 years after their first pregnancy. The combination reduces weight gain and fat deposition, maintains fitness, and improves exercise competence during and for 18–20 years after the index pregnancy.3 It also lowers indices of cardiovascular risk relative to both the population at large and women who maintain a lower level of recreational exercise performance later in life. However, the relationship between current exercise volume and many indices of cardiovascular risk was nonlinear, emphasizing that multiple factors other than exercise influence these indices.4,12,19 The same was true for metabolic risk as assessed using multiple indices of insulin sensitivity.
Another important finding is the confirmation2,4,5,19 that continuing even a low-volume, weight-bearing exercise program has benefit when compared with age-adjusted population norms for weight, weight gain, indices of fat deposition, bone density, cardiopulmonary fitness, cardiovascular function, and cardiovascular/metabolic risk. This is strongly supported by the findings that the wide range in current training volume did not alter a fairly consistent rate of fall in absolute VO2max over time in this group of physically active women but that even low exercise volumes reduced weight gain, multiple indices of fat deposition, and 2 mile run time while increasing weight-corrected VO2max.
Finally, the longitudinal data dealing with cardiovascular function and exercise competence indicate that the vascular remodeling and improved exercise competence induced by the hormonal changes of 2 or 3 pregnancies7,9 persists for many years and is associated with a lower cardiovascular risk profile in women who continue to engage in regular weight-bearing exercise during and after their pregnancies. The same does not appear to be true in the populace at large.22,23 The difference between their findings and ours suggests that the combination of several pregnancies and regular weight-bearing exercise enhances long-term cardiovascular function and lowers risk.
The major problem with the current study is that the large differences in current training regimens between the individuals in both the EX and NOEX groups was not anticipated. Therefore, it is impossible to separate the long-term effects of exercise during pregnancy from those of exercise after pregnancy. However, the correlations between current individual training volumes and parameters that reflect fitness and/or cardiovascular function indicate that current training regimens play a significant role. This suggests that the null hypothesis may be correct and that exercise during pregnancy alone has no long-term effect on fitness or cardiovascular risk. Additional studies are underway in an attempt to resolve this issue by studying a more focused NOEX subgroup (NOEX women who resumed and maintained a vigorous weight-bearing exercise program after pregnancy) for comparison.
An additional unanticipated issue is the small but significant between-group difference in prepregnancy VO2max values in the current study sample. As noted in previous text in the Results section, it is unlikely that this small weight-related difference would bias the current results because both groups were extremely fit and decremental changes in absolute VO2max over time were similar in the 2 groups.
In summary, women who continued to exercise throughout pregnancy have maintained their training regimens and level of fitness well above and indices of cardiovascular risk well below those present in both the general populace and women who temporarily stopped exercise during pregnancy.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health Grants HD21268 and RR00109 and funds from MetroHealth Medical Center.
The author thanks the subjects who willingly gave their time and effort to the project. He also thank, Nathan Kokinda, Lee Diveno, Diantha Howard, and the remaining staff of the General Clinical Research Center for their help and support of the project.21
REFERENCES
- 1.Wells CL, Boorman MA, Riggs DM. Effect of age and menopausal status on cardiorespiratory fitness in masters women runners. Med Sci Sports Exerc. 1992;24:1147–1154. [PubMed] [Google Scholar]
- 2.Astrand PO, Bergh U, Kilbom A. A 33-yr follow-up of peak oxygen uptake and related variables of former physical education students. J Appl Physiol. 1997;82:1844–1852. doi: 10.1152/jappl.1997.82.6.1844. [DOI] [PubMed] [Google Scholar]
- 3.Clapp JF. A clinical approach to exercise during pregnancy. Clin Sports Med. 1994;13:443–458. [PubMed] [Google Scholar]
- 4.Pearson TA, Blair SN, Daniels SR, et al. AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update: Consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases. Circulation. 2002;106:388–391. doi: 10.1161/01.cir.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- 5.Dunn AL, Marcus BH, Kampert JB, Garcia ME, Kohl HW, Blair SN. Comparison of lifestyle and structured interventions to increase physical activity and cardiorespiratory fitness. JAMA. 1999;281:327–334. doi: 10.1001/jama.281.4.327. [DOI] [PubMed] [Google Scholar]
- 6.Wolfe LA, Weissgerber TL. Clinical physiology of exercise in pregnancy: a literature review. J Obstet Gynaecol Can. 2003;25:473–483. doi: 10.1016/s1701-2163(16)30309-7. [DOI] [PubMed] [Google Scholar]
- 7.Clapp JF. The effects of maternal exercise on early pregnancy outcome. Am J Obstet Gynecol. 1989;161:1453–1457. doi: 10.1016/0002-9378(89)90903-4. [DOI] [PubMed] [Google Scholar]
- 8.Clapp JF. The course of labor after endurance exercise during pregnancy. Am J Obstet Gynecol. 1990;163:1799–1805. doi: 10.1016/0002-9378(90)90753-t. [DOI] [PubMed] [Google Scholar]
- 9.Clapp JF, Capeless EL. Neonatal morphometrics after endurance exercise during pregnancy. Am J Obstet Gynecol. 1990;163:1805–1811. doi: 10.1016/0002-9378(90)90754-u. [DOI] [PubMed] [Google Scholar]
- 10.Clapp JF, Capeless E. Cardiovascular function before, during and after the first and subsequent pregnancies. Am J Cardiol. 1997;80:1469–1473. doi: 10.1016/s0002-9149(97)00738-8. [DOI] [PubMed] [Google Scholar]
- 11.Clapp JF, Kim H, Burciu B, Schmidt S, Petry K, Lopez B. Continuing regular exercise during pregnancy: Effect of exercise volume on feto-placental growth. Am J Obstet Gynecol. 2002;187:142–147. doi: 10.1067/mob.2002.119109. [DOI] [PubMed] [Google Scholar]
- 12.Golding LA, Myers CR, Sinning WE. Champaign (IL): Human Kinetics; 1989. The Y’s way to physical fitness: The complete guide to fitness testing and instructions; pp. 68–89. [Google Scholar]
- 13.Clapp JF, Capeless EL. The VO2max of recreational athletes before and after pregnancy. Med Sci Sports Exerc. 1991;23:1128–1131. [PubMed] [Google Scholar]
- 14.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure monitoring in humans and experimental animals. Hypertension. 2005;45:142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 15.Devereux RB, Lutas EM, Casale PN, et al. Standardization of M-mode echocardiographic left ventricular anatomic measurements. J Am Coll Cardiol. 1984;4:1222–1230. doi: 10.1016/s0735-1097(84)80141-2. [DOI] [PubMed] [Google Scholar]
- 16.Teichholtz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: Echocardiographic-angiographic correlations in the presence or absence of asynergy. Am J Cardiol. 1976;37:7–11. doi: 10.1016/0002-9149(76)90491-4. [DOI] [PubMed] [Google Scholar]
- 17.Henry WL, Gardin JM, Ware JH. Echocardiographic measurements in normal subjects from infancy to old age. Circulation. 1980;62:1054–1061. doi: 10.1161/01.cir.62.5.1054. [DOI] [PubMed] [Google Scholar]
- 18.McDowell MA, Fryar CD, Hirsch R, Ogden CL. Advance data from vital health statistics; no. 361. Hyattsville (MD): National Center for Health Statistics; 2005. Anthropometric reference data for children and adults: US population, 1999–2002. [Google Scholar]
- 19.Pollock ML, Wilmore JH. Exercise in health and disease. Philadelphia: WB Saunders; 1990. pp. 1–81.pp. 668–669. [Google Scholar]
- 20.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 21.Linne Y, Dye L, Barkeling B, Rossner S. Weight development over time in parous women — the SPAWN study — 5 years follow-up. Int J Obes Relat Metab Disord. 2003;27:1516–1522. doi: 10.1038/sj.ijo.0802441. [DOI] [PubMed] [Google Scholar]
- 22.Sadaniantz A, Saint Laurent L, Parisi AF. Long-term effects of multiple pregnancies on cardiac dimensions and systolic and diastolic function. Am J Obstet Gynecol. 1996;174:1061–1064. doi: 10.1016/s0002-9378(96)70351-4. [DOI] [PubMed] [Google Scholar]
- 23.Ness RB, Harris T, Cobb J, et al. Number of pregnancies and the subsequent risk of cardiovascular disease. N Engl J Med. 1993;328:1528–1533. doi: 10.1056/NEJM199305273282104. [DOI] [PubMed] [Google Scholar]