Abstract
Event-related oscillations (EROs) have proved to be very useful in the understanding of a variety of neurocognitive processes including reward/outcome processing. In the present study, theta power (4.0–7.0 Hz) following outcome stimuli in the time window of the N2-P3 complex (200–500 ms) was analyzed in healthy normals (20 males and 20 females) while performing a gambling task that involved monetary loss and gain. The main aim was to analyze outcome processing in terms of event-related theta power in the context of valence, amount, gender, and impulsivity. The S-transform was used for the signal processing of the ERO data in terms of time-frequency-power. Results from filtered waveforms showed a partially consistent phase-alignment of the increased theta activity corresponding to N2 and P3 components following the outcome stimuli. Gain conditions produced more theta power than loss conditions. While there was anterior involvement in both gain and loss, posterior activation was stronger during gain conditions than during loss conditions. Females exhibited posterior maxima during gain conditions while males had an anterior maxima during both loss and gain conditions. The current source density of theta activity in females involved larger areas of the scalp including a bilateral frontal activity while males predominantly had a frontal midline activity. Theta power was significantly higher in females than males across all conditions. Low theta (4.0–5.5 Hz) predominantly contributed to the posterior activity during gain conditions. High theta (5.5–7.0 Hz) was more associated with impulsivity measures than low theta activity. These findings may offer valuable clues to understand outcome processing, impulsivity, and gender differences.
Keywords: Theta power, Outcome-related negativity, Outcome-related positivity, Error-related negativity, Medial Frontal Negativity, gambling task, impulsivity
1. Introduction
The human electroencephalogram (EEG) consists of the activity of an ensemble of generators producing oscillatory activity in several frequency ranges, and these oscillations are the basic responses of the brain (cf. Başar and Karakaş, 2006). Mountcastle (1992) indicated that the paradigm change introduced by using brain oscillations became one of the most important conceptual and analytic tools for the understanding of cognitive processes (cf. Başar et al., 2007). Cognitive functions consist of neural transactions within and between several brain networks (Fuster, 2006) and are mediated by spatial and temporal synchrony of oscillatory activity over multiple frequency bands (Nunez, 2000). While every frequency band of brain oscillations is shown to have functional significance (Başar, 1999), theta band oscillations have been found to correlate with a variety of behavioral, cognitive, and emotional variables (Knyazev, 2007).
In recent years, the neural mechanisms of outcome evaluation have received considerable attention in cognitive neuroscience (Cohen et al., 2007). Several event-related potential (ERP) studies have used gambling tasks to examine the outcome processing and have identified two major components: outcome-related negativity (ORN), a negative going component around 200–250 ms and outcome-related positivity (ORP), a positive going component at about 300–500 ms (e.g. Gehring and Willoughby, 2002; Nieuwenhuis et al., 2004; Holroyd et al., 2004; Hajcak et al., 2005). However, only a few studies have focused on the frequency characteristics of the ERP components related to outcome processing (Cohen et al., 2007). For instance, Gehring and Willoughby (2004) extracted theta oscillations using the Morlet wavelet transform and found a frontally focused theta (4–7 Hz) activity during a loss condition. In a recent study, Cohen et al. (2007) found that losses, compared to wins, were associated with enhanced power and phase coherence in the theta frequency band, while wins but not losses were modulated by reward probability (i.e. 25%, 50%, and 75%). Analysis of theta responses in these studies were motivated by the consistent findings that error-related negativity (ERN) in the error-paradigms, a closely related component to ORN, reflected activity primarily in the theta (4–7 Hz) frequency range (Gevins et al., 1989; Luu and Tucker, 2001; Luu et al., 2003, 2004; Makeig et al., 2002; Yordanova et al., 2004; Yeung et al., 2007; Trujillo and Allen, 2007; Hall et al., 2007).
In our previous study (Kamarajan et al., under re-review), we examined outcome-related ERP potentials (i.e. ORN and ORP) and gender differences in these components using a Single Outcome Gambling task. While many studies had used a two-choice-two-outcome paradigm based on Gehring and Willoughby (2002), we used a two-choice-single-outcome paradigm. In the two-outcome paradigms, since the outcome was shown for both chosen and unchosen stimuli (amounts), participants subjectively evaluated the ‘net-outcome’ of gain/loss in comparison to the outcome of the unchosen amount. On the other hand, our findings elicited clear distinctions between ERP responses to losses and gains without a confound of ‘relative loss’ and ‘relative gain’, as there was only a single outcome for the chosen amount (Kamarajan et al., under re-review). As the ERP waveform is itself shown to be generated by the superposition of brain oscillations of different frequency bands (Yordanova and Kolev, 1998; Başar, 1999), in the present study, we are extending our analysis to the evaluation of ERO measures, which have several advantages over the traditional ERP methods. The time-frequency characteristics obtained from ERO methods can augment the more traditional defining criteria of an ERP component (Donchin et al., 1978) and can also offer valuable information on specific frequency-dependent cognitive processing.
It is to be noted that the previous research has narrowed down the oscillatory activity associated with error as well as outcome processing specifically to theta band (4–7 Hz) activity, and many studies have suggested that both ERN and ORN are predominantly composed of theta oscillations (e.g. Luu et al., 2003, 2004; Gehring and Willoughby, 2004; Cohen et al., 2007). Makeig et al. (2002), using independent component analysis (ICA), reported that the largest independent contributors to the ERN was in the theta-frequency range. Motivated by Luu and Tucker’s (2001) observation that the ERN was mainly composed of theta response, Gehring and Willoughby (2004) examined a time-frequency representation of outcome processing in a gambling paradigm. They found that the maximal activation areas of theta (4–7 Hz) response at the frontal location extended up to 500 ms for the loss condition. While our earlier ERP study using the same gambling task (Kamarajan et al., under re-review) found that females produced higher amplitudes in both ORN and ORP especially during gain conditions, no study, to our knowledge, has as yet examined the role of gender in the oscillatory response related to outcome processing. Since recent studies have examined oscillatory responses in cognitive tasks with a special emphasis on gender differences (Doppelmayr et al., 2002; Güntekin and Başar, 2007a, 2007b) and since we had already demonstrated a specific gender difference in the ERP components of outcome processing, the current study was designed to focus on gender difference in oscillatory activity during outcome processing. Relating the ERP/ERO measures with impulsivity, Hall et al. (2007) reported that ERN as well as theta response to errors was reduced among high-impulsive or high-externalizing individuals. In a sample of actively drinking treatment-naïve alcoholics, using a Balloon Analogue Risk Task that involves rewards and measures risk-taking propensity, Fein and Chang (2008) reported that smaller feedback ERN amplitudes were associated with a greater family history density of alcohol problems. Further, in our earlier study using a gambling paradigm, we had also reported a strong relationship between task-related impulsivity scores and ERP measures in a gambling task (Kamarajan et al., under re-review). Therefore, as a continuation, the present study aims to examine the role of theta oscillatory responses in reward/outcome processing in the context of gender and impulsivity.
2. Results
2.1. Impulsivity measures
There were no statistically significant differences observed between male and female groups in any of the impulsivity measures as shown in Table 1. However, the canonical correlations elicited significant associations between impulsivity measures (of both BIS and task-related scores) and theta power at different scalp regions (Table 2). Inter-correlations among the variables obtained from correlation matrices (not shown) indicated that impulsivity variables were predominantly negatively correlated with theta power (i.e. higher the impulsivity lower the theta power). As there were several arrays of canonical coefficients and correlation matrices, they were not included here owing to space consideration and were beyond the scope of the present study. Predominantly, theta band power at frontal region and theta2 subband power at both frontal and central regions showed significant correlations. It appears that the significant correlations for the theta broadband are mostly due to the theta2 subband. The only significant correlation observed for the theta1 power was at the left-temporal region during −50 condition. The gain 10 condition (+10) was not significant in any of the regions. Occipital and right-temporal regions in any of the conditions were also not significant.
Table 1.
Comparison of impulsivity scores between male and female groups.
| Variables | Male | Female | F | P | ||
|---|---|---|---|---|---|---|
| M | SD | M | SD | |||
| BIS Total score | 58.95 | 11.83 | 60.15 | 9.25 | 0.13 | 0.72 |
| BIS Non-planning | 21.45 | 4.11 | 22.05 | 4.64 | 0.19 | 0.67 |
| BIS Motor Impulsivity | 23.30 | 8.15 | 22.95 | 3.91 | 0.03 | 0.86 |
| BIS Cognitive Impulsivity | 14.20 | 3.04 | 15.15 | 3.36 | 0.88 | 0.35 |
| Total number of selections/responses | 156.05 | 10.77 | 150.15 | 16.95 | 1.73 | 0.20 |
| Number of Impulsive Responses-1 (IR-1) | 38.10 | 3.60 | 35.90 | 4.99 | 2.56 | 0.12 |
| Number of Impulsive Responses-2 (IR-2) | 18.85 | 6.08 | 16.60 | 4.65 | 1.73 | 0.20 |
| RT of Impulsive Responses-1 (IR-1) | 349.01 | 77.94 | 370.42 | 101.95 | 0.56 | 0.46 |
| RT of Impulsive Responses-2 (IR-2) | 351.81 | 77.10 | 375.43 | 78.34 | 0.92 | 0.34 |
| Mean RT of Impulsive Responses | 362.96 | 80.54 | 380.69 | 90.01 | 0.43 | 0.52 |
| SF for 50 after a losing trend of previous 2 trials | 30.75 | 8.58 | 26.40 | 5.99 | 3.46 | 0.07 |
| SF for 10 after a losing trend of previous 2 trials | 27.55 | 6.83 | 26.60 | 8.04 | 0.16 | 0.69 |
| SF for 50 after a gaining trend of previous 2 trials | 49.40 | 12.70 | 50.05 | 8.49 | 0.04 | 0.85 |
| SF for 10 after a gaining trend of previous 2 trials | 47.35 | 12.79 | 46.10 | 7.87 | 0.14 | 0.71 |
| SF for 50 after a losing trend of previous 3 trials | 37.95 | 11.90 | 34.90 | 6.97 | 0.98 | 0.33 |
| SF for 10 after a losing trend of previous 3 trials | 36.65 | 10.69 | 35.75 | 10.76 | 0.07 | 0.79 |
| SF for 50 after a gaining trend of previous 3 trials | 41.55 | 8.32 | 41.00 | 6.69 | 0.05 | 0.82 |
| SF for 10 after a gaining trend of previous 3 trials | 37.90 | 11.06 | 36.50 | 7.35 | 0.22 | 0.64 |
| SF for 50 after a losing trend of previous 4 trials | 32.55 | 10.03 | 30.00 | 6.06 | 0.95 | 0.34 |
| SF for 10 after a losing trend of previous 4 trials | 30.35 | 8.73 | 27.05 | 9.48 | 1.31 | 0.26 |
| SF for 50 after a gaining trend of previous 4 trials | 46.40 | 11.58 | 45.50 | 8.54 | 0.08 | 0.78 |
| SF for 10 after a gaining trend of previous 4 trials | 43.75 | 13.63 | 44.60 | 8.74 | 0.06 | 0.82 |
| Mean RT following −50 trials | 347.19 | 90.88 | 365.38 | 81.08 | 0.45 | 0.51 |
| Mean RT following −10 trials | 334.33 | 81.34 | 373.23 | 77.36 | 2.40 | 0.13 |
| Mean RT following +50 trials | 350.29 | 81.18 | 371.48 | 76.29 | 0.72 | 0.40 |
| Mean RT following +10 trials | 349.01 | 74.54 | 369.35 | 76.20 | 0.73 | 0.40 |
| Mean RT following loss trials (−50 and −10) | 341.81 | 84.84 | 369.82 | 75.17 | 1.22 | 0.28 |
| Mean RT following gain trials (+50 and +10) | 349.13 | 75.60 | 370.54 | 75.21 | 0.81 | 0.37 |
| Mean RT following amount 50 trials (−50 and +50) | 348.15 | 84.04 | 369.00 | 76.98 | 0.67 | 0.42 |
| Mean RT following amount 10 trials (−10 and +10) | 341.85 | 77.24 | 371.45 | 74.39 | 1.52 | 0.22 |
BIS, Barratt Impulsivity Scale; IR-1, Impulsive response-1 (selecting 50 after a single event of loss); IR-2, Impulsive response-1 (selecting 50 after two consecutive events of loss); RT, Reaction time; SF, Selection frequency (refers to the number of times a particular amount was selected); M – Mean; SD – Standard Deviation.
Table 2.
The results of the canonical correlations between impulsivity measures (BIS and task-related impulsivity) and theta (θ) band (4.0–7.0 Hz) and its subbands – low theta, θ1 (4.0–5.5 Hz) and high theta, θ2 (5.5–7.0 Hz) power during each condition are shown. The p-values were derived from Wilks’ Lambda statistic. Significance level indicates the strength of correlation between theta power in a scalp region and impulsivity measures/scores as a whole.
| Measure | Condition | Frontal | Central | Parietal | Occipital | Left Temporal |
Right Temporal |
|---|---|---|---|---|---|---|---|
| θ band (4.0–7.0 Hz) |
−50 | 0.0375* | 0.11 | 0.13 | 0.18 | 0.0180* | 0.21 |
| −10 | 0.0298* | 0.22 | 0.0388* | 0.21 | 0.26 | 0.95 | |
| +50 | 0.0496* | 0.15 | 0.12 | 0.14 | 0.55 | 0.11 | |
| +10 | 0.53 | 0.38 | 0.26 | 0.11 | 0.57 | 0.20 | |
| θ1 band (4.0–5.5 Hz) |
−50 | 0.27 | 0.33 | 0.41 | 0.12 | 0.0465* | 0.65 |
| −10 | 0.22 | 0.75 | 0.19 | 0.08 | 0.38 | 0.94 | |
| +50 | 0.26 | 0.55 | 0.83 | 0.44 | 0.68 | 0.28 | |
| +10 | 0.89 | 0.62 | 0.38 | 0.06 | 0.40 | 0.81 | |
| θ2 band (5.5–7.0 Hz) |
−50 | 0.0393* | 0.0404* | 0.13 | 0.05 | 0.05 | 0.14 |
| −10 | 0.0155* | 0.0088** | 0.06 | 0.52 | 0.18 | 0.62 | |
| +50 | 0.13 | 0.0224* | 0.0057** | 0.64 | 0.52 | 0.81 | |
| +10 | 0.26 | 0.49 | 0.14 | 0.36 | 0.14 | 0.10 |
p < 0.05
p < 0.01
p < 0.001
2.2. Time course of theta activity
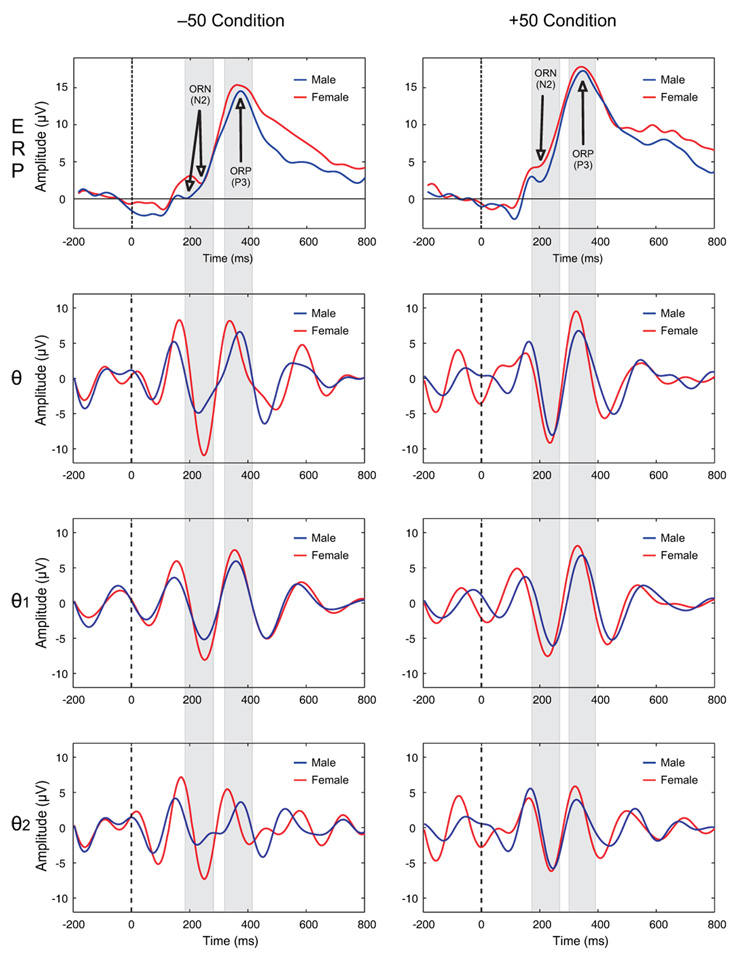
The waveforms of ERPs and corresponding theta bands during −50 and +50 conditions at FCZ electrode are shown in Figure 1. On visual inspection, a partial phase-alignment of the increased theta activity corresponding to N2 and P3 components can be observed. Females showed higher theta amplitude in all theta bands (broadband and subbands) and this difference was more pronounced during −50 condition around 200 ms. While theta power was greatest during the time window of 200–500 ms for both genders, males displayed a marked reduction in both broadband and subbands of theta activity (Figure 2).
Figure 1.
The grand-averaged waveforms of ERPs (1st row) and theta bands (rows 2–4) during −50 and +50 conditions at FCZ electrode. The region of ORN and ORP peaks and the corresponding theta activity during the time window are shaded in gray color. There is a partial phase-alignment of the theta activity corresponding to ORN and ORP components. Females showed higher amplitude in both broadband and subbands of theta activity and this difference was more pronounced during −50 condition around 200 ms. The dashed line represents the onset of an outcome stimulus. Time (in ms) is shown in X-axis while the amplitude (in µV) is represented in Y-axis.
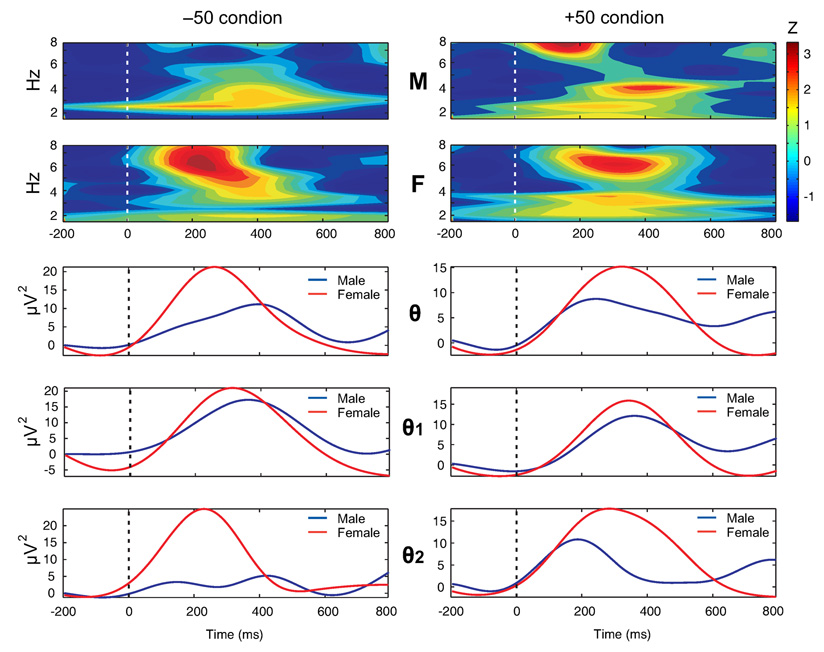
Figure 2.
The time-frequency plots (top 2 rows) and the power-curves obtained from amplitude envelope (bottom 3 rows) for theta bands during −50 (left column) and +50 (right column) conditions at FCZ electrode in male (M) and female (F) groups are shown. The female group had relatively more power in all theta bands. Time (in ms) is represented on the X-axis while power (in µV2) is represented on the Y-axis of the power curves. The color scale (top right) represents power in Z-score. The z-scores were calculated at each frequency (1.0 Hz width) for the epoch length of 1000 ms (200 ms prestimulus plus 800 ms poststimulus). The dashed line represents the onset of an outcome stimulus.
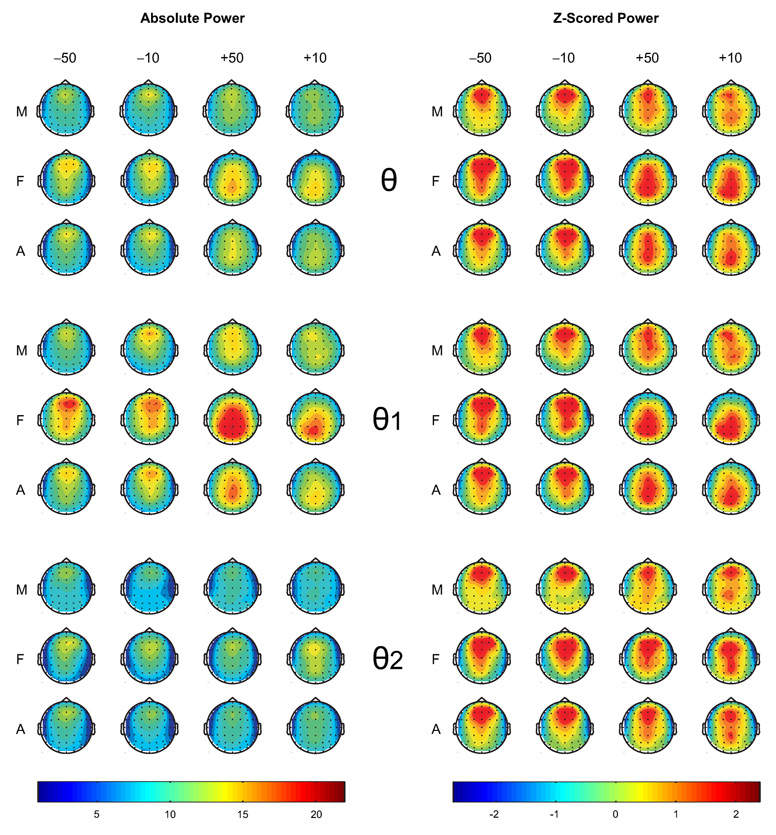
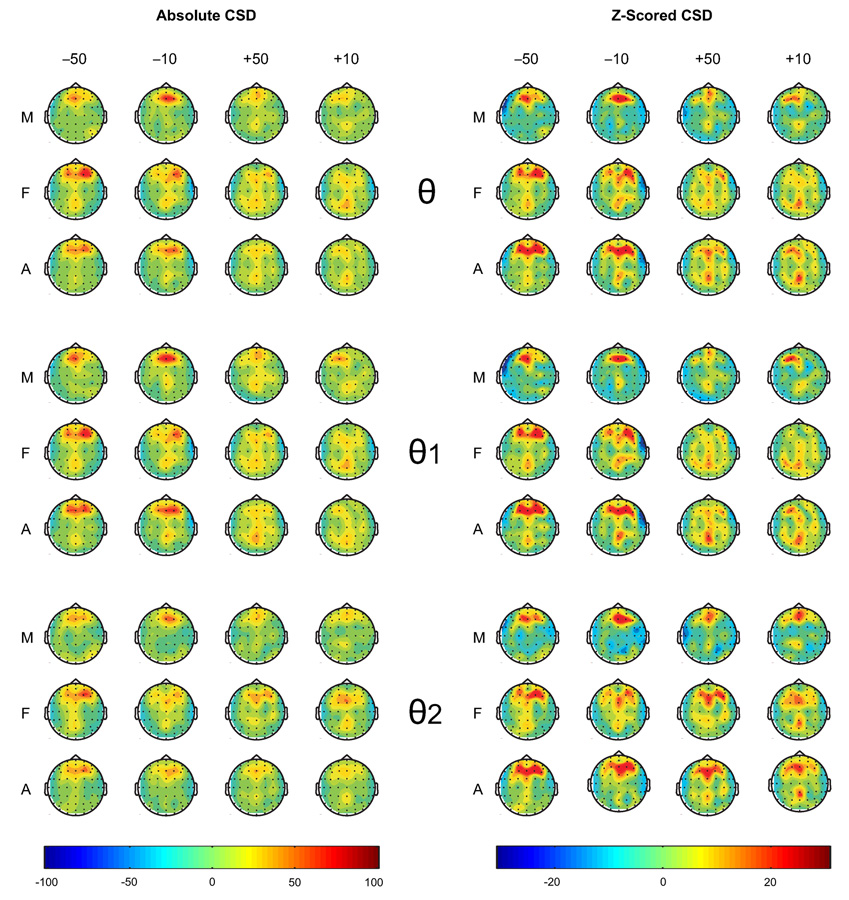
2.3. Topography of theta activity
The topographic maps of theta activity are shown in Figure 3. Visual inspection of the maps revealed that the spread of theta activity was primarily anterior, but was also extended to posterior areas during gain trials in contrast to loss trials. This posterior involvement was more evident in females than in males, especially during gain conditions. In low theta (θ1), the maximum activity was anterior in males and posterior in females during gain conditions. Further, females, in general, had increased theta activity than males in all conditions. The CSD topography of theta bands indicated that frontal and parietal activations were more widespread in females than males during the outcomes of loss and gain (see Figure 4). The involvement of posterior areas was more evident in females than males. The frontal activity in males was more concentrated at the midline while the females had a bilateral frontal activity in all conditions.
Figure 3.
The topographic maps on the left side show absolute theta power (in µV2) and those on the right side are based on the power values that were z-scored within each map for theta power and its subbands. The power values between 200–500 ms during different outcomes (−50, −10, +50, and +10) in males (M), females (F), and all subjects (A) are shown. Females, as compared to males, produced more theta power in each condition in general, and more posterior activity during gain conditions.
Figure 4.
The CSD maps of theta power (µV2/r2, where r = head radius in cm) between 200–500 ms during different outcomes (−50, −10, +50, and +10) in males (M), females (F), and all subjects (A). Females, as compared to males, activated more widespread frontal and parietal areas during the outcomes.
2.4. Mixed model ANOVA
The statistical results of the mixed model ANOVA are shown in Table 3 as well as in Fig. 7 & Fig. 8. The gain condition had significantly higher power than the loss condition in both broadband and subbands of theta activity (i.e. significant main effect for valence, p < 0.0001). Overall, females had significantly higher power in the theta broadband and the theta1 subband as shown by the gender main effects. For the objectives of the present study, 2-way interactions of Gender × Valence and Gender × Amount (Figure 5) as well as 3-way interactions of Gender × Valence × Region and Gender × Amount × Region (Figure 5) were considered.
Table 3.
The F-value, p-value, and significance level of main and interaction effects of theta power for Gender, Valence, Amount, and Region are shown. The statistical significance is marked with asterisks.
| θ band | θ1 band | θ2 band | ||||
|---|---|---|---|---|---|---|
| F | P | F | p | F | p | |
| Gender | 4.20 | 0.0473* | 7.34 | 0.0100* | 1.29 | 0.26 |
| Valence | 102.00 | < 0.0001** | 85.33 | < 0.0001*** | 29.37 | < 0.0001*** |
| Amount | 3.15 | 0.08 | 17.77 | 0.0001*** | 0.81 | 0.37 |
| Region | 199.02 | < 0.0001*** | 212.11 | < 0.0001*** | 135.83 | < 0.0001*** |
| Gender × Valence | 0.57 | 0.45 | 0.06 | 0.80 | 0.32 | 0.58 |
| Gender × Amount | 0.17 | 0.69 | 26.03 | < 0.0001*** | 39.02 | < 0.0001*** |
| Gender × Region | 11.19 | < 0.0001*** | 16.61 | < 0.0001*** | 8.13 | < 0.0001*** |
| Valence × Amount | 0.00 | 0.97 | 29.16 | < 0.0001*** | 16.88 | 0.0002*** |
| Valence × Region | 24.46 | < 0.0001*** | 26.57 | < 0.0001*** | 6.28 | < 0.0001*** |
| Amount × Region | 1.63 | 0.15 | 0.81 | 0.54 | 1.66 | 0.15 |
| Gender × Valence × Amount | 1.62 | 0.21 | 2.79 | 0.10 | 8.16 | 0.0069** |
| Gender × Valence × Region | 10.59 | < 0.0001*** | 10.99 | < 0.0001*** | 3.02 | 0.0119* |
| Gender × Amount × Region | 1.37 | 0.24 | 0.60 | 0.70 | 1.79 | 0.12 |
| Valence × Amount × Region | 1.89 | 0.10 | 4.10 | 0.0015** | 0.37 | 0.87 |
| Electrode (Region) | 17.56 | < 0.0001*** | 13.56 | < 0.0001*** | 11.30 | < 0.0001*** |
p < 0.05
p < 0.01
p < 0.001
Figure 7.
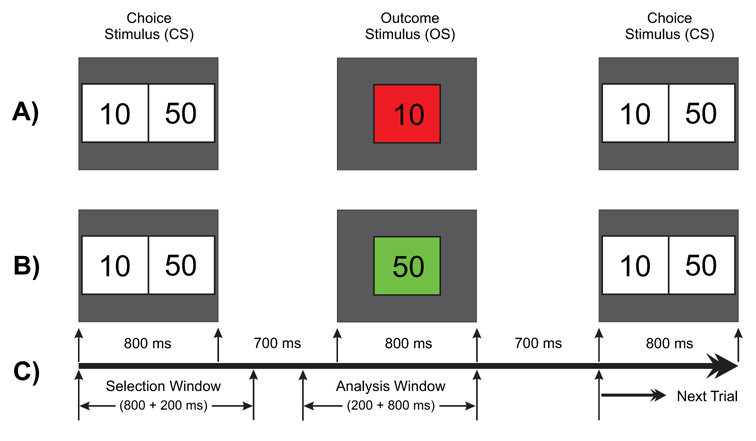
Schematic illustration of the gambling task used in this experiment. One of the two numbers (10 or 50) in the choice stimulus (800 ms) is to be selected by the subject. The selected amount appears as the outcome stimulus (800 ms) either in red (to indicate a loss) or in green (to indicate a gain). A) a typical trial showing a loss of 10 in red box; B) another trial having a gain of 50 in green box; and C) the time duration for the task events: the selection window (1000 ms) wherein the subject selects either of the numbers and the analysis window (200 ms prestimulus + 800 ms poststimulus) represents the time segment that was used for the ERP/ERO analysis.
Figure 8.
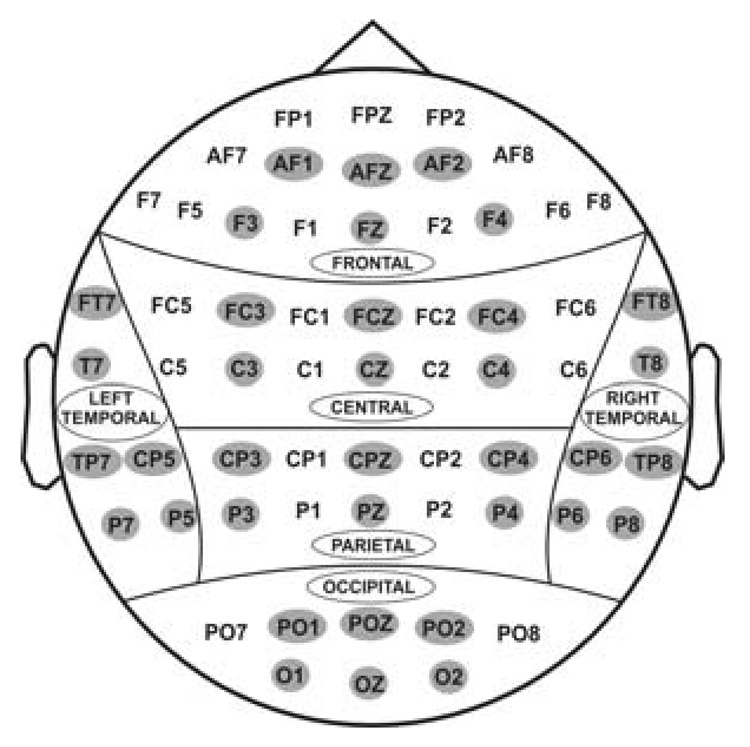
Sixty one electrodes as recorded from the surface of the scalp. For statistical analyses, 36 electrodes (as highlighted) were selected to represent 6 electrodes in 6 regions of the brain viz., frontal, central, parietal, occipital, left-temporal, and right-temporal.
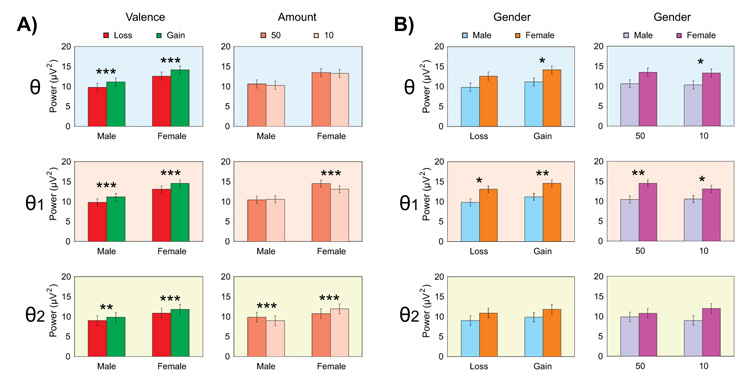
Figure 5.
The bar graphs show the least squares means of the Gender × Valence and Gender × Amount interactions for the within-group (panel-set A) and between-group (panel-set B) comparisons. The significant differences are marked with asterisks (*p < 0.05, **p < 0.01, and ***p < 0.001). The error bars represent 1 Standard Error.
2.4.1. Valence
Gender × Valence interaction was significant neither in the theta broadband nor in the theta subbands while Gender × Valence × Region was significant in both broadband and subbands (Table 3). Overall, the gain condition had significantly higher theta power than the loss condition in broad band and in subbands (Figure 5, 1st column), in several regions in males and females (Panel A, Figure 6) with the exception of frontal region showing an opposite pattern indicating that females had more power for the loss compared to gain (2nd column of panel A in Figure 6). In terms of gender differences for valence (Panel B, Figure 6), females had significantly higher power than males in the loss condition at anterior regions (left column) as well as in the gain conditions at posterior regions (right column).
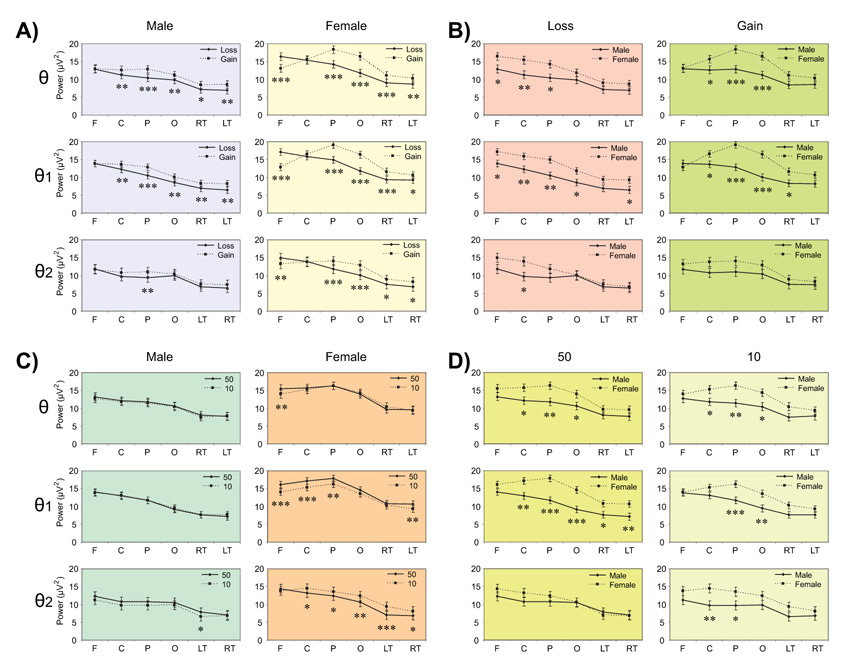
Figure 6.
The line graphs show the pairwise comparisons of least squares means of the Gender × Valence × Region (panel sets A and B) and Gender × Amount × Region (panel sets C and D) interactions in six regions viz. frontal (F), central (C), parietal (P), occipital (O), left-temporal (LT), and right-temporal (RT). There are significant within-group or condition differences within each gender (panel-sets A and C) as well as significant between-group or gender differences in loss and gain (panel-sets B and D). The level of significance is marked with asterisks (*p < 0.05, **p < 0.01, and ***p < 0.001). The error bars represent 1 Standard Error.
2.4.2. Amount
Gender × Amount interaction was highly significant in θ1 and θ2 bands (p < 0.0001), while Gender × Amount × Region was significant neither in broadband nor in subbands. The amount 50¢ had significantly higher power than the amount 10¢ in θ1 band of females as well in θ2 bands of both males and females (2nd column, Figure 5). Females had significantly higher power than males in both amounts for θ1 band, but this difference was not significant for θ2 band (4th column, Figure 5). Regionwise analysis (Figure 6, panel C) showed that only females showed significant differences while processing the amount; amount 50 had higher power than amount 10 in anterior regions in θ1 band, while the reverse was true for posterior regions in θ2 band. Further, gender differences in each amount were more prominent at the parietal region in θ and θ1 bands for both amounts, and at central region in θ2 band only for amount 10 (Figure 6, panel D).
3. Discussion
The main aim of the present study was to analyze outcome processing in terms of event-related theta power during a single outcome gambling task in the context of several factors/dimensions, viz., valence, amount, gender, and impulsivity. The major findings were as follows: 1) in the grand-averaged waveforms, a partial phase-alignment of the increased theta activity corresponding to N2 (200–250 ms) and P3 (300–500 ms) components was observed; 2) gain conditions produced more theta power than loss conditions; 3) in addition to anterior involvement during both gains and losses, posterior areas were more involved during gains than during losses; 4) females exhibited posterior maximum during gains while males primarily exhibited anterior maximum during gains and losses; 5) in the CSD topography, females activated more widespread frontal and parietal areas than males during the outcomes of loss and gain, besides midline frontal activity in males and a bilateral frontal activity in females; 6) there were significant associations between impulsivity measures and theta power at different scalp regions, especially at frontal (θ, θ2 bands) and central (θ2 band) regions; and 7) subdivision of theta band into its subbands (θ1 and θ2) yielded additional information, viz., a) topographically, θ1 primarily contributed to the posterior activity while θ2 mainly involved the anterior activity, b) gender differences across conditions were more robust for θ1 than for θ2, and c) θ2 was more associated with impulsivity measures than θ1.
3.1. Theta activity, ERP components and cognitive processing
The role of theta oscillations in a variety of cognitive processes has been well-documented (e.g. Schacter, 1977; Başar et al., 1999, 2000, 2001, 2006; Başar-Eroğlu and Demiralp, 2001; Kahana et al., 2001; ; Klimesch, 1996, 1999; Klimesch et al., 1996, 1997a, 1997b, 2001a, 2001b, 2005, 2006; Raghavachari et al., 2001, 2006; Luu et al., 2004; Trujillo and Allen, 2007; Cohen et al., 2007). Evidence suggests that increased theta activity (or synchronization) during cognitive/affective processing indicates more active task-related processing while a decrease (or desynchronization) may suggest a weaker/suppressed task processing in several paradigms related to memory (e.g. Klimesch, 1996, 1999; Doppelmayr et al., 1998a, 2000), working memory (e.g. Krause et al., 2000a; Raghavachari et al., 2001, 2006; Schmiedt et al., 2005), creative thinking (e.g. Razumnikova, 2007), intelligence (Doppelmayr et al., 2005), cognitive workload (e.g. Sammer et al., 2007), face perception (Başar et al., 2007), motor planning (Caplan et al., 2003), executive function (Gonzalez-Hernandez et al., 2002), response inhibition/execution (e.g. Kamarajan et al., 2004, 2006), visual target discrimination (Karakaş et al., 2000a; Jones et al., 2006b; Rangaswamy et al., 2007a), stroop effect (Hanslmayr et al., 2008), emotion processing (Krause et al., 2000b; Doppelmayr et al., 2002; Aftanas et al., 2003), error processing (e.g. Luu et al., 2004; Trujillo and Allen, 2007), and outcome processing (Gehring and Willoughby, 2004; Cohen et al., 2007). Our results add support to the phenomenon of theta enhancement during cognitive and/or evaluative processing. In comparison to prestimulus baseline, theta activity markedly increased in the active poststimulus processing of outcome evaluation, especially between 200–500 ms (see Fig. 3 & Fig. 4). A similar time-frequency region of maximal activity of event-related theta has been observed in the gambling paradigm (Gehring and Willoughby, 2004) as well as in the error paradigm (Trujillo and Allen, 2007). Although this activity in both paradigms extended up to 500 ms after the onset of outcome stimulus, theta enhancement, as observed in the time-frequency and waveform representations, commenced much earlier than 200 ms in the error paradigm (Luu et al., 2004; Trujillo and Allen, 2007) and started after 200 ms in the gambling paradigm (Gehring and Willoughby, 2004). The relatively early appearance of theta during the error paradigm can be expalained by the ERP findings that the ERN occurred much earlier than the ORN (Luu et al., 2003; Gehring and Willoughby, 2004).
As shown in Figure 1, the grand-averaged waveforms showed a partially consistent phase-alignment of the increased theta activity in the time window corresponding to N2 and P3 components. An identical phenomenon has been reported in the error-related paradigm wherein the negativity (ERN) and the positivity (Pe) were in line with the negative and positive phases of theta waveform respectively (Luu et al., 2004). Based on the findings from our study and from literature, it is evident that theta activity, to a large extent, forms the basis for both N2 and P3 components of ERPs in different paradigms and modalities. Karakaş et al. (2000a, 2000b) reported that the morphology and the amplitude of both N2 and P3 components are produced by the interplay between the theta and the delta oscillations, i.e. through the principle of superposition (Başar, 1980). Further, Luu et al. (2004) and Trujillo and Allen (2007) suggested that ERN emerges from phase-locking of ongoing theta-band activity. However, although evidence favors the involvement of the theta band in generating these ERP components (Gehring & Willoughby, 2004; Luu & Tucker, 2001; Trujillo & Allen, 2007; Yeung et al., 2007; Cohen et al., 2007), the exact mechanism is still not clear (Cohen et al., 2007; Yeung et al., 2007). According to Yordanova et al. (2002), a theta-dominated state is common across different stimulus modalities and may reflect an active processing stage that is obligatory for stimulus evaluation.
3.2. Theta activity and the processing of loss and gain
A wide variety of cognitive functions occur in the theta frequency range and at frontal locations (Başar et al., 1999; Klimesch et al., 2000) and several ERP studies have specifically attempted to elicit processing differences between losses and gains (Nieuwenhuis et al., 2004, 2005b; Yeung and Sanfey, 2004, Yeung et al., 2005; Hajcak et al., 2005, 2006; Toyomaki and Murohashi, 2005a, 2005b; Holroyd et al., 2006; Masaki et al., 2006). In terms of brain oscillations, Cohen et al. (2007) has provided strong evidence for the difference between the outcomes of losses and gains in terms of theta power and phase coherence. They found that enhanced theta power and phase coherence were more associated with losses than wins, which indicated that loss loomed larger than gains. However, in contrast to Cohen et al’s (2007) study, our results showed that gain conditions predominantly had higher theta power than loss conditions in both males and females, albeit with one exception: frontal theta power in females was higher during the losses than during the gains (see Figure 6, panel-A). That is, our finding suggest that loss looms larger only in females at frontal regions. The difference in findings between our study and Cohen et al’s study may be due to several differences in the task paradigms that were used in these two studies. For example, Cohen et al’s (2007) study had only one amount (0.10 Euros), three different probabilities (25%, 50%, 75%) and one gender (male) whereas our study involved two amounts (10¢, 50¢) with a uniform probability (50%) and both genders. Additionally, the theta activity reported in their study was a baseline adjusted relative measure while our study used absolute theta power. We analyzed the absolute power instead of baseline-adjusted power for several reasons. The relative measures such as event-related desynchronization/synchronization (ERD/ERS) responses are influenced by EEG characteristics in the pre-stimulus interval (Schacter, 1977; Doppelmayr et al., 1998b; Fingelkurts et al., 2002) and reflect EEG changes only in approximately 43% for the theta-oscillations of all trials (Fingelkurts et al., 2002). Further, the “active” baseline during task performance may also involve an attentional component that may be necessary even during the actual stimulus processing or outcome evaluation. Removing the theta activity at the magnitude equal to “non-significant baseline activity” from the “processing-related theta activity” may severely compromise the measurement of actual theta activity during cognitive processing.
In our previous ERP study using the same gambling task, it was found that the gain condition (as compared to the loss condition) had the higher amplitude in both males and females in several brain regions, specifically frontal and central areas for the N2 and central and parietal areas for the P3 component(Kamarajan et al., in revision). In the present study, the phenomenon of “gain greater than loss” has been replicated in theta band responses. The similarity in findings can be explained in the context that the N2-P3 complex largely involves theta band activity (Karakaş et al., 2000a, 2000b) and there could be a strong correlation between ERPs and theta power as evidenced in Cohen et al’s (2007) study. Another interesting finding of our current study is that involvement of posterior areas was more obvious during gains than during losses. The losses were predominantly anteriorly focused in both males and females while the gains were posteriorly focused in females and bi-focal representation of anterior and posterior in males (see Figure 3). In fact, the separation of theta band into θ1 (4.0–5.5 Hz) and θ2 (5.5–7.0 Hz) in our analyses was prompted by these phenomena of “anterior-loss” and “posterior-gain” in order to elicit the relative contribution of posterior activity (especially during the gain conditions). It was found that θ1 primarily contributed to the posterior activity while θ2 mainly involved the anterior activity (Figure 3). Visual inspection of topography reveals that θ2 may exercise an executive control (frontal profile) while θ1 may involve an evaluative control (frontal and parietal profile). It is possible that different task demands are correlated with topographical aspects of theta. For example, Deiber et al. (2007) reported that induced theta activity was recorded in the frontal region for active tasks such as detection and n-back memory tasks, while evoked theta activity (4–8 Hz) phase-locked to the visual stimulus was evidenced in the parietooccipital region for both active and passive tasks. Başar et al. (2006) demonstrated highly increased occipital theta responses in comparison to frontal theta activity during stimulation with angry faces. Further, Sauseng et al (2006) reported a significantly stronger theta coupling (in a range of 4–7 Hz) between prefrontal and posterior regions during trails of task switching and the long-range coherent oscillatory activity in the theta band reflected top-down activation. Regarding the topographic nature of theta activity during different outcomes in gambling paradigms, while there is a paucity of ERO studies, fMRI studies on reward (Nieuwenhuis et al., 2005a) as well as error processing (Marco-Pallares et al., 2007) have revealed a larger network involving multiple brain regions including the striatum, prefrontal cortex, anterior and posterior cingulate, inferior parietal lobule, as well as the midbrain. However, although a distributed network involving a wide variety of brain regions may be involved in the outcome processing as shown by fMRI studies, these findings may not exactly correspond to the millisecond-specific time-dependent oscillatory activity, considering the poor time resolution of imaging techniques (i.e. in seconds). For instance, Nieuwenhuis et al. (2005a), using fMRI, reported the reward sensitive activity between 4 and 8 seconds, while the theta activity in our study was as early as 200–500 ms.
Another interesting finding of the present study is that maximal theta activation (peak) was in the midline electrodes. This finding was not consistent with Gehring & Willoughby’s finding (2004) that frontal theta activity during the loss was lateralized on the right. The difference in the findings can be accounted for by difference in task and signal processing methods used in these tasks; i.e. our task involved a single outcome paradigm and an absolute total theta measure, while Gehring & Willoughby had used two outcomes (of both selected and unselected, which can account for additional frontal input for the relative evaluation) and a prestimulus-adjusted evoked theta measure. However, similar to our findings, both ERP and ERO studies on outcome and error paradigms consistently report a frontal maximal activation during loss and error conditions respectively (Gehring and Willoughby, 2004; Luu et al., 2004; Cohen et al., 2007; Yeung et al., 2007; Kamarajan et al., in revision). The studies using dipole and CSD analyses (e.g. Gehring and Willoughby, 2002; Ruchsow et al., 2002; Luu et al., 2003) as well as fMRI methods (e.g. Ullsperger and von Cramon, 2003) have confirmed the role of anterior cingulate and other medial frontal areas as the source of activation during reward and error processing. Our finding of stronger CSD theta activity at frontal locations during loss conditions (Figure 4) can be compared with Luu et al’s (2004) observation of a frontal (anterior cingulate) CSD activation following errors. On the other hand, during gain conditions, theta activity of both absolute and CSD power involved distributed posterior (or parietal) areas in addition to anterior involvement. This can be explained by the findings that the reward processing network, especially for the positive reward, is much larger to include multiple brain areas including posterior areas (Nieuwenhuis et al., 2005b; Volz et al., 2005).
3.3. Gender differences during outcome processing
There are only a few studies in the literature that have compared event-related brain oscillations between male and female subjects. In the context that brain oscillations are highly influenced by gender differences (Doppelmayr et al., 2002; Güntekin and Başar, 2007a, 2007b), our study has found that females exhibited differences in theta power as well as in topography (Fig. 3–Fig. 8). For example, females had significantly higher theta power than males. Topographically, females had a posterior maximum during gain trials while males exhibited anterior maxima during both gains and losses (Figure 3). In the CSD topography (Figure 4), it was clear that females activated more widespread frontal and parietal areas than males. Further, males displayed largely midline frontal activity while females showed a larger bilateral frontal activation (Figure 4). Doppelmayr et al. (2002) reported gender-related differences in theta power changes of the EEG during the presentation of arousal and emotional stimuli. Güntekin and Başar (2007a) reported that female subjects, compared to males, had higher peak-to-peak amplitude in the delta, beta and gamma frequency ranges at different locations. They did not find any gender difference in the theta band, particulary due to their task involving simple sensory stimulation through a serial presentation of visual stimuli. Further, using a more complex visual processing paradigm involving facial processing, Güntekin and Başar (2007b) reported that occipital beta response (15–24 Hz) was significantly larger for females than for males. Similar gender differences have been reported in several ERP paradigms as well (Orozco and Ehlers, 1998; Orozco et al., 1999; Guillem and Mograss, 2005; Proverbio et al., 2006a, 2006b). According to Hoffman and Polich (1999), females, as compared to males, produce larger P2, N2 and P3 components during an oddball paradigm. In our previous study using the same paradigm reported here, we found a strong gender difference in outcome processing: females produced higher amplitudes in both ORN (N2) and ORP (P3) especially during gain conditions (Kamarajan et al., in revision).
The gender difference in ERP and oscillatory responses for various cognitive paradigms are generally explained through underlying biological mechanisms. For instance, Hoffman and Polich (1999) explained the gender differences in ERP components in terms of differences in callosal size and the resultant inter-hemispheric transmission efficacy. de Courten-Myers (1999) has outlined gender differences in the structure and function of the human cerebral cortex and explained that sexually dimorphic features displayed by the cerebral cortices can be linked to a complex array of gender-specific advantages and limitations in cognitive functions. It has already been established that there are gender-specific differences in various neuropsychological functions, and this accounts for separate norms (Strauss et al., 2006). For example, Vecchi and Girelli (1998) reported gender difference in visuo-spatial abilities in active processing tasks. However, it should be mentioned that in our study, despite significant gender differences in theta activity, there were no significant differences in the impulsivity measures of either BIS or task performance. It is possible that neurocognitive measures such as ERPs and OROs may be more sensitive than the behavioral measures of impulsivity in order to lend a subtle differentiation. For instance, Kamarajan et al. (2006) reported that event-related theta activity was more sensitive than behavioral performance in a Go/NoGo task in differentiating the offspring of alcoholics from controls. Goudriaan et al. (2008) reported that endophenotypical neurocognitive characteristics were more promising in the prediction of relapse in pathological gambling than phenotypical (behavioral) personality characteristics. Previous studies and meta-analyses on gender differences as well as similarities in impulsivity and/or related disorders (Stein et al., 1995; Gaub and Carlson, 1997; Gershon, 2002; Soloff et al., 2003) may help explain our findings. For example, Soloff et al. (2003) concluded that gender differences in central serotonergic function may contribute to variations in impulsivity in borderline personality disorder. It is also possible that different cognitive strategies may be utilized by the genders (Shen, 2005; Frings et al., 2006). For instance, Njemanze (2005) reported that female subjects used a left hemisphere strategy, while males used a right hemisphere strategy to successfully solve tasks in the Raven’s Progressive Matrices. Taken together, difference in theta activity between males and females during outcome processing could reflect one or a combination of the following: a general difference in cognitive functioning, a specific difference in reward/outcome processing, and/or a diffrence in using a cognitive strategy. Only future studies that attempt to elicit the dissociation of different cognitive functions in the context of gender can further enlighten our understanding of gender differences in gross cognitive abilities as well as during specific task processing.
3.4. Impulsivity, theta activity and outcome processing
The present study has elicited significant associations between impulsivity measures and theta power at different scalp regions, especially at frontal and central regions. Analysis of inter-correlations revealed that the impulsivity variables especially the task-related variables (e.g. frequently selecting 50 in the face of losses) were negatively correlated with theta power (i.e. the higher the impulsivity, the lower the theta power). This indicates that theta power has an influential role on impulsivity or cognitive control. There is a vast literature on the role of theta activity in cognitive functions, especially frontal functions (e.g. Demiralp and Başar, 1992; Yordanova and Kolev, 1997; Krause et al., 2000a; Kamarajan et al., 2004; Raghavachari et al., 2006; Deiber et al., 2007; Hanslmayr et al., 2008). The studies that implicate theta oscillations with disorders of impulse control or disinhibition showed increased resting (EEG) theta (Bresnahan et al., 1999; Clarke et al., 2003; ) as well as decreased event-related theta activation (Kamarajan et al., 2004; Kamarajan et al., 2006; Rangaswamy et al., 2007b; Hall et al., 2007). The relationship between theta response and impulsivity/disinhibition can also be explained on the basis of biological/genetic markers (Forbes et al., 2007; Scheres et al., 2007; Strohle et al., 2008; Hall et al., 2007), the genetics of various neurotransmitter system (Porjesz and Rangaswamy, 2007) and the involvement of fronto-limbic circuitry (Luu et al., 2003).
Interestingly, while the loss conditions regardless of the amounts (−50 and −10) and the larger gain (+50) were significantly correlated with impulsivity, the smaller gain (+10) did not show any significant correlation (Table 2). In other words, while the impulsive individuals responded strongly to both bigger and smaller loss and to the bigger gain, they showed a total disregard of smaller gain. This poorly modulated responding for reward is a common diathesis underlying disinhibited behavior in externalizing disorders (alcohol/substance disorders, antisocial personality, ADHD, conduct disorder, impuse-control disorders, and borderline personality) as well as in nonpathological impulsivity (Gorenstein and Newman, 1980). In our earlier ERP study with this gambling paradigm (Kamarajan et al., in revision), we also found that regardless of gender, the subjects with more impulsive responses (e.g. frequently selecting 50 in the face of losses) had lower ORP amplitude, indicating a lack of cognitive control. Failure to learn from aversive feedback (i.e. risk-taking) is one of the characteristics of impulsive individuals (Patterson and Newman, 1993) and of persons with and/or at high-risk for externalizing disorders (Ernst et al., 2003; Slutske et al., 2005; Fein and Chang, 2008). Since our current study has convincingly demonstrated the relationship between anterior theta response and impulsivity, we suggest that frontal theta oscillations can potentially serve as a useful marker to differentiate people with high and low impulsivity. Further, it has already been suggested by Hall et al. (2007) that oscillatory correlates of cognitive control and/or impulsivity may assume a critical importance in identifying/establishing markers for the externalizing disorders associated with elevated impulsivity and disinhibition.
In conclusion, our study demonstrates strong evidence for the view that separate brain processes/circuitry may mediate loss and gain. Significant gender-specific differentiation of oscillatory responses highlights the importance of studying gender in separate groups in future studies. Analysis of oscillatory responses during reward processing in the context of impulsivity and gender may offer important clues to the understanding of the neurocognitive processes in normals and of the pathophysiology in various clinical/disinhibitory conditions. As our study has convincingly demonstrated, separation of the theta band into subbands will further refine the identified phenomena and may yield additional hints to explain outcome processing. Since our study involved a single outcome as a feedback and avoided the contamination of differential evaluation associated with two outcomes, future studies may consider using the single outcome gambling paradigms. It may be a limitation of the present study and a suggestion for future studies that a post-performance interview or a subjective evaluation of participants’ excitement or emotional response after the end of the task might throw further light on the comparison between behavioral and electrophysiological correlates of impulsivity and of outcome processing. It is further suggested that future studies also consider examining both power and coherence during the outcome processing across a wide spectrum of oscillatory responses, in the slower frequency (delta, theta, and alpha) bands as well as in the fast frequency (beta and gamma) bands. As the selectively distributed and selectively coherent oscillations of multiple frequencies are involved during cognitive, affective and motivational processing of the human brain, further analyses involving multiple frequency ranges may offer an integrative approach to understand the brain functions in the context of a given cognitive paradigm.
4. Experimental Procedure
4.1. Participants
The sample consisted of 40 healthy volunteers (20 males and 20 females) within the age range of 18–25 years. The subjects were matched for age and education. The age and education (in terms of mean ± standard deviation in years) were 20.95 ± 2.46 and 12.06 ± 2.34 for males, and 20.00 ± 2.00 and 13.18 ± 1.63 for females, respectively. The participants were recruited through advertisements, and the study was conducted at SUNY Downstate Medical Center at Brooklyn, NY, USA. Individuals with hearing or visual impairment, severe medical (e.g. liver diseases, recent surgery, and chronic pain conditions that may interfere with the performance of the task), neurological (e.g. dementia, delirium, head injury, degenerative diseases, and cerebrovascular diseases/conditions), psychiatric illnesses (e.g. schizophrenia, depression, bipolar disorders, and other psychotic conditions) or drug/alcohol dependence were excluded from the study. Subjects who had major childhood behavioral disorders (e.g. ADHD, conduct disorder, oppositional defiant disorder, and autism) and a family history of major psychiatric illnesses and alcohol/substance dependence were also excluded. Those who were on any medication that could affect the central nervous system at the time of the study were not included. Individuals with severe cognitive deficits based on their score on the mini mental state examination (MMSE) (Folstein et al., 1975) were excluded from the study. Experimental procedures and ethical guidelines were in accordance with approval from the institutional review board (IRB) at SUNY Downstate Medical Center.
4.2. The gambling task
The Single Outcome Gambling task used in the study is illustrated in Figure 7. At the start of each trial, a choice stimulus (CS) with two numbers 10 (left box) and 50 (right box), corresponding to equivalent monetary value in US cents, was displayed for 800 ms. The subject was instructed to select one number by pressing the left button for ‘10’ or the right button for ‘50’. The outcome stimulus (OS) appeared 700 ms after the CS offset and lasted 800 ms. The OS comprised the selected number inside a green box (to indicate a gain) or a red box (to indicate a loss). Thus, there were four possible outcomes: namely, gain 50 (+50), loss 50 (−50), gain 10 (+10), and loss 10 (−10). The subject had to respond by selecting either 10¢ or 50¢ within 1000 ms of CS onset. The OS would not appear if the subject did not respond/select within the specified time (1000 ms), and the next trial would commence. While the occurrence of loss (in red) or gain (in green) in the OS was maintained at equal probability (50%), the order of appearance was pseudo-randomized. The choice was always between 10 and 50 on every trial; 10 always occurred on the left and 50 on the right. The subjects were not made aware of the probability of loss/gain or sequence of the task prior to the experiment. There were a total of 172 trials and the inter-trial interval was 3000 ms throughout the experiment. The task was presented in two blocks with each block (with 86 trials) lasting for 4 minutes; the procedure was identical in both blocks. At the end of each block, the status of overall ‘loss’ or ‘gain’ for the entire block was displayed on the monitor screen. The next block was started by the operator when the subject was ready.
4.3. Measures of impulsivity
All the scores that were derived from impulsivity measures are listed in Table 1. There were two impulsivity measures used in the study: 1) Barratt impulsiveness scale, version 11 (BIS-11) (Barratt, 1985; Patton et al., 1995), a self-rated measure that assesses trait-related impulsivity, and 2) task-related impulsivity scores as derived from the performance of the gambling task. The BIS-11 consists of thirty items yielding a total score, and additional scores for three subcategories: motor impulsivity (acting without thinking), cognitive impulsivity (making decisions quickly), and non-planning (lack of prior planning or of future orientation). The scores were of 3 categories: 1) reaction time for the task conditions and responses, 2) number of impulsive responses (IR), viz. IR-1 (selecting 50 after a single event of loss) and IR-2 (selecting 50 after two consecutive events of loss) and 3) selection frequency that represents the number of times a particular amount (10 or 50) was chosen after a losing or gaining trend in the previous 2 to 4 trials. The gaining and losing trends were computed based on the resultant outcome of the cumulative account of the preceding outcomes. For example, if the previous three outcomes were of +10, +10, and −50, then the trend was considered loss (of 30¢), whereas if the previous three outcomes were −10, −10, and +50 then the trend would be a gain (of 30¢).
4.4. EEG data acquisition and signal analysis
The subjects were comfortably seated in front of the computer monitor placed 1m away. EEG activity was recorded on a Neuroscan system (Version 4.1 & 4.3) using a 61-channel electrode cap which included 19 electrodes of the 10–20 International System and 42 additional electrode sites. The electrodes were referenced to the tip of the nose and the ground electrode was at the forehead (frontal midline). Eye movements were recorded using a supraorbital vertical lead and a horizontal lead on the external canthus of the left eye. Electrode impedance was maintained below 5 kΩ. The EEG signals were recorded continuously with a bandpass at 0.02–100 Hz and amplified 10,000 times using a set of amplifiers (Sensorium, Charlotte, VT). The data consisted of different sampling rates (256, 512, and 500) and were resampled at 256 during the signal analysis.
The ERO data was extracted using the S-tranform method (Stockwell et al., 1996). This method has been applied in several recent studies on alcoholism in our laboratory (Jones et al., 2004, 2006a, 2006b; Kamarajan et al., 2006; Padmanabhapillai et al., 2006a, 2006b; Rangaswamy et al., 2007a). Code to calculate the time-frequency representation (TFR) is available at http://www.cora.nwra.com/~stockwell. To obtain an estimate of event related total power (stimulus onset phase locked plus non-phase locked oscillations), the squared instantaneous amplitudes (or power) of the S-transform TFR were averaged across single trials for each individual. The total power enhances events that occur in a similar time range as related to the stimulus onset, irrespective of their phase relations. Average values were calculated for analysis from the S-transform power TFR within time-frequency regions of interest (TFROI’s) (Lachaux et al., 2003). Specification of the time windows and further sub-windowing of the frequency bands was based on visual inspection of grand mean TFR data and the spatial distribution of grand mean ERO estimates over the scalp.
In the current study, subbands of the theta broadband (4.0–7.0 Hz), viz. low theta – θ1 (4.0–5.5 Hz) and high theta – θ2 (5.5–7.0 Hz) were also examined in order to analyze the specific contribution to the topography of theta activity during the loss and gain conditions. For each task condition, total power was acquired by the average of TFR data in individual trials of 1000 ms (pre-stimulus 200 ms and post-stimulus 800 ms). The filter setting to extract theta bands included a 5th order Chebyshev type I filter (two-step cascade type) with ripple factor (ε) 0.108 and ripple attenuation (Rp) 0.05. The mean theta power values were extracted from the amplitude envelope within the TFROI corresponding to 200–500 ms of post-stimulus time window within which both N2 and P3 components of outcome trials had their peaks (Kamarajan et al., in revision). A minimum response time set for the CS was 100 ms, and the trials with less than 100 ms were rejected as premature responses. Trials exceeding 100 µV were removed as artifacts, and a minimum number of 15 artifact-free trials was required for the analysis.
4.5. Statistical analysis of ERP data
Thirty six electrodes that represented 6 scalp regions (6 electrodes per region) were selected for the statistical analyses (see Figure 8). The theta power data were analyzed by performing a linear mixed model of the Analysis of Variance (ANOVA) using the Statistical Analysis System (SAS, version 9.2, SAS Institute Inc., NC 27513, USA). The values with ±4 standard deviations were considered as outliers and removed from the data before the analysis. The covariance structure used in the model was ‘Compound Symmetry’ which has a constant variance and constant covariance. The model included five factors as fixed effects: Valence (loss, gain), Amount (50¢, 10¢), Region (frontal, central, parietal, occipital, left-temporal, and right-temporal), Electrode (6 electrodes) as within-subjects factors, and Gender as a between-subjects factor. Electrodes were nested within the regions. The pairwise comparisons for each main and interaction effect were calculated using the least means square values.
The data on impulsivity measures (BIS and task-related impulsivity) were compared across genders using Univariate Analysis of Variance (ANOVA). Canonical correlations were performed to analyze the relationship of impulsivity measures with theta power values in each scalp region during each task condition.
Acknowledgements
In memory of Dr. Henri Begleiter, we acknowledge with great admiration his seminal scientific contributions to the field. We are indebted to his charismatic leadership and luminous guidance. We are truly inspired by his visions to carry forward the work he fondly cherished.
This study was supported by the National Institutes of Health (NIH) Grants #5 RO1 AA02686 and AA005524 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). We are grateful for the valuable technical assistance of Carlene Haynes, Joyce Alonzia, Chamion Thomas, Tracy Crippen, Glenn Murawski, Eric Talbert, Patrick Harvey, Cindy Lipper, and Gaby Wurzel.
Abbreviations
- ERO
Event-related Oscillation
- ORN
Outcome-related Negativity
- ORP
Outcome-related Positivity
- ERN
Error-related Negativity
- MFN
Medial Frontal Negativity
- CSD
Current Source Density
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aftanas LI, Pavlov SV, Reva NV, Varlamov AA. Trait anxiety impact on the EEG theta band power changes during appraisal of threatening and pleasant visual stimuli. Int J Psychophysiol. 2003;50(3):205–212. doi: 10.1016/s0167-8760(03)00156-9. [DOI] [PubMed] [Google Scholar]
- Barratt ES. Impulsiveness subtraits: Arousal and information processing. In: Spence JT, Izard CE, editors. Motivation, Emotion and Personality. New York: Elsevier; 1985. pp. 137–146. [Google Scholar]
- Başar-Eroğlu C, Demiralp T. Event-related theta oscillations: an integrative and comparative approach in the human and animal brain. Int J Psychophysiol. 2001;39(2–3):167–195. doi: 10.1016/s0167-8760(00)00140-9. [DOI] [PubMed] [Google Scholar]
- Başar E. EEG brain dynamics: Relation between EEG and brain evoked potentials. New York: Elsevier; 1980. [Google Scholar]
- Başar E. Vol. II: Integrative brain function, neurophysiology and cognitive processes. Berlin: Springer Verlag; 1999. Brain Function and Oscillations. [Google Scholar]
- Başar E, Başar-Eroğlu C, Karakaş S, Schurmann M. Are cognitive processes manifested in event-related gamma, alpha, theta and delta oscillations in the EEG? Neurosci Lett. 1999;259(3):165–168. doi: 10.1016/s0304-3940(98)00934-3. [DOI] [PubMed] [Google Scholar]
- Başar E, Başar-Eroğlu C, Karakaş S, Schurmann M. Brain oscillations in perception and memory. Int J Psychophysiol. 2000;35(2–3):95–124. doi: 10.1016/s0167-8760(99)00047-1. [DOI] [PubMed] [Google Scholar]
- Başar E, Başar-Eroğlu C, Karakaş S, Schurmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol. 2001;39(2–3):241–248. doi: 10.1016/s0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Başar E, Güntekin B, Öniz A. Principles of oscillatory brain dynamics and a treatise of recognition of faces and facial expressions. Prog Brain Res. 2006;159:43–62. doi: 10.1016/S0079-6123(06)59004-1. [DOI] [PubMed] [Google Scholar]
- Başar E, Karakaş S. Neuroscience is awaiting for a breakthrough: an essay bridging the concepts of Descartes, Einstein, Heisenberg, Hebb and Hayek with the explanatory formulations in this special issue. Int J Psychophysiol. 2006;60(2):194–201. doi: 10.1016/j.ijpsycho.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Başar E, ÖzgÖren M, Öniz A, Schmiedt C, Başar-Eroğlu C. Brain oscillations differentiate the picture of one's own grandmother. Int J Psychophysiol. 2007;64(1):81–90. doi: 10.1016/j.ijpsycho.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Bresnahan SM, Anderson JW, Barry RJ. Age-related changes in quantitative EEG in attention-deficit/hyperactivity disorder. Biol Psychiatry. 1999;46(12):1690–1697. doi: 10.1016/s0006-3223(99)00042-6. [DOI] [PubMed] [Google Scholar]
- Caplan JB, Madsen JR, Schulze-Bonhage A, Aschenbrenner-Scheibe R, Newman EL, Kahana MJ. Human theta oscillations related to sensorimotor integration and spatial learning. J Neurosci. 2003;23(11):4726–4736. doi: 10.1523/JNEUROSCI.23-11-04726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, McCarthy R, Selikowitz M, Clarke DC, Croft RJ. Effects of stimulant medications on children with attention-deficit/hyperactivity disorder and excessive beta activity in their EEG. Clin Neurophysiol. 2003;114(9):1729–1737. doi: 10.1016/s1388-2457(03)00112-3. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Elger CE, Ranganath C. Reward expectation modulates feedback-related negativity and EEG spectra. Neuroimage. 2007;35(2):968–978. doi: 10.1016/j.neuroimage.2006.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Courten-Myers GM. The human cerebral cortex: gender differences in structure and function. J Neuropathol Exp Neurol. 1999;58(3):217–226. doi: 10.1097/00005072-199903000-00001. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Missonnier P, Bertrand O, Gold G, Fazio-Costa L, Ibanez V, Giannakopoulos P. Distinction between perceptual and attentional processing in working memory tasks: a study of phase-locked and induced oscillatory brain dynamics. J Cogn Neurosci. 2007;19(1):158–172. doi: 10.1162/jocn.2007.19.1.158. [DOI] [PubMed] [Google Scholar]
- Demiralp T, Başar E. Theta rhythmicities following expected visual and auditory targets. Int J Psychophysiol. 1992;13(2):147–160. doi: 10.1016/0167-8760(92)90054-f. [DOI] [PubMed] [Google Scholar]
- Donchin E, Ritter W, McCallum C. Cognitive psychophysiology: The endogenous components of the ERP. In: Callaway E, Tueting P, Koslow S, editors. Brain event-related potentials in man. New York: Academic Press; 1978. pp. 349–441. [Google Scholar]
- Doppelmayr M, Klimesch W, Sauseng P, Hodlmoser K, Stadler W, Hanslmayr S. Intelligence related differences in EEG-bandpower. Neurosci Lett. 2005;381(3):309–313. doi: 10.1016/j.neulet.2005.02.037. [DOI] [PubMed] [Google Scholar]
- Doppelmayr M, Klimesch W, Schwaiger J, Auinger P, Winkler T. Theta synchronization in the human EEG and episodic retrieval. Neurosci Lett. 1998a;257(1):41–44. doi: 10.1016/s0304-3940(98)00805-2. [DOI] [PubMed] [Google Scholar]
- Doppelmayr M, Klimesch W, Schwaiger J, Stadler W, Rohm D. The time locked theta response reflects interindividual differences in human memory performance. Neurosci Lett. 2000;278(3):141–144. doi: 10.1016/s0304-3940(99)00925-8. [DOI] [PubMed] [Google Scholar]
- Doppelmayr M, Stadler W, Sauseng P, Rachbauer D, Klimesch W. Gender-related differences in theta bandpower changes of the EEG during the presentation of erotic and child related stimuli; Paper presented at the 12th annual conference emotions and the brain; Toronto, Canada. 2002. [Google Scholar]
- Doppelmayr MM, Klimesch W, Pachinger T, Ripper B. The functional significance of absolute power with respect to event-related desynchronization. Brain Topogr. 1998b;11(2):133–140. doi: 10.1023/a:1022206622348. [DOI] [PubMed] [Google Scholar]
- Ernst M, Grant SJ, London ED, Contoreggi CS, Kimes AS, Spurgeon L. Decision making in adolescents with behavior disorders and adults with substance abuse. Am J Psychiatry. 2003;160(1):33–40. doi: 10.1176/appi.ajp.160.1.33. [DOI] [PubMed] [Google Scholar]
- Fein G, Chang M. Smaller feedback ERN amplitudes during the BART are associated with a greater family history density of alcohol problems in treatment-naive alcoholics. Drug and Alcohol Dependence. 2008;92(1–3):141–148. doi: 10.1016/j.drugalcdep.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingelkurts AA, Fingelkurts AA, Krause CM, Sams M. Probability interrelations between pre-/post-stimulus intervals and ERD/ERS during a memory task. Clin Neurophysiol. 2002;113(6):826–843. doi: 10.1016/s1388-2457(02)00058-5. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frings L, Wagner K, Unterrainer J, Spreer J, Halsband U, Schulze-Bonhage A. Gender-related differences in lateralization of hippocampal activation and cognitive strategy. Neuroreport. 2006;17(4):417–421. doi: 10.1097/01.wnr.0000203623.02082.e3. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The cognit: a network model of cortical representation. Int J Psychophysiol. 2006;60(2):125–132. doi: 10.1016/j.ijpsycho.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Gaub M, Carlson CL. Gender differences in ADHD: a meta-analysis and critical review. J Am Acad Child Adolesc Psychiatry. 1997;36(8):1036–1045. doi: 10.1097/00004583-199708000-00011. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295(5563):2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. Are all medial frontal negativities created equal? Toward a richer empirical basis for theories of action monitoring. In: Ullsperger M, Falkenstein M, editors. Errors, Conflicts, and the Brain: Current Opinions on Performance Monitoring. Leipzig: Max Planck Institute of Cognitive Neuroscience; 2004. [Google Scholar]
- Gershon J. A meta-analytic review of gender differences in ADHD. J Atten Disord. 2002;5(3):143–154. doi: 10.1177/108705470200500302. [DOI] [PubMed] [Google Scholar]
- Gevins AS, Cutillo BA, Bressler SL, Morgan NH, White RM, Illes J, Greer DS. Event-related covariances during a bimanual visuomotor task. II. Preparation and feedback. Electroencephalogr Clin Neurophysiol. 1989;74(2):147–160. doi: 10.1016/0168-5597(89)90020-8. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Hernandez JA, Pita-Alcorta C, Cedeno I, Bosch-Bayard J, Galan-Garcia L, Scherbaum WA, Figueredo-Rodriguez P. Wisconsin Card Sorting Test synchronizes the prefrontal, temporal and posterior association cortex in different frequency ranges and extensions. Hum Brain Mapp. 2002;17(1):37–47. doi: 10.1002/hbm.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorenstein EE, Newman JP. Disinhibitory psychopathology: a new perspective and a model for research. Psychol Rev. 1980;87(3):301–315. [PubMed] [Google Scholar]
- Goudriaan AE, Oosterlaan J, De Beurs E, Van Den Brink W. The role of self-reported impulsivity and reward sensitivity versus neurocognitive measures of disinhibition and decision-making in the prediction of relapse in pathological gamblers. Psychol Med. 2008;38(1):41–50. doi: 10.1017/S0033291707000694. [DOI] [PubMed] [Google Scholar]
- Guillem F, Mograss M. Gender differences in memory processing: evidence from event-related potentials to faces. Brain Cogn. 2005;57(1):84–92. doi: 10.1016/j.bandc.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Güntekin B, Başar E. Brain oscillations are highly influenced by gender differences. Int J Psychophysiol. 2007a;65(3):294–299. doi: 10.1016/j.ijpsycho.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Güntekin B, Başar E. Gender differences influence brain's beta oscillatory responses in recognition of facial expressions. Neurosci Lett. 2007b;424(2):94–99. doi: 10.1016/j.neulet.2007.07.052. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Holroyd CB, Moser JS, Simons RF. Brain potentials associated with expected and unexpected good and bad outcomes. Psychophysiology. 2005;42(2):161–170. doi: 10.1111/j.1469-8986.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF. The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biol Psychol. 2006;71(2):148–154. doi: 10.1016/j.biopsycho.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Hall JR, Bernat EM, Patrick CJ. Externalizing psychopathology and the error-related negativity. Psychol Sci. 2007;18(4):326–333. doi: 10.1111/j.1467-9280.2007.01899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S, Pastotter B, Bauml KH, Gruber S, Wimber M, Klimesch W. The electrophysiological dynamics of interference during the Stroop task. J Cogn Neurosci. 2008;20(2):215–225. doi: 10.1162/jocn.2008.20020. [DOI] [PubMed] [Google Scholar]
- Hoffman LD, Polich J. P300, handedness, and corpus callosal size: gender, modality, and task. Int J Psychophysiol. 1999;31(2):163–174. doi: 10.1016/s0167-8760(98)00050-6. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Hajcak G, Larsen JT. The good, the bad and the neutral: Electrophysiological responses to feedback stimuli. Brain Res. 2006 doi: 10.1016/j.brainres.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Larsen JT, Cohen JD. Context dependence of the event-related brain potential associated with reward and punishment. Psychophysiology. 2004;41(2):245–253. doi: 10.1111/j.1469-8986.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Almasy L, Bierut L, Dick D, Goate A, Hinrichs A, Rice JP, Wang JC, Bauer LO, Crowe R, Foroud T, Hesselbrock V, Kuperman S, Nurnberger J, Jr, O'Connor SJ, Rohrbaugh J, Schuckit MA, Tischfield J, Edenberg HJ, Begleiter H. A Cholinergic Receptor Gene (CHRM2) Affects Event-related Oscillations. Behav Genet. 2006a doi: 10.1007/s10519-006-9075-6. [DOI] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Almasy L, Bierut L, Goate A, Wang JC, Dick DM, Hinrichs A, Kwon J, Rice JP, Rohrbaugh J, Stock H, Wu W, Bauer LO, Chorlian DB, Crowe RR, Edenberg HJ, Foroud T, Hesselbrock V, Kuperman S, Nurnberger J, Jr, O'Connor SJ, Schuckit MA, Stimus AT, Tischfield JA, Reich T, Begleiter H. Linkage and linkage disequilibrium of evoked EEG oscillations with CHRM2 receptor gene polymorphisms: implications for human brain dynamics and cognition. Int J Psychophysiol. 2004;53(2):75–90. doi: 10.1016/j.ijpsycho.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Chorlian D, Rangaswamy M, Kamarajan C, Padmanabhapillai A, Stimus A, Begleiter H. S-transform time-frequency analysis of P300 reveals deficits in individuals diagnosed with alcoholism. Clin Neurophysiol. 2006b;117(10):2128–2143. doi: 10.1016/j.clinph.2006.02.028. [DOI] [PubMed] [Google Scholar]
- Kahana MJ, Seelig D, Madsen JR. Theta returns. Curr Opin Neurobiol. 2001;11(6):739–744. doi: 10.1016/s0959-4388(01)00278-1. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones K, Chorlian D, Padmanabhapillai A, Rangaswamy M, Stimus A, Begleiter H. Event-related oscillations in offspring of alcoholics: neurocognitive disinhibition as a risk for alcoholism. Biol Psychiatry. 2006;59(7):625–634. doi: 10.1016/j.biopsych.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, Rangaswamy M, Stimus AT, Begleiter H. The role of brain oscillations as functional correlates of cognitive systems: a study of frontal inhibitory control in alcoholism. Int J Psychophysiol. 2004;51(2):155–180. doi: 10.1016/j.ijpsycho.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Rangaswamy M, Tang Y, Chorlian DB, Padmanabhapillai A, Pandey AK, Roopesh BN, Saunders R, Manz N, Stimus AT, Begleiter H. Brain signatures of monetary loss and gain: Outcome-related potentials in a single outcome gambling task. Behav Brain Res. doi: 10.1016/j.bbr.2008.08.011. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakaş S, Erzengin OU, Başar E. The genesis of human event-related responses explained through the theory of oscillatory neural assemblies. Neurosci Lett. 2000a;285(1):45–48. doi: 10.1016/s0304-3940(00)01022-3. [DOI] [PubMed] [Google Scholar]
- Karakaş S, Erzengin OU, Başar E. A new strategy involving multiple cognitive paradigms demonstrates that ERP components are determined by the superposition of oscillatory responses. Clin Neurophysiol. 2000b;111(10):1719–1732. doi: 10.1016/s1388-2457(00)00418-1. [DOI] [PubMed] [Google Scholar]
- Klimesch W. Memory processes, brain oscillations and EEG synchronization. Int J Psychophysiol. 1996;24(1–2):61–100. doi: 10.1016/s0167-8760(96)00057-8. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev. 1999;29(2–3):169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Pachinger T, Ripper B. Brain oscillations and human memory: EEG correlates in the upper alpha and theta band. Neurosci Lett. 1997a;238(1–2):9–12. doi: 10.1016/s0304-3940(97)00771-4. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Russegger H, Pachinger T. Theta band power in the human scalp EEG and the encoding of new information. Neuroreport. 1996;7(7):1235–1240. doi: 10.1097/00001756-199605170-00002. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schimke H, Ripper B. Theta synchronization and alpha desynchronization in a memory task. Psychophysiology. 1997b;34(2):169–176. doi: 10.1111/j.1469-8986.1997.tb02128.x. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schwaiger J, Winkler T, Gruber W. Theta oscillations and the ERP old/new effect: independent phenomena? Clin Neurophysiol. 2000;111(5):781–793. doi: 10.1016/s1388-2457(00)00254-6. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Wimmer H, Schwaiger J, Rohm D, Gruber W, Hutzler F. Theta band power changes in normal and dyslexic children. Clin Neurophysiol. 2001a;112(7):1174–1185. doi: 10.1016/s1388-2457(01)00545-4. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Yonelinas A, Kroll NE, Lazzara M, Rohm D, Gruber W. Theta synchronization during episodic retrieval: neural correlates of conscious awareness. Brain Res Cogn Brain Res. 2001b;12(1):33–38. doi: 10.1016/s0926-6410(01)00024-6. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Hanslmayr S, Sauseng P, Gruber W, Brozinsky CJ, Kroll NE, Yonelinas AP, Doppelmayr M. Oscillatory EEG correlates of episodic trace decay. Cereb Cortex. 2006;16(2):280–290. doi: 10.1093/cercor/bhi107. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schack B, Sauseng P. The functional significance of theta and upper alpha oscillations. Exp Psychol. 2005;52(2):99–108. doi: 10.1027/1618-3169.52.2.99. [DOI] [PubMed] [Google Scholar]
- Knyazev GG. Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neurosci Biobehav Rev. 2007;31(3):377–395. doi: 10.1016/j.neubiorev.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Krause CM, Sillanmaki L, Koivisto M, Saarela C, Haggqvist A, Laine M, Hamalainen H. The effects of memory load on event-related EEG desynchronization and synchronization. Clin Neurophysiol. 2000a;111(11):2071–2078. doi: 10.1016/s1388-2457(00)00429-6. [DOI] [PubMed] [Google Scholar]
- Krause CM, Viemero V, Rosenqvist A, Sillanmaki L, Astrom T. Relative electroencephalographic desynchronization and synchronization in humans to emotional film content: an analysis of the 4–6, 6–8, 8–10 and 10–12 Hz frequency bands. Neurosci Lett. 2000b;286(1):9–12. doi: 10.1016/s0304-3940(00)01092-2. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Chavez M, Lutz A. A simple measure of correlation across time, frequency and space between continuous brain signals. J Neurosci Methods. 2003;123(2):175–188. doi: 10.1016/s0165-0270(02)00358-8. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM. Regulating action: alternating activation of midline frontal and motor cortical networks. Clin Neurophysiol. 2001;112(7):1295–1306. doi: 10.1016/s1388-2457(01)00559-4. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychol Sci. 2003;14(1):47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Makeig S. Frontal midline theta and the error-related negativity: neurophysiological mechanisms of action regulation. Clin Neurophysiol. 2004;115(8):1821–1835. doi: 10.1016/j.clinph.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Makeig S, Westerfield M, Jung TP, Enghoff S, Townsend J, Courchesne E, Sejnowski TJ. Dynamic brain sources of visual evoked responses. Science. 2002;295(5555):690–694. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- Marco-Pallares J, Muller SV, Munte TF. Learning by doing: an fMRI study of feedback-related brain activations. Neuroreport. 2007;18(14):1423–1426. doi: 10.1097/WNR.0b013e3282e9a58c. [DOI] [PubMed] [Google Scholar]
- Masaki H, Takeuchi S, Gehring WJ, Takasawa N, Yamazaki K. Affective-motivational influences on feedback-related ERPs in a gambling task. Brain Res. 2006;1105(1):110–121. doi: 10.1016/j.brainres.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. Preface. In: Başar E, Bullock TH, editors. Induced Rhythms in the Brain. Boston, MA: Birkhäuser; 1992. pp. 217–231. [Google Scholar]
- Nieuwenhuis S, Heslenfeld DJ, von Geusau NJ, Mars RB, Holroyd CB, Yeung N. Activity in human reward-sensitive brain areas is strongly context dependent. Neuroimage. 2005a;25(4):1302–1309. doi: 10.1016/j.neuroimage.2004.12.043. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Slagter HA, von Geusau NJ, Heslenfeld DJ, Holroyd CB. Knowing good from bad: differential activation of human cortical areas by positive and negative outcomes. Eur J Neurosci. 2005b;21(11):3161–3168. doi: 10.1111/j.1460-9568.2005.04152.x. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, Holroyd CB, Schurger A, Cohen JD. Sensitivity of electrophysiological activity from medial frontal cortex to utilitarian and performance feedback. Cereb Cortex. 2004;14(7):741–747. doi: 10.1093/cercor/bhh034. [DOI] [PubMed] [Google Scholar]
- Njemanze PC. Cerebral lateralization and general intelligence: gender differences in a transcranial Doppler study. Brain Lang. 2005;92(3):234–239. doi: 10.1016/j.bandl.2004.06.104. [DOI] [PubMed] [Google Scholar]
- Nunez PL. Toward a quantitative description of large-scale neocortical dynamic function and EEG. Behav Brain Sci. 2000;23(3):371–398. doi: 10.1017/s0140525x00003253. discussion 399–437. [DOI] [PubMed] [Google Scholar]
- Orozco S, Ehlers CL. Gender differences in electrophysiological responses to facial stimuli. Biol Psychiatry. 1998;44(4):281–289. doi: 10.1016/s0006-3223(97)00487-3. [DOI] [PubMed] [Google Scholar]
- Orozco S, Wall TL, Ehlers CL. Influence of alcohol on electrophysiological responses to facial stimuli. Alcohol. 1999;18(1):11–16. doi: 10.1016/s0741-8329(98)00061-5. [DOI] [PubMed] [Google Scholar]
- Padmanabhapillai A, Porjesz B, Ranganathan M, Jones KA, Chorlian DB, Tang Y, Kamarajan C, Rangaswamy M, Stimus A, Begleiter H. Suppression of early evoked gamma band response in male alcoholics during a visual oddball task. Int J Psychophysiol. 2006a;60(1):15–26. doi: 10.1016/j.ijpsycho.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Padmanabhapillai A, Tang Y, Ranganathan M, Rangaswamy M, Jones KA, Chorlian DB, Kamarajan C, Stimus A, Kuperman S, Rohrbaugh J, O'Connor SJ, Bauer LO, Schuckit MA, Begleiter H, Porjesz B. Evoked gamma band response in male adolescent subjects at high risk for alcoholism during a visual oddball task. Int J Psychophysiol. 2006b;62(2):262–271. doi: 10.1016/j.ijpsycho.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Patterson CM, Newman JP. Reflectivity and learning from aversive events: toward a psychological mechanism for the syndromes of disinhibition. Psychol Rev. 1993;100(4):716–736. doi: 10.1037/0033-295x.100.4.716. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51(6):768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M. Neurophysiological endophenotypes, CNS disinhibition, and risk for alcohol dependence and related disorders. ScientificWorldJournal. 2007;7:131–141. doi: 10.1100/tsw.2007.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proverbio AM, Brignone V, Matarazzo S, Del Zotto M, Zani A. Gender and parental status affect the visual cortical response to infant facial expression. Neuropsychologia. 2006a;44(14):2987–2999. doi: 10.1016/j.neuropsychologia.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Proverbio AM, Brignone V, Matarazzo S, Del Zotto M, Zani A. Gender differences in hemispheric asymmetry for face processing. BMC Neurosci. 2006b;7:44. doi: 10.1186/1471-2202-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S, Kahana MJ, Rizzuto DS, Caplan JB, Kirschen MP, Bourgeois B, Madsen JR, Lisman JE. Gating of human theta oscillations by a working memory task. J Neurosci. 2001;21(9):3175–3183. doi: 10.1523/JNEUROSCI.21-09-03175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S, Lisman JE, Tully M, Madsen JR, Bromfield EB, Kahana MJ. Theta oscillations in human cortex during a working-memory task: evidence for local generators. J Neurophysiol. 2006;95(3):1630–1638. doi: 10.1152/jn.00409.2005. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Jones KA, Porjesz B, Chorlian DB, Padmanabhapillai A, Kamarajan C, Kuperman S, Rohrbaugh J, O'Connor SJ, Bauer LO, Schuckit MA, Begleiter H. Delta and theta oscillations as risk markers in adolescent offspring of alcoholics. Int J Psychophysiol. 2007a;63(1):3–15. doi: 10.1016/j.ijpsycho.2006.10.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaswamy M, Jones KA, Porjesz B, Chorlian DB, Padmanabhapillai A, Kamarajan C, Kuperman S, Rohrbaugh J, O'Connor SJ, Bauer LO, Schuckit MA, Begleiter H. Delta and theta oscillations as risk markers in adolescent offspring of alcoholics. Int J Psychophysiol. 2007b;63(1):3–15. doi: 10.1016/j.ijpsycho.2006.10.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razumnikova OM. Creativity related cortex activity in the remote associates task. Brain Res Bull. 2007;73(1–3):96–102. doi: 10.1016/j.brainresbull.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Ruchsow M, Grothe J, Spitzer M, Kiefer M. Human anterior cingulate cortex is activated by negative feedback: evidence from event-related potentials in a guessing task. Neurosci Lett. 2002;325(3):203–206. doi: 10.1016/s0304-3940(02)00288-4. [DOI] [PubMed] [Google Scholar]
- Sammer G, Blecker C, Gebhardt H, Bischoff M, Stark R, Morgen K, Vaitl D. Relationship between regional hemodynamic activity and simultaneously recorded EEG-theta associated with mental arithmetic-induced workload. Hum Brain Mapp. 2007;28(8):793–803. doi: 10.1002/hbm.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Freunberger R, Pecherstorfer T, Hanslmayr S, Doppelmayr M. Relevance of EEG alpha and theta oscillations during task switching. Exp Brain Res. 2006;170(3):295–301. doi: 10.1007/s00221-005-0211-y. [DOI] [PubMed] [Google Scholar]
- Schacter DL. EEG theta waves and psychological phenomena: a review and analysis. Biol Psychol. 1977;5(1):47–82. doi: 10.1016/0301-0511(77)90028-x. [DOI] [PubMed] [Google Scholar]
- Scheres A, Milham MP, Knutson B, Castellanos FX. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61(5):720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Schmiedt C, Brand A, Hildebrandt H, Başar-Eroğlu C. Event-related theta oscillations during working memory tasks in patients with schizophrenia and healthy controls. Brain Res Cogn Brain Res. 2005;25(3):936–947. doi: 10.1016/j.cogbrainres.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Shen X. Sex differences in perceptual processing: performance on the color-Kanji stroop task of visual stimuli. Int J Neurosci. 2005;115(12):1631–1641. doi: 10.1080/00207450590958484. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Caspi A, Moffitt TE, Poulton R. Personality and problem gambling: a prospective study of a birth cohort of young adults. Arch Gen Psychiatry. 2005;62(7):769–775. doi: 10.1001/archpsyc.62.7.769. [DOI] [PubMed] [Google Scholar]
- Soloff PH, Kelly TM, Strotmeyer SJ, Malone KM, Mann JJ. Impulsivity, gender, and response to fenfluramine challenge in borderline personality disorder. Psychiatry Res. 2003;119(1–2):11–24. doi: 10.1016/s0165-1781(03)00100-8. [DOI] [PubMed] [Google Scholar]
- Stein MA, Sandoval R, Szumowski E, Roizen N, Reinecke MA, Blondis TA, Klein Z. Psychometric characteristics of the Wender Utah Rating Scale (WURS): reliability and factor structure for men and women. Psychopharmacol Bull. 1995;31(2):425–433. [PubMed] [Google Scholar]
- Stockwell RG, Mansinha L, Lowe RP. Localization of the complex spectrum: The S-transform. IEEE Trans Sig Proc. 1996;44:998–1001. [Google Scholar]
- Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: administration, norms, and commentary. New York: Oxford University Press; 2006. [Google Scholar]
- Strohle A, Stoy M, Wrase J, Schwarzer S, Schlagenhauf F, Huss M, Hein J, Nedderhut A, Neumann B, Gregor A, Juckel G, Knutson B, Lehmkuhl U, Bauer M, Heinz A. Reward anticipation and outcomes in adult males with attention-deficit/ hyperactivity disorder. Neuroimage. 2008;39(3):966–972. doi: 10.1016/j.neuroimage.2007.09.044. [DOI] [PubMed] [Google Scholar]
- Toyomaki A, Murohashi H. Discrepancy between feedback negativity and subjective evaluation in gambling. Neuroreport. 2005a;16(16):1865–1868. doi: 10.1097/01.wnr.0000185962.96217.36. [DOI] [PubMed] [Google Scholar]
- Toyomaki A, Murohashi H. The ERPs to feedback indicating monetary loss and gain on the game of modified "rock-paper-scissors". International Congress Series. 2005b;1278:381–384. [Google Scholar]
- Trujillo LT, Allen JJ. Theta EEG dynamics of the error-related negativity. Clin Neurophysiol. 2007;118(3):645–668. doi: 10.1016/j.clinph.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Error monitoring using external feedback: specific roles of the habenular complex, the reward system, and the cingulate motor area revealed by functional magnetic resonance imaging. J Neurosci. 2003;23(10):4308–4314. doi: 10.1523/JNEUROSCI.23-10-04308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchi T, Girelli L. Gender differences in visuo-spatial processing: the importance of distinguishing between passive storage and active manipulation. Acta Psychol (Amst) 1998;99(1):1–16. doi: 10.1016/s0001-6918(97)00052-8. [DOI] [PubMed] [Google Scholar]
- Volz KG, Schubotz RI, von Cramon DY. Frontomedian activation depends on both feedback validity and valence: fMRI evidence for contextual feedback evaluation. Neuroimage. 2005 doi: 10.1016/j.neuroimage.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Yeung N, Bogacz R, Holroyd CB, Nieuwenhuis S, Cohen JD. Theta phase resetting and the error-related negativity. Psychophysiology. 2007;44(1):39–49. doi: 10.1111/j.1469-8986.2006.00482.x. [DOI] [PubMed] [Google Scholar]
- Yeung N, Holroyd CB, Cohen JD. ERP correlates of feedback and reward processing in the presence and absence of response choice. Cereb Cortex. 2005;15(5):535–544. doi: 10.1093/cercor/bhh153. [DOI] [PubMed] [Google Scholar]
- Yeung N, Sanfey AG. Independent coding of reward magnitude and valence in the human brain. J Neurosci. 2004;24(28):6258–6264. doi: 10.1523/JNEUROSCI.4537-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanova J, Falkenstein M, Hohnsbein J, Kolev V. Parallel systems of error processing in the brain. Neuroimage. 2004;22(2):590–602. doi: 10.1016/j.neuroimage.2004.01.040. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Kolev V. Developmental changes in the event-related EEG theta response and P300. Electroencephalogr Clin Neurophysiol. 1997;104(5):418–430. doi: 10.1016/s0168-5597(97)00054-3. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Kolev V. Single-sweep analysis of the theta frequency band during an auditory oddball task. Psychophysiology. 1998;35(1):116–126. [PubMed] [Google Scholar]
- Yordanova J, Kolev V, Rosso OA, Schurmann M, Sakowitz OW, Öniz M, Başar E. Wavelet entropy analysis of event-related potentials indicates modality-independent theta dominance. J Neurosci Methods. 2002;117(1):99–109. doi: 10.1016/s0165-0270(02)00095-x. [DOI] [PubMed] [Google Scholar]