A 2.5 Å resolution data set was collected from a crystal of a soluble chimeric form of NADPH-cytochrome P450 reductase (CPR) produced using a fusion gene composed of the yeast FMN and the human FAD domains. The chimeric protein was crystallized in a modified conformation compared with the previously solved structures.
Keywords: NADPH-cytochrome P450 reductase, chimeric proteins
Abstract
NADPH-cytochrome P450 reductase (CPR) is the favoured redox partner of microsomal cytochromes P450. This protein is composed of two flavin-containing domains (FMN and FAD) connected by a structured linker. An active CPR chimera consisting of the yeast FMN and human FAD domains has been produced, purified and crystallized. The crystals belonged to the monoclinic space group C2 and contained one molecule per asymmetric unit. Molecular replacement was performed using the published rat and yeast structures as search models. The initial electron-density maps revealed that the chimeric enzyme had crystallized in a conformation that differed from those of previously solved structures.
1. Introduction
NADPH-cytochrome P450 reductase (CPR) is a multidomain protein composed of at least three modules (Smith et al., 1994 ▶) that include an FMN-bound flavodoxin domain and an FAD-bound ferredoxin reductase domain. These two domains are linked via a connecting domain constituted mainly of α-helices. CPR is an electron-carrier protein that reduces microsomal cytochromes P450. The electronic flow from the electron source (NADPH) to P450 begins with the reduction of FAD. Two electrons are then sequentially transferred to FMN and finally to P450 (Gutierrez et al., 2001 ▶; Murataliev & Feyereisen, 1999 ▶). Regulation of intramolecular versus intermolecular electron transfer is critical for CPR activity.
Soluble oxidized forms of rat and yeast CPR have been crystallized and their three-dimensional structures have been determined (Lamb et al., 2006 ▶; Wang et al., 1997 ▶). The two structures are similar in their spatial organization: the FMN domain points into the FAD domain so that direct inter-flavin electron transfer is favoured. This closed conformation is clearly not optimized for electron delivery to external acceptors of CPR. Therefore, a major structural change during catalysis has been postulated (Murataliev et al., 2004 ▶).
CPR enzymes from distantly related organisms possess very different biochemical characteristics (electronic cycles, flavin redox potentials, enzymatic characteristics etc.). To understand how these differences have an impact on overall activity, we built a chimeric CPR composed of the yeast FMN domain (residues 44–211) and the human FAD domain (residues 232–677). Here, we report the successful purification, crystallization and preliminary X-ray analysis of this active chimeric yeast–human (YH) CPR.
2. Cloning, overproduction and purification
The YH chimeric gene was constructed as follows. A first PCR reaction, designed to separately amplify the yeast FMN and human FAD domains, was performed with the following oligonucleotides: primer 1, 5′-GACCTCGAGAGCTCGGGCAACAGAGACATGG, primer 2, 5′-GGTTTTGAAAGACGAACTGGGGGTGGAAGCCACTGGCGAGG, primer 3, 5′-GCGAGATCCACTAGCTCCACACGTCCAGGG, and primer 4, 5′-CCTCGCCAGTGGCTTCCACCCCCAGTTCGTCTTTCAAAACC. Primers 2 and 3 are compatible and allow the fusion of both PCR products during the second PCR (fusion PCR). Primary PCR products were purified with the Nucleo Spin Extract II kit (Macherey-Nagel) and were further used as matrices for a second amplification with oligonucleotides 1 and 4. The secondary PCR products were digested (XhoI and BglII) and cloned into pET-15b (Novagen). DNA sequencing confirmed the absence of any undesired mutations. The constructed plasmid was transformed into BL21 (DE3) competent Escherichia coli cells to allow YH CPR overproduction.
Protein overproduction was performed at 302 K in Terrific Broth medium complemented with 2.5 mg l−1 riboflavin and 100 µg ml−1 ampicillin during 36 h. Cultures were spun down for 10 min at 7500 rev min−1 and resuspended in 20 mM sodium/potassium phosphate buffer pH 7.4 (buffer A). Cell lysis was achieved in buffer A containing an antiprotease cocktail with a Constant Cell Disruption System (Constant Systems Ltd). Cell debris was removed by centrifugation at 8500 rev min−1 for 1 h at 277 K. His-tagged proteins were bound on Talon Polyhistidine-Tag Purification Resin (Clontech) equilibrated with buffer A containing 0.25 M NaCl and 0.25 M KCl and eluted with a 0.33 M imidazole step gradient. Fractions containing YH CPR were desalted using a HiPrep Desalting column (GE Healthcare). After concentration using a Vivaspin-15 centrifugal concentrator (Vivasciences), the protein solution was applied onto a Sephacryl S-400 gel-filtration column (GE Healthcare) equilibrated with buffer A containing 1 µM FMN, 1 µM FAD, 1 mM DTT and 1 mM EDTA. Sample purity was determined by SDS–PAGE and examination of the OD280nm/OD250nm ratio measured by optical spectroscopy (typically 5). The protein solution was completely oxidized after a 10 min incubation in the presence of 0.1 M K3[Fe(CN)6] and immediately desalted using a Hi-Prep desalting column.
2.1. Crystallization
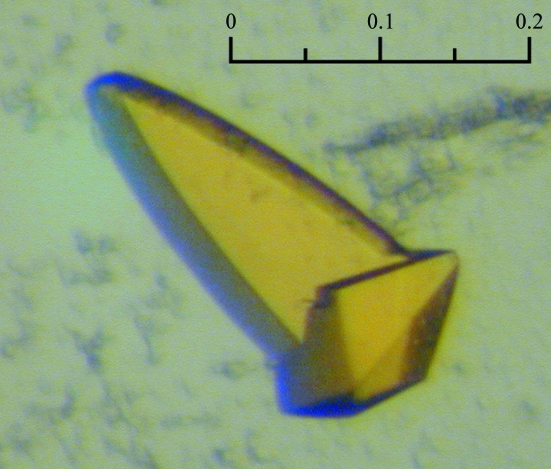
Prior to crystallization assays, soluble YH was concentrated to 10 mg ml−1 in a buffer consisting of 10 mM Tris pH 7.4 and 0.5 µM FMN. Commercial crystallization kits (the PEGs, PEGs II and Classics Suites from Qiagen) were used for screening in sitting-drop vapour-diffusion experiments using a Cartesian nanodrop robot (Genomic Solutions) at 293 K. Small crystals appeared within 5 d in only one condition [PEGs II condition G11: 0.1 M Tris–HCl pH 8.5, 28%(w/v) PEG 6000 and 0.1 M LiCl]. The crystals were improved using Additive Screen (Hampton Research) and were then manually optimized using home-made solutions. The crystal used for diffraction data measurement was obtained in the presence of 8% hexafluoroethanol after equilibration against a reservoir solution consisting of 0.01 M Tris–HCl pH 8.5, 20%(w/v) PEG 6000, 250 mM LiCl (Fig. 1 ▶).
Figure 1.
Yellow crystal of soluble YH CPR (scale bar: 0.2 mm)
2.2. Data collection and processing
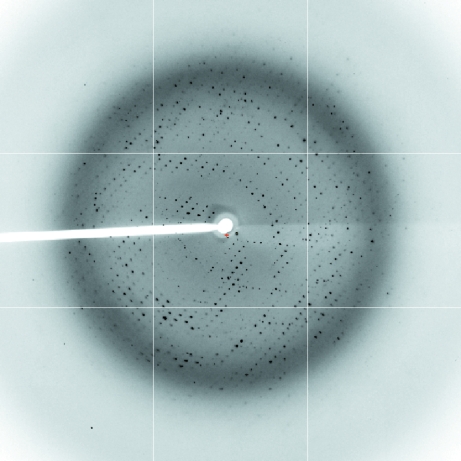
Crystals were flash-frozen in a cryoprotectant solution containing 0.01 M Tris–HCl pH 8.5, 250 mM LiCl, 20% PEG 6000 and 20% PEG 400. Data-collection experiments were carried out at 100 K on the PROXIMA I beamline at SOLEIL (Saint Aubin, France). 180 frames of 1° oscillation were collected with 1 s exposure per frame (Fig. 2 ▶). Diffraction intensities were evaluated with the program XDS (Kabsch, 1993 ▶) and further processed using the CCP4 program suite (Collaborative Computational Project, Number 4, 1994 ▶). The crystal diffracted to 2.5 Å resolution and belonged to the monoclinic space group C2, with unit-cell parameters a = 146.8, b = 60.7, c = 78.8 Å, β = 103.4°. Data-collection and processing statistics are given in Table 1 ▶.
Figure 2.
Diffraction pattern of YH CPR
Table 1. Data-collection statistics.
Values in parentheses are for the highest resolution shell (2.64–2.5 Å) of a total of nine.
| Space group | C2 |
| Unit-cell parameters (Å, °) | a = 146.8, b = 60.7, c = 78.8, β = 103.4 |
| Resolution (Å) | 20.0–2.5 |
| No. of observed reflections | 85573 |
| No. of unique reflections | 23129 |
| Completeness (%) | 97.4 (94.9) |
| Rmerge† (%) | 10.4 (50.9) |
| I/σ(I) | 12.7 (4.2) |
R
merge = 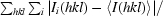
 , where I
i(hkl) is the ith observed amplitude of reflection hkl and 〈I(hkl)〉 is the mean amplitude for all observations i of reflection hkl.
, where I
i(hkl) is the ith observed amplitude of reflection hkl and 〈I(hkl)〉 is the mean amplitude for all observations i of reflection hkl.
2.3. Molecular replacement
Assuming that the asymmetric unit contains one chimeric YH molecule, the Matthews coefficient (Matthews, 1968 ▶) is 2.4 Å3 Da−1 and the solvent content is 49%. Molecular replacement with the program Phaser (Storoni et al., 2004 ▶) was attempted using two search models: the yeast FMN domain (residues 47–211 from PDB code 2bf4) and the rat FAD domain (residues 243–678 from PDB code 1amo), which shares 90% identity sequence with the human FAD domain. A copy of each domain was positioned in the asymmetric unit with good packing, high Z scores for the rotation function (RFZ = 24.7) and the translation function (TFZ = 35.6) and an excellent value for the log-likelihood gain (LLG = 1617). Examination of the resulting model composed of both FMN and FAD domains and density maps clearly showed that the soluble YH CPR is in a different conformation than those of the known structures. Refinement of this new YH CPR is under way.
Acknowledgments
We are grateful to Andrew Thompson for help during data collection on PROXIMA I.
References
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763.
- Gutierrez, A., Lian, L. Y., Wolf, C. R., Scrutton, N. S. & Roberts, G. C. (2001). Biochemistry, 40, 1964–1975. [DOI] [PubMed]
- Kabsch, W. (1993). J. Appl. Cryst.26, 795–800.
- Lamb, D. C., Kim, Y., Yermalitskaya, L. V., Yermalitsky, V. N., Lepesheva, G. I., Kelly, S. L., Waterman, M. R. & Podust, L. M. (2006). Structure, 14, 51–61. [DOI] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed]
- Murataliev, M. B. & Feyereisen, R. (1999). FEBS Lett.453, 201–204. [DOI] [PubMed]
- Murataliev, M. B., Feyereisen, R. & Walker, F. A. (2004). Biochim. Biophys. Acta, 1698, 1–26. [DOI] [PubMed]
- Smith, G. C., Tew, D. G. & Wolf, C. R. (1994). Proc. Natl Acad. Sci. USA, 91, 8710–8714. [DOI] [PMC free article] [PubMed]
- Storoni, L. C., McCoy, A. J. & Read, R. J. (2004). Acta Cryst. D60, 432–438. [DOI] [PubMed]
- Wang, M., Roberts, D. L., Paschke, R., Shea, T. M., Masters, B. S. & Kim, J. J. (1997). Proc. Natl Acad. Sci. USA, 94, 8411–8416. [DOI] [PMC free article] [PubMed]