Abstract
Objectives
Given the recent evidence that activity participation may reduce cognitive or functional decline, the effect of activity on residents’ ability to remain in assisted living (AL) is of interest. We examined the relationship between participation in activities and the length of time residents remain in AL.
Methods
The data reported here were gathered in the initial phase of the Maryland Assisted Living Study (MDAL), an epidemiologic study of dementia and other psychiatric disorders in AL. A stratified, random sample of 198 residents of 22 AL facilities in central Maryland were evaluated using a number of cognitive, behavioral, general health, and functional assessments. The total amount of time each resident spent in group and solitary activity in the prior month was recorded. The dependent variable, time to discharge (TTD), was the length of time between the resident’s evaluation by the study team and the time at which the resident died, or was discharged or administratively censored.
Results
Levels of activity participation at baseline were associated with longer TTD in an univariate Cox proportional hazards model. After adjusting for MMSE, GMHR, and the PGDRS mobility score in the multivariate model, activity participation remained associated with longer TTD, albeit with marginal statistical significance (p=0.057).
Conclusions
Higher activity participation was associated with longer TTD in AL. This suggests that engagement in activities may delay functional decline or the perception of decline observed by caregivers in AL. Further research is needed to replicate and understand this finding.
Keywords: Activity participation, assisted living, psychiatric disorders, dementia and activities
Objectives
Providing housing to over one million Americans, assisted living (AL) is the fastest-growing type of senior housing in the United States (Assisted Living Federation of America, 2004; Ball MM, et al., 2004). Since 2000, there has been a 14.5% increase in AL housing capacity in the United States of America, and this capacity continues to grow (Ball MM, et al., 2004). A primary factor in this growth is the increase in the number of older Americans (Assisted Living Federation of America, 2004). For example, between 2000 and 2010, the number of Americans ≥ 85 years old is expected to rise by 33% (Assisted Living Federation of America, 2004).
Unlike other forms of senior housing in the United States, AL promotes the philosophy of “aging in place” by fostering the independence of each resident (Samus QM, et al., 2005). According to the Assisted Living Federation of America (ALFA), AL facilities are defined as residences that provide a special combination of housing, personalized supportive services, and health care to meet the needs of those who require help with activities of daily living (Assisted Living Federation of America, 2004). To promote the well-being of their residents, many ALs provide services ranging from assistance with medications to meal preparation to access to medical care (Assisted Living Federation of America, 2004). AL facilities also provide social and recreational activities, including exercise programs (Assisted Living Federation of America, 2004).
While aging in place is a major goal of AL, it is not know whether this is occurring. This goal is under question, in part because an increasing number of studies have suggested that AL residents are more similar to nursing home residents than previously anticipated (Sloane PD, et al., 2003). For instance, nearly half of residents are over the age of 85, and three-quarters are women (Sloane PD, et al., 2003). Moreover, approximately 70% have a diagnosable dementia that may interfere with their ability to age in place (Rosenblatt A, et al., 2004). Furthermore, nearly one-third of residents remain in AL until death, suggesting that significant end-of-life care is being provided (Sloane PD, et al., 2003). Despite this, most providers view a resident’s stay in AL as a temporary placement designed to delay ultimate residence in a nursing home (Sloane PD, et al., 2003).
Given the temporary nature of AL habitation for most residents, many research studies are focusing on factors that influence how long residents remain in the AL setting before progression to death or transfer to another living environment. Several factors have been found to influence the length of a resident’s stay ranging from state regulatory requirements for admission and retention to the services provided by the facility (Sloane PD, et al., 2003).
We investigated whether participation in activities is a factor that influences how long a person resides in AL. To our knowledge, no data investigating the relationship between activity participation and the amount of time residents remain in nursing homes or AL have been published. Given the reports that mental, physical, and social activities have a protective effect against cognitive decline and dementia, that 70% of AL residents have a dementia, and evidence that activity participation may reduce cognitive or functional decline, the effect of activity on residents’ ability to remain in the AL setting is of interest (Rosenblatt A, et al., 2004; Fratiglioni L, et al., 2004; Podewils LJ, et al).
Methods
The data analyzed in this paper were gathered in the initial phase of the Maryland Assisted Living Study (MDAL), an epidemiologic study of dementia and other psychiatric disorders among residents of AL facilities. The specifics of the design and execution of the study are explained in detail elsewhere (Rosenblatt A, et al., 2004). The MDAL study procedures were reviewed and approved by the institutional review board at the Johns Hopkins University School of Medicine. In short, a stratified random sample of 22 AL facilities in central Maryland was selected from a state list of all AL facilities, either licensed or pending license. The facilities included 10 large facilities (≥ 16 beds) and 12 small facilities (≤ 15 beds). In each of the larger facilities, 15 residents were randomly selected from a list of occupied rooms provided by the facility director and approached for participation. All residents of the small facilities were approached for participation in the study.
Informed consent was obtained from each participant, or in the event of cognitive impairment affecting the participant’s capacity to consent, from a legal representative. Study participants received a comprehensive in-person clinical evaluation by a research team which included a geriatric psychiatrist, a nurse, and a psychometric technician. A detailed history and current status information were obtained from the participant, a family informant, medical records from the AL facility, and facility clinical staff. Measures of cognition and psychopathology were administered to the participants. The clinical data for each participant were evaluated at a consensus conference by a multidisciplinary team of experts who made diagnoses of dementia and psychiatric illness using DSM-IV-TR criteria.
The data and measures relevant to this analysis were:
Total number of hours in the past month spent engaged in solo and group activities: the study staff asked the residents, family informants, and AL staff to provide their best estimate in response to the following questions, “Number of hours spent in group activities per month on average by the participant (e.g. bingo, cards)” and “Number of hours spent in solitary activities per month (e.g. needlepoint, jigsaw puzzles)”
Cornell Scale for Depression in Dementia (CSDD) (Alexopoulos GS, et al., 1988);
Neuropsychiatric Inventory (NPI) to assess for neuropsychiatric and related behavioral disorders (Cummings JL, et al., 1994);
Psychogeriatric Dependency Rating Scale (PGDRS) mobility sub-scale with scores of 1 (full mobility), 2 (some mobility), and 3 (poor mobility) (Wilkinson IM, et al., 1980);
General Medical Health Rating (GMHR) Scale, a measure of medical co-morbidity, with scores ranging from 1 (poor health) to 4 (excellent health) (Lyketsos CG, et al., 1999);
Mini-Mental State Exam (MMSE), a measure of global cognitive functioning; scores range from 0 to 30 (higher scores suggest better functioning) (Folstein MF, et al., 1975);
DSM-IV Dementia Diagnosis (American Psychiatric Association, 1994)
The overall activity participation level of each AL resident was approximated as the sum of the number of hours each participant was reported to have spent in the previous month engaged in solitary activity and group activity. After baseline assessment, the residents were followed every six months by telephone contact with the facility to assess their vital and residence status. If a resident was discharged from AL or had died during the follow-up interval, the date of the event was recorded.
The dependent variable, time to discharge (TTD), was the length of time between the resident’s date of first assessment by the MDAL team and the final day in AL due to discharge, death, or administrative censoring. Patients with incomplete demographic data, incomplete scores for any of the scales described above, or insufficient data to tabulate the study participant’s overall activity level or TTD were excluded from the analyses (n=10), yielding an analytic sample of 188 participants from the original sample of 198.
Univariate and multivariate Cox proportional hazard models were adopted to examine the relative contribution of total activity level on TTD without, and then with, an adjustment for relevant covariates. Only variables that were associated with TTD at p<0.05 in the univariate models were included in the multivariate models. All data analyses were performed using Stata 8.0 (2003, Stata Corporation, College Station, TX).
Results
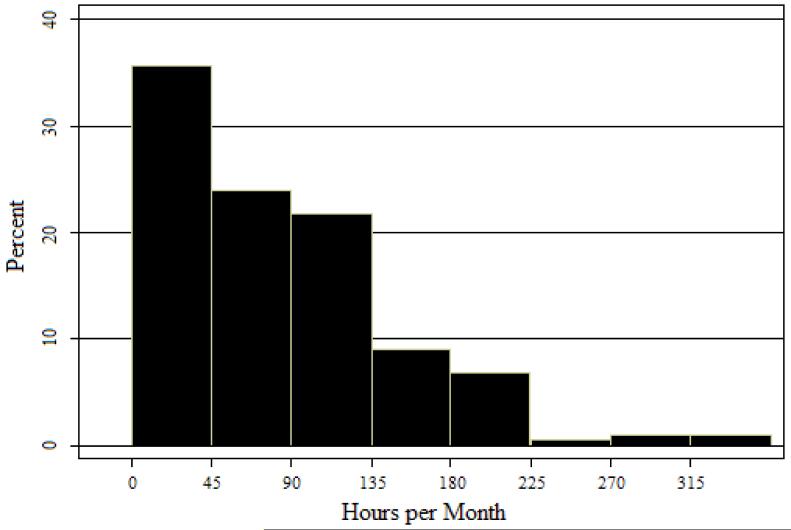
Figure 1 shows the distribution of activity participation in the past month among the AL residents studied. Demographic and other baseline characteristics of the participants are presented in Table 1.
Figure 1.
Distribution of Activity Participation.
Table 1.
Characteristics of Individuals Surveyed in the Maryland Assisted Living Study.*
| Characteristic | Total |
|---|---|
| Age, years, at survey (mean, SD) | 85.6 (8.3) |
| Gender | |
| Female | 146 (77.7) |
| Male | 42 (22.3) |
| Marital Status | |
| Married | 13 (6.9) |
| Unmarried | 175 (93.1) |
| Race | |
| Caucasian | 158 (84.0) |
| Other | 30 (16.0) |
| Years of education (mean, SD) | 13.6 (3.1) |
| CSDD total (0-38), (mean, SD) | 5.2 (4.6) |
| MMSE total (0-30), (mean, SD) | 18.7 (8.6) |
| Dementia diagnosis (DSM IV) | |
| Dementia absent | 62 (33) |
| Dementia present | 126 (67) |
| GMHR (1-4) | |
| 4 (Excellent) | 35 (18.6) |
| 3 (Good) | 80 (42.6) |
| 2 (Fair) | 67 (35.6) |
| 1 (Poor) | 6 (3.2) |
| NPI total (mean, SD) | 11.4 (14.3) |
| PGDRS Mobility score (1-3) | |
| 1 (Full mobility) | 49 (26.1) |
| 2 (Some mobility) | 15 (8.0) |
| 3 (Poor mobility) | 124 (66.0) |
| Time to discharge (mean, SD) | 453.4 (255.3) |
Legend: Data are presented as No. (%) unless otherwise indicated. SD=Standard deviation; CSDD=Cornell Scale for Depression in Dementa; GMHR= General Medical Health Rating; MMSE= Mini-Mental State Exam; NPI= Neuropsychiatric Inventory; PGDRS = Psychogeriatric Dependency Rating Scale
In the univariate proportional hazards models, total activity level, age, CSDD, MMSE, dementia status, GMHR, and PGDRS mobility score were each significantly associated with time to discharge (Table 2). When these seven variables were used in a multivariate Cox proportional hazards model, only MMSE, GMHR, and PGDRS mobility score remained significant predictors (Table 3). The multivariate model reveals that better general health, mobility, and cognitive functioning favor longer intervals of time in AL (Table 3). The p-value from the multivariate model for total activity level was close to statistically significant (p=0.057), although the point estimate for the effect size of activity participation was largely unchanged from the univariate analysis. From the multivariate model, there was, on average, an estimated 9.3% decrease in the rate of discharge from AL for each one-hour increase in activity per day (30 hours/month).
Table 2.
Results of the Univariate Cox Proportional Hazards Survival Regression
| Independent Variable | Hazard Ratio |
SE | 95% Confidence Interval |
P-value |
|---|---|---|---|---|
| Total Activity Level (per hour increase) | 0.996 | 0.002 | (0.993, 0.999) | 0.016 |
| Age (per year increase) | 1.027 | 0.013 | (1.001, 1.052) | 0.038 |
| Gender (male versus female) | 0.7458 | 0.1992 | (0.442, 1.259) | 0.272 |
| Marital Status (white versus other) | 0.6843 | 0.2703 | (0.315, 1.484) | 0.337 |
| Race (per year increase) | 0.9301 | 0.2626 | (0.535, 1.617) | 0.797 |
| Education (per year increase) | 0.9717 | 0.0324 | (0.910, 1.037) | 0.390 |
| CSDD total (per point increase) | 1.0659 | 0.0220 | (1.024, 1.110) | 0.002 |
| MMSE total (per point increase) | 0.9730 | 0.0116 | (0.951, 0.996) | 0.022 |
| Dementia diagnosis (yes versus no) | 1.5847 | 0.3600 | (1.015, 2.474) | 0.043 |
| GMHR (reference: GMHR=4) | ||||
| GMHR= 1 | 3.6387 | 2.0385 | (1.214, 10.910) | 0.021 |
| GMHR= 2 | 2.7489 | 0.9283 | (1.418, 5.328) | 0.003 |
| GMHR= 3 | 1.6233 | 0.5561 | (0.829, 3.177) | 0.157 |
| NPI Total (per point increase) | 1.0046 | 0.0061 | (0.993, 1.017) | 0.448 |
| PGDRS Mobility score | ||||
| (reference: mobility=3) | ||||
| Mobility= 1 | 0.3694 | 0.1050 | (0.212, 0.645) | < 0.001 |
| Mobility= 2 | 0.7335 | 0.2602 | (0.366, 1.470) | 0.382 |
Legend: SE= Standard Error; CSDD=Cornell Scale for Depression in Dementa; GMHR= General Medical Health Rating; MMSE= Mini-Mental State Exam; NPI= Neuropsychiatric Inventory; PGDRS = Psychogeriatric Dependency Rating Scale
Table 3.
Results of the Multivariate Cox Proportional Hazards Regression: Significant Correlates of Time to Discharge
| Independent Variable | Hazard Ratio |
SE | 95% Confidence Interval |
P-value |
|---|---|---|---|---|
|
Total Activity Level (per hour increase) |
0.997 | 0.002 | (0.993, 1.000) | 0.057 |
| GMHR (reference: GMHR=4) | ||||
| GMHR= 1 | 3.452 | 1.923 | (1.158, 10.288) | 0.026 |
| GMHR= 2 | 1.964 | 0.694 | (0.983, 3.925) | 0.056 |
| GMHR= 3 | 1.225 | 0.431 | (0.615, 2.440) | 0.563 |
|
PGDRS Mobility Score (reference: mobility=3) |
||||
| Mobility= 1 | 0.365 | 0.114 | (0.198, 0.674) | 0.001 |
| Mobility= 2 | 0.724 | 0.260 | (0.358, 1.465) | 0.369 |
| MMSE total (per point increase) | 0.967 | 0.012 | (0.944, 0.990) | 0.006 |
Legend: SE= Standard Error; GMHR= General Medical Health Rating; MMSE= Mini-Mental State Exam; PGDRS = Psychogeriatric Dependency Rating Scale
Conclusions
The aim of this study was to investigate the association between participation in activity, both solitary and group, and the length of time residents remained in AL. There was an association between participation in activity and time to discharge in that residents who spent more hours in activities, on average, had a longer TTD. This association was independent of better general health, mobility, and cognitive functioning, all of which favored longer lengths of stay in AL.
Although this study examined the association between activity participation and TTD in the AL setting, it did not address the relationship between activity and general cognitive and physical health. That is, it is not known whether higher levels of activity lead to better mental and physical health, or if good overall health leads to a more active lifestyle. Alternatively, continued participation in activities might influence the attitudes and decisions of care providers and surrogates regarding the continued appropriateness of a resident remaining in AL, a process that might in turn be independent of the resident’s cognitive and physical health. Future research is needed to elucidate these hypotheses, as they cannot be addressed in this dataset.
The MDAL study offers several advantages over previous studies of AL residents. Its examination of a random sample of nearly 200 participants from 22 facilities in Maryland is likely representative of AL populations nationally, as it includes small and large facilities. A major limitation, however, is that monthly activity level was based on retrospective staff and resident report. Moreover, the measure of total activity participation did not partition the amount of time each participant spent engaged in mental activity from that spent in physical activity. Finally, the cross-sectional nature of these analyses provides correlational support for a relationship between activity and remaining in AL, but it does not allow for causal attribution. Prospective, standardized evaluations of residents’ monthly activity levels will be needed in future studies to examine this question further.
One goal of the MDAL study is to provide data to guide AL facilities as they strive to maximize functioning and quality of life of their residents. If higher activity level correlates with longer stays in AL, facilities should consider devoting more resources and funding to programs that engage residents in physically and mentally stimulating activities as these may extend TTD. This would help to meet the goal of “aging in place.” Whether it would also improve the functioning and quality of life of AL residents requires further study.
This study suggests that activity participation is associated with time to discharge in the AL setting. Recent studies suggest that mental and physical activity have a beneficial effect on cognition and a protective effect against dementia, which raises the possibility that activity participation would have an even wider benefit (Fratiglioni L, et al., 2004; Podewils LJ, et al). These data are consistent with the finding in this study, but this analysis has not shown any association between longer TTD and improvements in cognition. As noted earlier, there are competing explanations for the positive effect of activity on TTD in this study. These alternatives represent hypotheses that warrant further exploration in future studies. Given the nature of the intervention, however, it may be reasonable to argue that this intervention is a reasonable one for AL care, although it is necessary for the finding reported in this study to be replicated in other samples.
Acknowledgements
This study was supported by a fellowship from the American Federation for Aging Research, a grant from the Office of the Dean of Students at the Johns Hopkins University School of Medicine, and an R01MH 60626 grant from the National Institutes of Mental Health (NIMH) and the National Institute on Aging. Ms. Leoutsakos is funded through the Biostatistics Mental Health/Psychiatry Training Program sponsored by the NIMH. A poster describing the findings of this study were presented at the 2005 Annual Scientific Meeting of the American Geriatrics Society held in Orlando, Fl from May 11-15, 2005 (Abstract Citation: PAPER ABSTRACTS. Journal of the American Geriatrics Society 53 (s1), 21-S217).
We are grateful to the study participants and their families for their participation and to the management and caregivers of the AL facilities.
References
- Alexopoulos GS, Abrams RC, Young RC, et al. Cornell Scale for Depression in Dementia. Biol Psychiatry. 1988;23:271–284. doi: 10.1016/0006-3223(88)90038-8. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders Text Revision. 4th American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Assisted Living Federation of America Available at www.alfa.org Accessed November 30, 2004
- Ball MM, Perkins MM, Whittington FJ, et al. Managing decline in assisted living: the key to aging in place. J Gerontol B Psychol Sci Soc Sci. 2004;59:S202–S212. doi: 10.1093/geronb/59.4.s202. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein S, McHugh P. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Paillard-Borg S, Winblad B.An active and socially integrated lifestyle in late life might protect against dementia Lancet Neurol 20043343–353. DOI: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Galik E, Steele C, et al. The General Medical Health Rating: a bedside global rating of medical comorbidity in patients with dementia. J Am Geriatr Soc. 1999;47:487–491. doi: 10.1111/j.1532-5415.1999.tb07245.x. [DOI] [PubMed] [Google Scholar]
- Podewils LJ, Guallar E, Kuller LH, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study Am J Epidemiol 2005161639–651. DOI: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- Rosenblatt A, Samus QM, Steele CD, et al. The Maryland Assisted Living Study: prevalence, recognition, and treatment of dementia and other psychiatric disorders in the assisted living population of central Maryland J Am Geriatr Soc 2004521618–1625. DOI: 10.1111/j.1532-5415.2004.52452.x. [DOI] [PubMed] [Google Scholar]
- Samus QM, Rosenblatt A, Steele C, et al. The association of neuropsychiatric symptoms and environment with quality of life in assisted living residents with dementia. Gerontologist. 2005;45:19–26. doi: 10.1093/geront/45.suppl_1.19. [DOI] [PubMed] [Google Scholar]
- Sloane PD, Zimmerman S, Hanson L, et al. End-of-life care in assisted living and related residential care settings: comparison with nursing homes J Am Geriatr Soc 2003511587–1594. DOI: 10.1046/j.1532-5415.2003.51511.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson IM, Graham-White J. Psychogeriatric dependency rating scales (PGDRS): a method of assessment for use by nurses. Br J Psychiatry. 1980;137:558–565. doi: 10.1192/bjp.137.6.558. [DOI] [PubMed] [Google Scholar]