Abstract
Background
Sirolimus plays a critical role in facilitating steroid-free immunosuppression, in conjunction with low dose tacrolimus, in current islet transplantation. Although several studies have investigated the effects of sirolimus on islet cells, conflicting results have been reported. In this study, we assessed the effects of sirolimus supplementation in culture media on human islet preparations, focusing on the anti-pro-inflammatory aspects.
Methods
Human islet preparations were divided into four groups: pure (purity >90%) sirolimus (30ng/ml); pure control (0ng/ml); impure (purity 40–60%) sirolimus and impure control. All groups were cultured for 3 days and assessed regarding glucose stimulated insulin release, fractional β-cell viability, β-cell and macrophage content. Cytokine/chemokine production from islet preparations and sorted pancreatic ductal cells were also examined.
Results
Stimulated insulin release in the impure sirolimus group was significantly increased (p=0.024), as previously reported. Although fractional β-cell viability showed no significant differences, β-cell survival during culture significantly increased in impure sirolimus group when compared to the impure control group (p=0.015). TNF-α, IL-1β, MCP-1 and MIP-1β production from the impure sirolimus group significantly decreased (p<0.05). Furthermore, TNF-α and MIP-1β production from sorted ductal cells significantly decreased in the sirolimus group (p<0.05). The number of macrophages contained in islet preparations significantly decreased in the impure sirolimus group when compared to the impure control group (p<0.05).
Conclusions
Sirolimus improved not only stimulated insulin release, but also β-cell survival during culture. The anti-inflammatory effects of sirolimus also appear beneficial to islet cells in culture and may be a useful strategy in improving islet transplantation outcomes.
Keywords: islet, transplantation, rapamycin, cytokine, chemokine
Introduction
Improved islet isolation techniques and immunosuppressive regimens have made islet transplantation a therapeutic option for patients with type 1 diabetes mellitus (1–4). This success is likely attributable in part to the novel immunosuppression protocol. Sirolimus (rapamycin) has played a critical role to facilitate steroid-free and low dose tacrolimus in the immunosuppressive protocols. Maintaining long-term islet graft function is an urgent issue to solve in current islet transplantation trials. Reduction or impairment of islet cell regeneration might potentially cause the loss of islet function over time in islet graft recipients. This phenomenon may be associated with adverse effects of immunosuppressive drugs.
Sirolimus is a macrolide that inhibits mRNA translation and protein synthesis via inhibition of the mammalian target of rapamycin (mTOR) (5). The mTOR is a serine and threonine protein kinase that regulates numerous cellular functions including the initiation of protein translation. The initiation of protein translation by mTOR is essential for enhanced protein synthesis resulting in increased cell cycle progression and proliferation (6). Although several studies have investigated the effects of sirolimus on islets in vivo or in vitro, the conclusions have often been conflicting (7–12).
The regulation of cytokine/chemokine production from human islet preparations (HIP) is highly desired for improving islet transplantation outcomes before and after transplantation. Cytokines/chemokines are produced by not only endocrine cells but also non-endocrine cells including ductal cells and macrophages. However, the anti-pro-inflammatory effects of sirolimus on HIP are not well studied.
The purpose of the present study is to assess the effects of sirolimus supplementation in culture, on fractional β-cell viability, cellular composition, insulin secretion and pro-inflammatory cytokine/chemokine production. This study indicates that sirolimus improves not only insulin secretion but also β-cell survival in vitro. Moreover, the number of macrophages contained in HIP and pro-inflammatory cytokine/chemokine production significantly decreased with the addition of sirolimus to culture media. These results suggest that sirolimus supplementation to pre-transplant culture media may be a beneficial strategy in modulating islet preparations optimally suited for islet transplantation.
Materials and Method
Human islet isolation
All HIP were isolated using a modified automated method (13) and purified using continuous Ficoll-based density gradients and semiautomated cell processor (Cobe 2991; COBE Laboratories, Inc. Lakewood, CO) at the Human Cell Processing Facility of the Diabetes Research Institute, University of Miami, School of Medicine. Nine independent human islet preparations were included in this study.
Islet culture with or without sirolimus
Pure (>90%) and impure (40–60%) HIP were prepared for the experiments. Miami-defined medium 1 (MM1, Mediatech Inc., Herndon, VA) supplemented with 20µg/ml ciprofloxacin (Cipro® I.V., Schering Co., Kenilworth, NJ) and 100u/ml heparin (Pharmaceutical Products, Schaumburg, IL) was used for this study (14). HIP were cultured at a density of 3,000 × % purity × 10−2 IEQ with or without sirolimus (30 ng/ml) (Rapamune, Wyeth, Madison, NJ) in a 37°C humidified incubator, 5% CO2 for 72 hours.
The concentration of sirolimus was decided based on the target trough level (10–15 ng/ml) used in clinical immunosuppression protocol (1, 4). The peak drug concentration of sirolimus in portal vein is generally double that of systemic level after oral administration (15, 16). Therefore, 30ng/ml of sirolimus was used for this study.
Human pancreatic ductal cell sorting
The cells from impure HIP were dispersed by incubation with 1ml of Accutase solution (Innovative Cell Technologies, Inc., San Diego, CA) for 10 minutes and incubated with anti-mouse CA19-9 (1:50; Dako North America, Inc, Carpinteria, CA) antibody for 30 minutes. Subsequently, the cells were incubated for an additional 10 minutes with magnetic beads coated with anti-mouse IgG (Miltenyi Biotec, Auburn, CA). The cell suspension was then passed through a MACS separation column (Miltenyi Biotec, Auburn, CA) to obtain positive selection of CA19-9+ cells. The efficiency of sorting was confrimed by FACS analysis (17).
Ductal cell culture with or without sirolimus
After sorting, 1.0 × 106 of ductal cells were incubated on a coated dish at 37 °C in DMEM containing 10% FBS and 20µg/ml ciprofloxacin for 3–5 days until growing to 50–80 % of confluent. The medium was changed every 2 days. Ductal cells were then cultured at 37°C for 72 hours with or without sirolimus (30ng/ml).
Glucose stimulated insulin release
Aliquots of islets were analyzed for their response to a dynamic stimulation assay in vitro (18). Islets were pre-perifused in a chromatography column (Bio-gel Fine 45–90nm; Bio-Rad) with a buffer containing 125mM NaCl, 5.9 mM KCl, 1.28mM CaCl2, 1.2 mM Mg Cl2, 25mM HEPES, 0.1% bovine serum albumin and 3mM glucose for 30 min, at 37°C. 100IEQ of islets were perifused in the same buffer for 10 min and then sequentially exposed to 11mM and 3mM glucose. Fractions of the perifusate were collected every 1 minutes during perifusion with 3mM glucose, and every minute during stimulation. The collected fractions were then assayed for human insulin concentrations by ELISA (Mercodia Inc., Winston Salem, NC).
Fractional β- and ductal cell viability assay
Fractional β- and ductal cell viability were determined using the modified method reported previously (19). Dispersed cell suspensions were stained using Newport Green PDX acetoxymethylether (NG; Molecular Probes, Eugene, OR), for the identification of β-cells; CA19-9, for the identification of ductal cell; tetramethylrhodamine ethyl ester (TMRE; Molecular Probes), for the evaluation of mitochondrial membrane potential; 7-aminoactinomycin D (7-AAD; Molecular Probes), for the exclusion of dead cells; and assessed with FACScan cytometer (Becton Dickinson, Mountain View, CA). Fractional β- and ductal cell viability was evaluated with the CellQuest software by the percentage of TMRE positive cells in 7-AAD negative and NG positive or CA19-9 positive population.
Assessment of cellular composition
The content of macrophage, β- and ductal cell was determined with laser scanning cytometer (LSC/iCys) (CompuCyte, Cambrige, MA) as described previously (19). Dispersed cells were fixed on glass slides with 2.5% paraformaldehyde (Electron Microscopy Sciences, Washington, PA). After incubation with Protein Block (BioGenex, San Ramon, CA) at room temperature for 30 min to reduce non-specific binding, cells were incubated at room temperature for 2 hours with the following primary antibodies; mouse monoclonal antibody to c-peptide (1:100 dilution, Abcam Inc., Cambridge, MA), Cytokeratin 19 (1:50; Dako North America, Inc, Carpinteria, CA) and CD68 (KP1, 1:100; Dako North America, Inc). After washing in Optimax Wash Buffer (Bio-Genex, San Ramon, CA), cells were incubated at room temperature for 1 hour with Alexa Fluor 488 goat antimouse IgG (1:200 dilution, Molecular Probes, Eugene, OR), Alexa Fluor 647 goat antimouse IgG (1:200 dilution, Molecular Probes) antibodies and 4’, 6-diamidino-2-phenylindole (DAPI). The number of macrophages, β-, and ductal cells was automatically counted with iCys software.
Measurement of pro-inflammatory cytokine and chemokine production
Aliquots of islets (350 IEQ) were cultured in MM1 for 72 hours. Concentrations of pro-inflammatory cytokines/chemokines such as interleukin-1 β (IL-1β), tumor necrosis factor alpha (TNF-α), interferon gamma (IFN-γ), IL-6, macrophage inflammatory protein 1β (MIP-1β) and monocyte chemoattractant-1 (MCP-1) in the supernatants were determined using Multi-Plex cytokine kits following the manufacturer’s protocol (Bio-Plex; Bio-Rad Laboratories, Hercules, CA). Aliquots of islets were lysed with 1% Triton X-200 in order to determine total protein. Total protein was measured with an assay kit (BCA Protein Assay Kit, PERBIO, Rockford, IL). The amount of cytokines/chemokines was normalized by total protein content (pg).
Calculation of absolute β- and ductal cell mass
Absolute β-and ductal cell mass were calculated with following a formula;
Absolute β-cell mass = β-cell content (%) × protein content (µg).
Absolute ductal cell mass = ductal cell content (%) × protein content (µg).
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM). Data were analyzed using Excel for Windows software for descriptive statistics and data plotting. Two samples were compared a using Student’s t-test; statistical significance was considered for p-values <0.05.
Results
Effects of sirolimus supplementation to culture media on insulin secretion in pure or impure islet preparations
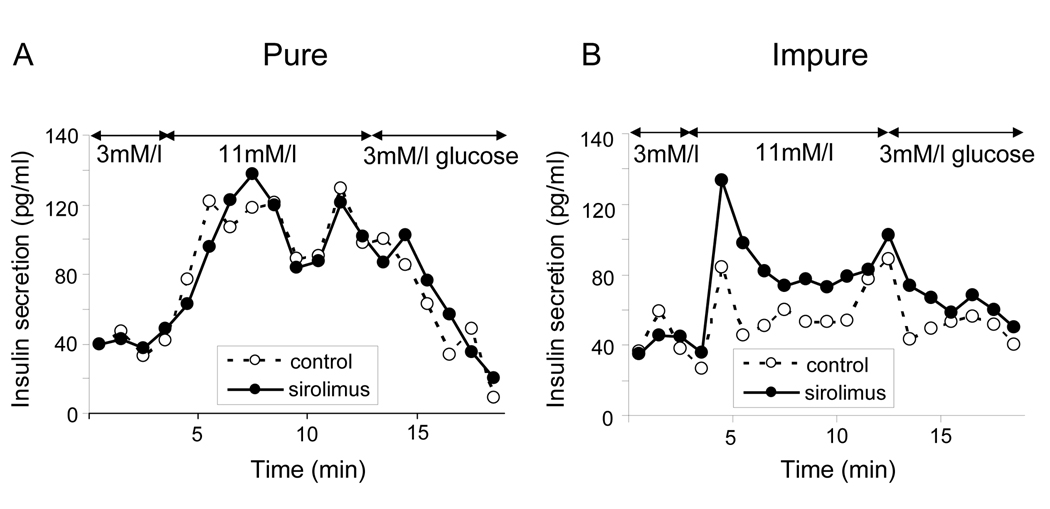
Pure (90% purity) and impure (40–60% purity) HIP were cultured with sirolimus (30ng/ml) and without sirolimus (control, 0ng/ml). Dynamic glucose stimulation assay showed that the insulin response to glucose as area under the curve (AUC) was significantly higher in the impure sirolimus group than in the impure control group (1365.3 ± 112.7 pg/ml and 1161.5 ± 105.8 pg/ml, respectively, p=0.007, Fig. 1 B). However, it was comparable between the pure sirolimus and control groups (1283.5 ± 190.9 pg/ml and 1228.2 ± 165.7 pg/ml, respectively, p=NS, Fig. 1 A). As the previous studies reported, sirolimus improved glucose stimulated insulin release in impure HIP in vitro.
Figure 1. Sirolimus increases insulin secretion in impure HIP.
HIP were divided into two groups based on the islet purity: pure group (90–95% purity, A) and impure group (40–60%purity, B). HIP were cultured with or without 30ng/ml sirolimus for 72 hours. Glucose stimulated insulin release was examined using dynamic stimulation assay. A and B showed dynamic insulin secretion at 3 and 11 mM glucose. The data shown represent three independent experiments.
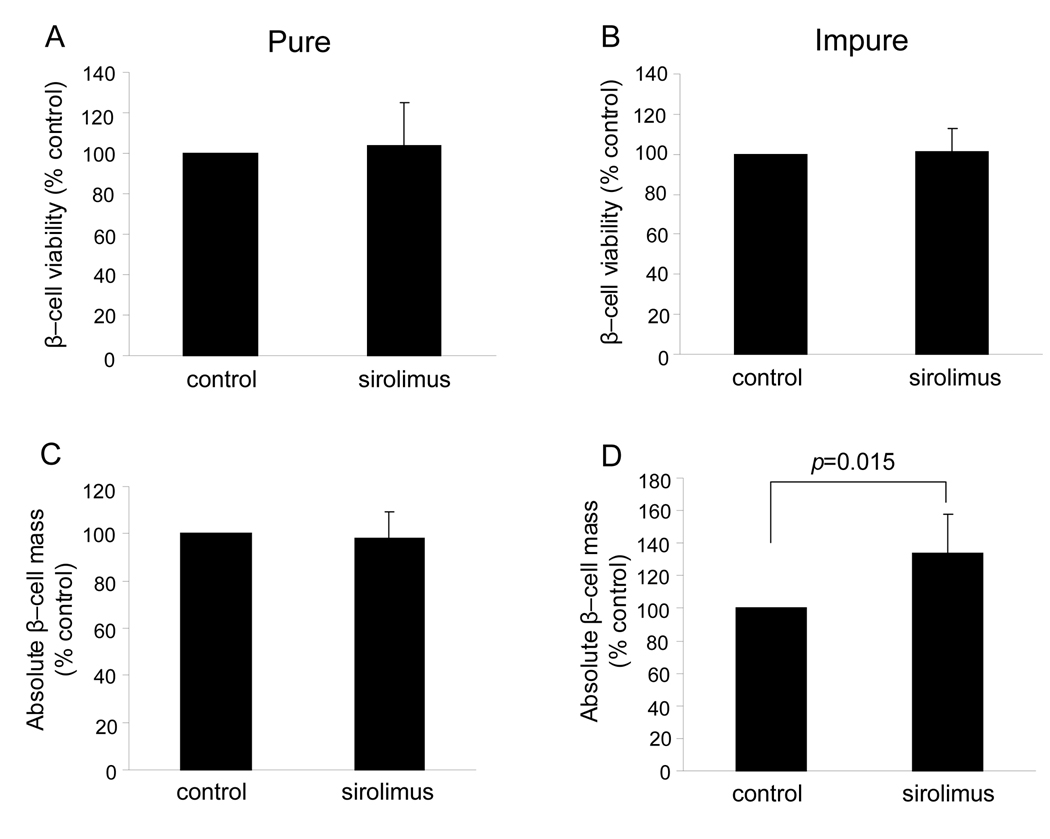
Fractional β-cell viability and absolute β-cell mass
To examine the effect of sirolimus on β-cell specific viability, fractional β-cell viability was assessed using FACS. Fractional β-cell viability indicated no significant difference between the sirolimus and the control groups in either the pure and impure HIP (103.9 ± 21.5 % control, p = NS in the pure sirolimus group, 101.0 ± 11.8 % control, p =NS in the impure sirolimus group) (Fig. 2 A and B). In the pure HIP, absolute β-cell mass was comparable between two groups (98.2 ± 11.0 % control, p =NS) (Fig. 2C). However, absolute β-cell mass in the impure sirolimus group significantly increased over the impure control group (133.6 ± 24.2 % control, p=0.015) (Fig. 2D). These results indicate that sirolimus has no deleterious effect on β-cell viability and is capable of improving β-cell survival after 3 days of culture in impure HIP.
Figure 2. Assessment of β-cell viability and content in HIP cultured with or without sirolimus.
A and B: After dispersion of HIP, islet cells were stained with 7-AAD, NG and TMRE. The viability of β-cells expressed the percentage of TMRE+ in 7-AAD− and NGbright population. C and D: Absolute β-cell mass was calculated as following formula; absolute β-cell mass = β-cell content (%) × protein content (µg). The value was expressed as % control. Results were shown the means ± SEM of five independent experiments.
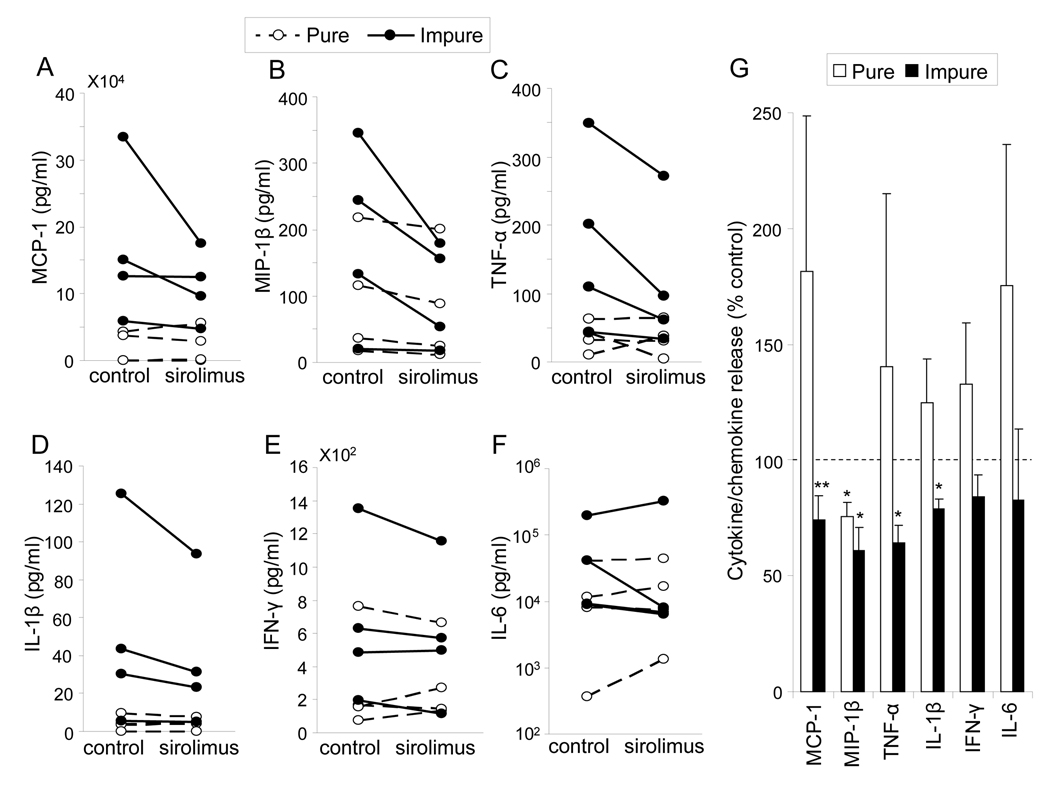
Cytokine/chemokine release from HIP cultured with or without sirolimus
To investigate the effect of sirolimus on pro-inflammatory cytokine/chemokine production from HIP, the amount of cytokines/chemokines in the supernatant was measured after 72 hrs culture (Figure 3A–F, Table 1). TNF-α, IL-1β, MCP-1 and MIP-1β production in the impure sirolimus group and MIP-1β production in the pure sirolimus group significantly decreased (64.4 ± 7.3 % control; p=0.003, 79.0 ± 4.3% control; p =0.003, 74.3 ± 10.2 % control; p = 0.046, 60.9 ± 9.8 % control; p=0.007 and 75.4 ± 6.2 % control; p=0.007, respectively). However, there were no significant difference of TNF-α, IL-1β and MCP-1 in the pure HIP (140.5 ± 74.6 % control; p = NS, 124.8 ± 37.7 % control; p = NS and 181.5 ± 67.3 % control; p=NS, respectively) (Fig. 3G). Sirolimus supplementation to culture media significantly reduced the productions of TNF-α, IL-1β, MCP-1 and MIP-1β in impure HIP and MIP-1β in pure HIP.
Figure 3. Cytokine / chemokine production in pure or impure HIP cultured with or without sirolimus.
After culturing HIP with or without sirolimus, cytokines/chemokines in the supernatant were determined using Bio-Plex in four independent experiments. A–F: The concentrations of cytokines / chemokines from pure (dotted lines) or impure (solid lines) HIP cultured with or without (control) sirolimus were shown. The values were normalized by the protein content of HIP in each group. G: Cytokine/chemokine productions in pure (white bars) or impure (black bars) HIP with sirolimus were shown as a percent control. Results were shown the means ± SEM of four independent experiments in each group. * p<0.01, ** p<0.05
Table1.
Cytokine/chemokine production in pure, impure HIP, and ductall cells
| pure islet preparations | impure islet preparations | ductal cells | ||||
|---|---|---|---|---|---|---|
| control | sirolimus | control | sirolimus | control | sirolimus | |
| MCP-1 | 20,359.0 ± 11,660.0 | 21,698.6 ± 13,221.8 | 167,075.1 ± 58,872.3 | 110,888.4 ± 26,624.1 | 37,204.9 ± 10,809.4 | 42,200.7 ± 13,659.8 |
| MIP-1β | 96.7 ± 45.7 | 81.4 ± 43.4 | 185.7 ± 70.1 | 101.9 ± 39.2 | 1,936.6 ± 1,225.9 | 769.4 ± 442.7 |
| TNF-α | 37.0 ± 10.8 | 34.2 ± 12.4 | 176.1 ± 66.1 | 116.0 ± 53.7 | 1,596.1 ± 993.0 | 1,163.0 ± 787.4 |
| IL-1β | 4.3 ± 1.9 | 4.3 ± 1.6 | 51.1 ± 25.9 | 38.4 ± 19.2 | 1,926.7 ± 1,849.4 | 1,886.2 ± 1,857.2 |
| IFN-γ | 290.6 ± 159.1 | 304.9 ± 124.5 | 666.3 ± 245.5 | 585.5 ± 215.2 | 1,567.4 ± 747.2 | 1,144.7 ± 509.6 |
| IL-6 | 15,195.8 ± 8,788.3 | 17,536.8 ± 9,555.0 | 63,226.5 ± 43,978.3 | 85,587.2 ± 78,470.8 | 77,985.8 ± 30,292.7 | 49,442.5 ± 24,399.2 |
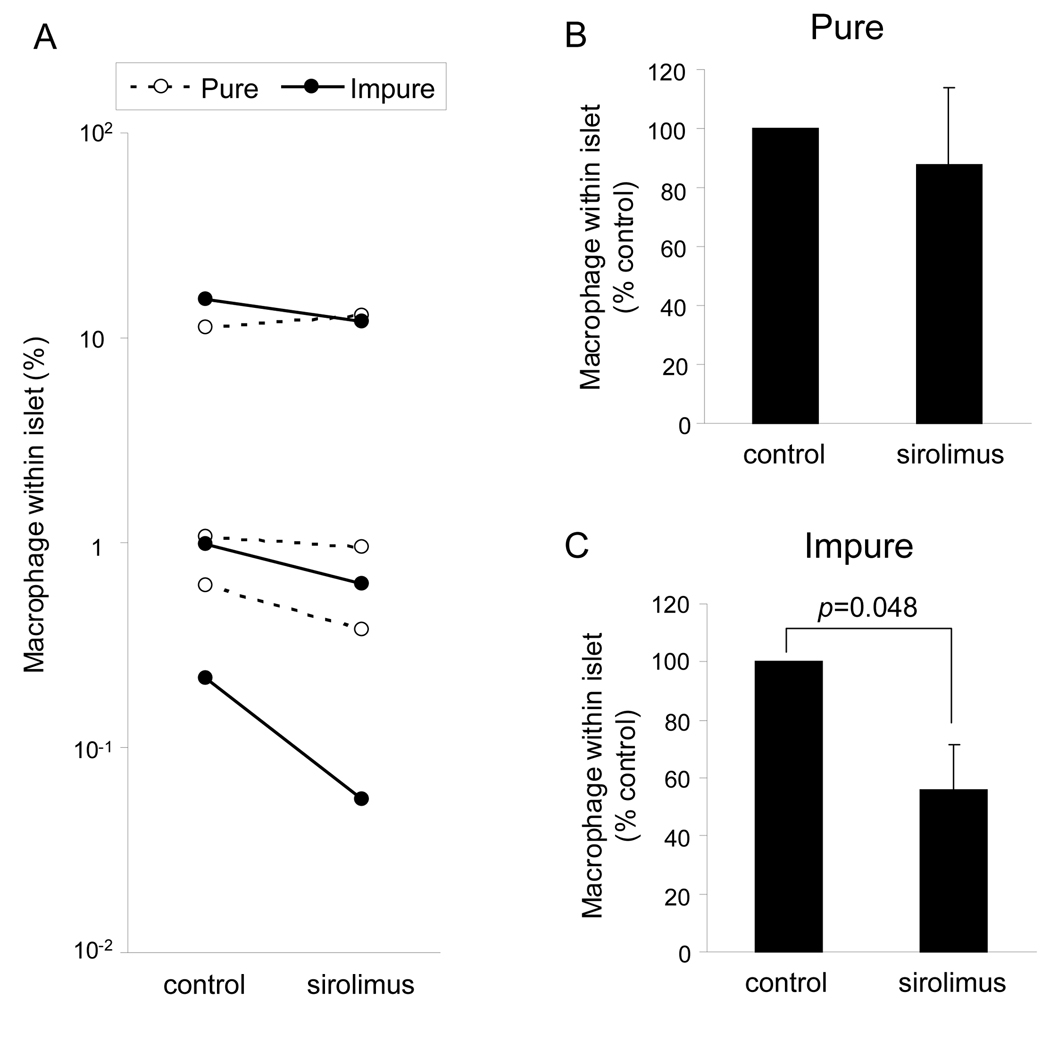
Macrophage content contained in human islet preparations decreased with sirolimus
Macrophages are considered as one of the main pro-inflammatory cytokine/chemokine producers in HIP. To examine the effect of sirolimus on the cellular composition of islet preparations, the content of macrophages (CD68+ cells) was examined using LSC/iCys. The number of macrophage in the impure sirolimus group significantly decreased compared to the impure control group (55.9 ± 27.0 % control; p = 0.048) (Fig. 5A –C). There was no significant difference in macrophages content between the pure sirolimus and pure control group (87.9 ± 15.1 % control; p = NS). A significant reduction in the quantity of macrophages in impure HIP by sirolimus may contribute to a sirolimus induced reduction of cytokine/chemokine release from HIP.
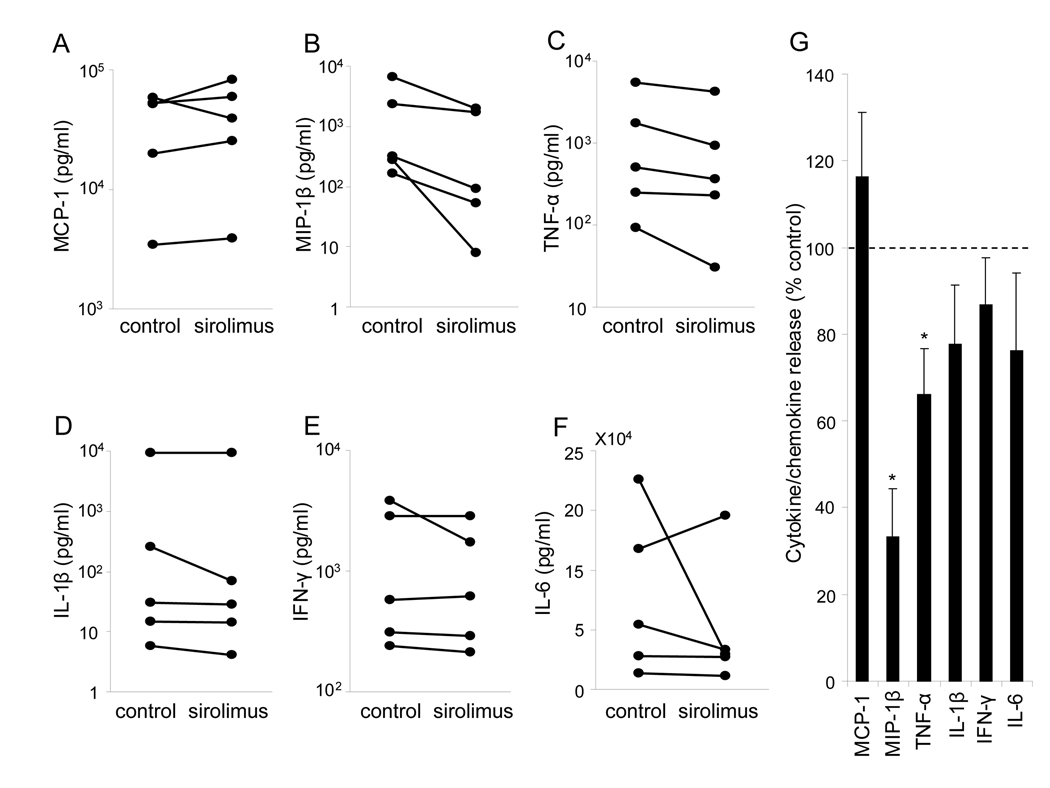
Figure 5. Cytokine/chemokine production in sorted ductal cells cultured with or without sirolimus.
After sorted ductal cells were cultured with or without sirolimus, cytokines/chemokines levels in the supernatant were determined using Bio-Plex in four independent experiments. (A–F) The concentrations of each cytokines/chemokines are shown. The values were normalized by the protein content in each group. (G) The % control of cytokine/chemokine in the sirolimus group was shown the means ± SEM. * p<0.05.
Effects of sirolimus on ductal cell viability and content
Ductal cell viability and content was examined using FACS or LSC/iCys. Pancreatic ductal cell viability showed no difference between in the cooperating sirolimus and control group in both pure and impure HIP (103.2 ± 2.8 % control in the pure sirolimus group; p=0.283, 101.3 ± 1.3 % control in the impure sirolimus group; p=0.357, respectively). Absolute ductal cell mass was also comparable between the sirolimus and control group in both pure and impure HIP (113.6 ± 16.0 % control in the pure sirolimus group; p=0.428, 93.4 ± 16.1 % control in the impure sirolimus group; p=0.704, respectively). Results were shown the means ± SEM of five independent experiments.
Effects of sirolimus on cytokine/chemokine production from pancreatic ductal cells
To examine the effect of sirolimus on cytokine/ chemokine production from pancreatic ductal cells, they were sorted from HIP using CA19-9 antibodies. The production of cytokine/chemokine from the sorted ductal cells was measured after 3 days culture with or without sirolimus (Figure 5A–F, Table 1). The production of TNF-α and MIP-1β significantly decreased in the presence of sirolimus as compared to the corresponding control group (66.1 ± 10.5 % control; p=0.012, 33.2 ± 11.2 % control; p<0.001, respectively) (Figure 5G). The data suggest that sirolimus affects cytokine/chemokine productions from ductal cells.
Discussion
Islet transplantation has been developed as a therapeutic option for patients with Type 1 diabetes thanks to significant advances in islet isolation methods and immunosuppressive protocols (1–4). Sirolimus (rapamycin) has played a critical role to facilitate steroid-free and low dose tacrolimus in recent islet transplant immunosuppressive regimens. The present study showed sirolimus improved not only insulin secretion but also β-cell survival during culture, as well as a significant reduction in macrophage content, and a significant reduction in cytokine/chemokine production in impure HIP.
Although several studies have investigated the effects of sirolimus on β-cells in vivo or in vitro, the conclusions have often been conflicting (7–12). In vivo studies, Kneteman et al. have shown an improvement of blood insulin levels with sirolimus injection in canines (7). However, it have recently been reported sirolimus reduced islet graft and insulin release in allo-transplant model using mice (11). Sirolimus is also known to have direct toxicity to β-cells (10, 20). We have recently reported that β-cell proliferation is reduced by sirolimus using the pregnant mouse model (21). In vitro studies, Bell et al. have reported that sirolimus reduces the viability of MIN-6 cells, in rat and human islets, assessed by the MTT metabolism assay (9). In addition, sirolimus has been reported to have a negative effect on the glucose-dependent insulin secretion of isolated human islet cells (10). On the other hand, Fuhrer et al. have reported that sirolimus improved insulin secretion of hamster HIT-T15 cells (8). More recently, Marcelli-Tourvieille et al. have reported that sirolimus has no deleterious effect to insulin secretion on human islets using clinical transplantation concentrations (12).
This study shows no deleterious effects of sirolimus on β-cell viability in both pure and impure HIP. In fact, sirolimus supplementation to culture media significantly improved β-cell survival and stimulated insulin secretion in impure HIP. The results are consistent with the study showing a sirolimus induced improvement of insulin secretion using 54 ± 4 % pure HIP (12). Although many investigators have the paradoxical effects of sirolimus on HIP, reconsidering the variability in the purity of HIP used in those experiments could more adequately elucidate the discrepancies and allow a better comparison with these results.
The evidences suggest that sirolimus supplementation in culture media decreased TNF-α, IL-1β and MCP-1 production in impure HIP but not in pure HIP. IL-1β and TNF-α administrated with IFN-γ induce apoptosis in human islets (22, 23), and the elevation of those cytokines are confirmed in the islet transplantation model using mice (24). The β-cell death associated with a non-specific inflammation appears to be mediated by TNF-α and IL-1β in the grafted islets (25). IL-1β has been reported to injure islets in patients with type 1 diabetes mellitus (26). Administrations of drugs that inhibit cytokine actions improve islet graft function after transplantation (27). Blocking MCP-1 prevents allograft rejection in islet transplantation in mice (28) and low MCP-1 results a relevant outcome in clinical islet transplantation (29). These findings suggest the reduction of pro-inflammatory cytokine/chemokine production with sirolimus supplementation in culture media might indirectly contribute to improved stimulated insulin secretion and β-cell survival.
Peak portal drug concentration of sirolimus is generally double that of systemic level after oral administration (15, 16). Morphological changes have been reported on islets exposed to super high concentrations of sirolimus (9). Sirolimus could passively have an adverse effect on β-cells. The benefits from the reduction of cytokines/chmokines release could be offset by the direct toxicity to β-cell by sirolimus.
IL-1β and TNF-α are mainly produced by macrophages (30–32). Depletion or inactivation of macrophages prevents rejection or primary non-function of the islet graft by the reduction of cytokine release (33–35). Mercalli et al. have reported that sirolimus induces caspase-independent cell death on monocytes (36). In the present study, the number of macrophages (CD68+ cells) significantly decreased with sirolimus. The data suggest that sirolimus directly reduced the number of macrophages in HIP, leading to the reduction of cytokines/chemokines release. Sirolimus supplementation to culture media could be a potential strategy to control the inflammatory cells contained in HIP before transplantation.
Pancreatic ductal cells are possibly a main source of pro-inflammatory cytokines/chemokines. Sirolimus significantly reduced pro-inflammatory cytokines/chemokines from pancreatic ductal cells. The assessments of ductal cell viability and content demonstrated no differences. Three days culture might be too short to detect the effects of sirolimus on the proliferation of ductal cells. This data suggests sirolimus directly reduced the cytokine/chemokine production from ductal cells.
Sirolimus significantly decreased MCP-1 production in impure HIP but not ductal cells nor pure HIP. Moreover, amount of MCP-1 production in impure HIP was higher when compared to pure HIP (Fig.3 A). Pancreatic acinar cells as well as macrophages are known to produce MCP-1 (37). Therefore, sirolimus might affect MCP-1 production from acinar cells and/or macrophages. Alternatively, it has been published that MCP-1 production was stimulated by IL-1β and/or TNF-α (29, 38, 39). The reduction of MCP-1 might be caused by the reductions of other cytokines such as TNF-α and IL-1β.
Interestingly, MIP-1β production was significantly reduced by sirolimus supplementation in both pure and impure HIP. Macrophage inflammatory protein (MIP)-1 is known to have four members of the MIP-1 CC chemokine subfamily, which termed CCL3 (MIP-1α), CCL4 (MIP-1β), CCL9/10 (MIP-1δ), and CCL15 (MIP-1γ). These chemokines are produced by many cells, particularly macrophages, dendritic cells, and lymphocytes. MIP-1β attracts other proinflammatory cells, which are also crucial in recruiting macrophages themselves to sites of inflammation (40). However, the effects of MIP-1β on islet transplantation have not been well investigated. We recently reported that pancreatic β-cells produce MIP-1β, which are elevated by CD40-CD40L interaction (41). The present study clearly shows that ductal cells also produce MIP-1β, and sirolimus supplementation to culture media significantly reduced the production. The further research regarding cytokines/chemokines produced from each cell subset composing of human islet preparation will be helpful for improving clinical islet transplantation outcomes.
The reduction of cytokine/chemokine production from HIP might contribute to the dramatic improvements in the success rate of 1 year insulin independence of sirolimus-based steroid-free immunosuppressive regimens in the Edmonton protocol (1, 42, 43). However, sirolimus inhibits the proliferation of β-and pancreatic ductal cells, which might lead to loss of long-term islet-graft function by decreasing β-cell regeneration and neogenesis from ductal cells (21, 44). Although the effects of long-term treatment of sirolimus on β- and ductal cells were not assessed in this study, the reduction of cytokine/chemokine by sirolimus may affect islet grafts, especially immediately after transplantation. In current islet transplantation, substantial amounts of non-endocrine cells, including ductal cells, are implanted as part of the islet graft. The benefits of reducing cytokine/chemokine release and depleting macrophages from HIP by short-term use of sirolimus during pre-transplant culture may exceed the negative effects such as inhibition of β-and ductal cell proliferation.
Conclusion
Our in vitro studies suggest sirolimus, at plasma-drug concentrations, is beneficial in several ways to 40–60% pure HIP, which are typically used in clinical trials. First, sirolimus increases glucose stimulated insulin release. Second, sirolimus improves β-cell survival during culture. Third, sirolimus reduces the quantity of macrophages contained in the HIP. Finally, sirolimus reduces cytokine/chemokine production from HIP, especially from ductal cells. The anti-pro-inflammatory effects of sirolimus on HIP might partially contribute to the improved islet transplantation outcomes in the Edmonton protocol. Sirolimus supplementation to the pre-transplant culture media may be of assistance in further developing the strategy to increase islet suitability for transplantation. A clinical study will be proposed to further delineate these findings as to how they translate to the clinic.
Figure 4. Reduction of macrophage content within HIP by sirolimus supplementation.
After dispersion of HIP into single cell suspensions, cells were fixed and stained with anti-CD68 antibody. The content of CD68+ macrophage in pure (dotted lines) or impure (solid lines) HIP was shown in A. The % control of macrophage content (CD68+) in pure (B) or impure (C) HIP were also shown.
Acknowledgements
The authors are grateful to the members of the Human Cell Processing Facility, Preclinical Cell Processing Laboratory of the Cell Transplant Center, Clinical Islet Transplant Program, General Clinical Research Center, Imaging Core at the Diabetes Research Institute, Administrative Offices at the Diabetes Research Institute, and Organ Procurement Organizations for the continuous enthusiasm and support to our program.
This work was supported in part by NIH-NCRR, GCRC MO1RR16587, NIDDK RO1-DK55347-IU42RR016603, 5R01 DK25802, ICR 5U42RR016603, JDRFI 4-200-946 and 4-2004-361, and the Diabetes Research Institute Foundation.
Abbreviations
- 7-AAD
7-aminoactinomycin D
- DAPI
4’, 6-diamidino-2-phenylindole
- HIP
human islet preparations
- IFN-γ
interferon gamma
- IL-1β
interleukin-1 β
- IL-6
interleukin-6
- MCP-1
monocyte chemoattractant-1
- MIP-1β
macrophage inflammatory protein 1β
- MM1
Miami-defined medium 1
- mTOR
mammalian target of rapamycin
- NG
Newport Green PDX acetoxymethylether
- SEM
standard error of the mean
- TMRE
tetramethylrhodamine ethyl ester
- TNF-α
tumor necrosis factor alpha
References
- 1.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Ryan EA, Lakey JR, Rajotte RV, et al. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes. 2001;50(4):710. doi: 10.2337/diabetes.50.4.710. [DOI] [PubMed] [Google Scholar]
- 3.Markmann JF, Deng S, Huang X, et al. Insulin independence following isolated islet transplantation and single islet infusions. Ann Surg. 2003;237(6):741. doi: 10.1097/01.SLA.0000072110.93780.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Froud T, Ricordi C, Baidal DA, et al. Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: Miami experience. Am J Transplant. 2005;5(8):2037. doi: 10.1111/j.1600-6143.2005.00957.x. [DOI] [PubMed] [Google Scholar]
- 5.Abraham RT. Mammalian target of rapamycin: immunosuppressive drugs uncover a novel pathway of cytokine receptor signaling. Curr Opin Immunol. 1998;10(3):330. doi: 10.1016/s0952-7915(98)80172-6. [DOI] [PubMed] [Google Scholar]
- 6.Terada N, Lucas JJ, Szepesi A, Franklin RA, Domenico J, Gelfand EW. Rapamycin blocks cell cycle progression of activated T cells prior to events characteristic of the middle to late G1 phase of the cycle. J Cell Physiol. 1993;154(1):7. doi: 10.1002/jcp.1041540103. [DOI] [PubMed] [Google Scholar]
- 7.Kneteman NM, Lakey JR, Wagner T, Finegood D. The metabolic impact of rapamycin (sirolimus) in chronic canine islet graft recipients. Transplantation. 1996;61(8):1206. doi: 10.1097/00007890-199604270-00015. [DOI] [PubMed] [Google Scholar]
- 8.Fuhrer DK, Kobayashi M, Jiang H. Insulin release and suppression by tacrolimus, rapamycin and cyclosporin A are through regulation of the ATP-sensitive potassium channel. Diabetes Obes Metab. 2001;3(6):393. doi: 10.1046/j.1463-1326.2001.00150.x. [DOI] [PubMed] [Google Scholar]
- 9.Bell E, Cao X, Moibi JA, et al. Rapamycin has a deleterious effect on MIN-6 cells and rat and human islets. Diabetes. 2003;52(11):2731. doi: 10.2337/diabetes.52.11.2731. [DOI] [PubMed] [Google Scholar]
- 10.Hui H, Khoury N, Zhao X, et al. Adenovirus-mediated XIAP gene transfers reverses the negative effects of immunosuppressive drugs on insulin secretion and cell viability of isolated human islets. Diabetes. 2005;54(2):424. doi: 10.2337/diabetes.54.2.424. [DOI] [PubMed] [Google Scholar]
- 11.Zhang N, Su D, Qu S, et al. Sirolimus is associated with reduced islet engraftment and impaired beta-cell function. Diabetes. 2006;55(9):2429. doi: 10.2337/db06-0173. [DOI] [PubMed] [Google Scholar]
- 12.Marcelli-Tourvieille S, Hubert T, Moerman E, et al. In vivo and in vitro effect of sirolimus on insulin secretion. Transplantation. 2007;83(5):532. doi: 10.1097/01.tp.0000255679.81792.dd. [DOI] [PubMed] [Google Scholar]
- 13.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37(4):413. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 14.Ichii H, Sakuma Y, Pileggi A, et al. Shipment of human islets for transplantation. Am J Transplant. 2007;7(4):1010. doi: 10.1111/j.1600-6143.2006.01687.x. [DOI] [PubMed] [Google Scholar]
- 15.Desai NM, Goss JA, Deng S, et al. Elevated portal vein drug levels of sirolimus and tacrolimus in islet transplant recipients: local immunosuppression or islet toxicity? Transplantation. 2003;76(11):1623. doi: 10.1097/01.TP.0000081043.23751.81. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro AM, Gallant HL, Hao EG, et al. The portal immunosuppressive storm: relevance to islet transplantation? Ther Drug Monit. 2005;27(1):35. doi: 10.1097/00007691-200502000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Gmyr V, Belaich S, Muharram G, et al. Rapid purification of human ductal cells from human pancreatic fractions with surface antibody CA19-9. Biochem Biophys Res Commun. 2004;320(1):27. doi: 10.1016/j.bbrc.2004.05.125. [DOI] [PubMed] [Google Scholar]
- 18.Ichii H, Pileggi A, Molano RD, et al. Rescue purification maximizes the use of human islet preparations for transplantation. Am J Transplant. 2005;5(1):21. doi: 10.1111/j.1600-6143.2005.00698.x. [DOI] [PubMed] [Google Scholar]
- 19.Ichii H, Inverardi L, Pileggi A, et al. A novel method for the assessment of cellular composition and beta-cell viability in human islet preparations. Am J Transplant. 2005;5(7):1635. doi: 10.1111/j.1600-6143.2005.00913.x. [DOI] [PubMed] [Google Scholar]
- 20.Ricordi C, Zeng YJ, Alejandro R, et al. In vivo effect of FK506 on human pancreatic islets. Transplantation. 1991;52(3):519. doi: 10.1097/00007890-199109000-00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zahr E, Molano RD, Pileggi A, et al. Rapamycin Impairs In Vivo Proliferation of Islet Beta-Cells. Transplantation. 2007;84(12):1576. doi: 10.1097/01.tp.0000296035.48728.28. [DOI] [PubMed] [Google Scholar]
- 22.Delaney CA, Pavlovic D, Hoorens A, Pipeleers DG, Eizirik DL. Cytokines induce deoxyribonucleic acid strand breaks and apoptosis in human pancreatic islet cells. Endocrinology. 1997;138(6):2610. doi: 10.1210/endo.138.6.5204. [DOI] [PubMed] [Google Scholar]
- 23.Hanley S, Liu S, Lipsett M, et al. Tumor necrosis factor-alpha production by human islets leads to postisolation cell death. Transplantation. 2006;82(6):813. doi: 10.1097/01.tp.0000234787.05789.23. [DOI] [PubMed] [Google Scholar]
- 24.Bottino R, Fernandez LA, Ricordi C, et al. Transplantation of allogeneic islets of Langerhans in the rat liver: effects of macrophage depletion on graft survival and microenvironment activation. Diabetes. 1998;47(3):316. doi: 10.2337/diabetes.47.3.316. [DOI] [PubMed] [Google Scholar]
- 25.Berney T, Molano RD, Cattan P, et al. Endotoxin-mediated delayed islet graft function is associated with increased intra-islet cytokine production and islet cell apoptosis. Transplantation. 2001;71(1):125. doi: 10.1097/00007890-200101150-00020. [DOI] [PubMed] [Google Scholar]
- 26.Arnush M, Heitmeier MR, Scarim AL, Marino MH, Manning PT, Corbett JA. IL-1 produced and released endogenously within human islets inhibits beta cell function. J Clin Invest. 1998;102(3):516. doi: 10.1172/JCI844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tran PO, Gleason CE, Robertson RP. Inhibition of interleukin-1beta-induced COX-2 and EP3 gene expression by sodium salicylate enhances pancreatic islet beta-cell function. Diabetes. 2002;51(6):1772. doi: 10.2337/diabetes.51.6.1772. [DOI] [PubMed] [Google Scholar]
- 28.Lee I, Wang L, Wells AD, et al. Blocking the monocyte chemoattractant protein-1/CCR2 chemokine pathway induces permanent survival of islet allografts through a programmed death-1 ligand-1-dependent mechanism. J Immunol. 2003;171(12):6929. doi: 10.4049/jimmunol.171.12.6929. [DOI] [PubMed] [Google Scholar]
- 29.Piemonti L, Leone BE, Nano R, et al. Human pancreatic islets produce and secrete MCP-1/CCL2: relevance in human islet transplantation. Diabetes. 2002;51(1):55. doi: 10.2337/diabetes.51.1.55. [DOI] [PubMed] [Google Scholar]
- 30.Giri JG, Lomedico PT, Mizel SB. Studies on the synthesis and secretion of interleukin 1. I. A 33,000 molecular weight precursor for interleukin 1. J Immunol. 1985;134(1):343. [PubMed] [Google Scholar]
- 31.March CJ, Mosley B, Larsen A, et al. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985;315(6021):641. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- 32.Urban JL, Rothstein JL, Shephard MH, Schreiber H. Tumor necrosis factor: a potent mediator of macrophage-dependent tumor-cell killing. Haematol Blood Transfus. 1987;31:351. doi: 10.1007/978-3-642-72624-8_73. [DOI] [PubMed] [Google Scholar]
- 33.Juang JH, Hsu BR, Kuo CH. 15-Deoxyspergualin protects the islet graft from macrophage-mediated injury. Transplant Proc. 2002;34(5):1458. doi: 10.1016/s0041-1345(02)02928-7. [DOI] [PubMed] [Google Scholar]
- 34.Kaufman DB, Gores PF, Field MJ, et al. Effect of 15-deoxyspergualin on immediate function and long-term survival of transplanted islets in murine recipients of a marginal islet mass. Diabetes. 1994;43(6):778. doi: 10.2337/diab.43.6.778. [DOI] [PubMed] [Google Scholar]
- 35.Matsuda T, Omori K, Vuong T, et al. Inhibition of p38 pathway suppresses human islet production of pro-inflammatory cytokines and improves islet graft function. Am J Transplant. 2005;5(3):484. doi: 10.1046/j.1600-6143.2004.00716.x. [DOI] [PubMed] [Google Scholar]
- 36.Mercalli A, Sordi V, Ponzoni M, et al. Rapamycin induces a caspase-independent cell death in human monocytes. Am J Transplant. 2006;6(6):1331. doi: 10.1111/j.1600-6143.2006.01332.x. [DOI] [PubMed] [Google Scholar]
- 37.Grady T, Liang P, Ernst SA, Logsdon CD. Chemokine gene expression in rat pancreatic acinar cells is an early event associated with acute pancreatitis. Gastroenterology. 1997;113(6):1966. doi: 10.1016/s0016-5085(97)70017-9. [DOI] [PubMed] [Google Scholar]
- 38.Baker MS, Chen X, Rotramel A, Nelson J, Kaufman DB. Proinflammatory cytokines induce NF-kappaB-dependent/NO-independent chemokine gene expression in MIN6 beta cells. J Surg Res. 2003;110(1):295. doi: 10.1016/s0022-4804(03)00027-1. [DOI] [PubMed] [Google Scholar]
- 39.Sun LK, Reding T, Bain M, Heikenwalder M, Bimmler D, Graf R. Prostaglandin E2 modulates TNF-alpha-induced MCP-1 synthesis in pancreatic acinar cells in a PKA-dependent manner. Am J Physiol Gastrointest Liver Physiol. 2007;293(6):G1196. doi: 10.1152/ajpgi.00330.2007. [DOI] [PubMed] [Google Scholar]
- 40.Maurer M, von Stebut E. Macrophage inflammatory protein-1. Int J Biochem Cell Biol. 2004;36(10):1882. doi: 10.1016/j.biocel.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 41.Barbe-Tuana FM, Klein D, Ichii H, et al. CD40-CD40 ligand interaction activates proinflammatory pathways in pancreatic islets. Diabetes. 2006;55(9):2437. doi: 10.2337/db05-1673. [DOI] [PubMed] [Google Scholar]
- 42.Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54(7):2060. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 43.Hering BJ, Kandaswamy R, Ansite JD, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA. 2005;293(7):830. doi: 10.1001/jama.293.7.830. [DOI] [PubMed] [Google Scholar]
- 44.Bussiere CT, Lakey JR, Shapiro AM, Korbutt GS. The impact of the mTOR inhibitor sirolimus on the proliferation and function of pancreatic islets and ductal cells. Diabetologia. 2006;49(10):2341. doi: 10.1007/s00125-006-0374-5. [DOI] [PubMed] [Google Scholar]